Mechanically Reinforced Gelatin Hydrogels by Introducing Slidable Supramolecular Cross-Linkers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Carboxymethyl Ether Group-Modified PRXs (CME-PRXs)
2.3. Characterization of CME-PRXs
2.4. Preparation of Gelatin Hydrogels Cross-Linked by CME-PRXs
2.5. TNBS Assay
2.6. Swelling Test of Gelatin Hydrogels Cross-Linked by CME-PRXs
2.7. Tensile Strength Test
2.8. Cyclic Tensile Test
2.9. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of CME-PRXs
3.2. Preparation of CME-PRX Cross-Linked Gelatin Hydrogels
3.3. Mechanical Properties of Gelatin Hydrogels Cross-Linked by CME-PRXs
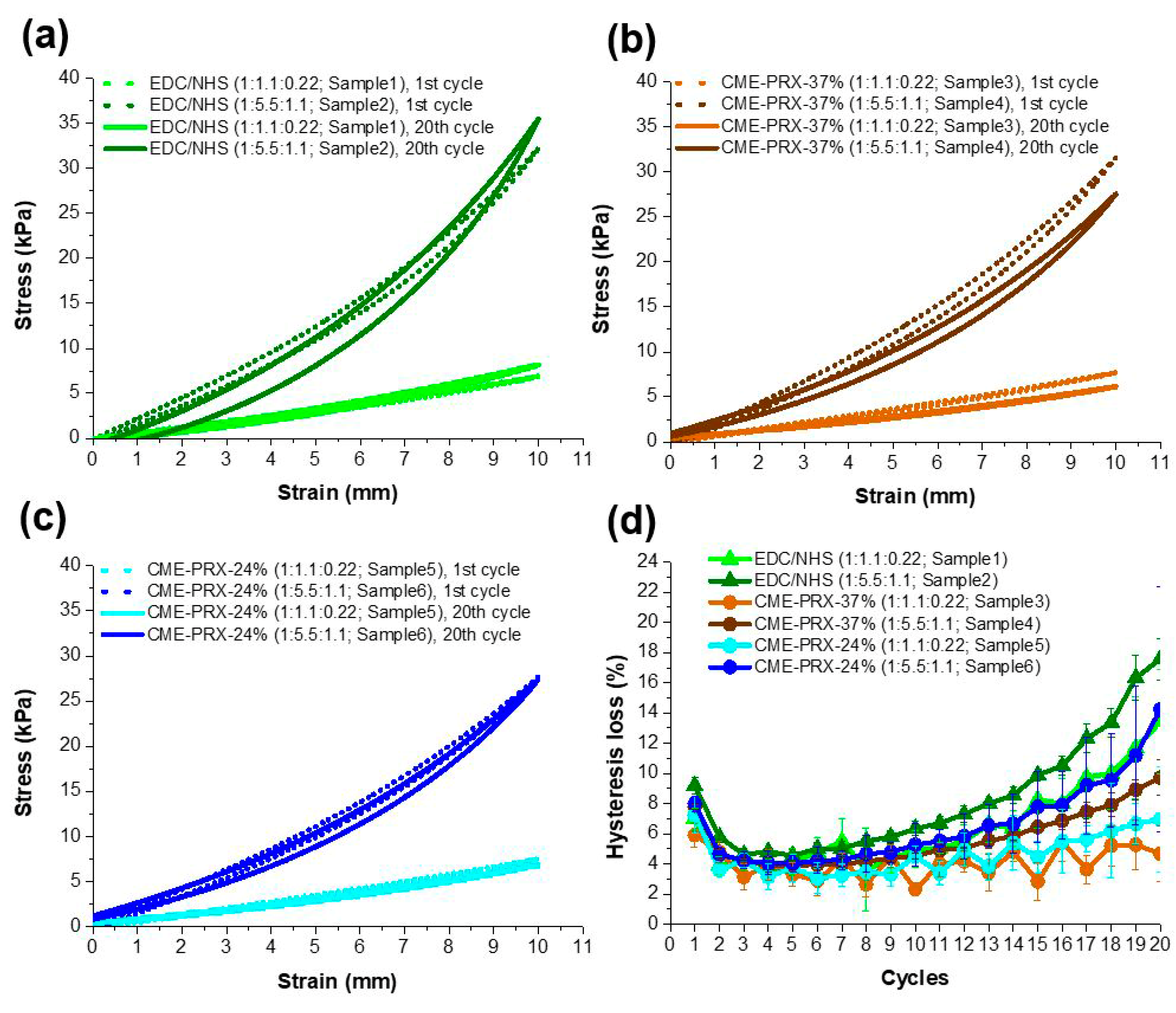
3.4. Effect of Hysteresis in Gelatin Hydrogels Cross-Linked by CME-PRXs Under Cyclic Stretching
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kook, Y.J.; Tian, J.; Jeon, Y.S.; Choi, M.J.; Song, J.E.; Park, C.H.; Reis, R.L.; Khang, G. Nature-derived epigallocatechin gallate/duck’s feet collagen/hydroxyapatite composite sponges for enhanced bone tissue regeneration. J. Biomater. Sci. Polym. Ed. 2017, 29, 984–996. [Google Scholar] [CrossRef] [PubMed]
- Torgersen, J.; Qin, X.-H.; Li, Z.; Ovsianikov, A.; Liska, R.; Stampfl, J. Hydrogels for Two-Photon Polymerization: A Toolbox for Mimicking the Extracellular Matrix. Adv. Funct. Mater. 2013, 23, 4542–4554. [Google Scholar] [CrossRef] [Green Version]
- Stammen, J.A.; Williams, S.; Ku, D.N.; Guldberg, R.E. Mechanical properties of a novel PVA hydrogel in shear and unconfined compression. Biomaterials 2001, 22, 799–806. [Google Scholar] [CrossRef]
- Kushner, A.M.; Gabuchian, V.; Johnson, E.G.; Guan, Z. Biomimetic Design of Reversibly Unfolding Cross-Linker to Enhance Mechanical Properties of 3D Network Polymers. J. Am. Chem. Soc. 2007, 129, 14110–14111. [Google Scholar] [CrossRef] [Green Version]
- Jeon, O.; Song, S.J.; Lee, K.-J.; Park, M.H.; Lee, S.-H.; Hahn, S.K.; Kim, S.; Kim, B.-S. Mechanical properties and degradation behaviors of hyaluronic acid hydrogels cross-linked at various cross-linking densities. Carbohydr. Polym. 2007, 70, 251–257. [Google Scholar] [CrossRef]
- Temenoff, J.S.; Athanasiou, K.A.; Lebaron, R.G.; Mikos, A.G. Effect of Poly(ethylene glycol) Molecular Weight on Tensile and Swelling Properties of Oligo(poly(ethylene glycol) fumarate) Hydrogels for Cartilage Tissue Engineering. J. Biomed. Mater. Res. 2002, 59, 429–437. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Zheng, X.; Zhang, A.; Zhang, X.; Tang, K. Pullulan dialdehyde crosslinked gelatin hydrogels with high strength for biomedical applications. Carbohydr. Polym. 2019, 216, 45–53. [Google Scholar] [CrossRef]
