A Review on Styrene Substitutes in Thermosets and Their Composites
Abstract
:1. Introduction
2. Traditionally Crosslinkable Monomers
2.1. Vinyl Monomers
2.1.1. Divinylbenzene
2.1.2. Other Vinyl Monomers
2.2. (Methyl)acrylate Monomers
2.3. Other Monomers
3. Novel Monomers Synthesized from Renewable Materials
3.1. Grafting Acrylate
3.2. Grafting Methacrylate
3.3. Others
4. Properties of Styrene-Free Thermosets and Their Composites
4.1. Processibility of Thermosets
4.2. Properties of Thermosets and Their Composites
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Li, K.; Qiu, R.; Liu, W. Improvement of interfacial adhesion in natural plant fiber-reinforced unsaturated polyester composites: A critical review. Rev. Adhes. Adhes. 2015, 3, 98–120. [Google Scholar] [CrossRef]
- Xie, T.; Liu, W.; Chen, T.; Qiu, R. Mechanical and thermal properties of hemp fiber-unsaturated polyester composites toughened by butyl methacrylate. BioResources 2015, 108, 2744–2754. [Google Scholar] [CrossRef]
- Can, E.; La Scala, J.J.; Sands, J.M.; Palmese, G.R. The synthesis of 9–10 dibromo stearic acid glycidyl methacrylate and its use in vinyl ester resins. J. Appl. Polym. Sci. 2007, 106, 3833–3842. [Google Scholar] [CrossRef]
- Mao, M.; Zhou, K.; Xiong, J. Study on the cure kinetics and technology of epoxy vinyl ester resin. J. Zhejiang Sci-Tech Univ. 2008, 25, 392–395. [Google Scholar]
- Andjelkovic, D.D.; Valverde, M.; Henna, P.; Li, F.; Larock, R.C. Novel thermosets prepared by cationic copolymerization of various vegetable oils–synthesis and their structure–property relationships. Polymer 2005, 46, 9674–9685. [Google Scholar] [CrossRef]
- Sousa, A.F.; Ferreira, S.; Lopez, A.; Borges, L.; Pinto, R.J.B.; Silvestre, A.J.D.; Freire, C.S.R. Thermosetting AESO-bacterial cellulose nanocomposite foams with tailored mechanical properties obtained by pickering emulsion templating. Polymer 2017, 118, 127–134. [Google Scholar] [CrossRef]
- Ansari, F.; Skrifvars, M.; Berglund, L. Nanostructured biocomposites based on unsaturated polyester resin and a cellulose nanofiber network. Compos. Sci. Technol. 2015, 117, 298–306. [Google Scholar] [CrossRef] [Green Version]
- Rosu, L.; Cascaval, C.N.; Rosu, D. Effect of UV radiation on some polymeric networks based on vinyl ester resin and modified lignin. Polym. Test. 2009, 28, 296–300. [Google Scholar] [CrossRef]
- Scala, J.J.L.; Orlicki, J.A.; Winston, C.; Robinette, E.J.; Sands, J.M.; Palmese, G.R. The use of bimodal blends of vinyl ester monomers to improve resin processing and toughen polymer properties. Polymer 2005, 46, 2908–2921. [Google Scholar] [CrossRef]
- Ahn, B.K.; Kraft, S.; Wang, D.; Sun, X.S. Thermally stable, transparent, pressure-sensitive adhesives from epoxidized and dihydroxyl soybean oil. Biomacromolecules 2011, 12, 1839–1843. [Google Scholar] [CrossRef]
- Yang, X.; Li, S.; Xia, J.; Song, J.; Huang, K.; Li, M. Renewable myrcene-based UV-curable monomer and its copolymers with acrylated epoxidized soybean oil: Design, preparation, and characterization. BioResources 2015, 10, 2130–2142. [Google Scholar] [CrossRef]
- Compston, P.; Schiemer, J.; Cvetanovska, A. Mechanical properties and styrene emission levels of a UV-cured glass-fibre/vinylester composite. Compos. Struct. 2008, 86, 22–26. [Google Scholar] [CrossRef]
- Maurin, R.; Perrot, Y.; Bourmaud, A.; Davies, P.; Baley, C. Seawater ageing of low styrene emission resins for marine composites: Mechanical behaviour and nano-indentation studies. Compos. Part A Appl. Sci. Manuf. 2009, 40, 1024–1032. [Google Scholar] [CrossRef] [Green Version]
- Can, E.; Wool, R.P.; Kusefoglu, S. Soybean and castor oil based monomers: Synthesis and copolymerization with styrene. J. Appl. Polym. Sci. 2006, 102, 2433–2447. [Google Scholar] [CrossRef]
- Campanella, A.; Scala, J.J.L.; Wool, R.P. Fatty acid-based comonomers as styrene replacements in soybean and castor oil-based thermosetting polymers. J. Appl. Polym. Sci. 2010, 119, 1000–1010. [Google Scholar] [CrossRef]
- Gu, J.; Lu, J.M.; Huang, X.C.; Wang, X.L.; Zheng, Z. Polystyrene/vinyl ester resin/styrene thermoplastic elastomer composites: Miscibility, morphology, and mechanical properties. J. Appl. Polym. Sci. 2011, 119, 93–101. [Google Scholar] [CrossRef]
- Cousinet, S.; Ghadban, A.; Fleury, E.; Lortie, F.; Pascault, J.; Portinha, D. Toward replacement of styrene by bio-based methacrylates in unsaturated polyester resins. Eur. Polym. J. 2015, 67, 539–550. [Google Scholar] [CrossRef]
- Luo, H.; Luo, Y. Unsaturated polyester resin with low styrene emission. Thermosett. Resins 1995, 4, 29–34. [Google Scholar]
- National emissions standards for hazardous air pollutants: Reinforced plastic composites production, Environmental Protection Agency. Federal Register 2003, 68, 19375–19443.
- U. S. Department of Health and Human Services, Public Health Services, National Toxicology Program 14th Report on Carcinogens. 2016. Available online: https://ntp.niehs.nih.gov/whatwestudy/assessments/cancer/roc/index.html?utm_source=direct&utm_medium=prod&utm_campaign=ntpgolinks&utm_term=roc (accessed on 3 November 2016).
- Yao, K.; Tang, C. Controlled polymerization of next-generation renewable monomers and beyond. Macromolecules 2013, 46, 1689–1721. [Google Scholar] [CrossRef]
- Werpy, T.; Holladay, J.E.; White, J.F. Top Value Added Chemicals from Biomass; Volume 1-Results of Screening for Potential Candidates from Sugars and Synthesis Gas; Department of Energy: Washington, DC, USA, 2004.
