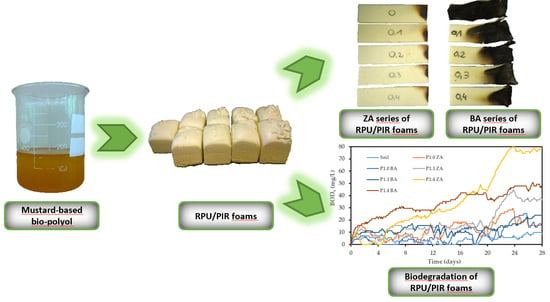
Biodegradable, Flame-Retardant, and Bio-Based Rigid Polyurethane/Polyisocyanurate Foams for Thermal Insulation Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of RPU/PIR Foams
2.3. Characterization of RPU/PIR Foams
2.3.1. Processing Times
2.3.2. Physico-Mechanical Properties
2.3.3. Aging Resistance Properties
2.3.4. Cell Structure
2.3.5. Thermal Insulation Properties
2.3.6. Flammability Tests
2.3.7. Susceptibility to Biodegradation
3. Results and Discussion
3.1. Foaming Process and Reactivity of Bio-Polyol
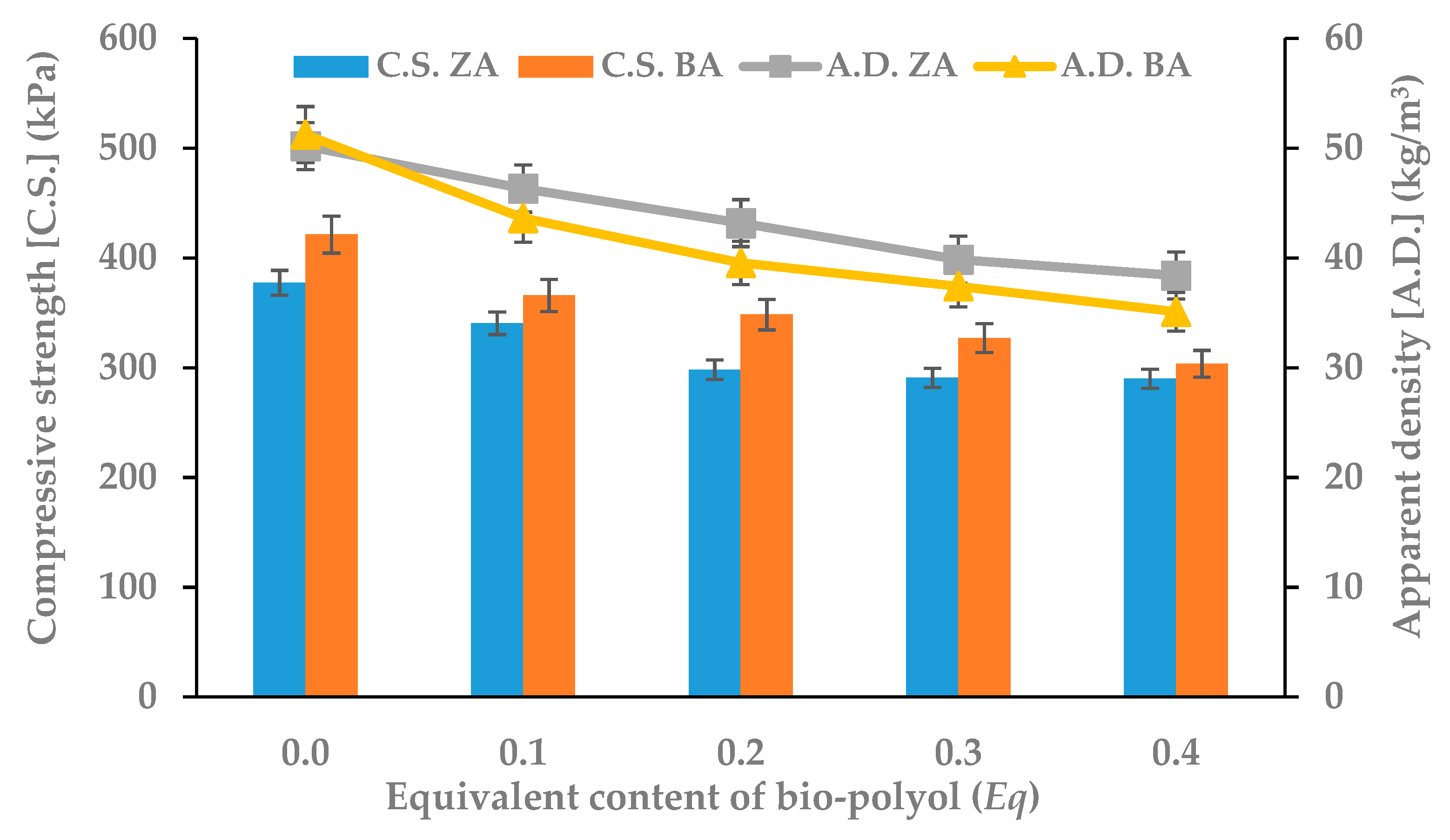
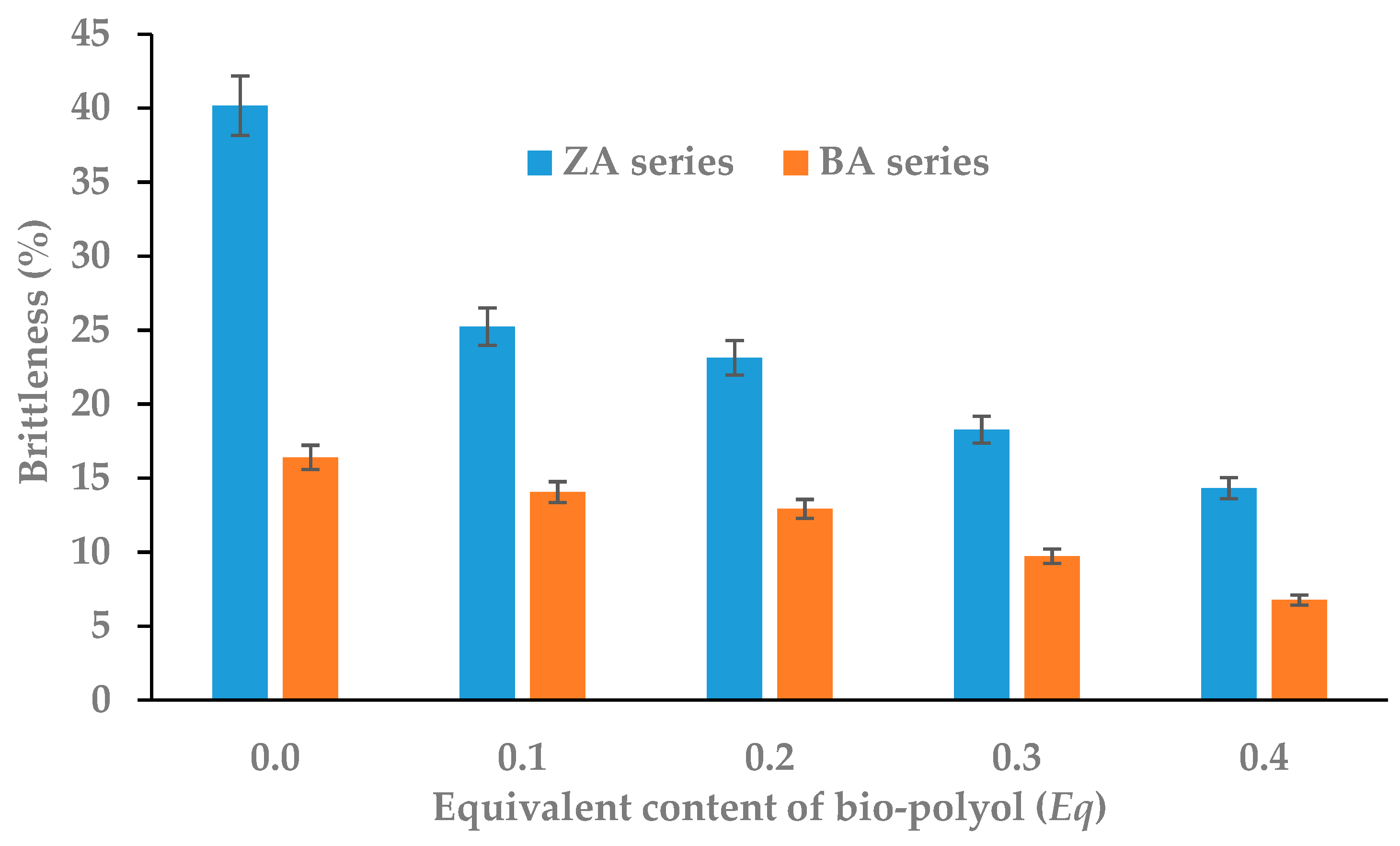
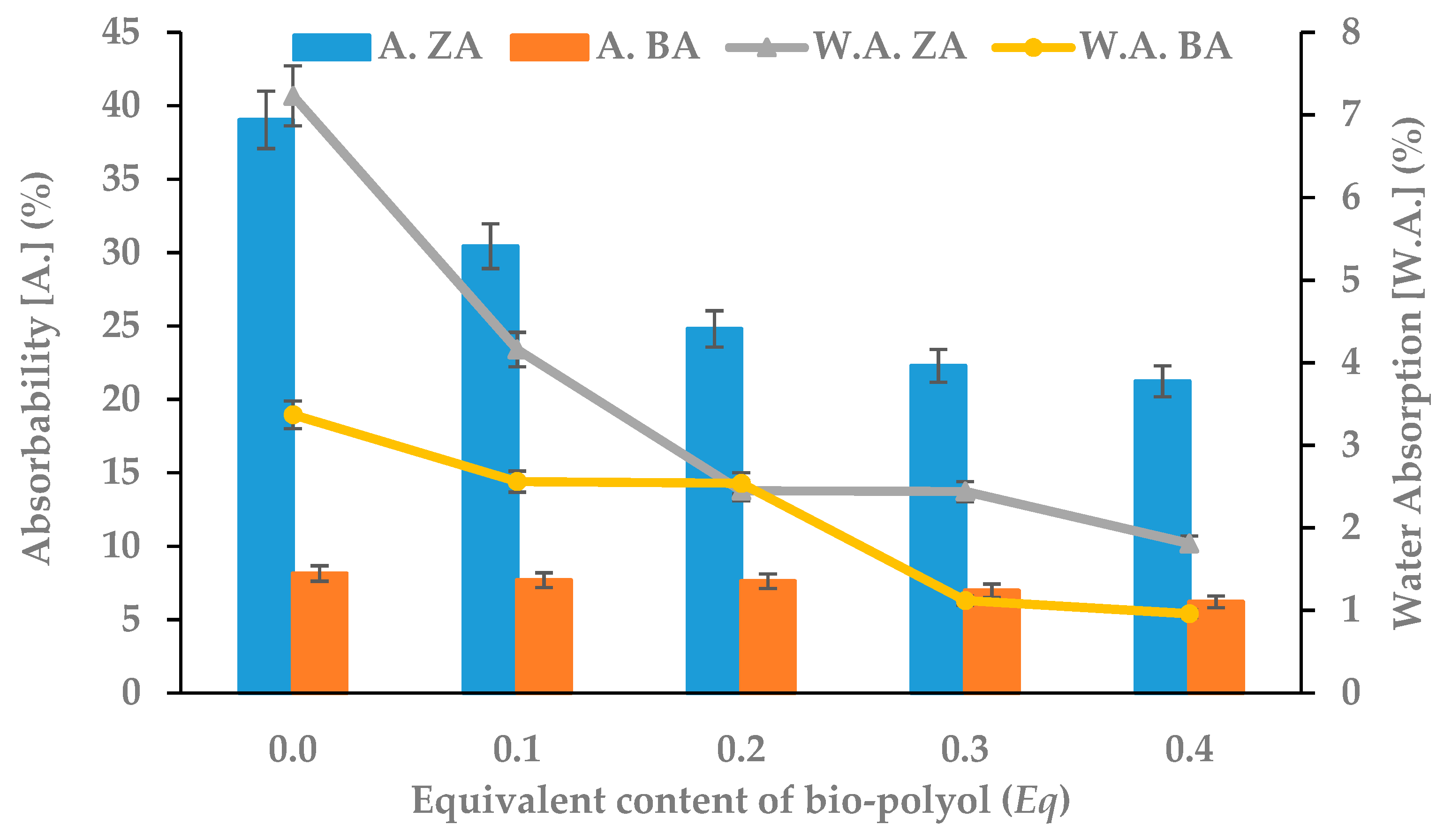
3.2. Physico-Mechanical Properties
