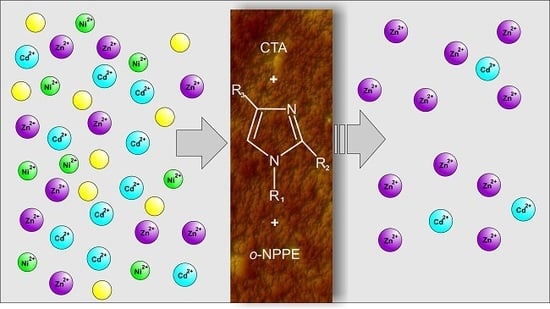
Polymer Inclusion Membranes (PIMs) Doped with Alkylimidazole and their Application in the Separation of Non-Ferrous Metal Ions
Abstract
:1. Introduction
2. Experimental
2.1. Reagents
The Preparation and Characteristics of Polymer Inclusion Membranes
2.2. Transport Studies
3. Results and Discussion
3.1. Membrane Characterization
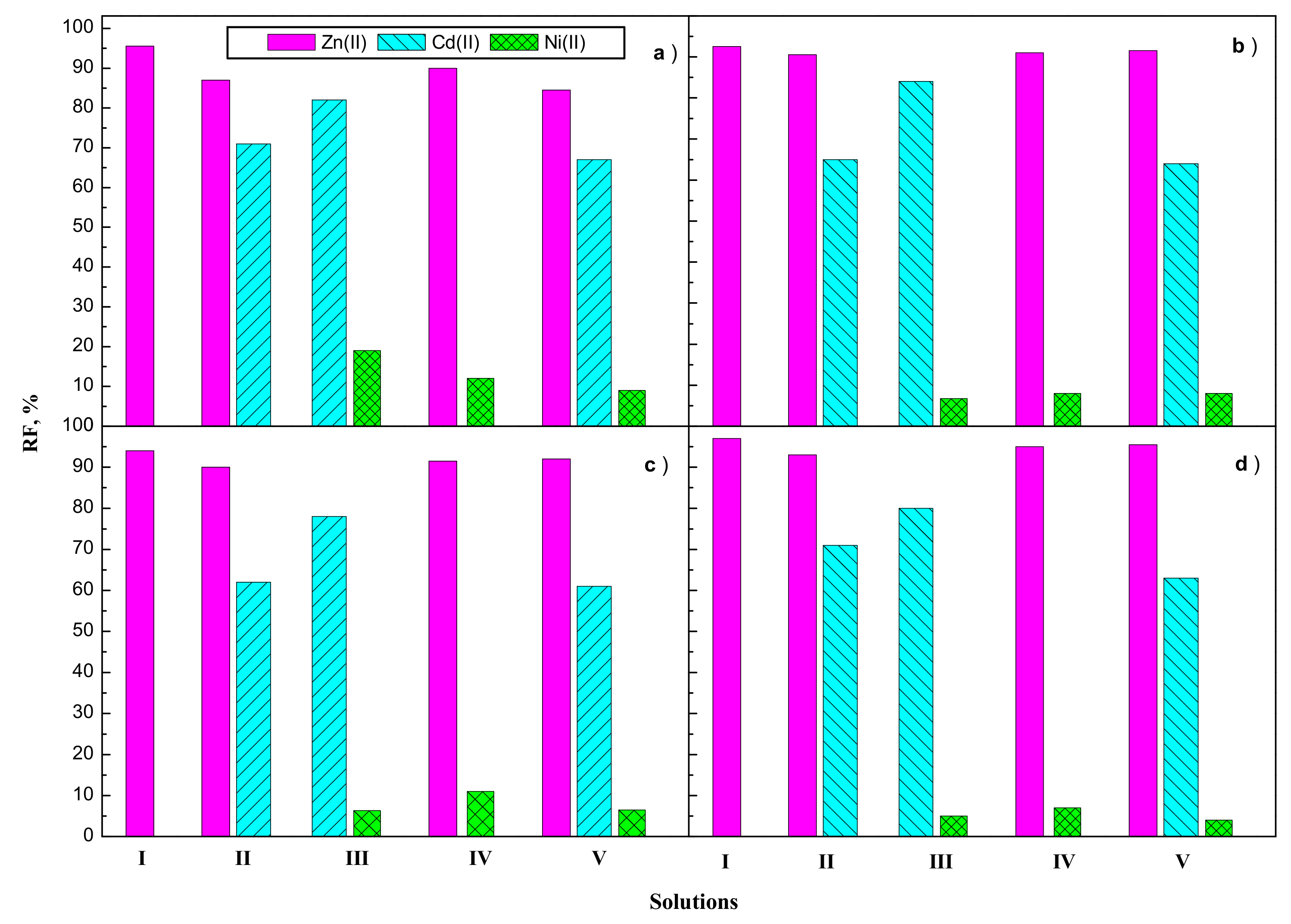
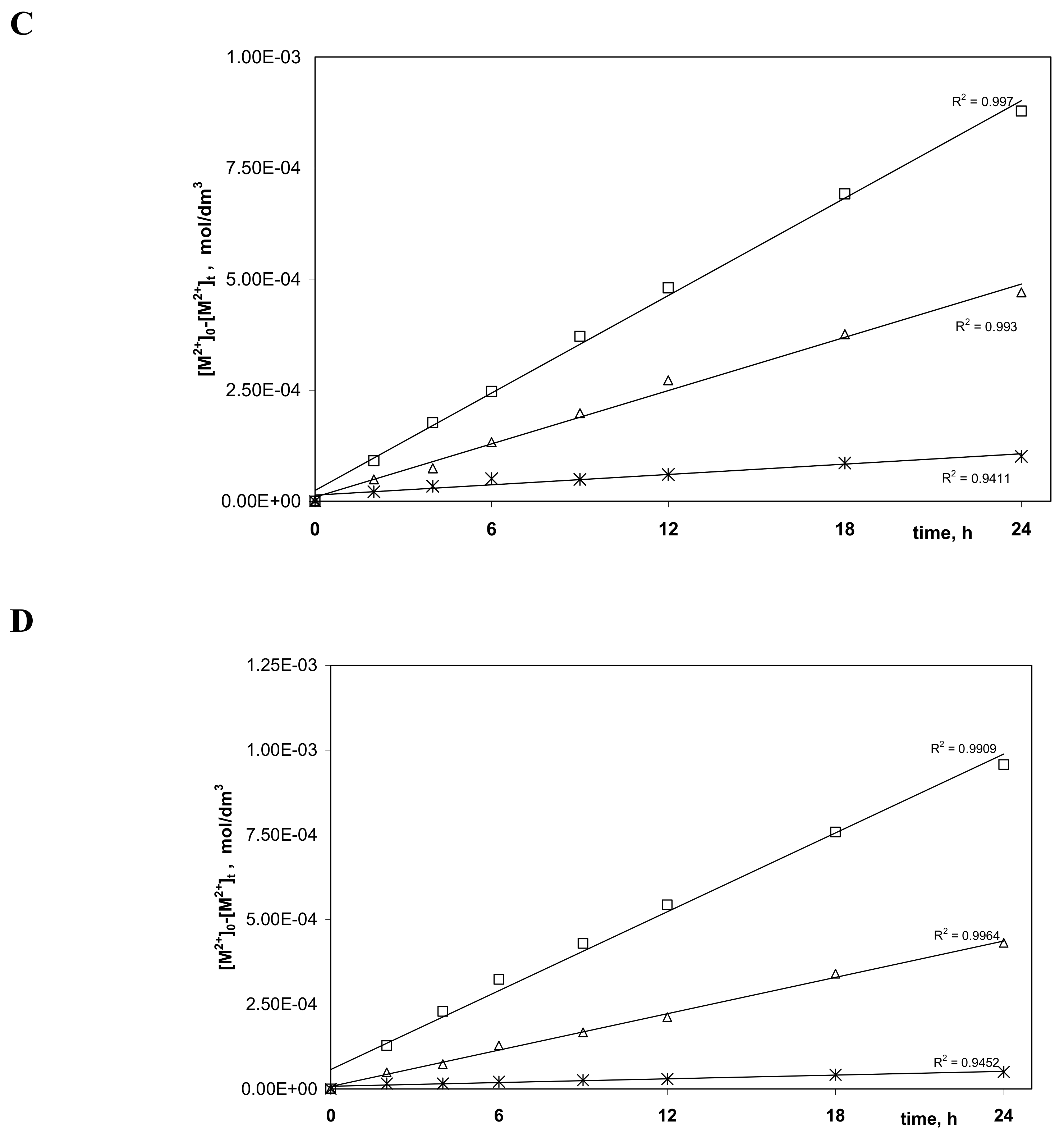
3.2. Transport Zn(II), Cd(II), and Ni(II) across PIMs
3.3. Recovery of Metal
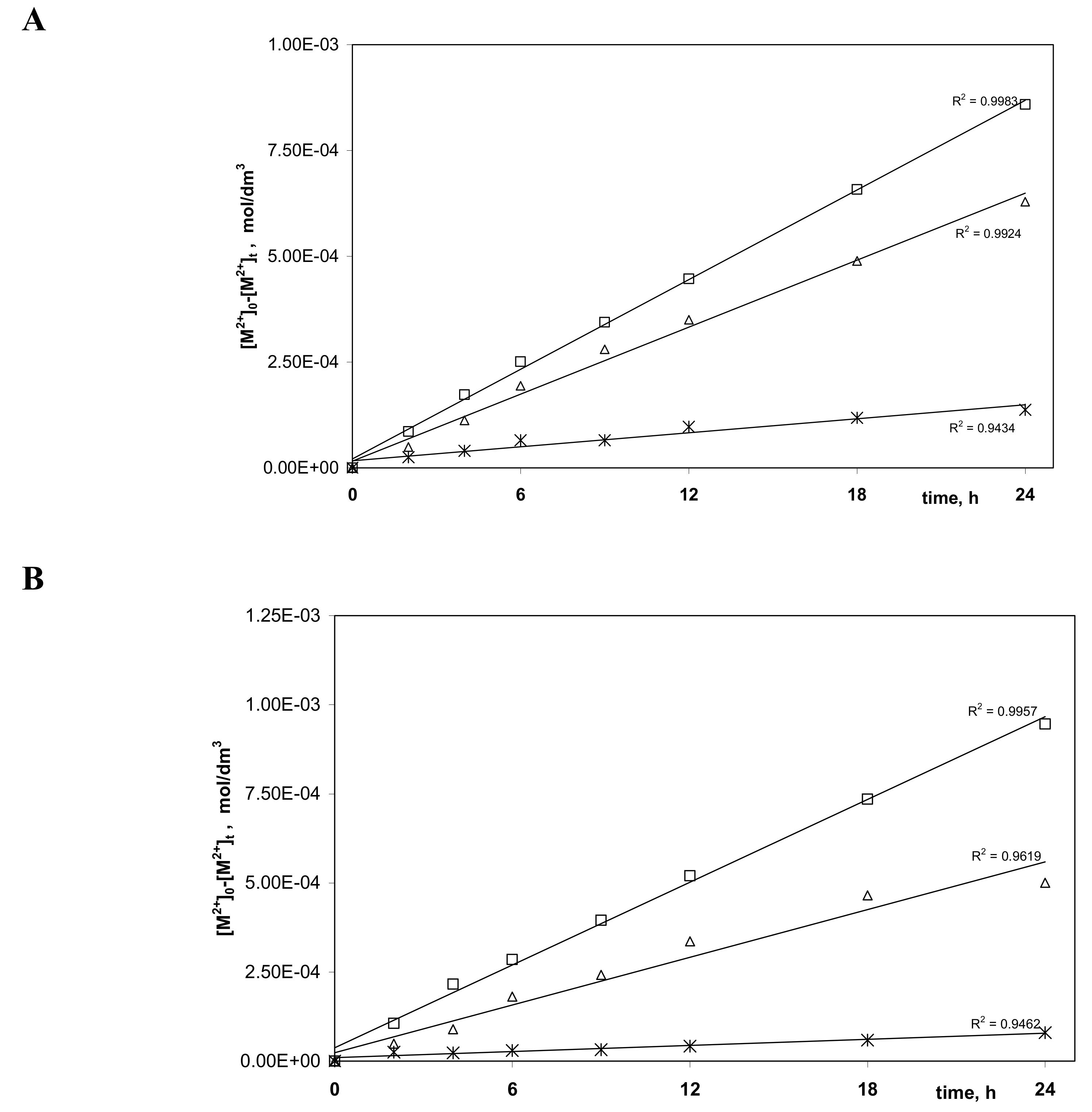
3.4. Membrane Diffusion Coefficients of Zn(II), Cd(II), and Ni(II) Complexes with Alkylimidazole (1–4)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cote, G. Hydrometallurgy of strategic metals. Solvent Extr. Ion Exch. 2000, 18, 703–727. [Google Scholar] [CrossRef]
- Habashi, F. Textbook of Hydrometallurgy, 2nd ed.; Metallurgie Extractive Quebec: Quebec, QC, Canada, 2007. [Google Scholar]
- Rao, S.R. Resource, Recovery and Recycling from Metallurgical Wastes; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Pretz, T.; Julius, J. Metal Waste; Waste—A Handbook for Management; Academic Press: Cambridge, MA, USA, 2011; Chapter 6; pp. 89–99. [Google Scholar]
- Wang, L.K.; Shammas, N.K.; Hung, Y.-T. Waste Treatment in the Metal Manufacturing, Forming, Coating and Finishing Industries; CRC Press Taylor and Francis group: Boca Raton, FL, USA, 2009. [Google Scholar]
- Rydberg, J.; Cox, M.; Musakis, C.; Choppin, G.R. Principles and Practices of Solvent Extraction. Revised and Expanded, 2nd ed.; M. Dekker, Inc.: New York, NY, USA, 2004; ISBN 9780824750633. [Google Scholar]
- Kislik, V.S. Solvent Extraction—Classical and Novel Approaches; Elsevier B.V.: Amsterdam, The Netherlands, 2012; ISBN 978-0-444-53778-2. [Google Scholar] [CrossRef]
- Mapatac, L.C. Handbook of Separation Process Technology; Delve Publishing LLC: Oakville, Canada, 2017; ISBN 9781680957303. [Google Scholar]
- Schlesinger, M.E.; King, M.J.; Sole, K.C.; Davenport, W.G. Solvent Extraction, Extractive Metallurgy of Copper, 5th ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2011; pp. 323–347. ISBN 9780080967899. [Google Scholar]
- Jha, M.K.; Kumar, V.; Singh, R.J. Solvent extraction of zinc from chloride solutions. Solvent Extr. Ion Exch. 2002, 20, 389–405. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumar, V.; Jeong, J.; Lee, J. Review on solvent extraction of cadmium from various solutions. Hydrometallurgy 2012, 111, 1–9. [Google Scholar] [CrossRef]