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates-The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Li, F.; Hanson, M.V.; Larock, R.C. Soybean oil–divinylbenzene thermosetting polymers: Synthesis, structure, properties and their relationships. Polymer 2001, 42, 1567–1579. [Google Scholar] [CrossRef]
- Zhan, M.J.; Wool, R.P. Biobased composite resins design for electronic materials. J. Appl. Polym. Sci. 2010, 118, 3274–3283. [Google Scholar] [CrossRef]
- Badrinarayanan, P.; Lu, Y.; Larock, R.C.; Kessler, M.R. Cure characterization of soybean oil-styrene-divinylbenzene thermosetting copolymers. J. Appl. Polym. Sci. 2009, 113, 1042–1049. [Google Scholar] [CrossRef]
- Pfister, D.P.; Larock, R.C. Cationically cured natural oil-based green composites: Effect of the natural oil and the agricultural fiber. J. Appl. Polym. Sci. 2012, 123, 1392–1400. [Google Scholar] [CrossRef]
- Hu, S.; Li, Y.; Wu, Q.; Li, Z. Development of low styrene emission unsaturated polyester resin. Guangdong Chem. Ind. 2002, 29, 2–5. [Google Scholar]
- Liu, W.; Xie, T.; Qiu, R. Styrene-free unsaturated polyesters for hemp fibre composites. Compos. Sci. Technol. 2015, 120, 66–72. [Google Scholar] [CrossRef]
- Liu, W.; Chen, T.; Xie, T.; Qiu, R. Soybean oil-based thermosets with N-vinyl-2-pyrrolidone as crosslinking agent for hemp fiber composites. Compos. Part A Appl. Sci. Manuf. 2016, 82, 1–7. [Google Scholar] [CrossRef]
- Liu, W.; Xie, T.; Qiu, R. Improvement of properties for biobased composites from modified soybean oil and hemp fibers: Dual role of diisocyanate. Compos. Part A Appl. Sci. Manuf. 2016, 90, 278–285. [Google Scholar] [CrossRef]
- Kim, H.; Oh, D.; Lee, H.; Mi, K. Effect of reactive diluents on properties of unsaturated polyester/montmorillonite nanocomposites. J. Appl. Polym. Sci. 2004, 92, 238–242. [Google Scholar] [CrossRef]
- Fonseca, A.C.; Lopes, I.M.; Coelho, J.F.J.; Serra, A.C. Synthesis of unsaturated polyesters based on renewable monomers: Structure/properties relationship and crosslinking with 2-hydroxyethyl methacrylate. React. Funct. Polym. 2015, 97, 1–11. [Google Scholar] [CrossRef]
- Sousa, A.F.; Fonseca, A.C.; Serra, A.C.; Freire, C.S.R.; Silvestre, A.J.D.; Coelho, J.F.J. New unsaturated copolyesters based on 2,5-furandicarboxylic acid and their crosslinked derivatives. Polym. Chem. 2016, 7, 1049–1058. [Google Scholar] [CrossRef]
- Zhang, Y.; Chu, D.; Zheng, M.; Kissel, T.; Agarwal, S. Biocompatible and degradable poly(2-hydroxyethylmethacrylate) based polymers for biomedical applications. Polym. Chem. 2012, 3, 2752–2759. [Google Scholar] [CrossRef]
