Novel Solid-State Potentiometric Sensors Using Polyaniline (PANI) as A Solid-Contact Transducer for Flucarbazone Herbicide Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
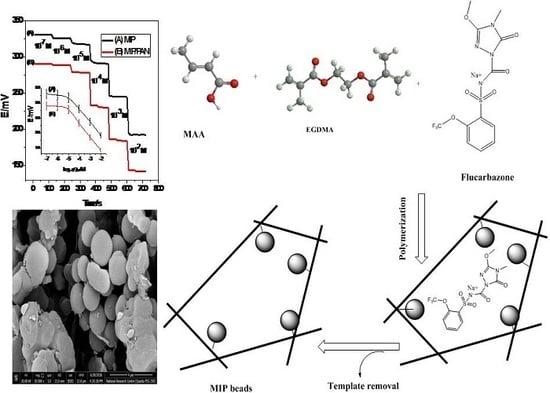
2.2. MIPs Synthesis
2.3. Electrode Fabrication
2.4. Electromotive Force (EMF) Measurements
2.5. Electrochemical Characterization
2.6. Applications to Real Samples
3. Results and Discussions
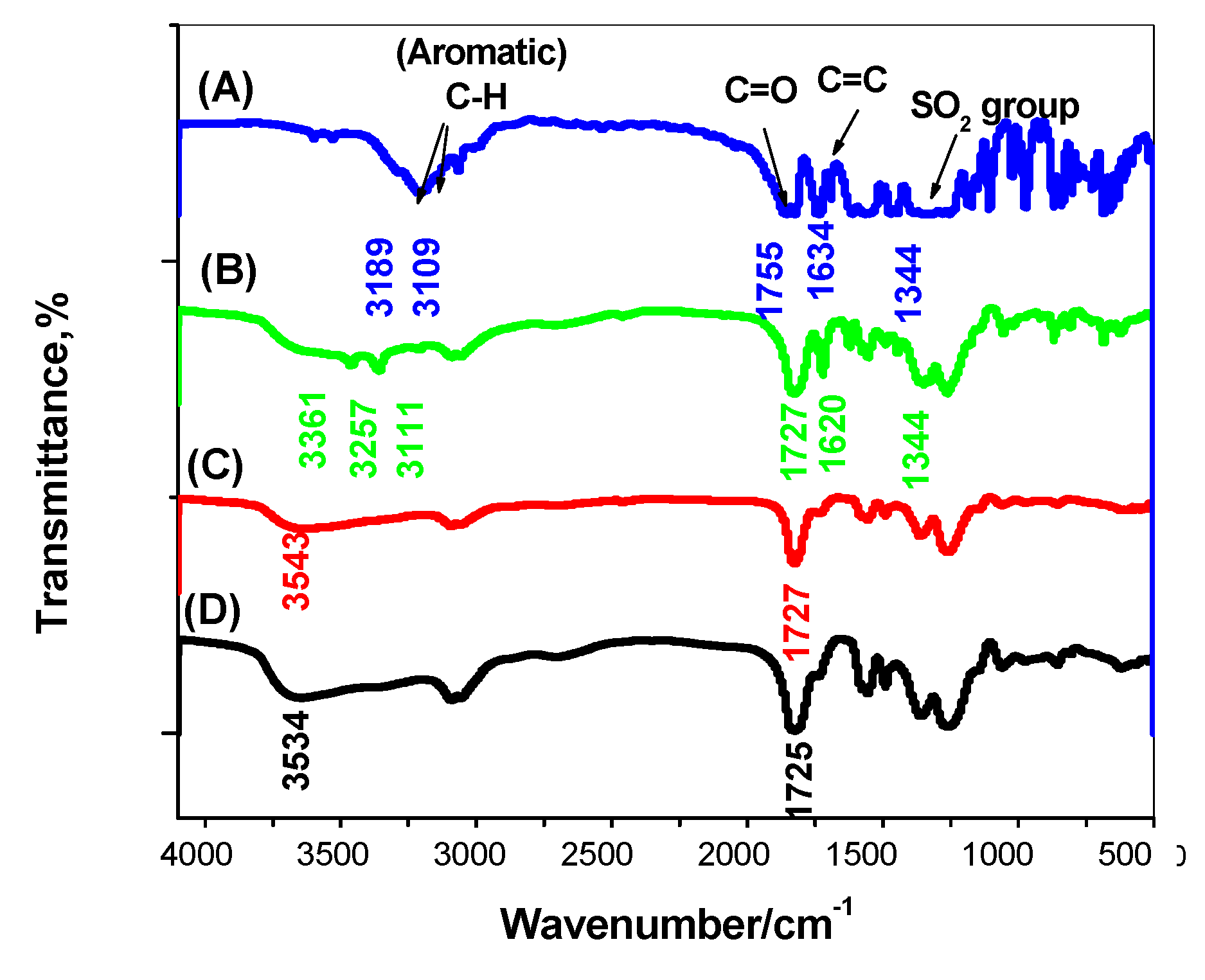
3.1. Characterization of MIP Beads
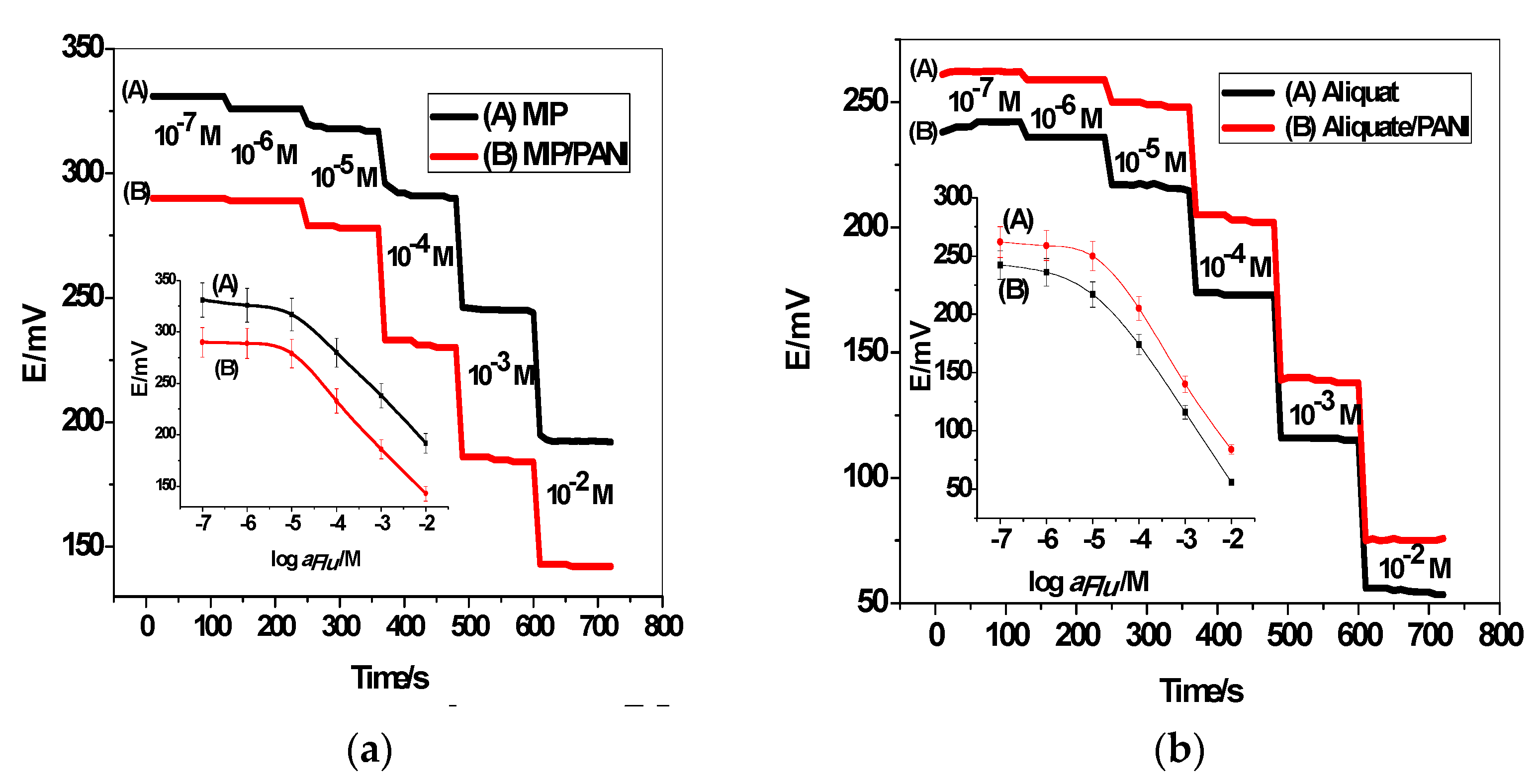
3.2. Potentiometric Performance of the All-Solid-State ISEs
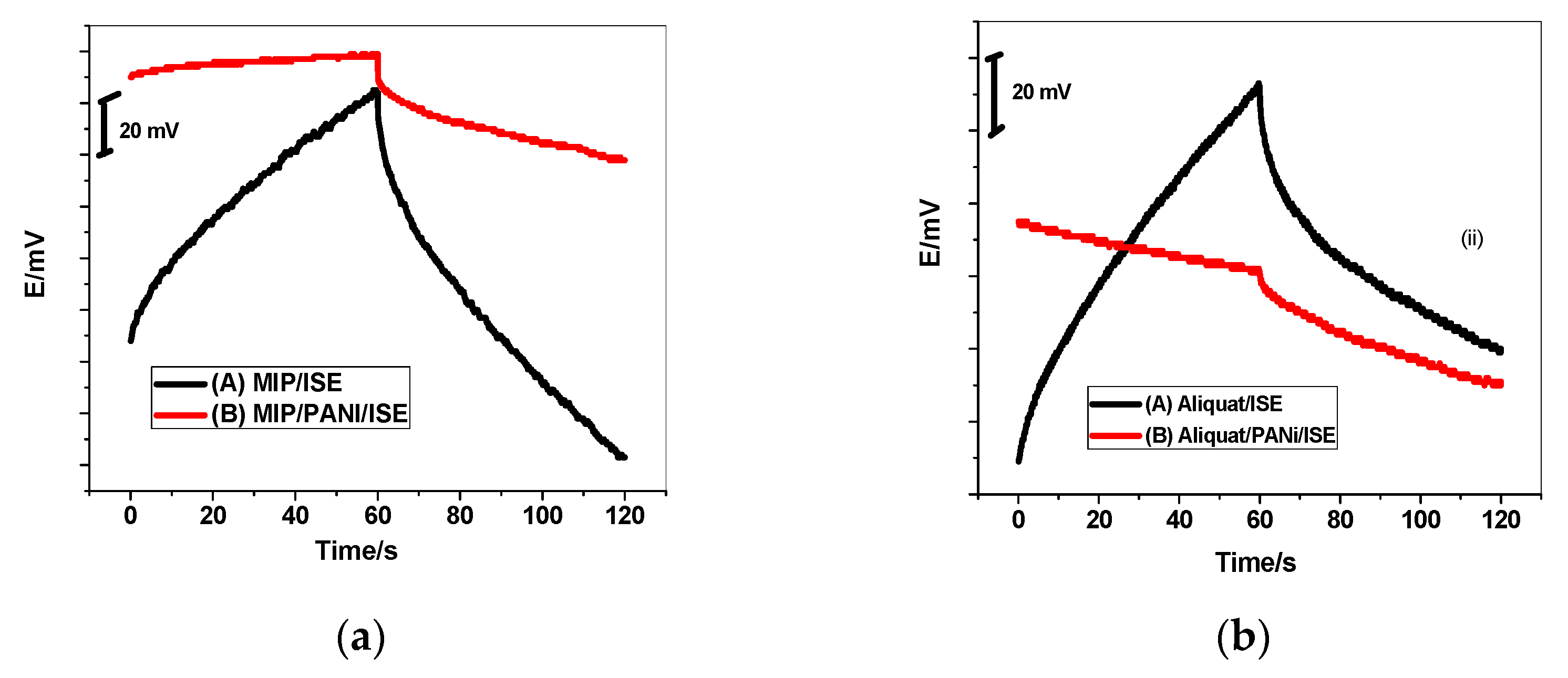
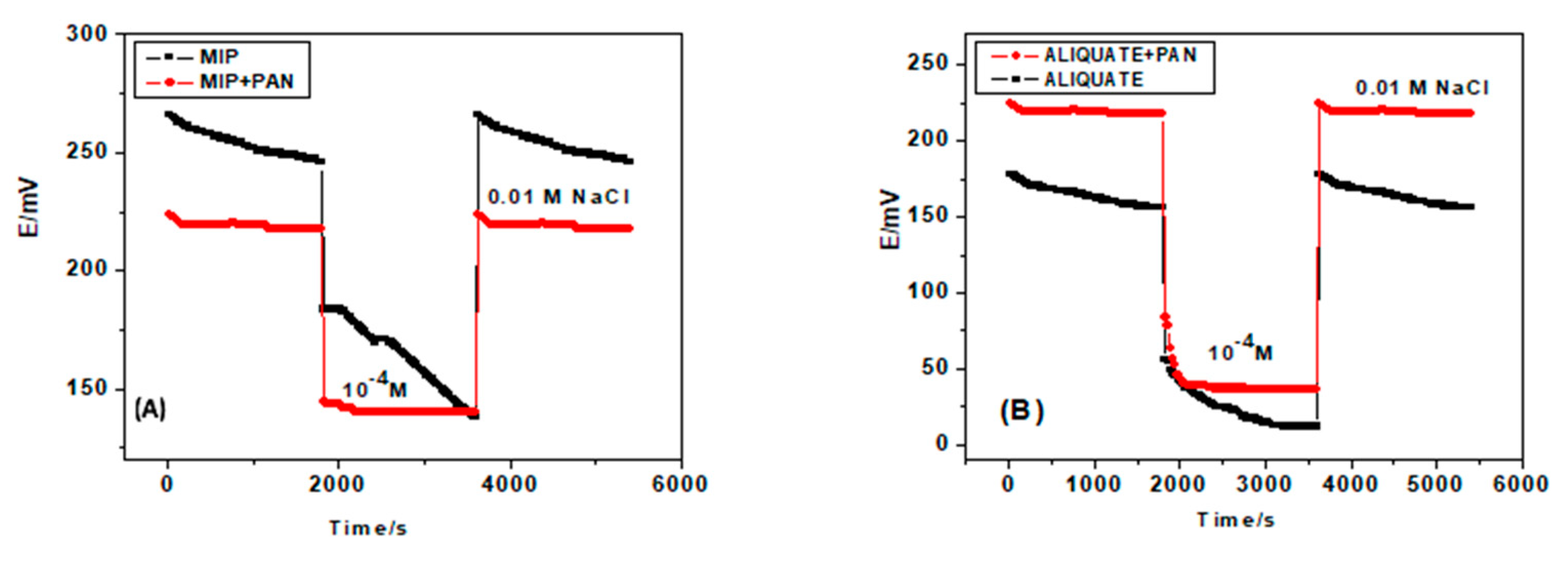
3.3. Potential Stability
3.4. Water Layer Test
3.5. Selectivity
3.6. Flucarbazone Assessment in Real Samples
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goto, T.; Ito, Y.; Oka, H.; Saito, I.; Matsumoto, H.; Nakazawa, H. Simple and rapid determination of N-methylcarbamate pesticides in citrus fruits by electrospray ionization tandem mass spectrometry. Anal. Chim. Acta 2003, 487, 201–209. [Google Scholar] [CrossRef]
- Torres, C.M.; Pico, Y.; Manes, J. Determination of pesticide residues infruits and vegetables. J. Chromatogr. A 1996, 754, 17–31. [Google Scholar] [CrossRef]
- Bayer Technical Bulletin. Everest™: Solutions for Wheat Production Headaches, Bayer Technical Bulletin, Kaiser-Wilhelm-Allee 151373 Leverkusen Germany. 1999.
- US Government Printing Office. Electronic Code of Federal Regulation. 2013. [Google Scholar]
- Malte, A.M.; Lilani, A.T. Riegel’s Handbook of Industrial Chemistry, 10th ed.; Kluwer, K., Ed.; Academic/Plenwn Publishers: New York, NY, USA, 2003. [Google Scholar]
