Self-Assembly Supramolecular Systems Based on Guanidinium Salts Modified Hyperbranched Polyamidoamine and Cationic Acrylamide Copolymers
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Instrumentation and Analytical Methods
2.3. Synthetic Methods
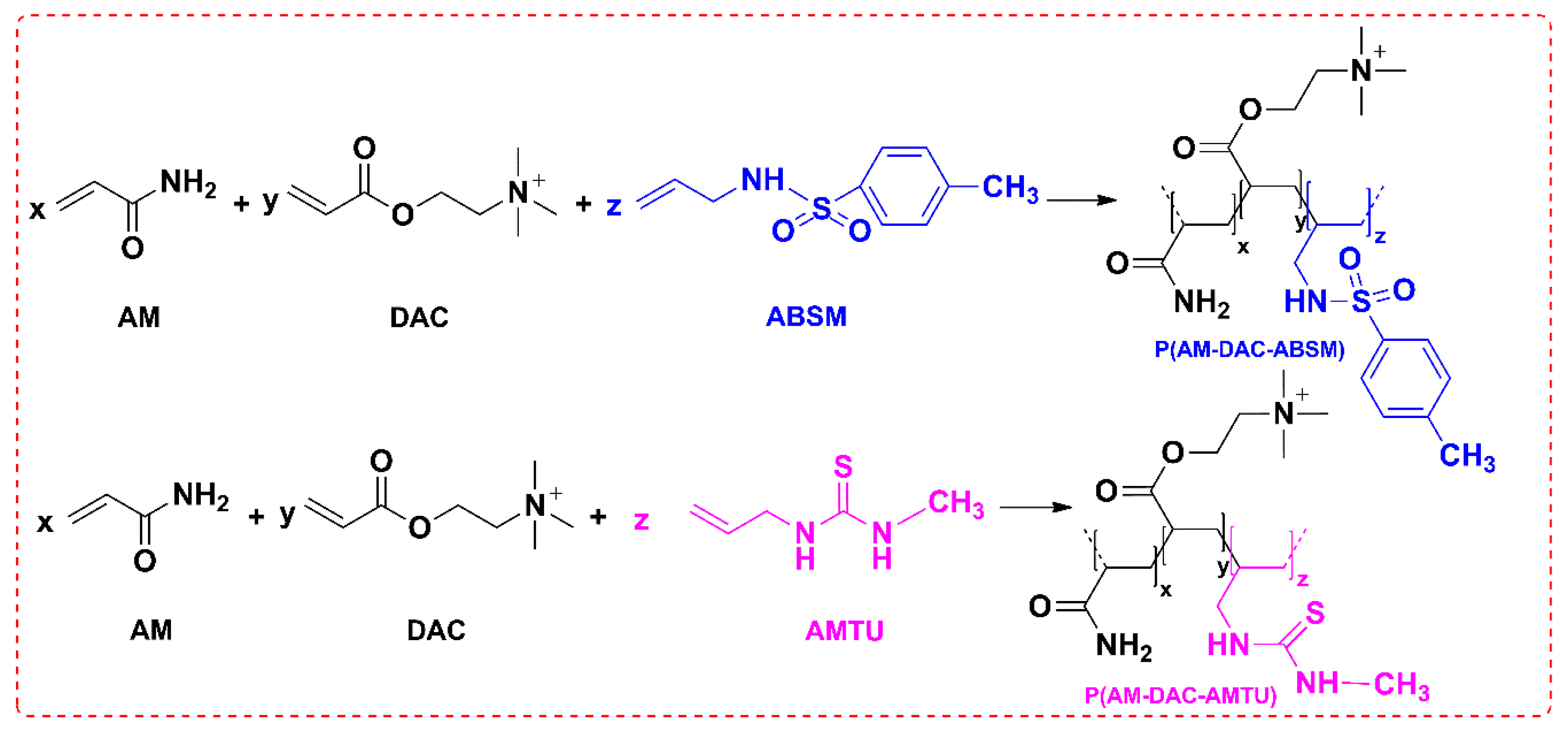
2.3.1. Preparation of Copolymer P(AM-DAC-ABSM)
2.3.2. Preparation of Copolymer P(AM-DAC-AMTU)