- Mehta, L.B.; Wadgaonkar, K.K.; Jagtap, R.N. Synthesis and characterization of high bio-based content unsaturated polyester resin for wood coating from itaconic acid: Effect of various reactive diluents as an alternative to styrene. J. Dispers. Sci. Technol. 2019, 40, 756–765. [Google Scholar] [CrossRef]
- Nebioglu, A.; Soucek, M.D. Reaction kinetics and microgel particle size characterization of ultraviolet-curing unsaturated polyester acrylates. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 6544–6557. [Google Scholar] [CrossRef]
- Nebioglu, A.; Soucek, M.D. Optimization of UV curable acrylated polyester-polyurethane/polysiloxane ceramer coatings using a response surface methodology. J. Coat. Technol. Res. 2006, 3, 61–68. [Google Scholar] [CrossRef]
- Fei, M.; Liu, W.; Jia, A.; Ban, Y.; Qiu, R. Bamboo fibers composites based on styrene-free soybean-oil thermosets using methacrylates as reactive diluents. Compos. Part A Appl. Sci. Manuf. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Greenfield, J. Tung oil. J. Am. Oil Chem. Soc. 1959, 36, 565–574. [Google Scholar] [CrossRef]
- Fu, M.; Chen, Y. Fatty acid groups of tung oil from major producing areas in China. Chin. Oil 1985, 1, 2–8. [Google Scholar]
- Meiorin, C.; Aranguren, M.I.; Mosiewicki, M.A. Polymeric networks based on tung oil: Reaction and modification with green oil monomers. Eur. Polym. J. 2015, 67, 551–560. [Google Scholar] [CrossRef]
- Mistri, E.; Routh, S.; Ray, D.; Sahoo, S.; Misra, M. Green composites from maleated castor oil and jute fibres. Ind. Crop. Prod. 2011, 34, 900–906. [Google Scholar] [CrossRef]
- Hu, Y.; Shang, Q.; Tang, T.; Wang, C.; Zhang, F.; Jia, P.; Feng, G.; Wu, Q. Use of cardanol-based acrylate as reactive diluent in UV-curable castor oilbased polyurethane acrylate resins. Ind. Crop. Prod. 2018, 117, 295–302. [Google Scholar] [CrossRef]
- Liu, C.; Hu, L.; Lei, W.; Zhou, Y. Castor oil-based polyfunctional acrylate monomers: Synthesis and utilization in UV-curable materials. Prog. Org. Coat. 2018, 121, 236–246. [Google Scholar] [CrossRef]
- Auclair, N.; Kaboorani, A.; Riedl, B.; Landry, V. Acrylated betulin as a comonomer for bio-based coatings. Part I: Characterization, photo-polymerization behavior and thermal stability. Ind. Crop. Prod. 2015, 76, 530–537. [Google Scholar] [CrossRef]
- Auclair, N.; Kaboorani, A.; Riedl, B.; Landry, V. Acrylated betulin as a comonomer for bio-based coatings. Part II: Mechanical and optical properties. Ind. Crop. Prod. 2016, 82, 118–126. [Google Scholar] [CrossRef]
- Jaillet, F.; Nouailhas, H.; Boutevin, B.; Caillol, S. Synthesis of novel biobased vinyl ester from dicyclopentadiene prepolymer, cashew nut shell liquid and soybean oil. Eur. J. Lipid Sci. Technol. 2016, 118, 1336–1349. [Google Scholar] [CrossRef]