- Hong, J.; Shin, Y.; Kim, S.; Lee, J.; Cha, C. Complex Tuning of Physical Properties of Hyperbranched Polyglycerol-Based Bioink for Microfabrication of Cell-Laden Hydrogels. Adv. Funct. Mater. 2019, 29, 1808750. [Google Scholar] [CrossRef]
- Lv, X.; Liu, C.; Shao, Z.; Sun, S. Tuning Physical Crosslinks in Hybrid Hydrogels for Network Structure Analysis and Mechanical Reinforcement. Polymers 2019, 11, 352. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Y.; Ferracci, G.; Zheng, J.; Cho, N.-J.; Lee, B.H. Gelatin methacryloyl and its hydrogels with an exceptional degree of controllability and batch-to-batch consistency. Sci. Rep. 2019, 9, 6863. [Google Scholar] [CrossRef]
- Zhou, M.; Lee, B.H.; Tan, Y.J.; Tan, L.P. Microbial transglutaminase induced controlled crosslinking of gelatin methacryloyl to tailor rheological properties for 3D printing. Biofabrication 2019, 11, 025011. [Google Scholar] [CrossRef]
- Natu, M.V.; Sardinha, J.P.; Correia, I.J.; Gil, M.H. Controlled release gelatin hydrogels and lyophilisates with potential application as ocular inserts. Biomed. Mater. 2007, 2, 241–249. [Google Scholar] [CrossRef]
- Amadori, S.; Torricelli, P.; Rubini, K.; Fini, M.; Panzavolta, S.; Bigi, A. Effect of sterilization and crosslinking on gelatin films. J. Mater. Sci. Mater. Electron. 2015, 26, 69. [Google Scholar] [CrossRef]
- Dash, R.; Foston, M.; Ragauskas, A.J. Improving the mechanical and thermal properties of gelatin hydrogels cross-linked by cellulose nanowhiskers. Carbohydr. Polym. 2013, 91, 638–645. [Google Scholar] [CrossRef]
- Shibaguchi, K.; Tamura, A.; Terauchi, M.; Matsumura, M.; Miura, H.; Yui, N. Mannosylated Polyrataxanes for Increasing Cellular Uptake Efficiency in Macrophages through Receptor-Mediated Endocytosis. Molecules 2019, 24, 439. [Google Scholar] [CrossRef]
- Tamura, A.; Yui, N. Rational Design of Stimuli-Cleavable Polyrataxanes for Therapeutic Applications. Polym. J. 2017, 49, 527–534. [Google Scholar] [CrossRef]
- Tamura, A.; Yui, N. Threaded macromolecules as a versatile framework for biomaterials. Chem. Commun. 2014, 50, 13433–13446. [Google Scholar] [CrossRef] [Green Version]
- Tamura, A.; Tonegawa, A.; Arisaka, Y.; Yui, N. Versatile Synthesis of End-Reactive Polyrataxanes Applicable to Fabrication of Supramolecular Biomaterials. Beilstein J. Org. Chem. 2016, 12, 2883–2892. [Google Scholar] [CrossRef]
- Seo, J.-H.; Kakinoki, S.; Yamaoka, T.; Yui, N. Directing Stem Cell Differentiation by Changing the Molecular Mobility of Supramolecular Surfaces. Adv. Healthc. Mater. 2015, 4, 215–222. [Google Scholar] [CrossRef]
- Seo, J.-H.; Kakinoki, S.; Inoue, Y.; Nam, K.; Yamaoka, T.; Ishihara, K.; Kishida, A.; Yui, N. The significance of hydrated surface molecular mobility in the control of the morphology of adhering fibroblasts. Biomaterials 2013, 34, 3206–3214. [Google Scholar] [CrossRef]