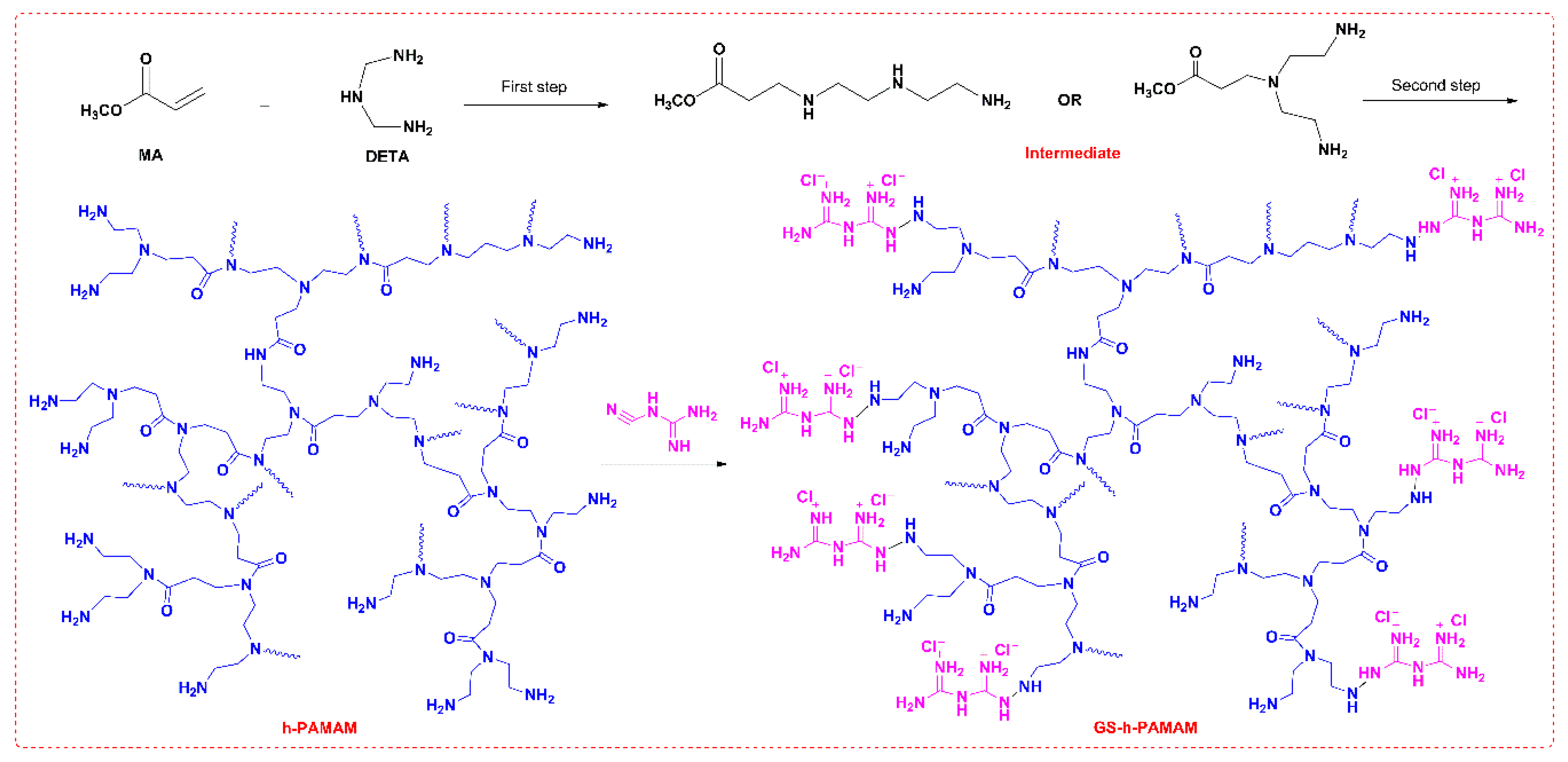
2.3.3. Preparation of h-PAMAM
2.3.4. Preparation of GS-h-PAMAM
3. Results and Discussion
3.1. Synthesis and Characterization
3.1.1. Monomers and Copolymers