- Can, E.; Kinaci, E.; Palmese, G.R. Preparation and characterization of novel vinyl ester formulations derived from cardanol. Eur. Polym. J. 2015, 72, 129–147. [Google Scholar] [CrossRef]
- Li, S.; Yang, X.; Huang, K.; Li, M.; Xia, J. Design, preparation and properties of novel renewable UV-curable copolymers based on cardanol and dimer fatty acids. Prog. Org. Coat. 2014, 77, 388–394. [Google Scholar] [CrossRef]
- Lima, M.S.; Costa, C.S.M.F.; Coelho, J.F.J.; Fonseca, A.C.; Serraa, A.C. A simple strategy toward the substitution of styrene by sobrerol-based monomers in unsaturated polyester resins. Green Chem. 2018, 20, 4880–4890. [Google Scholar] [CrossRef]
- Liu, W.; Xie, T.; Qiu, R. Biobased thermosets prepared from rigid isosorbide and flexible soybean oil derivatives. ACS Sustain. Chem. Eng. 2016, 5, 774–783. [Google Scholar] [CrossRef]
- Stanzione, J.F., III; Sadler, J.M.; La Scala, J.J.; Wool, R.P. Lignin model compounds as bio-based reactive diluents for liquid molding resins. ChemSusChem 2012, 5, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Stanzione, J.F., III; Giangiulio, P.A.; Sadler, J.M.; Scala, J.J.; Wool, R.P. Lignin-based bio-oil mimic as biobased resin for composite applications. ACS Sustain. Chem. Eng. 2013, 1, 419–426. [Google Scholar] [CrossRef]
- Zhang, C.; Madbouly, S.A.; Kessler, M.R. Renewable polymers prepared from vanillin and its derivatives. Macromol. Chem. Phys. 2015, 216, 1816–1822. [Google Scholar] [CrossRef]
- Sadler, J.M.; Nguyen, A.P.; Greer, S.M.; Plamese, G.R.; La Scale, J.J. Synthesis and characterization of a novel bio-based reactive diluent as a styrene replacement. J. Biobased Mater. Bioenergy 2012, 6, 86–93. [Google Scholar] [CrossRef]
- Dai, J.; Liu, X.; Ma, S.; Wang, J.; Shen, X.; You, S.; Zhu, J. Soybean oil-based UV-curable coatings strengthened by crosslink agent derived from itaconic acid together with 2-hydroxyethyl methacrylate phosphate. Prog. Org. Coat. 2016, 97, 210–215. [Google Scholar] [CrossRef]
- Liu, R.; Zhu, J.; Luo, J.; Liu, X. Synthesis and application of novel UV-curable hyperbranched methacrylates from renewable natural tannic acid. Prog. Org. Coat. 2014, 77, 30–37. [Google Scholar] [CrossRef]
- Shen, L.; Wang, Y.; Zhao, Q.; Lou, F.; Chen, J.; Lu, M.; Liang, L.; Wu, K.; Shi, J. Influence of a long-side-chain-containing reactive diluent on the structure and mechanical properties of UV-cured films. Polym. Int. 2016, 65, 1150–1156. [Google Scholar] [CrossRef]