3.3. Aging Resistance Properties of RPU/PIR Foams
3.4. Foam Structure
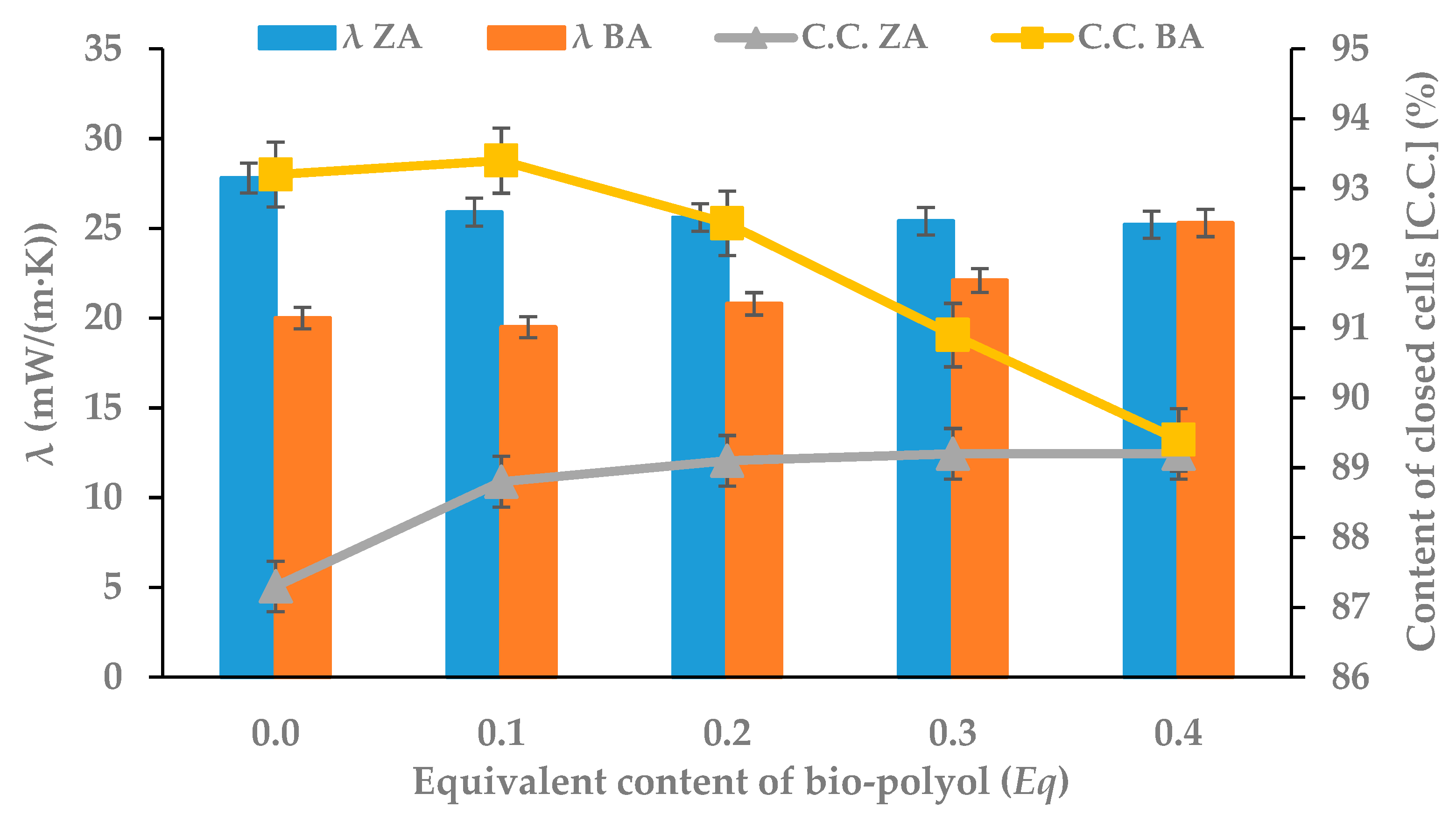
3.5. Thermal Insulation Properties
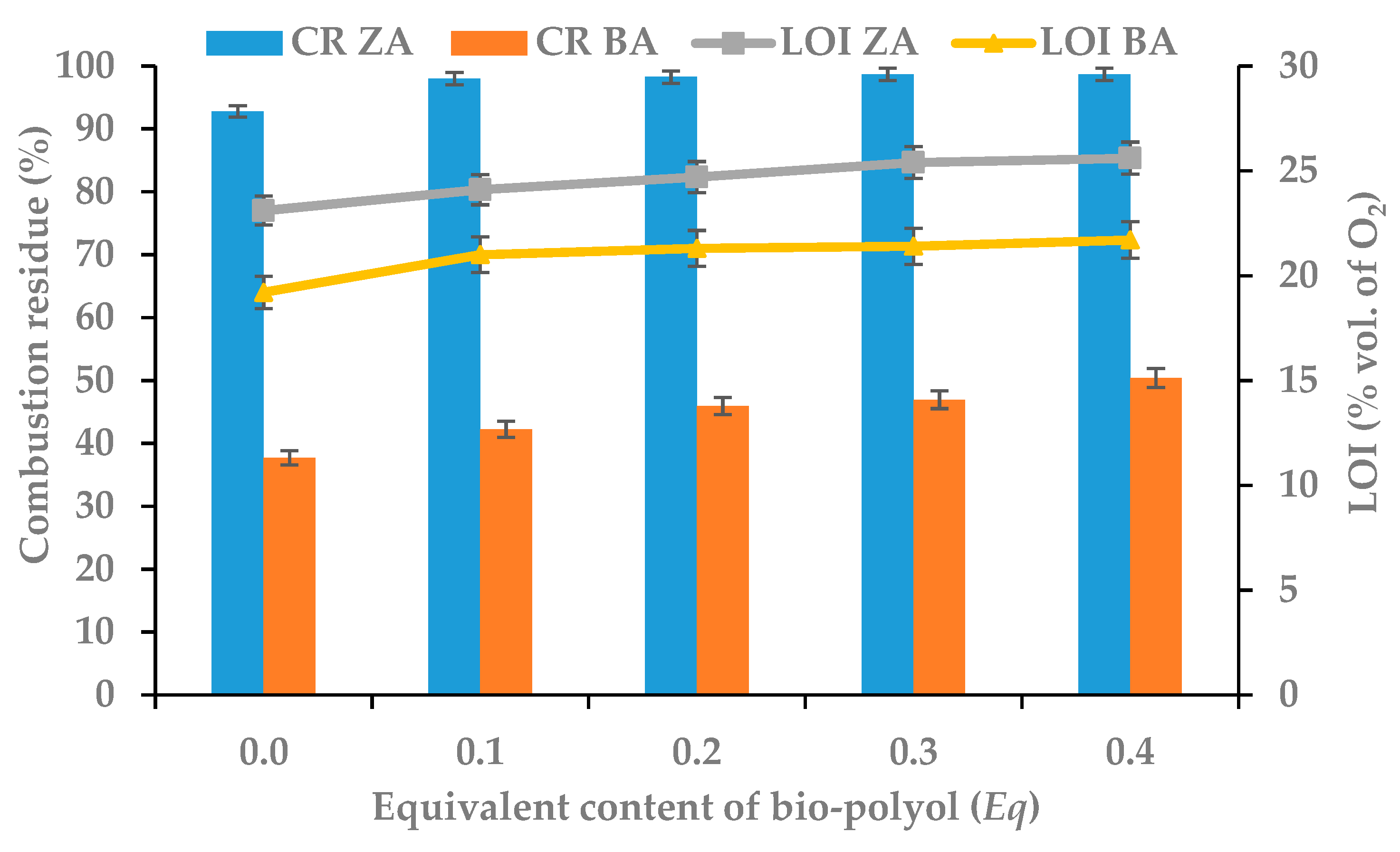
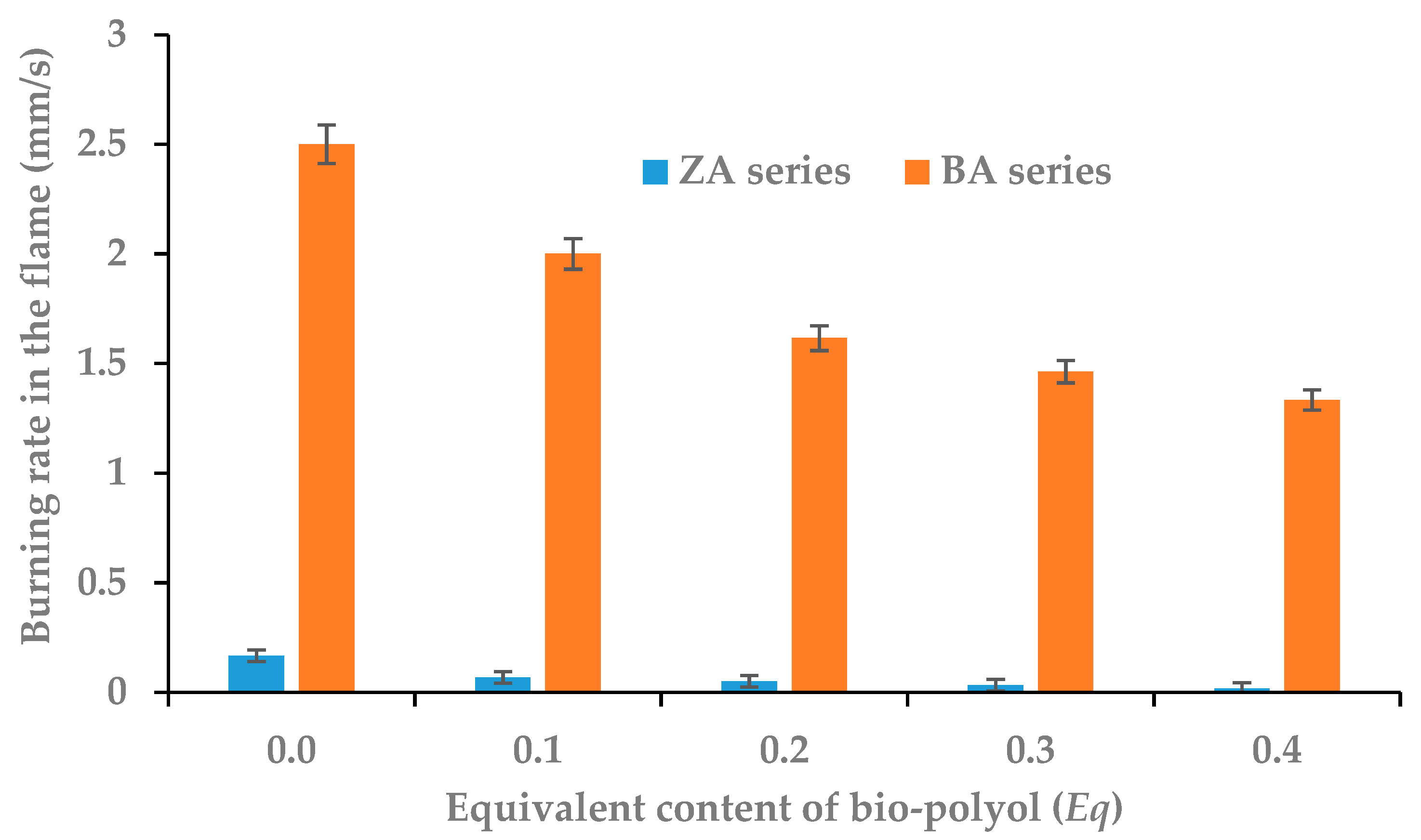
3.6. Flammability of RPU/PIR Foams
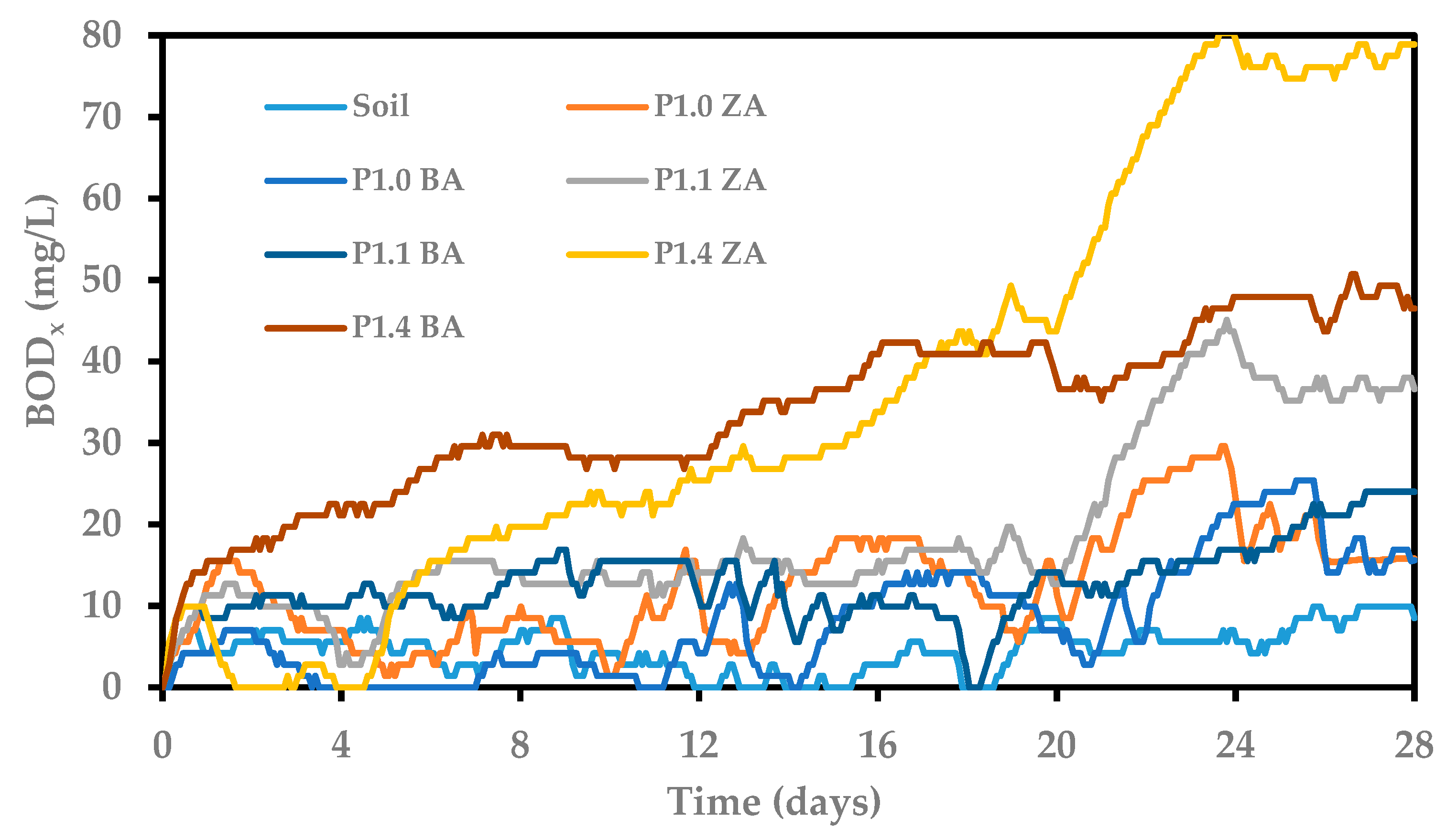
3.7. Susceptibility to Biodegradation
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bukowski, Z. Sustainable Development in the Law System; TNOIK Dom Organiztora: Toruń, Poland, 2009. (In Polish) [Google Scholar]
- Report of the World Commission on Environment and Development: Our Common Future. 1987. Available online: http://www.un-documents.net/our-common-future.pdf (accessed on 19 September 2019).