- Schimmel, K.A.; Ilias, S.; Akella, S. Nondispersive liquid-liquid extraction of Zn(II), Cu(II), Co(II), and Cd(II) from dilute solution with DEHPA in a hollow-fiber membrane module. Sep. Sci. Technol. 2001, 36, 805–821. [Google Scholar] [CrossRef]
- Wassink, B.; Dreisinger, D.; Howard, J. Solvent extraction separation of zinc and cadmium from nickel and cobalt using Aliquat 336, a strong base anion exchanger, in the chloride and thiocyanate forms. Hydrometallurgy 2000, 57, 235–252. [Google Scholar] [CrossRef]
- Wejman-Gibas, K.; Pilsniak-Rabiega, M.; Ochromowicz, K. Solvent extraction of zinc(II) from ammonia leaching solution by LIX 54-100, LIX 84I and TOA. Physicochem. Probl. Miner. Process. 2017, 53, 202–211. [Google Scholar]
- Szymanowski, J. Kinetics and interfacial phenomena. Solvent Extr. Ion Exch. 2000, 18, 728–751. [Google Scholar] [CrossRef]
- Fleitlikh, I.U.; Grigorieva, M.A.; Logutenko, O.A. Extraction of non-ferrous metals and iron with systems based on bis(2,4,4-trimethylpentyl) dithiophosphinic acid (CYANEX 301). A review. Solvent Extr. Ion Exch. 2018, 36, 1–21. [Google Scholar] [CrossRef]
- Aguilar, M.; Cortina, J.L. Solvent Extraction and Liquid Membranes: Fundamentals and Applications in New Materials; CRC Pres Taylor and Francis group LLC: Baca Raton, FL, USA, 2010; ISBN 9780824740153. [Google Scholar]
- Kislik, V. Liquid Membranes—Principles and Applications in Chemical Separations and Wastewater Treatment; Elsevier: Burlington, NY, USA, 2010; ISBN 9780444532183. [Google Scholar]
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef] [Green Version]
- Sgarlata, C.; Arena, G.; Longo, E.; Zhang, D.; Yang, Y.; Bartsch, R.A. Heavy metal separation with polymer inclusion membranes. J. Membrane Sci. 2008, 323, 444–451. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Bringas, E.; Tan, N.R.; Ortiz, I.; Ghahramani, M.; Shahmirzadi, M.A.A. Recent progress in development of high performance polymeric membranes and materials for metal plating wastewater treatment: A review. J. Water Proc. Eng. 2016, 9, 78–110. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Ulewicz, R.; Ulewicz, M. Zinc recovery from model and waste solutions using polymer inclusion membrane (PIMs) with 1-octyl-4-methylimidazole. Desalin. Water Treat. 2018, 102, 211–219. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Ulewicz, M. Selective transport of Cu(II) across a polymer inclusion membrane with 1-alkylimidazole from nitrate solutions. Sep. Sci. Technol. 2012, 47, 1113–1118. [Google Scholar] [CrossRef]
- Bryan, Y.O.; Truong, Y.B.; Cattrall, R.W.; Kyratzis, I.L.; Kolev, S.D. A new generation of highly stable and permeable polymer inclusion membranes (PIMs) with their carrier immobilized in a crosslinked semi-interpenetrating polymer network. Application to the transport of thiocyanate. J. Membr. Sci. 2017, 529, 55–62. [Google Scholar] [CrossRef]