3.1.2. GS-h-PAMAM and h-PAMAM
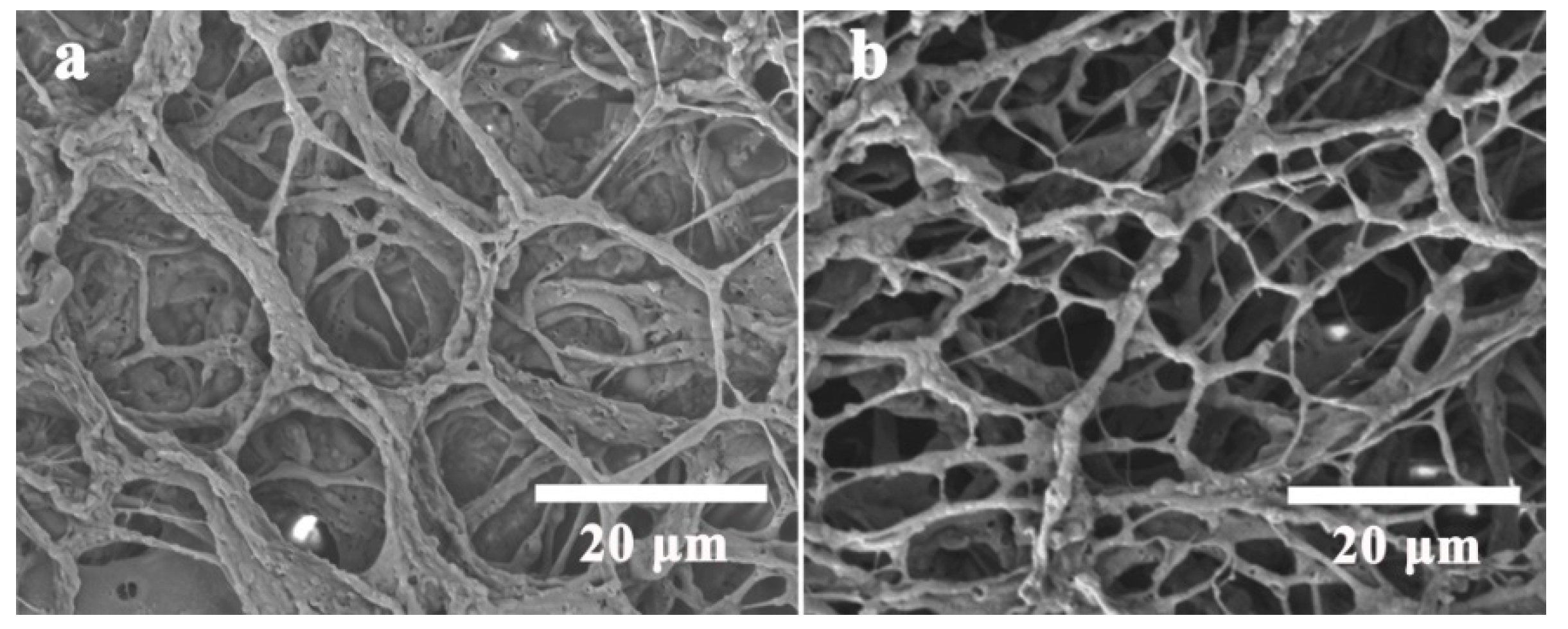
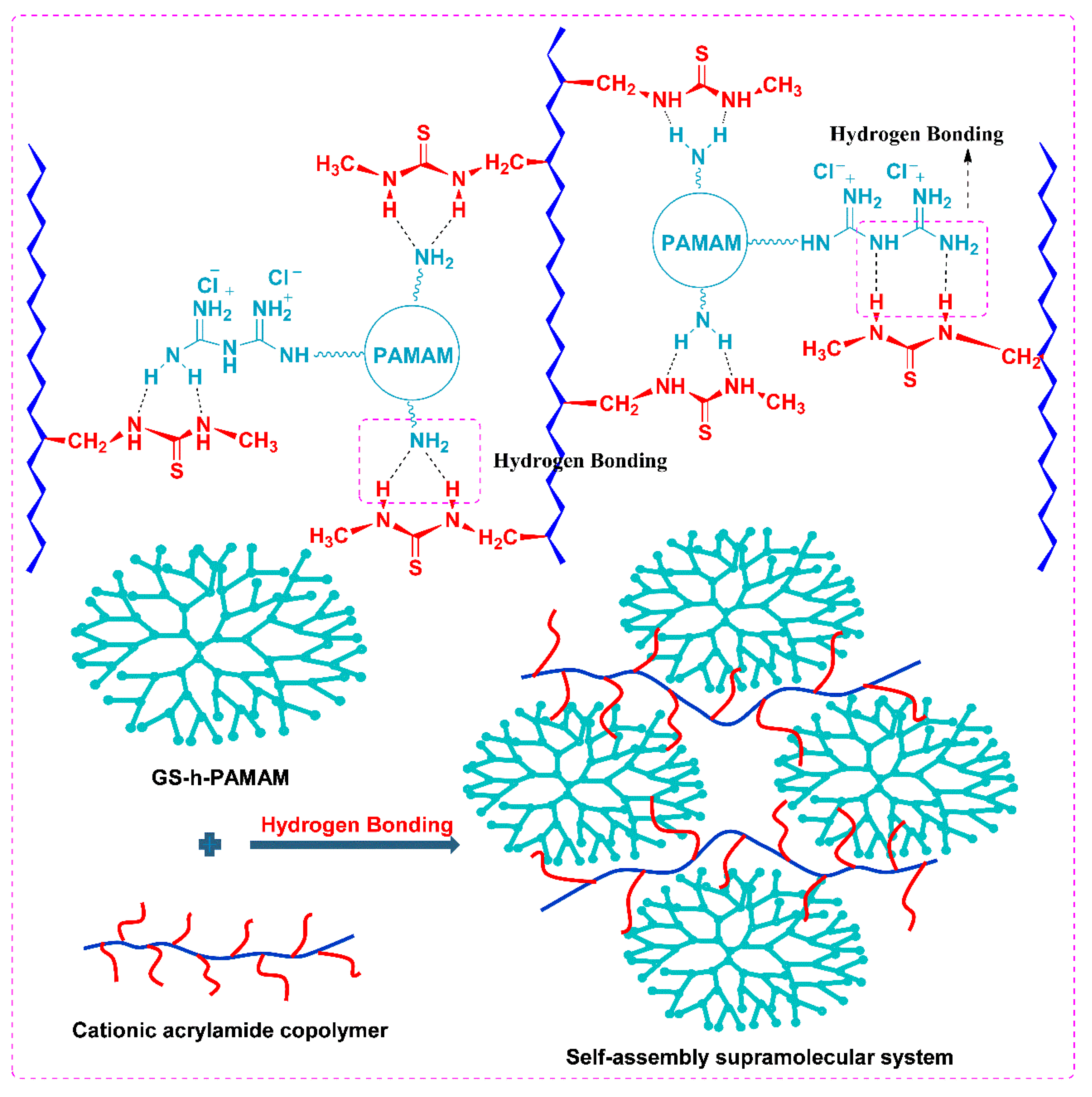
3.1.3. Self-Assembly Supramolecular Systems