- Żmihorska-Gotfryd, A. Selected Aspects of Biological Degradation of Polymers; Politechniki Rzeszowskiej: Rzeszów, Poland, 2015. (In Polish) [Google Scholar]
- Dobrzyńska, R. The toxicity of products of thermal decomposition and combustion of polyurethane foams used in manufacturing of upholstered furniture. Saf. Fire Tech. 2012, 4, 53–58. [Google Scholar]
- Prociak, A.; Rokicki, G.; Ryszkowska, J. Polyurethane Materials; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2014. (In Polish) [Google Scholar]
- Septevani, A.A.; Evans, D.A.C.; Chaleat, C.; Martin, D.J.; Annamalai, P.K. A systematic study substituting polyether polyol with palm kernel oil based polyester polyol in rigid polyurethane foam. Ind. Crop. Prod. 2015, 66, 16–26. [Google Scholar] [CrossRef]
- Chian, K.S.; Gan, L.H. Development of a rigid polyurethane foam from palm oil. J. Appl. Polym. Sci. 1998, 68, 509–515. [Google Scholar] [CrossRef]
- Kurańska, M.; Prociak, A. The influence of rapeseed oil-based polyols on the foaming process of rigid polyurethane foams. Ind. Crop. Prod. 2016, 89, 182–187. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Czupryński, B.; Borowicz, M.; Liszkowska, J. New polyurethane materials containing biofiller. Polimery 2015, 9, 586–591. [Google Scholar] [CrossRef]
- Miao, S.; Sun, L.; Wang, P.; Liu, R.; Su, Z.; Zhang, S. Soybean oil-based polyurethane networks as candidate biomaterials: Synthesis and biocompability. Eur. J. Lipid Sci. Technol. 2012, 114, 1165–1174. [Google Scholar] [CrossRef]
- Miao, S.; Zhang, S.; Su, Z.; Wang, P. Synthesis of bio-based polyurethanes from epoxidized soybean oil and isopropanolamine. J. Appl. Polym. Sci. 2013, 10, 1929–1936. [Google Scholar] [CrossRef]
- Miao, S.; Callow, N.; Wang, P.; Liu, Y.; Su, Z.; Zhang, S. Soybean oil-based polyurethane networks: Shape-memory effects and Surface morphologies. J. Am. Oil Chem. Soc. 2013, 90, 1415–1421. [Google Scholar] [CrossRef]
- Veronese, V.B.; Menger, R.K.; de, C.; Forte, M.M.; Petzhold, C.L. Rigid polyurethane foam based on vegetable oil. J. Appl. Polym. Sci. 2011, 120, 530–537. [Google Scholar] [CrossRef]
- Dhaliwal, G.S.; Anandan, S.; Chandrashekhara, K.; Lees, J.; Nam, P. Development and characterization of polyurethane foams with substitution of polyether polyol with soy-based polyol. Eur. Polym. J. 2018, 107, 105–117. [Google Scholar] [CrossRef]
- Dhaliwal, G.S.; Anandan, S.; Chandrashekhara, K.; Dudenhoeffer, N.; Nam, P. Fabrication and Testing of Soy-Based Polyurethane Foam for Insulation and Structural Applications. J. Polym. Environ. 2019, 27, 1897–1907. [Google Scholar] [CrossRef]
- Prociak, A.; Kurańska, M.; Cabulis, U.; Kirpluks, M. Rapeseed oil as main component in synthesis of bio-polyurethane-polyisocyanurate porous materials modified with carbon fibers. Polym. Test. 2017, 59, 478–486. [Google Scholar] [CrossRef]
- Malewska, E.; Prociak, A. The effect of nanosilica filler on the foaming process and properties of flexible polyurethane foams obtained with rapeseed oil-based polyol. Polimery 2015, 60, 472–479. [Google Scholar] [CrossRef]
- Malewska, E.; Bąk, S.; Kurańska, M.; Prociak, A. The effect of various rapeseed oil-based polyols on selected properties of flexible polyurethane foams. Polimery 2016, 61, 799–806. [Google Scholar] [CrossRef]
- Badri, K.H.; Redhwan, A.M. Effect of Phosphite Loading on the Mechanical and Fire Properties of Palm-Based Polyurethane. Sains Malays. 2010, 39, 769–774. [Google Scholar]
- Badri, K.H. Biobased polyurethane from palm kernel oil-based polyol. In Polyurethane; Zafar, F., Sharmin, E., Eds.; InTechOpen: Rijeka, Croatia, 2012; pp. 447–470. [Google Scholar] [CrossRef]
- Su’ait, M.S.; Ahmad, A.; Badri, K.H.; Mohamed, N.S.; Rahman, M.Y.A.; Ananza Ricardo, C.L.; Scardi, P. The potential of polyurethane bio-based solid polymer electrolyte for photoelectrochemical cell application. Int. J. Hydrog. Energy 2014, 39, 3005–3017. [Google Scholar] [CrossRef]
- Adnan, S.; Tuan Noor, M.T.I.; Ain, N.H.; Devi, K.P.P.; Mohd, N.S.; Kian, Y.S.; Idris, Z.B.; Campara, I.; Schiffman, C.M.; Pietrzyk, K.; et al. Impact of the hard-segment concentration on highly resilient polyurethane foams based on palm olein polyol. J. Appl. Polym. Sci. 2017, 134, 45440. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Borowicz, M.; Czupryński, B.; Liszkowska, J. The Method of Obtaining Polyol Raw Material for the Synthesis of Rigid Polyurethane-Polyisocyanurate Foams and the Method of Synthesis of Rigid Polyurethane-Polyisocyanurate Foams. Poland Patent Application No. 233221, 17 September 2017. (In Polish). [Google Scholar]