- Cousinet, S.; Ghadban, A.; Allaoua, I.; Lortie, F.; Portinha, D.; Drockenmuller, E.; Pascault, J. Biobased vinyl levulinate as styrene replacement for unsaturated polyester resins. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 3356–3364. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, X.; Zhang, R.; Zhu, J.; Jiang, Y. Synthesis and properties of full bio-based thermosetting resins from rosin acid and soybean oil: The role of rosin acid derivatives. Green Chem. 2013, 15, 1300–1310. [Google Scholar] [CrossRef]
- Liu, W.; Chen, T.; Fei, M.; Qiu, R.; Yu, D.; Fu, T.; Qiu, J. Properties of natural fiber-reinforced biobased thermoset biocomposites: Effects of fiber type and resin composition. Compos. Part B Eng. 2019, 171, 87–95. [Google Scholar] [CrossRef]
- Sun, Z.; Fridrich, B.; Santi, A.D.; Elangovan, S.; Barta, K. Bright side of lignin depolymerization: Toward new platform chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef] [PubMed]
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijin, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 10, 1039. [Google Scholar] [CrossRef] [PubMed]
- Stanzione, J.F., III; Sadler, J.M.; La Scala, J.J.; Reno, K.H.; Wool, R.P. Vanillin-based resin for use in composite applications. Green Chem. 2012, 14, 2346–2352. [Google Scholar] [CrossRef]
- Bassett, A.W.; Rogers, D.P.; Sadler, J.M.; Scala, J.J.; Wool, R.P.; Stanzione, J.F., III. The effect of impurities in reactive diluents prepared from lignin model compounds on the properties of vinyl ester resins. J. Appl. Polym. Sci. 2016, 133, 43817. [Google Scholar] [CrossRef]
- Zhang, Y.; Thakur, V.K.; Li, Y.; Garrison, T.F.; Gao, Z.; Gu, J.; Kessler, M.R. Soybean-oil-based thermosetting resins with methacrylated vanillyl alcohol as bio-based, low-viscosity comonomer. Macromol. Mater. Eng. 2018, 303, 1700278. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Wang, L.; Gao, Z.; Kessler, M.R. Synthesis and characterization of methacrylated eugenol as a sustainable reactive diluent for a maleinated acrylated epoxidized soybean oil resin. ACS Sustain. Chem. Eng. 2017, 5, 8876–8883. [Google Scholar] [CrossRef]
- Liu, K.; Madbouly, S.A.; Kessler, M.R. Biorenewable thermosetting copolymer based on soybean oil and eugenol. Eur. Polym. J. 2015, 69, 16–28. [Google Scholar] [CrossRef]
- Dai, J.; Ma, S.; Teng, N.; Dai, X.; Shen, X.; Wang, S.; Liu, X.; Zhu, J. 2,5-Furandicarboxylic acid-and itaconic acid-derived fully biobased unsaturated polyesters and their cross-linked networks. Ind. Eng. Chem. Res. 2017, 56, 2650–2657. [Google Scholar] [CrossRef]
- Ding, R.; Du, Y.; Goncalves, R.B. Sustainable near UV-curable acrylates based on natural phenolics for stereolithography 3D printing. Polym. Chem. 2019, 10, 1067–1077. [Google Scholar] [CrossRef]