- Senhadji-Kebiche, O.; Belaid, T.; Benamor, M. Polymer inclusion membrane (PIM) as competitive material for applications in SPE for water treatment process. Open Access Libr. J. 2014, 1, e758. [Google Scholar] [CrossRef]
- Ulewicz, M.; Radzyminska-Lenarcik, E. Application of polymer and supported membranes with 1-decyl-4-methylimidazole for pertraction of transition metal ions. Sep. Sci. Technol. 2014, 49, 1713–1721. [Google Scholar] [CrossRef]
- Altin, S.; Alemdar, S.; Atlin, A.; Yildririm, Y. Facilitated transport of Cd(II) through a supported liquid membrane with Aliquat 336 as a carrier. Sep. Sci. Technol. 2011, 46, 754–764. [Google Scholar] [CrossRef]
- Aguilar, J.C.; Sanches-Castellanos, M.; de San Miguel, E.R.; de Gyves, J. Cd(II) and Pb(II) extraction and transport modeling in SLM and PIM systems using Kelex 100 as carrier. J. Membr. Sci. 2001, 190, 107–114. [Google Scholar] [CrossRef]
- Ulewicz, M.; Radzymińska-Lenarcik, E. Application of polymer inclusion membranes doped with 1-hexyl-4-methylimidazole for pertraction of zinc(II) and other transition metal ions. Physicochem. Probl. Miner. Process. 2015, 51, 447–460. [Google Scholar]
- Ulewicz, M.; Radzyminska-Lenarcik, E. Application of supported and polymer membrane with 1-decyl-2-methylimidazole for separation of transition metal ions. Physicochem. Probl. Miner. Process. 2012, 48, 91–102. [Google Scholar]
- Ncib, S.; Barhoumi, A.; Bouguerra, W.; Larchet, C.; Dammak, L.; Hamrouni, B.; Elaloui, E. Preparation and characterization of cellulose triacetate polymer inclusion membrane blended with acetylated kraft lignin: Effect of AKL and application to the copper(II) extraction from acidic media. Desalin. Water Treat. 2018, 104, 263–272. [Google Scholar] [CrossRef]
- Annane, K.; Sahmoune, A.; Montels, P.; Tingry, S. Polymer inclusion membrane extraction of cadmium(II) with Aliquat 336 in micro-channel cell. Chem. Eng. Res. Des. 2015, 94, 605–610. [Google Scholar] [CrossRef]
- Baczynska, M.; Regel-Rosocka, M.; Nowicki, M.; Wiśniewski, M. Effect of the structure of polymer inclusion membranes on Zn(II) transport from chloride aqueous solutions. J. Appl. Polym. Sci. 2015, 132, 42319–42329. [Google Scholar] [CrossRef]
- Inês, M.; Almeida, G.S.; Cattrall, R.W.; Kolev, S.D. Recent trends in extraction and transport of metal ions using polymer inclusion membranes (PIMs). J. Membr. Sci. 2012, 415, 9–23. [Google Scholar]