- Tamura, A.; Ohashi, M.; Yui, N. Oligo(ethylene glycol)-Modified β-cyclodextrin-Based Polyrataxanes for Simultaneously Modulating Solubility and Cellular Internalization Efficiency. J. Biomater. Sci. Polym. Ed. 2017, 28, 1124–1139. [Google Scholar] [CrossRef]
- Ito, K. Slide-ring materials using topological supramolecular architecture. Curr. Opin. Solid State Mater. Sci. 2010, 14, 28–34. [Google Scholar] [CrossRef]
- Okumura, Y.; Ito, K. The Polyrotaxane Gel: A Topological Gel by Figure-of-Eight Cross-links. Adv. Mater. 2001, 13, 485–487. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, C.; Mayumi, K.; Kato, K.; Yokoyama, H.; Ito, K. Highly Stretchable and Instantly Recoverable Slide-Ring Gels Consisting of Enzymatically Synthesized Polyrotaxane with Low Host Coverage. Chem. Mater. 2018, 30, 5013–5019. [Google Scholar] [CrossRef]
- Kato, K.; Hori, A.; Ito, K. An efficient synthesis of low-covered polyrotaxanes grafted with poly(ε-caprolactone) and the mechanical properties of its cross-linked elastomers. Polymer 2018, 147, 67–73. [Google Scholar] [CrossRef]
- Minato, K.; Mayumi, K.; Maeda, R.; Kato, K.; Yokoyama, H.; Ito, K. Mechanical properties of supramolecular elastomers prepared from polymer-grafted polyrotaxane. Polymer 2017, 128, 386–391. [Google Scholar] [CrossRef]
- Ito, K. Novel Cross-Linking Concept of Polymer Network: Synthesis, Structure, and Properties of Slide-Ring Gels with Freely Movable Junctions. Polym. J. 2007, 39, 489–499. [Google Scholar] [CrossRef]
- Araki, J.; Zhao, C.; Ito, K. Efficient Production of Polyrotaxanes from α-Cyclodextrin and Poly(ethylene glycol). Macromolecules 2005, 38, 7524–7527. [Google Scholar] [CrossRef]
- Araki, J. Polyrataxane Derivatives. II. Preparation and Characterization of Ionic Polyrotaxanes and Ionic Slide-Ring Gels. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 2199–2209. [Google Scholar] [CrossRef]
- Matsui, H.; Tamura, A.; Osawa, M.; Tonegawa, A.; Arisaka, Y.; Matsumura, M.; Miura, H.; Yui, N. Scavenger Receptor A-Mediated Targeting of Carboxylated Polyrotaxanes to Macrophages and the Impacts of Supramolecular Structure. Macromol. Biosci. 2018, 18, 1800059. [Google Scholar] [CrossRef]
- Layman, H.; Spiga, M.-G.; Brooks, T.; Pham, S.; Webster, K.A.; Andreopoulos, F.M. The effect of the controlled release of basic fibroblast growth factor from ionic gelatin-based hydrogels on angiogenesis in a murine critical limb ischemic model. Biomaterials 2007, 28, 2646–2654. [Google Scholar] [CrossRef] [Green Version]