- Li, P.; Ma, S.; Dai, J.; Liu, X.; Jiang, Y.; Wang, S.; Wei, J.; Chen, J.; Zhu, J. Itaconic acid as a green alternative to acrylic acid for producing a soybean oil-based thermoset: Synthesis and properties. ACS Sustain. Chem. Eng. 2016, 5, 1228–1236. [Google Scholar] [CrossRef]
- Campanella, A.; Zhan, M.; Watt, P.; Grous, A.T.; Shen, C.; Wool, R.P. Triglyceride-based thermosetting resins with different reactive diluents and fiber reinforced composite applications. Compos. Part A Appl. Sci. Manuf. 2015, 72, 192–199. [Google Scholar] [CrossRef]
- Campanella, A.; La Scala, J.J.; Wool, R.P. The use of acrylated fatty acid methyl esters as styrene replacements in triglyceride-based thermosetting polymers. Polym. Eng. Sci. 2009, 49, 2384–2392. [Google Scholar] [CrossRef]
- La Scala, J.; Boyd, S.; Andrews, K.; Goldek, T.; Lochner, C.; Myers, P.; Levine, F.; De Bonis, D.; Hayes, R.; Sands, J. Cost and Performance Report: Low-Hazardous Air Pollutant(HAP)/Volatile Organic Compound(VOC)-Compliant Resins for Military Applications; Army Research Lab Aberdeen Proving Ground Md Weapons and Materials Research Directorate: Cambridge, MA, USA, 2012. [Google Scholar]
- Dey, T. Properties of vinyl ester resins containing methacrylated fatty acid comonomer: The effect of fatty acid chain length. Polym. Int. 2010, 56, 853–859. [Google Scholar] [CrossRef]
- Cai, J.; Shu, W.; Fu, L.; Zan, L. Influence of reactive diluents on UV curing acrylic epoxidized soybean oil system. Paint Coat. Ind. 2006, 36, 12–15. [Google Scholar]
- Liu, Y.; Li, C.; Dai, J.; Jiang, Y.; Liu, X.; Zhu, J. Synthesis of multifunctional monomers from rosin for the properties enhancement of soybean-oil based thermosets. Sci. China Technol. Sci. 2017, 60, 1332–1338. [Google Scholar] [CrossRef]
- Yu, A.; Serum, E.M.; Renner, A.C.; Sahouani, J.M.; Sibi, M.P.; Webster, D.C. Renewable reactive diluents as practical styrene replacements in biobased vinyl ester thermosets. ACS Sustain. Chem. Eng. 2018, 6, 12586–12592. [Google Scholar] [CrossRef]
- Bowden, S.T.; Butler, E.T. Intermolecular forces in liquid systems. Part II. Viscosity, surface tension, and parachor relationships in binary systems. J. Chem. Soc. 1939, 78–83. [Google Scholar] [CrossRef]
- Qiu, R.; Liu, W.; Wu, Y.; Li, K. Development of a reinforced styrene-free unsaturated polyester composite based on bamboo fibers. Holzforschung 2019, 73, 689–694. [Google Scholar] [CrossRef]
- Odian, G. Principles of Polymerization; Wileyintersecience: New York, NY, USA, 2014. [Google Scholar]
- Liu, W.; Fei, M.; Ban, Y.; Jia, A.; Qiu, R.; Qiu, J. Concurrent improvements in crosslinking degree and interfacial adhesion of hemp fibers reinforced acrylated epoxidized soybean oil composites. Compos. Sci. Technol. 2018, 160, 60–68. [Google Scholar] [CrossRef]