- Kozlowski, C.A.; Walkowiak, W. Applicability of liquid membranes in chromium(VI) transport with amines as ion carriers. J. Membr. Sci. 2005, 266, 143–150. [Google Scholar] [CrossRef]
- Gherrou, A.; Kerdjoudj, H.; Molinari, R.; Seta, P.; Drioli, E. Fixed sites plasticized cellulose triacetate membranes containing crown ethers for silver(I), copper(II) and gold(III) ions transport. J. Membr. Sci. 2004, 228, 149–157. [Google Scholar] [CrossRef]
- Gherrou, A.; Kerdjoudj, H. Specific membrane transport of silver and copper as Ag(CN)32− and Cu(CN)43− ions through a supported liquid membrane using K+-crown ether as a carrier. Desalination 2003, 151, 87–94. [Google Scholar] [CrossRef]
- Arous, O.; Amara, M.; Kerdjoudj, H. Synthesis and characterization of cellulose triacetate and poly(ethylene imine) membranes containing a polyether macrobicyclic: Their application to the separation of copper(II) and silver(I) ions. J. Appl. Polym. Sci. 2004, 93, 1401–1410. [Google Scholar] [CrossRef]
- Xu, J.; Wang, L.; Shen, W.; Paimin, R.; Wang, X. The Influence of the Interior Structure of Aliquat 336/PVC membranes to their extraction behavior. Sep. Sci. Technol. 2005, 39, 3527–3539. [Google Scholar] [CrossRef]
- Gherrou, A.; Kerdjoudj, H.; Molinari, R.; Seta, P. Preparation and characterization of polymer plasticized membranes (PPM) embedding a crown ether carrier application to copper ions transport. Mat. Sci. Eng. C 2005, 25, 436–443. [Google Scholar] [CrossRef]
- Arous, O.; Kerdjoudj, H.; Seta, P. Comparison of carrier-facilitated silver(I) and copper(II) ions transport mechanisms in a supported and in a plasticized cellulose triacetate membrane. J. Membr. Sci. 2004, 241, 177–185. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Ulewicz, M. The application of polymer inclusion membranes based on CTA with 1-alkylimidazole for the separation of zinc(II) and manganese(II) ions from aqueous solutions. Polymers 2019, 11, 242. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.S.; Walker, J.O.; Lamb, J.D. Permeability and durability effects of cellulose polymer variation in polymer inclusion membranes. J. Membr. Sci. 2004, 229, 87–93. [Google Scholar] [CrossRef]
- Pernak, J.; Krysinski, J.; Skrzypczak, A. Bakterizide wirkung von iminiumverbindungen. A Tenside Surfact Det. 1987, 27, 276–286. [Google Scholar]
- Lenarcik, B.; Ojczenasz, P. The influence of the size and position of the alkyl groups in alkylimidazole molecules on their acid – base properties. J. Heterocycl. Chem. 2002, 39, 287–290. [Google Scholar] [CrossRef]