- Xue, J.; Li, H.; Liu, F.; Jian, Q.; Peng, W.; Wang, S. Determination and dissipation of flucarbazone-sodium and its metabolite in wheat and soils by LC-MS/MS. Int. J. Environ. Anal. Chem. 2014, 94, 479–492. [Google Scholar] [CrossRef]
- Klaffenbach, P.; Holland, P.T. Analysis of sulfonylurea herbicides by gas–liquid chromatography: Determination of chlorsulfuron and metsulfuron-methyl in soil and water samples. J. Agric. Food Chem. 1993, 41, 396–401. [Google Scholar] [CrossRef]
- Galletti, G.C.; Bonnetti, A.; Dinelli, G. High performance liquid chromatographic determination of sulfonylureas in soil and water. J. Chromatogr. A 1995, 692, 27–37. [Google Scholar] [CrossRef]
- Horowitz, M. Application of bioassay techniques to herbicide investigations. Weed Res. 1976, 16, 209–215. [Google Scholar] [CrossRef]
- Vencill, W.K. Flucarbazone-Sodium. In Herbicide Handbook, 8th ed.; Weed Science Society of America: Champaign, IL, USA, 2002. [Google Scholar]
- Szmigielski, A.M.; Schoenau, J.J.; Irvine, A.; Schilling, B. Evaluating a Mustard Root-Length Bioassay for Predicting Crop Injury from Soil Residual Flucarbazone. Commun. Soil Sci. Plant Anal. 2008, 39, 413–420. [Google Scholar] [CrossRef]
- Mikhelson, K.N. Ion-Selective Electrodes. In Lecture Notes in Chemistry; Springer: Berlin/Heidelberg, Germany, 2013; Volume 81. [Google Scholar]
- Hassan, S.S.M.; Mahmoud, W.H.; Mohamed, A.H.K.; Kelany, A.E. Mercury(II) Ion-Selective Polymeric Membrane Sensors for Analysis of Mercury in Hazardous Wastes. Anal. Sci. 2006, 22, 877–881. [Google Scholar] [CrossRef] [Green Version]
- Hassan, S.S.M.; Marzouk, S.A.M.; Mohamed, A.H.K.; Badawy, N.M. Novel dicyanoargentate polymeric membrane sensors for selective determination of cyanide ions. Electroanalysis 2004, 16, 298–303. [Google Scholar] [CrossRef]