- Ma, S.Q.; Liu, W.Q.; Hu, C.H.; Wang, Z.F.; Tang, C.Y. Toughening of epoxy resin system using a novel dendritic polysiloxane. Macromol. Res. 2010, 18, 392–398. [Google Scholar] [CrossRef]
- Ma, S.Q.; Liu, W.Q.; Yu, D.; Wang, Z.F. Modification of epoxy resin with polyether-grafted-polysiloxane and epoxy-miscible polysiloxane particles. Macromol. Res. 2010, 18, 22–28. [Google Scholar] [CrossRef]
- Ma, S.Q.; Webster, D.C. Naturally occurring acids as cross-linkers to yield VOC-free, high-performance, fully bio-based, degradable thermosets. Macromolecules 2015, 48, 7127–7137. [Google Scholar] [CrossRef]
- Ma, S.Q.; Webster, D.C.; Jabeen, F. Hard and flexible, degradable thermosets from renewable bioresources with the assistance of water and ethanol. Macromolecules 2016, 49, 3780–3788. [Google Scholar] [CrossRef]
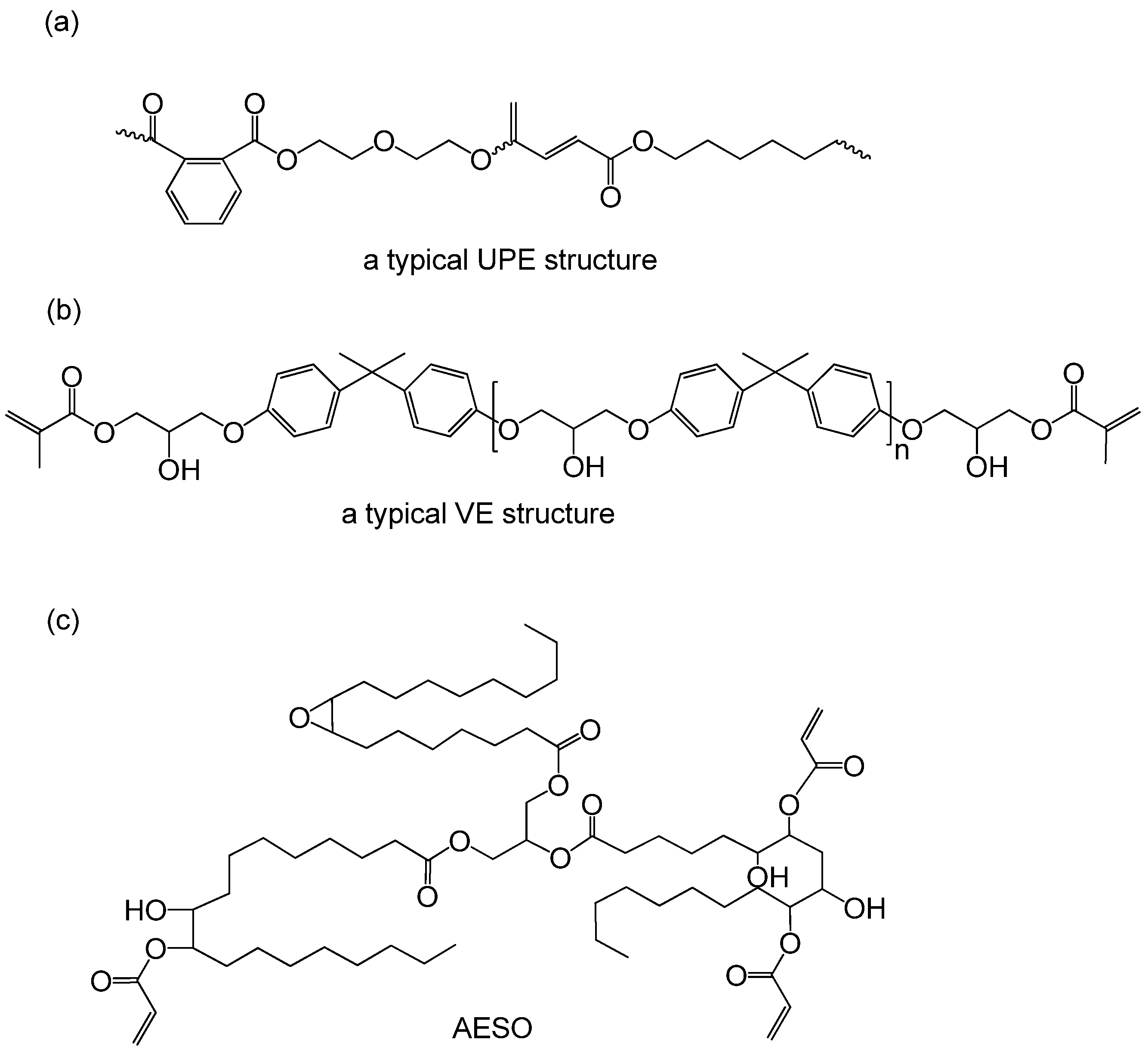
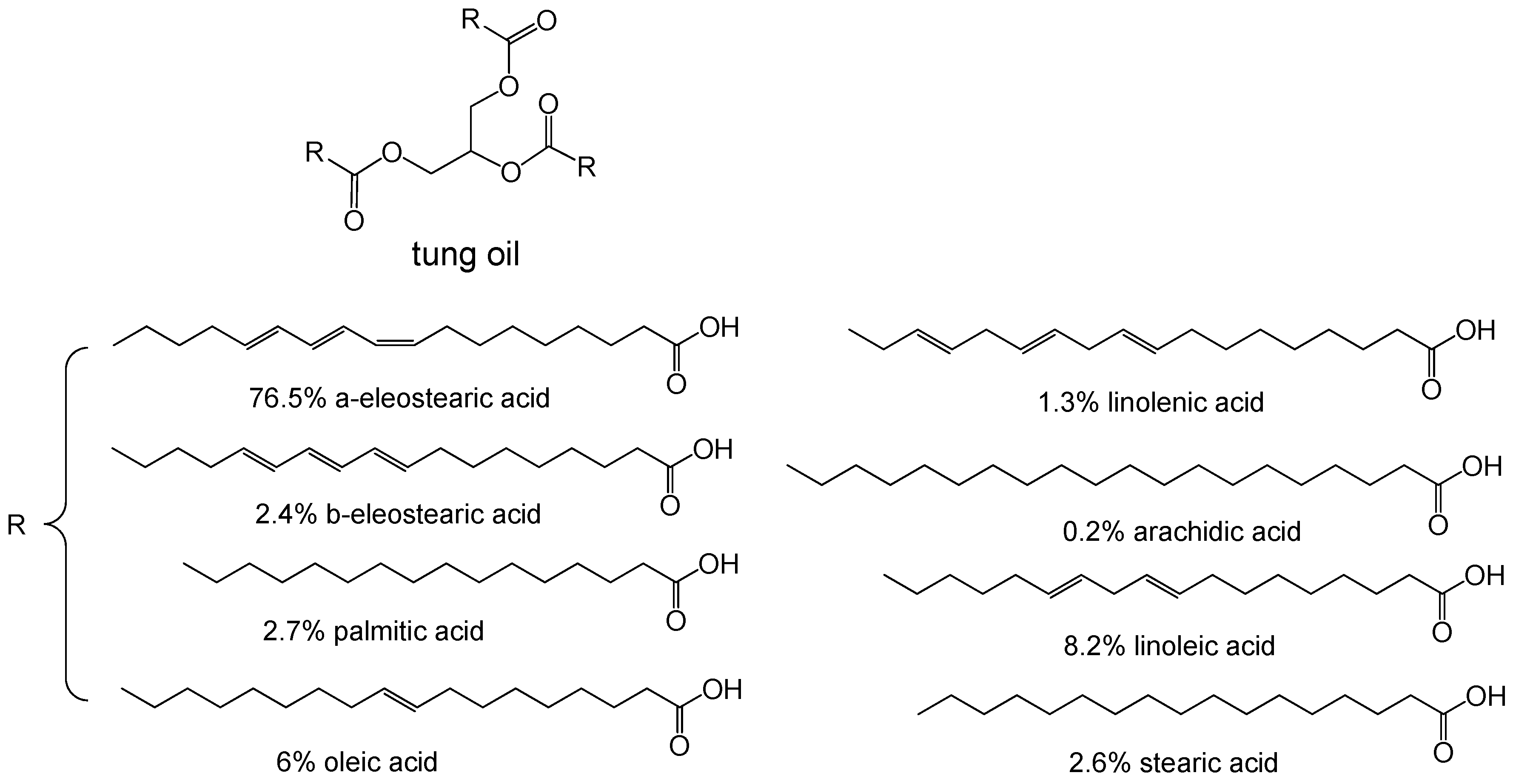



| Monomers | Molecular Weight (g/cm3) | Boiling Point (°C) | Toxicity | Chemical Structure |
|---|---|---|---|---|
| Styrene | 104.15 | 145–146 | acute toxicity |  |
| Divinylbenzene (DVB) | 130.19 | 195 | acute toxicity |  |
| α-Methylstyrene | 118.18 | 165–169 | acute toxicity |  |
| Fluorostyrene | 122.14 | 67 | - |  |
| Vinyltoluene | 118.18 | 169–171 | acute toxicity |  |
| N-Vinyl-2-pyrrolidone (NVP) | 111.14 | 92–95 | acute toxicity |  |
| tri(Ethylene-glycol)divinyl ether (TDE) | 202.25 | 120–126 | - |  |
| Monomer | Molecular Weight (g/cm3) | Boiling Point (°C) | Toxicity | Chemical Structure |
|---|---|---|---|---|
| Butyl methacrylate (BMA) | 140.20 | 162–165 | acute toxicity |  |
| Hydroxypropylacrylate (HPA) | 130.14 | 77 | acute toxicity |  |
| 2-Hydroxyethylmethac-rylate (HEMA) | 130.14 | 85 | danger |  |
| Isobornyl methacrylate (IBOMA) | 222.32 | 127–129 | environmental hazards |  |
| Methyl methacrylate (MMA) | 100.12 | 100 | acute toxicity |  |
| Trimethylolpro-panetri-acrylate (TMPTA) | 296.32 | - | acute toxicity |  |
| Trimethylolpropane trimethacrylate (TMPTMA) | 338.40 | - | environmental hazards |  |
| 1,4-Butanediol dimethacrylate (BDDMA) | 226.27 | 132–134 | acute toxicity |  |
| Methods | Renewable Monomers | Reactive Agents | Novel Monomer | Sources of Monomer |
|---|---|---|---|---|
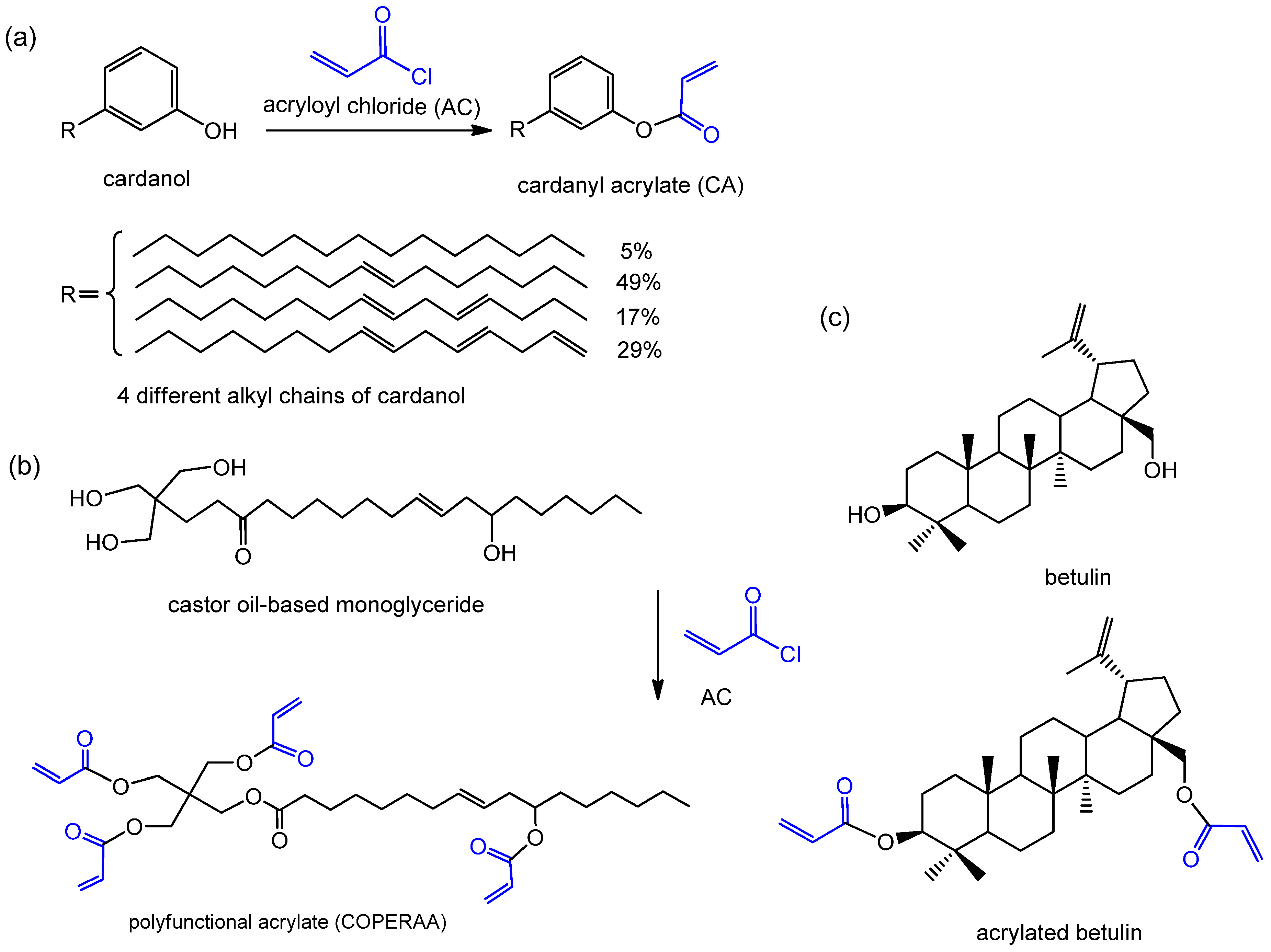
| Grafting acrylate | cardanol | acryloyl chloride (AC) | cardanyl acrylate (CA) [44] | cashew nut |
| castor oil | AC | a new polyfunctional acrylate monomer (COPERAA) [45] | castor | |
| betulin | AC | acrylated betulin [46,47] | rosin | |
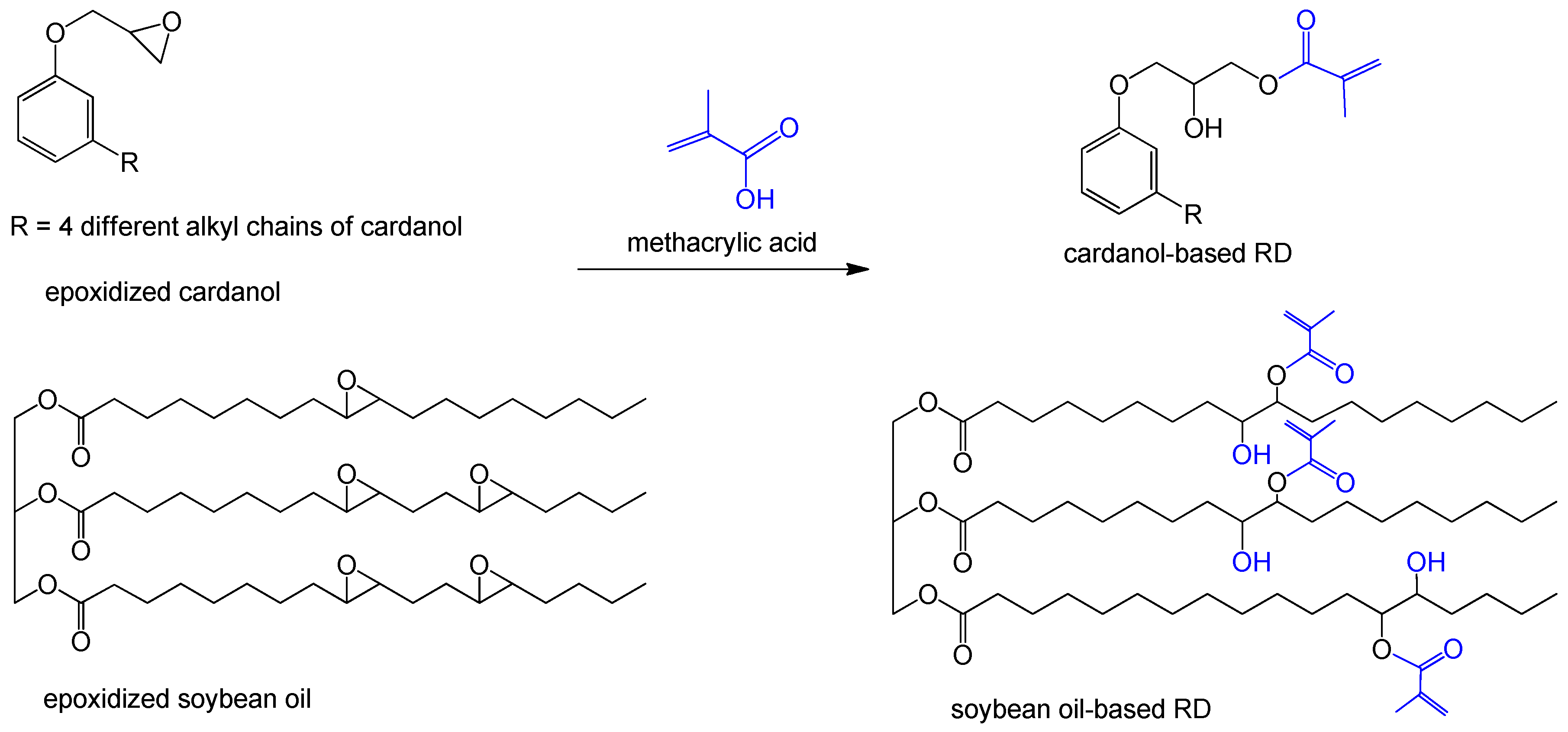
| Grafting methacr-ylate | cardanol | methacrylic acid | methacrylated cardanol (MC) [48,49] | cashew nut |