- Paciorek-Sadowska, J.; Borowicz, M.; Czupryński, B.; Liszkowska, J. New bio-polyol based on white mustard seed (Sinapis alba) as an alternative raw material for the polyurethane industry. Polimery 2018, 63, 38–43. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Borowicz, M.; Czupryński, B.; Isbrandt, M. Effect of evening primrose oil-based polyol on the properties of rigid polyurethane–polyisocyanurate foams for thermal insulation. Polymers 2018, 10, 1334. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Borowicz, M.; Czupryński, B.; Tomaszewska, E.; Liszkowska, J. New bio-polyol based on white mustard seed oil for rigid PUR-PIR foams. Pol. J. Chem. Tech. 2018, 20, 24–31. [Google Scholar] [CrossRef]
- ASTM International. Standard Practice for Polyurethane Raw Materials: Polyurethane Foam Cup Test; ASTM Standard D7487—13e1, 2008; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- ISO Standard, no. 17556:2012. Plastics-Determination of the Ultimate Aerobic Biodegradability of Plastic Materials in Soil by Measuring the Oxygen Demand in a Respirometer or the Amount of Carbon Dioxide Evolved; International Organization for Standardization: Geneva, Switzerland, 2012. [Google Scholar]
- Li, Y.; Luo, X.; Hu, S. (Eds.) Polyols and Polyurethanes from Vegetable Oils and Their Derivatives. In Bio-Based Polyols and Polyurethanes; Springer: New York, NY, USA, 2015; Volume 1, Chapter 2; pp. 15–43. [Google Scholar]
- Beltran, A.A.; Boyaca, L.A. Production of rigid polyurethane foams from soy-based polyols. Lat. Am. Appl. Res. 2011, 41, 75–80. [Google Scholar]
- Prociak, A.; Kurańska, M.; Malewska, E. Porous polyurethane plastics synthetized using bio-polyols from renewable raw materials. Polimery 2017, 62, 353–363. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Borowicz, M.; Czupryński, B.; Liszkowska, J. Composites of rigid polyurethane-polyisocyanurate foams with oak bark. Polimery 2017, 62, 666–672. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Borowicz, M.; Czupryński, B.; Liszkowska, J.; Tomaszewska, E. Application of halloysite as filler in the production of rigid PUR-PIR foams. Polimery 2018, 63, 185–190. [Google Scholar] [CrossRef]
- Kurańska, M.; Prociak, A. Bio-Based Polyurethane Foams for Heat-Insulating Applications. In Nano and Biotech Based Materials for Energy Building Efficiency; Pacheco, T., Buratti, F.C., Kalaiselvam, S., Granqvist, C., Ivanov, V., Eds.; Springer Nature Switzerland AG: Basel, Switzerland, 2016; Volume 1, pp. 357–374. [Google Scholar]
- Chuang, W.; Su, Y.; Huang, Y.; Sheen, Y.; Chiang, C. Bio-polyol Composition and Bio-Polyurethane Foam. U.S. Patent Application No. 20170158802, 8 June 2017. [Google Scholar]
- Lorusso, C.; Vergaro, V.; Conciauro, F.; Ciccarella, G.; Congedo, P.M. Thermal and mechanical performance of rigid polyurethane foam added with commercial nanoparticles. Nanomater. Nanotechnol. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- PIANEX Co. Catalogue of Products. Parameters of PUR-PIR Foams. Available online: http://www.pianex.pl/pur-pir-parametry,48.html (accessed on 19 September 2019).
- Stirna, U.; Sevastyanova, I.; Misane, M.; Cabulis, U.; Beverte, I. Structure and properties of polyurethane foams obtained from rapeseed oil polyols. Proc. Estonian Acad. Sci. Chem. 2006, 55, 101–110. [Google Scholar]
- Murayama, S.; Fukuda, K.; Kimura, T.; Sasahara, T. Water-blown Polyurethane Rigid Foam Modified with Maleate. J. Cell. Plast. 2005, 41, 373–387. [Google Scholar] [CrossRef]
- Sripathy, M.; Sharma, K.V. Flammability and Moisture absorption test of rigid polyurethane foam. Int. J. Sci. Eng. Res. 2013, 2, 1–8. [Google Scholar]
- Paciorek-Sadowska, J. Synthesis of rigid polyurethane-polyisocyanurate foams. In Studies on Influence of Derivatives of N,N′-di(methyleneoxy-hydoxyalkyl)urea and Boric Acid on Properties of Rigid Polyurethane-Polyisocyanurate Foams; WU Uniwersytetu Kazimierza Wielkiego: Bydgoszcz, Poland, 2011; Volume 1, pp. 93–124. (In Polish) [Google Scholar]
- Paciorek-Sadowska, J.; Borowicz, M.; Isbrandt, M. New Poly(lactide-urethane-isocyanurate) Foams Based on Bio-Polylactide Waste. Polymers 2019, 11, 481. [Google Scholar] [CrossRef]
- Radziszewska-Zielina, E. Comparative analysis of parameters of thermal insulation materials using as an external insulation of wall. Przegląd Budowlany 2009, 4, 32–37. (In Polish) [Google Scholar]
- Yarahmadi, N.; Vega, A.; Jakubowicz, I. Accelerated ageing and degradation characteristics of rigid polyurethane foam. J. Polym. Degrad. Stab. 2017, 138, 192–200. [Google Scholar] [CrossRef]
- Xie, J.; Qi, J.; Hse, C.; Shupe, T.F. Effect of lignin derivatives in the bio-polyols from microwave liquefied bamboo on the properties of polyurethane foams. BioResources 2014, 9, 578–588. [Google Scholar] [CrossRef]
- Zhang, C.; Kessler, M.R. Bio-based Polyurethane Foam Made from Compatible Blends of Vegetable-Oil-based Polyol and Petroleum-based Polyol. ACS Sustain. Chem. Eng. 2015, 3, 743–749. [Google Scholar] [CrossRef]