- Ulewicz, M.; Radzyminska-Lenarcik, E. Supported liquid (SLM) and polymer inclusion (PIM) membranes pertraction of copper(II) from aqueous nitrate solutions by 1-hexyl-2-methylimidazole. Sep. Sci. Technol. 2012, 47, 1383–1389. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Ulewicz, M. The use of 1-alkylimidazoles for selective separation of zinc ions in the transport process across polymer inclusion membrane. Physicochem. Probl. Miner. Process. 2014, 50, 131–142. [Google Scholar]
- Ulewicz, M.; Sadowska, K.; Biernat, J.F. Selective transport of Pb(II) across polymer inclusion membrane using imidazole azocrown ethers as carriers. Physicochem. Probl. Miner. Process. 2007, 41, 133–143. [Google Scholar]
- Ulewicz, M.; Szczygelska-Tao, J.; Biernat, J.F. Selectivity of Pb(II) transport across polymer inclusion membranes doped with imidazole azothiacrown ethers. J. Membr. Sci. 2009, 344, 32–38. [Google Scholar] [CrossRef]
- Salazar-Alvarez, G.; Bautista-Flores, A.N.; San Miguel, E.R.; Muhammed, M.; Gyves, J. Transport characterization of a PIM system used for the extraction of Pb(II) using D2EHPA as carrier. J. Membr. Sci. 2005, 250, 247–257. [Google Scholar] [CrossRef]
- Tor, A.; Arslan, G.; Muslu, H.; Celikas, A.; Cengeloglu, Y.; Ersoz, M. Facilitated transport of Cr(III) thought polymer inclusion membrane with di(2-ethylhexyl) phosphoric acid (DEHPA). J. Membr. Sci. 2009, 329, 169–174. [Google Scholar] [CrossRef]
- Danesi, P.R. Separation of metal species by supported liquid membranes. Sep. Sci. Technol. 1984, 19, 857–894. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Witt, K. The application of membrane extraction on the separation of zinc and cadmium ions. Desalin. Water Treat. 2018, 128, 140–147. [Google Scholar] [CrossRef]
- Wolf, J.R.; Strieder, W. Surface and void tortuosities for a random fiber bed: Overlapping, parallel cylinders of several radii. J. Membr. Sci. 1990, 49, 103–115. [Google Scholar] [CrossRef]
- Lenarcik, B.; Kierzkowska, A. The influence of alkyl chain length and steric effect on extraction of zinc(II) complexes with 1-alkyl-2-methylimidazoles. Solvent Ext. Ion Exch. 2006, 24, 433–445. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Lenarcik, B. Determination of stability constants of some 1-Alkylimidazole complexes with Cd(II) by extraction method. In Proceedings of the XXIVth International Symposium on Physicochemical Methods of Separations “Ars Separatoria 2009”, Kudowa-Zdroj, Poland, 14–18 June 2009; pp. 187–188. [Google Scholar]
- Lenarcik, B.; Rauckyte, T. The influence of alkyl chain length on extraction equilibria of Ni(II) complexes with 1-alkylimidazoles in aqueous solution/organic solvent systems. Sep. Sci. Technol. 2004, 39, 3353–3372. [Google Scholar] [CrossRef]