- Kamel, A.H.; Jiang, X.; Li, P.; Liang, R. A paper-based potentiometric sensing platform based on molecularly imprinted nanobeads for determination of bisphenol A. Anal. Methods 2018, 10, 3890–3895. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Elnemma, E.M.; Mohamed, A.H.K. Novel Biomedical Sensors for Flow Injection Potentiometric Determination of Creatinine in Human Serum. Electroanalysis 2005, 17, 2246–2253. [Google Scholar] [CrossRef]
- Kamel, A.H.; Hassan, A.M.E. Solid Contact Potentiometric Sensors Based on Host-Tailored Molecularly Imprinted Polymers for Creatine Assessment. Int. J. Electrochem. Sci. 2016, 11, 8938–8949. [Google Scholar] [CrossRef]
- Jaramillo, A.; Lopez, S.; Justice, J.B. Acetycholine and choline ion-selective microelectrodes. Anal. Chem. Acta 1983, 146, 149–159. [Google Scholar] [CrossRef]
- Moreira, F.T.C.; Guerreiro, J.R.L.; Azevedo, V.L.; Kamel, A.H.; Sales, M.G.F. New biomimetic sensors for the determination of tetracycline in biological samples: Batch and flow mode operations. Anal. Methods 2010, 2, 2039–2045. [Google Scholar] [CrossRef] [Green Version]
- Kamel, A.H.; Mohammad, S.G.; Awwad, N.S.; Mohammed, Y.Y. Survey on the Integration of Molecularly Imprinted Polymers as Artificial Receptors in Potentiometric Transducers for pharmaceutical Drugs. Int. J. Electrochem. Sci. 2019, 14, 2085–2124. [Google Scholar] [CrossRef]
- Kamel, A.H.; Mahmoud, W.H.; Mostafa, M.S. Biomimetic ciprofloxacin sensors made of molecularly imprinted network receptors for potential measurements. Anal. Methods 2011, 3, 957–964. [Google Scholar] [CrossRef]