- Hejna, A.; Kirpluks, M.; Kosmela, P.; Cabulis, U.; Haponiuk, J.; Piszczyk, Ł. The influence of crude glycerol and castor oil-based polyol on the structure and performance of rigid polyurethane-polyisocyanurate foams. Ind. Crop. Prod. 2017, 95, 113–125. [Google Scholar] [CrossRef]
- Kang, J.W.; Kim, J.M.; Kim, M.S.; Kim, Y.H.; Kim, W.N.; Jang, W.; Shin, D.S. Effects of nucleating agents on the morphological, mechanical and thermal insulating properties of rigid polyurethane foams. Macromol. Res. 2009, 17, 856–862. [Google Scholar] [CrossRef]
- Dolomanova, V.; Jens, C.M.R.; Jensen, L.R.; Pyrz, R.; Timmons, A.B. Mechanical properties and morphology of nano-reinforced rigid PU foam. J. Cell. Plast. 2011, 47, 81–93. [Google Scholar] [CrossRef]
- Niyogi, D.; Kumar, R.; Gandhi, K.S. Water blown free rise polyurethane foams. Polym. Eng. Sci. 1999, 39, 199–209. [Google Scholar] [CrossRef]
- Zhang, H.; Fang, W.; Li, Y.; Tao, W. Experimental study of the thermal conductivity of polyurethane foams. J. Appl. Therm. Eng. 2016, 115, 528–538. [Google Scholar] [CrossRef]
- Kuhn, J.; Ebert, H.P.; Arduini-Shuster, M.C.; Buttner, D.; Fricke, J. Thermal transport in polystyrene and polyurethane foam insulations. Int. J. Heat Mass Transf. 1992, 35, 1795–1801. [Google Scholar] [CrossRef]
- Kurańska, M.; Cabulis, U.; Auguścik, M.; Prociak, A.; Ryszkowska, J.; Kirpluks, M. Bio-based polyurethane-polyisocyanurate composites with an intumescent flame retardant. Polym. Deg. Stab. 2016, 127, 11–19. [Google Scholar] [CrossRef]
- Modesti, M.; Lorenzetti, A.; Simioni, F.; Checchin, M. Influence of different flame retardants on fire behaviour of modified PIR/PUR polymers. Polym. Deg. Stab. 2001, 74, 475–479. [Google Scholar] [CrossRef]
- Sankowska, M.; Gajek, A.; Celiński, M. Analysis of toxic products of thermal decomposition and combustion of substrates used in the synthesis of polyurethanes. Przem. Chem. 2016, 95, 1237–1242. [Google Scholar] [CrossRef]
- Chen, M.J.; Wang, X.; Tao, M.C.; Liu, X.Y.; Liu, Z.G.; Zhang, Y.; Zhao, C.S.; Wang, J.S. Full substitution of petroleum-based polyols by phosphorus-containing soy-based polyols for fabricating highly flame-retardant polyisocyanurate foams. Polym. Deg. Stab. 2018, 154, 312–322. [Google Scholar] [CrossRef]
- Janowska, G.; Rybiński, P.; Krauze, S. The effect of the curing agent type on the flammability of butadiene-acrylonitrile rubbers. Polimery 2006, 51, 735–741. [Google Scholar] [CrossRef]
- Rasmussen, C.L.; Glarborg, P.; Marshall, P. Mechanisms of radical removal by SO2. Peoc. Combust. Inst. 2007, 31, 339–347. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J. Polyol containing boron atoms as a compound which reduces flammability of rigid polyurethane-polyisocyanurate foam. In Aspects of Polyurethanes; Yilmaz, F., Ed.; InTech: Rijeka, Croatia, 2017; pp. 111–130. [Google Scholar] [CrossRef][Green Version]
- Chmiel, E.; Lubczak, J. Synthesis of oligoetherols from mixtures of melamine and boric acid and polyurethane foams formed from these oligoetherols. Polym. Bull. 2019, 76, 2253–2275. [Google Scholar] [CrossRef]
- Chmiel, E.; Oliwa, R.; Lubczak, J. Boron-containing non-flammable polyurethane foams. Polym. Plast. Technol. Eng. 2019, 58, 394–404. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Borowicz, M.; Isbrandt, M.; Czupryński, B.; Apiecionek, Ł. The Use of Waste from the Production of Rapeseed Oil for Obtaining of New Polyurethane Composites. Polymers 2019, 11, 1431. [Google Scholar] [CrossRef]
- Bhoyate, S.; Ionescu, M.; Kahol, P.K.; Gupta, R.K. Sustainable flame-retardant polyurethanes using renewable resources. Ind. Crop. Prod. 2018, 123, 480–488. [Google Scholar] [CrossRef]
- Bhoyate, S.; Ionescu, M.; Kahol, P.K.; Chen, J.; Mishra, S.R.; Gupta, R.K. Highly flame-retardant polyurethane foam based on reactive phosphorus polyol and limonene-based polyol. J. Appl. Polym. Sci. 2018, 135, 46224. [Google Scholar] [CrossRef]
- Czupryński, B. Issues From Chemistry and Technology of Polyurethanes; Wydawnictwo Akademii Bydgoskiej: Bydgoszcz, Poland, 2004. [Google Scholar]
- Paciorek-Sadowska, J. Chemical recycling of rigid PUR-PIR foams with boronitrile flame retardant. Inż. Ap. Chem. 2010, 49, 93–94. (In Polish) [Google Scholar]
- Das, N.; Chandran, P. Microbial Degradation of Petroleum Hydrocarbon Contaminants: An Overview. Biotechnol. Res. Int. 2011, 2011, 941810. [Google Scholar] [CrossRef] [PubMed]
- Klotz, M.G.; Bryant, D.A.; Hanson, T.E. The microbial sulfur cycle. Front. Microbiol. 2011, 2, 241. [Google Scholar] [CrossRef] [PubMed]
- Borowicz, M. Synthesis and Application of New Bio-Polyols Aased on Vegetable Raw Materials for the Production of Bio-Composites in the Form of Rigid Polyurethane-Polyisocyanurate Foams. Ph.D. Thesis, West Pomeranian University of Technology, Szczecin, Poland, 2019. [Google Scholar]