- Lenarcik, B.; Kierzkowska, A. The influence of alkyl chain length on stability Constants of Zn(II) Complexes with 1-Alkylimidazoles in Aqueous Solutions and their partition between aqueous phase and organic solvent. Solvent Ext. Ion Exch. 2004, 22, 449–471. [Google Scholar] [CrossRef]
- Lenarcik, B.; Adach, A.; Radzyminska-Lenarcik, E. The influence of steric effect and alkyl chain length on the extraction of the complexes of Co(II), Ni(II), Cu(II) Zn(II) and Cd(II) with 1-alkyl-2-methylimidazole. Pol. J. Chem. 1999, 73, 1273–1281. [Google Scholar]
- Radzyminska-Lenarcik, E. Search for the possibility of utilizing the differences in complex-forming capacities of alkylimidazoles for selective extraction of some metal ions from aqueous solutions. Pol. J. Chem. Technol. 2008, 10, 73–78. [Google Scholar] [CrossRef] [Green Version]
- Lenarcik, B.; Kierzkowska, A. The influence of alkyl chain length and steric effect on stability constants and extractability of Zn(II) complexes with 1-alkyl-4-methylimidazoles. Solv. Ext. Ion Exch. 2004, 39, 3485–3508. [Google Scholar] [CrossRef]
- Lenarcik, B.; Kurdziel, K.; Czopek, R. Search for optimum conditions of extraction of metal complexes with alkylimidazoles. III. Structure—extractability relationships for 1,4-dimethylimidazole complexes of Co(II), Ni(II), Cu(II), Zn(II), and Cd(II). Solvent Ext. Ion Exch. 1986, 4, 165–182. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Witt, K. Studies on the separation of some transition metals using trialkylimidazole as selective extractant. In Proceedings of the E3S Web of Conferences, MEC2017, Wisła, Poland, 20–23 September 2017; Volume 18, p. 01017. [Google Scholar]
- Ulewicz, M.; Walkowiak, W.; Gega, J.; Pospiech, B. Zinc(II) selective removal from other transition metal ions by solvent extraction and transport through polymer inclusion membranes with D2EHPA. Ars Sep. Acta 2003, 2, 47–55. [Google Scholar]
- Ulewicz, M.; Walkowiak, W. Selective removal of transition metal ions by transport through polymer inclusion membranes with organophosphous acids. Environ. Prot. Eng. 2005, 31, 73–81. [Google Scholar]
- Kozlowska, J.; Kozlowski, C.; Koziol, J.J. Transport of Zn(II), Cd(II),and Pb(II) cross CTA plasticized membranes containing organophosphorous acids as ion carriers. Sep. Purif. Technol. 2007, 57, 430–434. [Google Scholar] [CrossRef]
- Kozlowski, C.A.; Girek, T.; Walkowiak, W.; Koziol, J.J. Application of hydrophobic â-cyclodextrin polymer in separation of metal ions by plasticized membranes. Sep. Purif. Technol. 2005, 46, 136–144. [Google Scholar] [CrossRef]