- Lindner, E.; Gyurcsányi, R.J. Quality control criteria for solid-contact, solvent polymeric membrane ion-selective electrodes. Solid State Electrochem. 2009, 13, 51–68. [Google Scholar] [CrossRef]
- Cuartero, M.; Crespo, G.A. All-solid-state potentiometric sensors: A new wave for in situ aquatic research. Curr. Opin. Electrochem. 2018, 10, 98–106. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef] [PubMed]
- Gui, R.; Jin, H.; Guo, H.; Wang, Z. Recent advances and future prospects in molecularly imprinted polymers-based electrochemical biosensors. Biosens. Bioelectron. 2018, 100, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, S.; Li, J. Recent advances in molecular imprinting technology: Current status, challenges and highlighted applications. Chem. Soc. Rev. 2011, 40, 2922–2942. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Liu, B.; Wang, Z.; Zhang, Z. Imprinting of molecular recognition sites on nanostructures and its applications in chemosensors. Sensors 2008, 8, 8291–8320. [Google Scholar] [CrossRef]
- Bossi, A.; Bonini, F.; Turner, A.P.F.; Piletsky, S.A. Molecularly imprinted polymers for the recognition of proteins: The state of the art. Biosens. Bioelectron. 2007, 22, 1131–1137. [Google Scholar] [CrossRef]
- Farooq, S.; Nie, J.; Cheng, Y.; Yan, Z.; Li, J.; Bacha, S.A.S.; Mushtaq, A.; Hui Zhang, H. Molecularly imprinted polymers’ application in pesticide residue detection. Analyst 2018, 143, 3971–3989. [Google Scholar] [CrossRef]
- Wang, X.; Yu, J.; Wu, X.; Fu, J.; Kang, Q.; Shen, D.; Li, J.; Chen, L. A molecular imprinting-based turn-on Ratiometric fluorescence sensor for highly selective and sensitive detection of 2,4-dichlorophenoxyacetic acid (2,4-D). Biosens. Bioelectron. 2016, 81, 438–444. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Dang, Y.; Chen, Z.; Zhang, R.; Li, Y.; Ye, B. A robust electrochemical sensing of molecularly imprinted polymer prepared by using bifunctional monomer and its application in detection of cypermethrin. Biosens. Bioelectron. 2019, 127, 207–214. [Google Scholar] [CrossRef]
- Qi, J.; Li, B.; Zhou, N.; Wang, X.; Deng, D.; Luo, L.; Chen, L. The strategy of antibody-free biomarker analysis by in-situ synthesized molecularly imprinted polymers on movable valve paper-based device. Biosens. Bioelectron. 2019, 142, 111533. [Google Scholar] [CrossRef]
- Kamel, A.H.; Al Hamid, F.A.; Soror, T.Y.; Galal, H.R.; Gendy, F.A. Solid contact biosensor based on man-tailored polymers for acetylcholine detection: Application to acetycholinesterase assay. Eur. Chem. Bull. 2016, 5, 266–273. [Google Scholar]
- Moon, J.; Thapliyal, N.; Hussain, K.K.; Goyal, R.N.; Shim, Y. Conducting polymer-based electrochemical biosensors for neurotransmitters: A review. Biosens. Bioelectron. 2018, 102, 540–552. [Google Scholar] [CrossRef] [PubMed]
- El-Kosasy, A.; Kamel, A.H.; Hussin, L.; Ayad, M.F.; Fares, N. Mimicking new receptors based on molecular imprinting and their application to potentiometric assessment of 2,4-dichlorophenol as a food taint. Food Chem. 2018, 250, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, N.S.; Amr, A.E.; El-Tantawy, A.S.M.; Al-Omar, M.A.; Kamel, A.H.; Khalifa, N.W. Tailor-Made Specific Recognition of Cyromazine Pesticide Integrated in a Potentiometric Strip Cell for Environmental and Food Analysis. Polymers 2019, 11, 1526. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, N.S.; Youssef, M.A.; Algarni, H.; Nasser, S.; Awwad, N.S.; Kamel, A.H. All Solid-State Poly (Vinyl Chloride) Membrane Potentiometric Sensor Integrated with Nano-Beads Imprinted Polymers for Sensitive and Rapid Detection of Bispyribac Herbicide as Organic Pollutant. Molecules 2019, 24, 712. [Google Scholar] [CrossRef]
- Stejskal, J.; Gilbert, R.G. Polyaniline. Preparation of a conducting polymer (IUPAC Technical Report). Pure Appl. Chem 2002, 74, 857–867. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, Z.; Wan, M. Nanostructures of Polyaniline Doped with Inorganic Acids. Macromolecules 2002, 35, 5937–5942. [Google Scholar] [CrossRef]
- Stejskal, J.; Sapurin, I.; Prokeš, J.; Zemek, J. In-situ polymerized polyaniline films. Synth. Met. 1999, 105, 195–202. [Google Scholar] [CrossRef]
- Sapurina, I.; Fedorova, S.; Stejskal, J. Surface Polymerization and Precipitation Polymerization of Aniline in the Presence of Sodium Tungstate. Langmuir 2003, 19, 7413–7416. [Google Scholar] [CrossRef]
- Buck, R.P.; Lindner, E. Recommendations for nomenclature of ion selective electrodes (IUPAC Recommendations 1994). Pure Appl. Chem. 1994, 66, 2527–2536. [Google Scholar] [CrossRef]
- Lindner, E.; Umezawa, Y. Performance evaluation criteria for preparation and measurement of macro- and microfabricated ion-selective electrodes (IUPAC Technical Report). Pure Appl. Chem. 2008, 80, 85–104. [Google Scholar] [CrossRef]
- Bobacka, J. Potential stability of all-solid-state ion-selective electrodes using conducting polymers as ion-to-electron transducers. Anal. Chem. 1999, 71, 4932–4937. [Google Scholar] [CrossRef] [PubMed]
- Veder, J.P.; De Marco, R.; Clarke, G.; Chester, R.; Nelson, A.; Prince, K. Elimination of undesirable water layers in solid-contact polymeric ion-selective electrodes. Anal. Chem. 2008, 80, 6731–6740. [Google Scholar] [CrossRef] [PubMed]