| Foam Symbol | Rokopol RF-551 (Eq) (g) | PG1 (Eq) (g) | Tegostab 8460 (g) | 33% DABCO (g) | 33% Potassium Acetate (g) | Antiblaze TCMP (g) | Solkane HFC 365/227 (g) | Purocyn B (Eq) (g) |
|---|---|---|---|---|---|---|---|---|
| P1.0 ZA | 1.0 | 0.0 | 4.59 | 2.70 | 6.75 | 45.90 | 32.40 | 3.0 |
| 66.80 | 0.00 | 203.2 | ||||||
| P1.1 ZA | 0.9 | 0.1 | 4.60 | 2.71 | 6.77 | 46.04 | 32.75 | 3.0 |
| 60.12 | 9.59 | 203.2 | ||||||
| P1.2 ZA | 0.8 | 0.2 | 4.62 | 2.71 | 6.79 | 46.19 | 33.10 | 3.0 |
| 53.44 | 19.18 | 203.2 | ||||||
| P1.3 ZA | 0.7 | 0.3 | 4.63 | 2.72 | 6.81 | 46.34 | 33.45 | 3.0 |
| 46.76 | 28.78 | 203.2 | ||||||
| P1.4 ZA | 0.6 | 0.4 | 4.64 | 2.73 | 6.84 | 46.48 | 33.80 | 3.0 |
| 40.08 | 38.37 | 203.2 | ||||||
| P1.0 BA | 1.0 | 0.0 | 4.59 | 2.70 | 6.75 | 0.00 | 32.40 | 3.0 |
| 66.80 | 0.00 | 203.2 | ||||||
| P1.1 BA | 0.9 | 0.1 | 4.60 | 2.71 | 6.77 | 0.00 | 32.75 | 3.0 |
| 60.12 | 9.59 | 203.2 | ||||||
| P1.2 BA | 0.8 | 0.2 | 4.62 | 2.71 | 6.79 | 0.00 | 33.10 | 3.0 |
| 53.44 | 19.18 | 203.2 | ||||||
| P1.3 BA | 0.7 | 0.3 | 4.63 | 2.72 | 6.81 | 0.00 | 33.45 | 3.0 |
| 46.76 | 28.78 | 203.2 | ||||||
| P1.4 BA | 0.6 | 0.4 | 4.64 | 2.73 | 6.84 | 0.00 | 33.80 | 3.0 |
| 40.08 | 38.37 | 203.2 |
| Foam Symbol | Cream Time (s) | String Gel Time (s) | Tack-Free Time (s) | Free Rise Time (s) |
|---|---|---|---|---|
| P1.0 ZA | 6 | 21 | 24 | 34 |
| P1.1 ZA | 7 | 22 | 25 | 37 |
| P1.2 ZA | 7 | 23 | 27 | 44 |
| P1.3 ZA | 9 | 24 | 29 | 46 |
| P1.4 ZA | 9 | 31 | 35 | 47 |
| P1.0 BA | 9 | 20 | 23 | 29 |
| P1.1 BA | 9 | 20 | 23 | 29 |
| P1.2 BA | 9 | 20 | 23 | 31 |
| P1.3 BA | 9 | 20 | 23 | 33 |
| P1.4 BA | 9 | 20 | 23 | 34 |
| Parameter | Apparent Density (kg/m3) | Compressive Strength (kPa) | Water Absorption after 24 h (%) |
|---|---|---|---|
| G40 * | 36.0 - 42.0 | > 250.00 | < 3.00 |
| P1.4 ZA | 38.4 ± 1.1 | 289.95 ± 7.46 | 1.81 ± 0.06 |
| P1.4 BA | 35.9 ± 1.3 | 303.67 ± 5.32 | 0.96 ± 0.03 |
| Foam Symbol | Cell Size (μm) | Thickness of Cell Wall (μm) | Content of Cells per area unit (cells/mm2) |
|---|---|---|---|
| P1.0 ZA | 248 ± 26 | 18 ± 2 | 15 ± 3 |
| P1.1 ZA | 263 ± 31 | 22 ± 2 | 13 ± 3 |
| P1.4 ZA | 296 ± 41 | 27 ± 3 | 11 ± 4 |
| P1.0 BA | 273 ± 34 | 19 ± 2 | 13 ± 2 |
| P1.1 BA | 294 ± 29 | 22 ± 3 | 12 ± 4 |
| P1.4 BA | 317 ± 43 | 27 ± 2 | 11 ± 4 |
| Foam Symbol | TTI (s) | TTF (s) | HRR (kW/m2) | THR (MJ/m2) |
|---|---|---|---|---|
| P1.0 ZA | 10 ± 1 | 128 ± 2 | 78.9 ± 0.8 | 16.9 ± 0.5 |
| P1.4 ZA | 21 ± 1 | 69 ± 2 | 114.8 ± 1.3 | 9.7 ± 0.3 |
| P1.0 BA | 6 ± 1 | 175 ± 3 | 69.4 ± 0.7 | 23.1 ± 0.9 |
| P1.4 BA | 12 ± 1 | 125 ± 1 | 86.7 ± 0.7 | 15.6 ± 0.3 |
| Element | C | H | O | Si | N | S | P | Cl | K | F |
|---|---|---|---|---|---|---|---|---|---|---|
| PG1.0 ZA | 0.599 | 0.057 | 0.168 | 0.005 | 0.059 | 0.000 | 0.012 | 0.041 | 0.002 | 0.058 |
| PG1.1 ZA | 0.6 | 0.059 | 0.164 | 0.005 | 0.058 | 0.002 | 0.012 | 0.041 | 0.002 | 0.058 |
| PG1.4 ZA | 0.603 | 0.063 | 0.153 | 0.005 | 0.056 | 0.007 | 0.012 | 0.041 | 0.002 | 0.058 |
| PG1.0 BA | 0.638 | 0.057 | 0.164 | 0.005 | 0.067 | 0.000 | 0.000 | 0.000 | 0.003 | 0.066 |
| PG1.1 BA | 0.639 | 0.059 | 0.159 | 0.005 | 0.066 | 0.002 | 0.000 | 0.000 | 0.003 | 0.066 |
| PG1.4 BA | 0.643 | 0.065 | 0.146 | 0.005 | 0.064 | 0.008 | 0.000 | 0.000 | 0.003 | 0.066 |
| Foam symbol | PG1.0 ZA | PG1.1 ZA | PG1.4 ZA | PG1.0 BA | PG1.1 BA | PG1.4 BA |
|---|---|---|---|---|---|---|
| BOD28 (mg/L) | 7.0 | 28.1 | 70.4 | 7.1 | 15.5 | 38.0 |
| TOD (mg/L) | 87.2 | 86.7 | 95.0 | 90.3 | 94.6 | 97.5 |
| Dt (%) | 8.0 | 32.4 | 74.1 | 7.9 | 16.4 | 39.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borowicz, M.; Paciorek-Sadowska, J.; Lubczak, J.; Czupryński, B. Biodegradable, Flame-Retardant, and Bio-Based Rigid Polyurethane/Polyisocyanurate Foams for Thermal Insulation Application. Polymers 2019, 11, 1816. https://doi.org/10.3390/polym11111816
Borowicz M, Paciorek-Sadowska J, Lubczak J, Czupryński B. Biodegradable, Flame-Retardant, and Bio-Based Rigid Polyurethane/Polyisocyanurate Foams for Thermal Insulation Application. Polymers. 2019; 11(11):1816. https://doi.org/10.3390/polym11111816
Chicago/Turabian StyleBorowicz, Marcin, Joanna Paciorek-Sadowska, Jacek Lubczak, and Bogusław Czupryński. 2019. "Biodegradable, Flame-Retardant, and Bio-Based Rigid Polyurethane/Polyisocyanurate Foams for Thermal Insulation Application" Polymers 11, no. 11: 1816. https://doi.org/10.3390/polym11111816
APA StyleBorowicz, M., Paciorek-Sadowska, J., Lubczak, J., & Czupryński, B. (2019). Biodegradable, Flame-Retardant, and Bio-Based Rigid Polyurethane/Polyisocyanurate Foams for Thermal Insulation Application. Polymers, 11(11), 1816. https://doi.org/10.3390/polym11111816