- Kozlowski, C.A.; Kozlowska, J. PNP-16-crown-6 derivatives as ion carriers for Zn(II), Cd(II) and Pb(II) transport across polymer inclusion membranes. J. Membr. Sci. 2009, 326, 215–221. [Google Scholar] [CrossRef]
- Kozłowski, C.; Walkowiak, W. Transport of Cr(VI), Zn(II) and Cd(II) ions across polymer inclusion membranes with tridecyl(pyridine) oxide and tri-n-octylamine. Sep. Sci. Technol. 2004, 39, 3127–3141. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Alonso, M. Separation of zinc(II) from cobalt(II) solutions using supported liquid membrane with DP-8R as a carrier. Sep. Purif. Technol. 2005, 41, 179–184. [Google Scholar] [CrossRef]
- Rehman, H.U.; Akhtar, G.; Rashid, H.U.; Ali, N.; Ahmad, I.; Rehman, S.U.; Khan, K.; Arshad, M. Transport of Zn (II) by TDDA-Polypropylene Supported Liquid Membranes and Recovery from Waste Discharge Liquor of Galvanizing Plant of Zn (II). J. Chem. 2017, 2017, 7569354. [Google Scholar] [CrossRef]
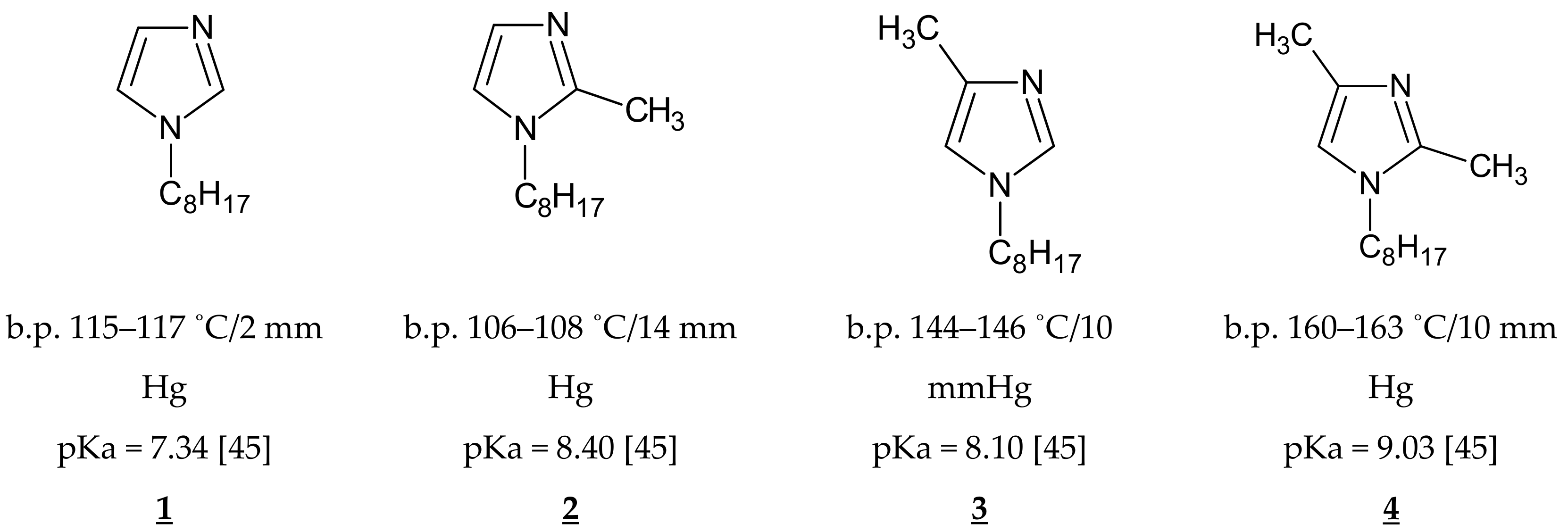


| Carrier in the CTA–o-NPPE Membrane | Effective Pore Size (µm) | Tortuosity | Roughness (Rq) (nm) | Ref. |
|---|---|---|---|---|
| 1-decyl-2-methylimidazole | 0.057 | 2.81 | 7.2 | [30] |
| 1-decyl-4-methylimidazole | 0.060 | 2.85 | 6.7 | [26] |
| 1-decyl-2,4-dimethylimidazole | 0.065 | 2.45 | 5.8 | [53] |
| 1-octylimidazole (1) | 0.051 | 2.15 | 5.4 | this work |
| 1-octyl-2-methylimidazole (2) | 0.054 | 2.38 | 6.1 | this work |
| 1-octyl-4-methylimidazole (3) | 0.058 | 2.75 | 6.5 | this work |
| 1-octyl-2,4-dimethylimidazole (4) | 0.062 | 2.60 | 6.0 | this work |
| Carrier | Solutions | Metal Ions | J0 (µmol/m2∙s) | Selectivity Order Selectivity Coefficients SZn(II)/M(II) |
|---|---|---|---|---|
| 1 | I | Zn(II) | 16.32 | - |
| II | Zn(II) Cd(II) | 11.30 7.96 | Zn(II) > Cd(II) 1.4 | |
| III | Cd(II) Ni(II) | 8.56 2.42 | Cd(II) > Ni(II) 3.5 | |
| IV | Zn(II Ni(II) | 10.83 1.07 | Zn(II) > Ni(II) 10.1 | |
| V | Zn(II) Cd(II) Ni(II) | 10.76 6.61 1.18 | Zn(II) > Cd(II) > Ni(II) SZn(II)/Cd(II)—1.6, SZn(II)/Ni(II)—9.1 | |
| 2 | I | Zn(II) | 12.45 | - |
| II | Zn(II) Cd(II) | 10.13 6.24 | Zn(II) > Cd(II) 1.6 | |
| III | Cd(II) Ni(II) | 7.16 0.45 | Cd(II) > Ni(II) 15.9 | |
| IV | Zn(II) Ni(II) | 9.82 0.43 | Zn(II) > Ni(II) 22.7 | |
| V | Zn(II) Cd(II) Ni(II) | 8.49 5.83 0.30 | Zn(II) > Cd(II) > Ni(II) SZn(II)/Cd(II)—1.5, SZn(II)/Ni(II)—18.0 | |
| 3 | I | Zn(II) | 11.68 | - |
| II | Zn(II) Cd(II) | 9.53 7.01 | Zn(II) > Cd(II) 1.4 | |
| III | Cd(II) Ni(II) | 6.92 0.35 | Cd(II) > Ni(II) 19.8 | |
| IV | Zn(II) Ni(II) | 10.02 0.29 | Zn(II) > Ni(II) 34.6 | |
| V | Zn(II) Cd(II) Ni(II) | 8.97 5.61 0.37 | Zn(II) > Cd(II) > Ni(II) SZn(II)/Cd(II)—1.6, SZn(II)/Ni(II)—22.9 | |
| 4 | I | Zn(II) | 28.13 | - |
| II | Zn(II) Cd(II) | 26.08 9.24 | Zn(II) > Cd(II) 2.8 | |