- Fibbioli, M.; Morf, W.E.; Badertscher, M.; de Rooij, N.F.; Pretsch, E. Potential drifts of solid-contacted ion-selective electrodes due to zero-current ion fluxes through the sensor membrane. Electroanalysis 2000, 12, 1286–1292. [Google Scholar] [CrossRef]
- Bakker, E. Determination of improved selectivity coefficients of polymer membrane ion-selective electrodes by conditioning with a discriminated ion. J. Electrochem. Soc. 1996, 143, L83–L85. [Google Scholar] [CrossRef]

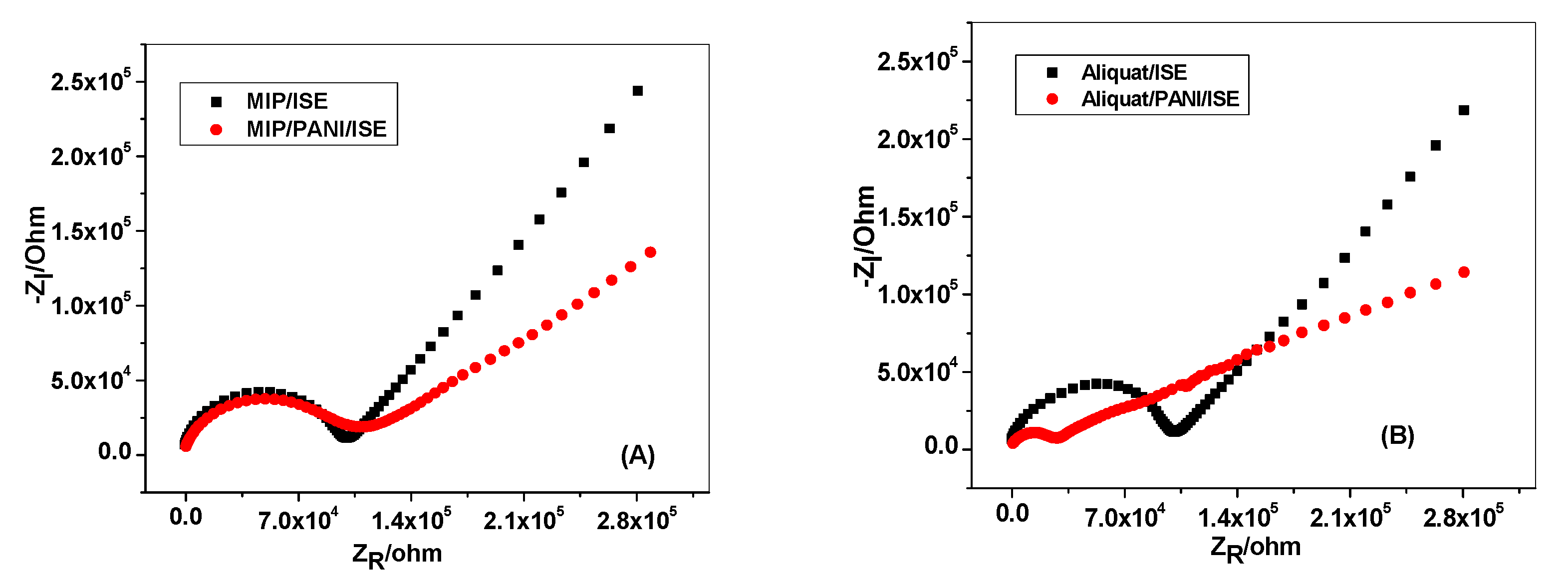
| Parameter | GC/MIP/FLU−-ISE | GC/PANI-MIP/FLU−-ISE | GC/Aliquat/FLU−-ISE | GC/PANI-Aliquat/FLU−-ISE |
|---|---|---|---|---|
| Slope (mV/decade) | −41.7 ± 2.2 | −45.5 ± 1.3 | −54.1 ± 1.1 | −56.3 ± 1.5 |
| Correlation coefficient (r2) | 0.9988 | 0.9998 | 0.9972 | 0.9977 |
| Linear range (M) | 10−2–10−5 | 10−2–10−5 | 10−2–5 × 10−5 | 10−2–3 × 10−5 |
| Detection limit (M) | 7.4 × 10−6 | 5.8 × 10−6 | 1.7 × 10−5 | 8.5 × 10−6 |
| Working pH range (pH) | 4–9 | 4–9 | 4–9 | 4–9 |
| Time response (s) | <10 | <10 | <10 | <10 |
| Accuracy (%) | 98.6 | 99.1 | 99.2 | 99.5 |
| Precision (%) | 1.1 | 0.9 | 0.7 | 0.8 |
| Standard deviation (mV%) | 1.1 | 1.3 | 0.9 | 1.1 |
| Interfering ion, J | (Log Kpot i,j)* | |
|---|---|---|
| GC/PANI-MIP/FLU−-ISE | GC/PANI-Aliquat/FLU−-ISE | |
| Diquat | −3.55 ± 0.07 | −3.62 ± 0.02 |
| Cyromazine | −2.95 ± 0.08 | −4.21 ± 0.06 |
| Bispyribac | −2.42 ± 0.08 | −4.54 ± 0.07 |
| Dinotefuran | −3.43 ± 0.06 | −4.36 ± 0.03 |
| Cl− | −3.47 ± 0.01 | −4.58 ± 0.03 |
| Br− | −3.79 ± 0.02 | −3.38 ± 0.04 |
| SO42− | −3.45 ± 0.09 | −4.93 ± 0.07 |