- Wissink, M.J.B.; Beernink, R.; Pieper, J.S.; Poot, A.A.; Engbers, G.H.M.; Beugeling, T.; Aken, W.G.; Feijen, J. Immobilization of Heparin to EDC/NHS-Crosslinked Collagen. Characterization and In Vitro Evaluation. Biomaterials 2001, 22, 151–163. [Google Scholar] [CrossRef]
- Li, Z.; Qu, T.; Ding, C.; Ma, C.; Sun, H.; Li, S.; Liu, X. Injectable Gelatin Derivative Hydrogels with Sustained Vascular Endothelial Growth Factor Release for Induced Angiogenesis. Acta Biomater. 2015, 13, 88–100. [Google Scholar] [CrossRef]
- Kim, M.S.; Jun, I.; Shin, Y.M.; Jang, W.; Kim, S.I.; Shin, H. The Development of Genipin-Crosslinked Poly(caprolactone) (PCL)/ Gelatin Nanofibers for Tissue Engineering Applications. Macromol. Biosci. 2010, 10, 91–100. [Google Scholar] [CrossRef]
- Nam, K.; Kimura, T.; Kishida, A. Controlling Coupling Reaction of EDC and NHS for Preparation of Collagen Gels Using Ethanol/Water Co-Solvents. Macromol. Biosci. 2008, 8, 32–37. [Google Scholar] [CrossRef]
- Long, R.; Hui, C.-Y. Fracture toughness of hydrogels: Measurement and interpretation. Soft Matter 2016, 12, 8069–8086. [Google Scholar] [CrossRef]
- Xu, L.; Wang, C.; Cui, Y.; Li, A.; Qiao, Y.; Qiu, D. Conjoined-network rendered stiff and tough hydrogels from biogenic molecules. Sci. Adv. 2019, 5, eaau3442. [Google Scholar] [CrossRef] [Green Version]
- Bin Imran, A.; Esaki, K.; Gotoh, H.; Seki, T.; Ito, K.; Sakai, Y.; Takeoka, Y. Extremely stretchable thermosensitive hydrogels by introducing slide-ring polyrotaxane cross-linkers and ionic groups into the polymer network. Nat. Commun. 2014, 5, 5124. [Google Scholar] [CrossRef]
- Okazumi, Y.; Ito, K.; Kato, K.; Okabe, Y. A significant impact of host–guest stoichiometry on the extensibility of polyrotaxane gels. Chem. Commun. 2015, 51, 16180–16183. [Google Scholar]
- Li, G.; Kato, K.; Mayumi, K.; Yokoyama, H.; Ito, K. Efficient Mechanical Toughening of Polylactic Acid without Substantial Decreases in Stiffness and Transparency by the Reactive Grafting of Polyrotaxanes. J. Incl. Phenom. Macrocycl. Chem. 2019, 93, 107–116. [Google Scholar] [CrossRef]
- Kato, K.; Ikeda, Y.; Ito, K. Direct Determination of Cross-Link Density and Its Correlation with the Elastic Modulus of a Gel with Slidable Cross-Links. ACS Macro Lett. 2019, 8, 700–704. [Google Scholar] [CrossRef]
- Broderick, E.P.; O’Halloran, D.M.; Rochev, Y.A.; Griffin, M.; Collighan, R.J.; Pandit, A.S. Enzymatic Stabilization of Gelatin-Based Scaffolds. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2005, 72B, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, M.L.; Nielsen, L.H.; Boisen, A.; Almdal, K.; Dufva, M. Characterization of Thin Gelatin Hydrogel Membranes with Balloon Properties for Dynamic Tissue Engineering. Biopolymers 2019, 110, e23241. [Google Scholar] [CrossRef] [PubMed]