| III | Cd(II) Ni(II) | 8.47 0.36 | Cd(II) > Ni(II) 23.5 | |
| IV | Zn(II) Ni(II) | 27.12 0.25 | Zn(II) > Ni(II) 104.5 | |
| V | Zn(II) Cd(II) Ni(II) | 25.44 7.62 0.29 | Zn(II) > Cd(II) > Ni(II) SZn(II)/Cd(II)—3.3, SZn(II)/Ni(II)—87.7 |
| Ligand/Carrier | Metal Ion | log β1 | log β2 | log β3 | log β4 | Ref. |
|---|---|---|---|---|---|---|
| 1 | Zn(II) | 2.73 | 4.15 | 6.64 | 10.18 | [55] |
| Cd(II) | 1.95 | 2.81 | 4.00 | 5.20 | [56] | |
| Ni(II) | 0.79 | 3.90 | 6.20 | 8.10 | [57] | |
| 2 | Zn(II) | 1.96 | 4.45 | 6.80 | 9.10 | [58] |
| Cd(II) | 1.03 | 2.20 | 3.45 | 5.05 | [58] | |
| Ni(II) | 0.11 | 0.47 | 1.19 | 2.63 | [59] | |
| 3 | Zn(II) | 2.04 | 3.50 | 6.20 | 6.90 | [60] |
| Cd(II) | 1.26 | 2.20 | 3.93 | 5.11 | [61] | |
| Ni(II) | 0.69 | 1.04 | 2.00 | 2.92 | [61] | |
| 4 | Zn(II) | 1.65 | 2.17 | 4.48 | 6.39 | [62] |
| Cd(II) | 1.17 | 2.53 | 4.21 | 5.68 | [62] | |
| Ni(II) | 0.09 | 0.22 | 1.11 | 2.05 | [62] |
| Carrier | Metal Ion | Δo (s/m) | Do (cm2/s) | Do,n (cm2/s) |
|---|---|---|---|---|
| 1 | Zn(II) | 107.25 | 2.04·10−8 | 2.11·10−9 |
| Cd(II) | 108.49 | 7.16·10−9 | 7.40·10−10 | |
| Ni(II) | 1010.11 | 4.39·10−11 | 4.53·10−12 | |
| 2 | Zn(II) | 106.14 | 1.92·10−8 | 2.01·10−9 |
| Cd(II) | 107.63 | 6.76·10−9 | 7.14·10−10 | |
| Ni(II) | 1010.65 | 4.15·10−11 | 4.27·10−12 | |
| 3 | Zn(II) | 106.67 | 1.72·10−8 | 1.98·10−9 |
| Cd(II) | 108.02 | 6.34·10−9 | 6.75·10−10 | |
| Ni(II) | 1010.50 | 4.59·10−11 | 4.83·10−12 | |
| 4 | Zn(II) | 106.08 | 1.53·10−8 | 1.74·10−9 |
| Cd(II) | 107.15 | 6.06·10−9 | 6.58·10−10 | |
| Ni(II) | 1010.61 | 4.72·10−11 | 4.91·10−12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radzyminska-Lenarcik, E.; Ulewicz, M. Polymer Inclusion Membranes (PIMs) Doped with Alkylimidazole and their Application in the Separation of Non-Ferrous Metal Ions. Polymers 2019, 11, 1780. https://doi.org/10.3390/polym11111780
Radzyminska-Lenarcik E, Ulewicz M. Polymer Inclusion Membranes (PIMs) Doped with Alkylimidazole and their Application in the Separation of Non-Ferrous Metal Ions. Polymers. 2019; 11(11):1780. https://doi.org/10.3390/polym11111780
Chicago/Turabian StyleRadzyminska-Lenarcik, Elzbieta, and Malgorzata Ulewicz. 2019. "Polymer Inclusion Membranes (PIMs) Doped with Alkylimidazole and their Application in the Separation of Non-Ferrous Metal Ions" Polymers 11, no. 11: 1780. https://doi.org/10.3390/polym11111780
APA StyleRadzyminska-Lenarcik, E., & Ulewicz, M. (2019). Polymer Inclusion Membranes (PIMs) Doped with Alkylimidazole and their Application in the Separation of Non-Ferrous Metal Ions. Polymers, 11(11), 1780. https://doi.org/10.3390/polym11111780