| cardanol | methacrylic anhydride (MA) | methacrylated cardanol (MC) [50] | cashew nut | |
| sobrerol | MA | sobrerol methacrylate (SoMet) [51] | α-pinene | |
| isosorbide | MA | methacrylated isosorbide (MI) [52] | starch/cellulose | |
| vanillin | MA | methacrylated vanillin (MV) [53] | lignin | |
| guaiacol | MA | methacrylated guaiacol (MG) [53] | lignin | |
| eugenol | MA | methacrylated eugenol (ME) [53] | lignin | |
| phenol | MA | phenyl methacrylate (PM) [54] | lignin | |
| creosol | MA | methacrylated creosol (MCre) [54] | lignin | |
| 4-ethylguai-acol | MA | methacrylated 4-ethylguaiacol (MEG) [54] | lignin | |
| 4-propylgu-aiacol | MA | methacrylated 4-propylguaiacol (MPG) [54] | lignin | |
| catechol | MA | methacrylated catechol (MCat) [54] | lignin | |
| 4-methylcat-echol | MA | methacrylated 4-methylcatechol (MMCat) [54] | lignin | |
| vanillin alcohol | MA | methacrylated vanillyl alcohol (MVA) [55] | lignin | |
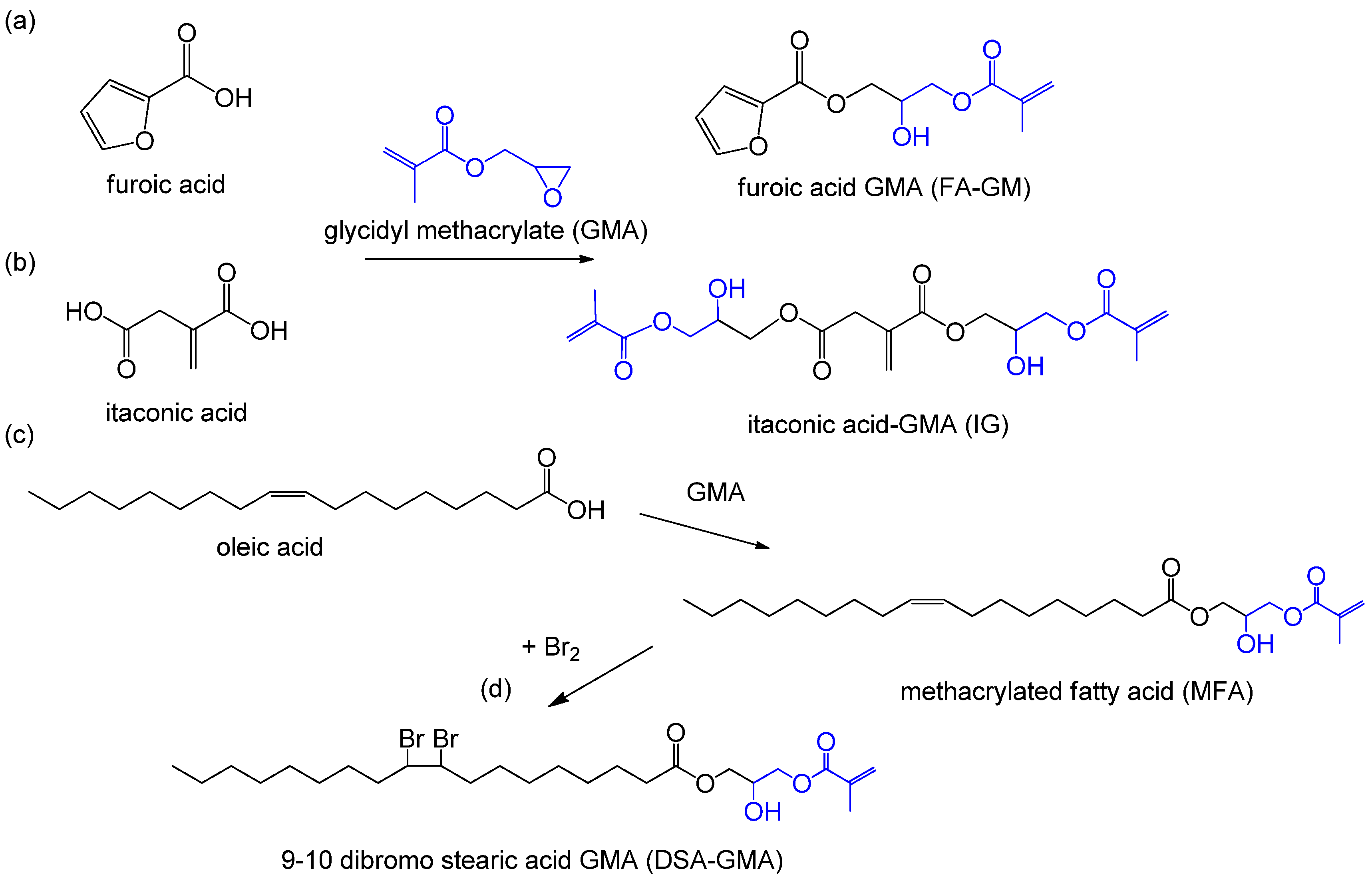
| furoic acid | glycidyl methacrylate (GMA) | furoic acid glycidyl methacrylate (FA-GM) [56] | cellulose | |
| itaconic acid | GMA | a UV curable unsaturated monomer (IG) [57] | starch/cellulose | |
| oleic acid | Br2/GMA | 9-10 dibromo stearic acid glycidyl methacrylate [3] | extractives | |
| oleic acid | GMA | methacrylated fatty acid (MFA) | extractives | |
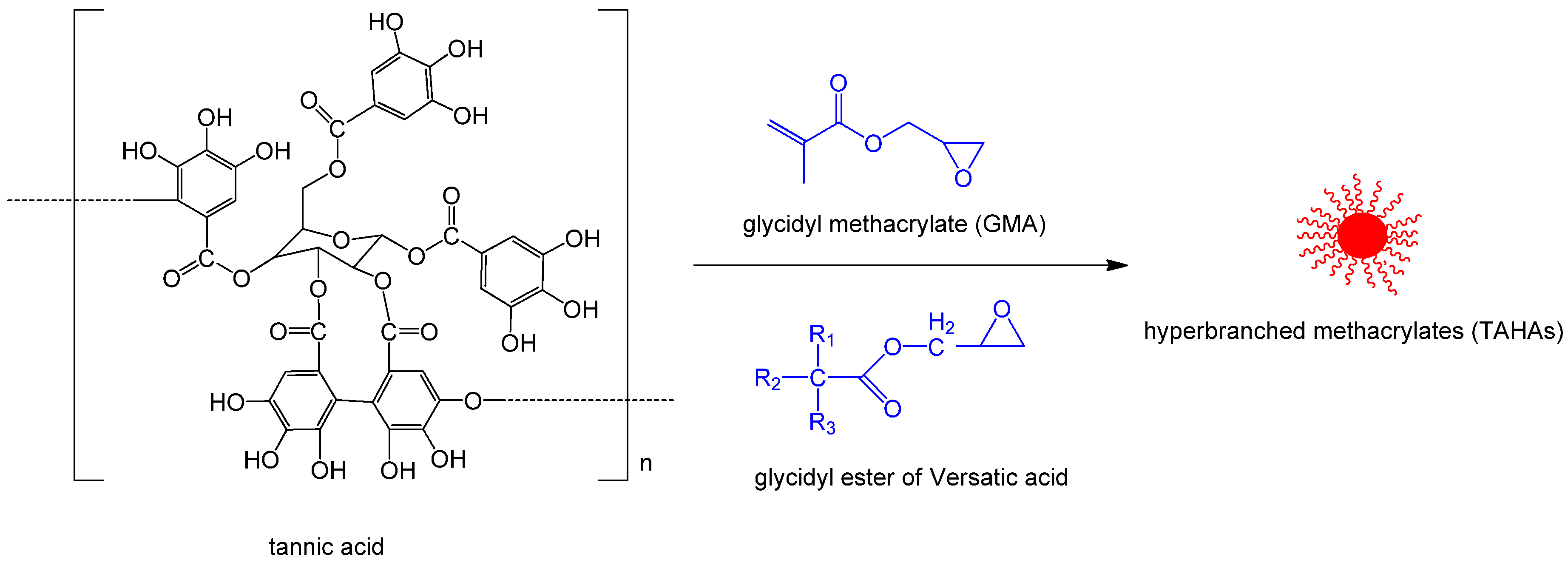
| tannic acid | GMA/glycidyl ester of versatic acid (CE10) | hyperbranched methacrylates [58] | starch/cellulose | |
| Others | Hydroxyeth-ylmethacry-late (HEMA) | 2-dodecen-1-ylsuccinic anhydride | a dimethacrylate reactive diluent (HEMA-DDSA) [59] | - |
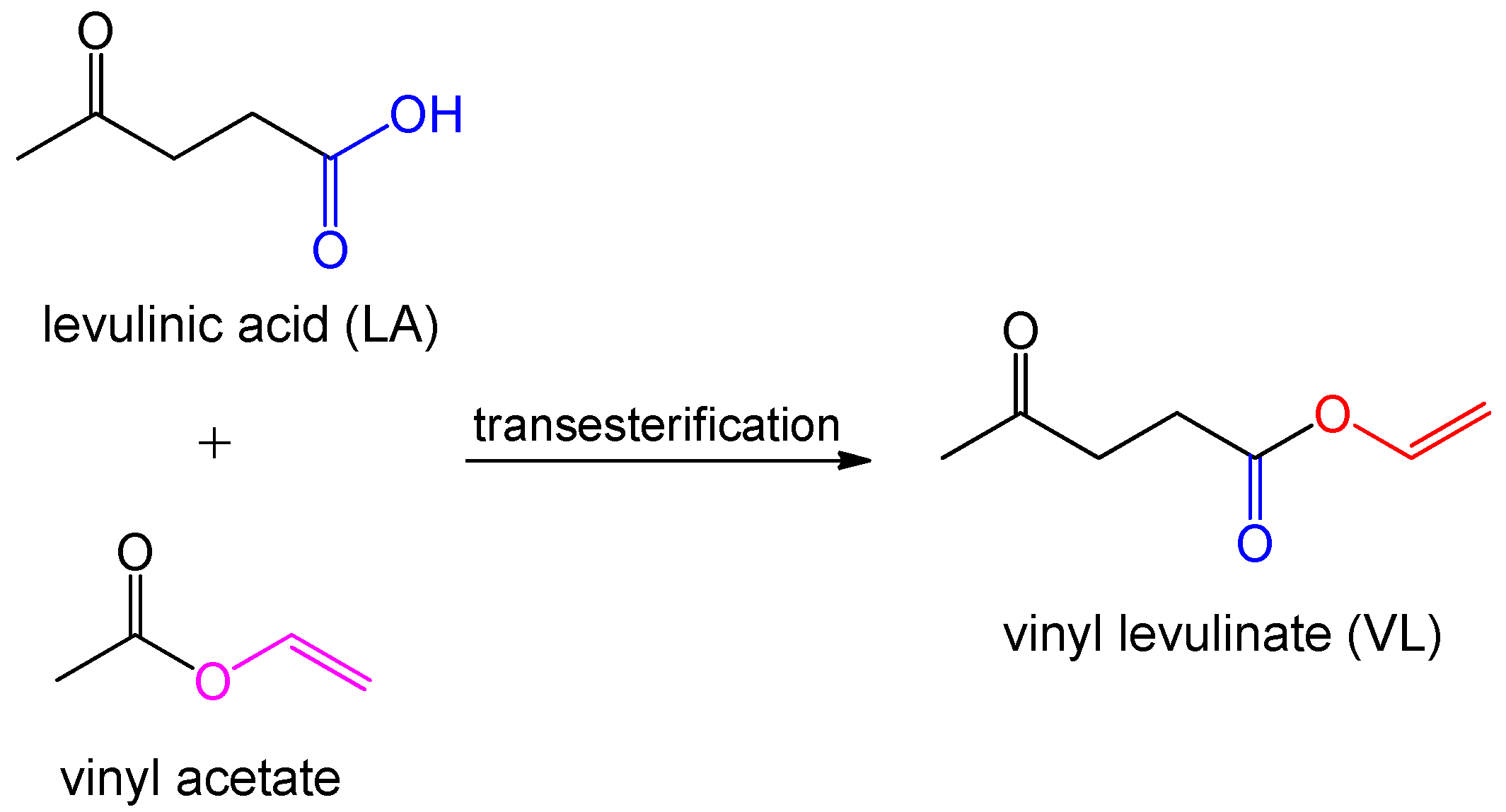
| levulinic acid (LA) | vinyl acetate | vinyl levulinate (VL) [60] | starch/cellulose | |
| rosin derivatives | allyl bromide | divinyl rosin/trivinyl rosin [61] | rosin |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Fei, M.; Qiu, R.; Liu, W.; Qiu, J. A Review on Styrene Substitutes in Thermosets and Their Composites. Polymers 2019, 11, 1815. https://doi.org/10.3390/polym11111815
Wu Y, Fei M, Qiu R, Liu W, Qiu J. A Review on Styrene Substitutes in Thermosets and Their Composites. Polymers. 2019; 11(11):1815. https://doi.org/10.3390/polym11111815
Chicago/Turabian StyleWu, Yuchao, Mingen Fei, Renhui Qiu, Wendi Liu, and Jianhui Qiu. 2019. "A Review on Styrene Substitutes in Thermosets and Their Composites" Polymers 11, no. 11: 1815. https://doi.org/10.3390/polym11111815
APA StyleWu, Y., Fei, M., Qiu, R., Liu, W., & Qiu, J. (2019). A Review on Styrene Substitutes in Thermosets and Their Composites. Polymers, 11(11), 1815. https://doi.org/10.3390/polym11111815