- Bitoh, Y.; Akuzawa, N.; Urayama, K.; Takigawa, T.; Kidowaki, M.; Ito, K. Peculiar Nonlinear Elasticity of Polyrotaxane Gels with Movable Cross-Links Revealed by Multiaxial Stretching. Macromolecules 2011, 44, 8661–8667. [Google Scholar] [CrossRef]
- Konda, A.; Mayumi, K.; Urayama, K.; Takigawa, T.; Ito, K. Influence of Structural Characteristics on Stretching-Driven Swelling of Polyrotaxane Gels with Movable Cross Links. Macromolecules 2012, 45, 6733–6740. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, D.; Yang, J.; Nishi, T.; Ito, K.; Zhao, X.; Zhang, L. Novel Slide-Ring Material/Natural Rubber Composites with High Damping Property. Sci. Rep. 2016, 6, 22810. [Google Scholar] [CrossRef]




| Sample | Mn of the PEG Axle | Number of Threaded α-CDs onto PRX 1 | Number of CME Groups on PRX 2 | Mn3 |
|---|---|---|---|---|
| CME-PRX-24% | 35,000 | 96 (24.1 %) | 294 (3.06) | 157,000 |
| CME-PRX-37% | 35,000 | 147 (37.0 %) | 498 (3.39) | 227,000 |
| Sample | Cross-Linker Type | Weight Ratio of Gelatin/CME-PRX/EDC/NHS 1 | Cross-Linking Degree (%) | Young’s Modulus (kPa) | |
|---|---|---|---|---|---|
| 1% Strain | Fracture Point | ||||
| 1 | EDC/NHS | 100:0:0.46:0.06 (0:1.1:0.22) | 8.41 ± 4.86 | 0.11 ± 0.01 | 1.24 ± 0.20 |
| 2 | EDC/NHS | 100:0:2.31:0.28 (0:5.5:1.1) | 18.41 ± 2.09 | 0.27 ± 0.01 | 1.79 ± 0.14 |
| 3 | CME-PRX-37% | 100:1:0.46:0.06 (1:1.1:0.22) | 9.69 ± 3.66 | 0.11 ± 0.01 | 1.31 ± 0.09 |
| 4 | CME-PRX-37% | 100:1:2.31:0.28 (1:5.5:1.1) | 17.64 ± 0.51 | 0.30 ± 0.01 | 1.84 ± 0.24 |
| 5 | CME-PRX-24% | 100:1.17:0.46:0.06 (1:1.1:0.22) | 11.88 ± 3.21 | 0.11 ± 0.02 | 1.61 ± 0.30 |
| 6 | CME-PRX-24% | 100:1.17:2.31:0.28 (1:5.5:1.1) | 21.69 ± 4.00 | 0.32 ± 0.02 | 1.83 ± 0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.H.; Tamura, A.; Arisaka, Y.; Seo, J.-H.; Yui, N. Mechanically Reinforced Gelatin Hydrogels by Introducing Slidable Supramolecular Cross-Linkers. Polymers 2019, 11, 1787. https://doi.org/10.3390/polym11111787
Lee DH, Tamura A, Arisaka Y, Seo J-H, Yui N. Mechanically Reinforced Gelatin Hydrogels by Introducing Slidable Supramolecular Cross-Linkers. Polymers. 2019; 11(11):1787. https://doi.org/10.3390/polym11111787
Chicago/Turabian StyleLee, Dae Hoon, Atsushi Tamura, Yoshinori Arisaka, Ji-Hun Seo, and Nobuhiko Yui. 2019. "Mechanically Reinforced Gelatin Hydrogels by Introducing Slidable Supramolecular Cross-Linkers" Polymers 11, no. 11: 1787. https://doi.org/10.3390/polym11111787
APA StyleLee, D. H., Tamura, A., Arisaka, Y., Seo, J.-H., & Yui, N. (2019). Mechanically Reinforced Gelatin Hydrogels by Introducing Slidable Supramolecular Cross-Linkers. Polymers, 11(11), 1787. https://doi.org/10.3390/polym11111787