| NO3− | −3.62 ± 0.02 | −3.79 ± 0.08 |
| CH3COO− | −3.19 ± 0.06 | −5.10 ± 0.09 |
| CN− | −2.09 ± 0.05 | −3.58 ± 0.03 |
| Sample | Amount of Flucarbazone (µg/g) | ||
|---|---|---|---|
| GC/PANI-MIP/FLU−-ISE | GC/PANI-Aliquat/FLU−-ISE | (LC-MS/MS) Method [6] * | |
| 1 | 8.5 ± 0.3 | 8.1 ± 0.4 | 7.8 ± 0.1 |
| 2 | 15.6 ± 0.7 | 14.7 ± 0.7 | 15.2 ± 0.4 |
| 3 | 12.3 ± 0.9 | 11.8 ± 0.6 | 11.1 ± 0.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamel, A.H.; Amr, A.E.-G.E.; Abdalla, N.S.; El-Naggar, M.; Al-Omar, M.A.; Alkahtani, H.M.; Sayed, A.Y.A. Novel Solid-State Potentiometric Sensors Using Polyaniline (PANI) as A Solid-Contact Transducer for Flucarbazone Herbicide Assessment. Polymers 2019, 11, 1796. https://doi.org/10.3390/polym11111796
Kamel AH, Amr AE-GE, Abdalla NS, El-Naggar M, Al-Omar MA, Alkahtani HM, Sayed AYA. Novel Solid-State Potentiometric Sensors Using Polyaniline (PANI) as A Solid-Contact Transducer for Flucarbazone Herbicide Assessment. Polymers. 2019; 11(11):1796. https://doi.org/10.3390/polym11111796
Chicago/Turabian StyleKamel, Ayman H., Abd El-Galil E. Amr, Nashwa S. Abdalla, Mohamed El-Naggar, Mohamed A. Al-Omar, Hamad M. Alkahtani, and Ahmed Y. A. Sayed. 2019. "Novel Solid-State Potentiometric Sensors Using Polyaniline (PANI) as A Solid-Contact Transducer for Flucarbazone Herbicide Assessment" Polymers 11, no. 11: 1796. https://doi.org/10.3390/polym11111796
APA StyleKamel, A. H., Amr, A. E.-G. E., Abdalla, N. S., El-Naggar, M., Al-Omar, M. A., Alkahtani, H. M., & Sayed, A. Y. A. (2019). Novel Solid-State Potentiometric Sensors Using Polyaniline (PANI) as A Solid-Contact Transducer for Flucarbazone Herbicide Assessment. Polymers, 11(11), 1796. https://doi.org/10.3390/polym11111796