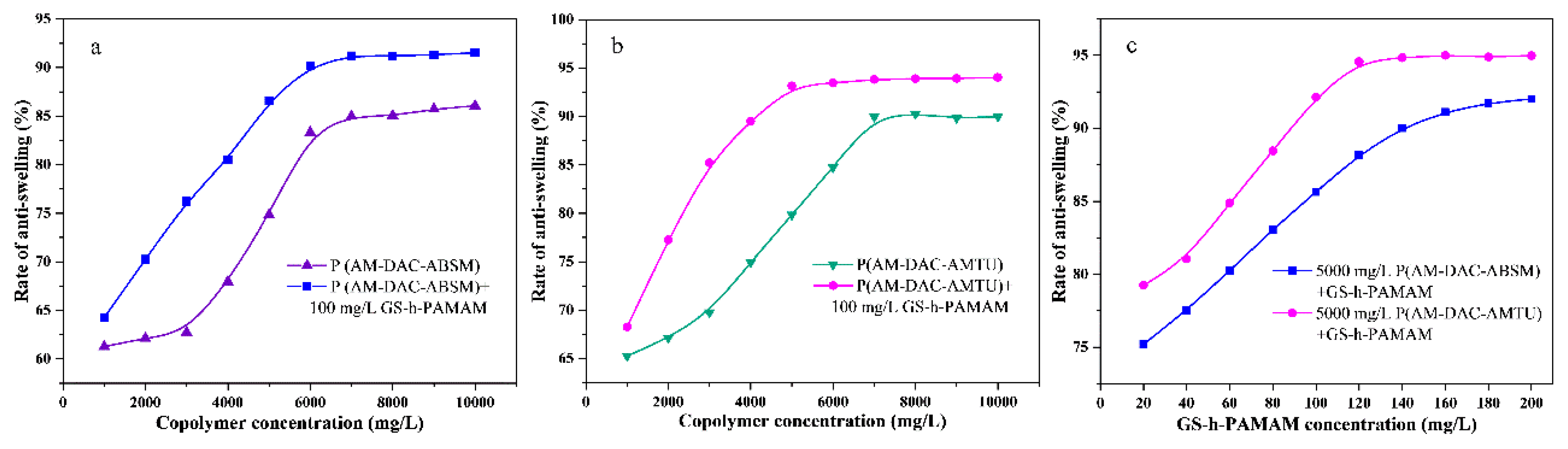
3.2. Solution Properties of Copolymers and Supramolecular Systems
3.2.1. Effect of Inorganic Salts
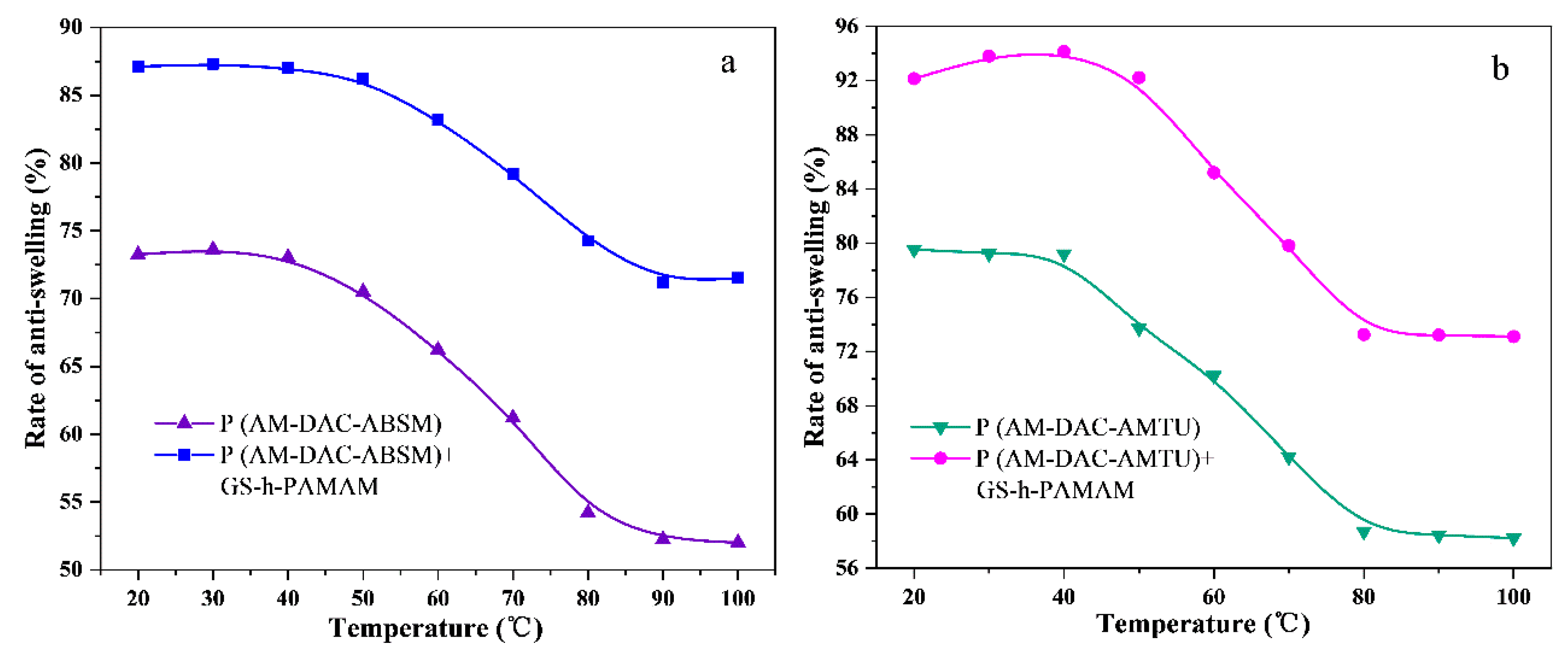
3.2.2. Effect of Temperature
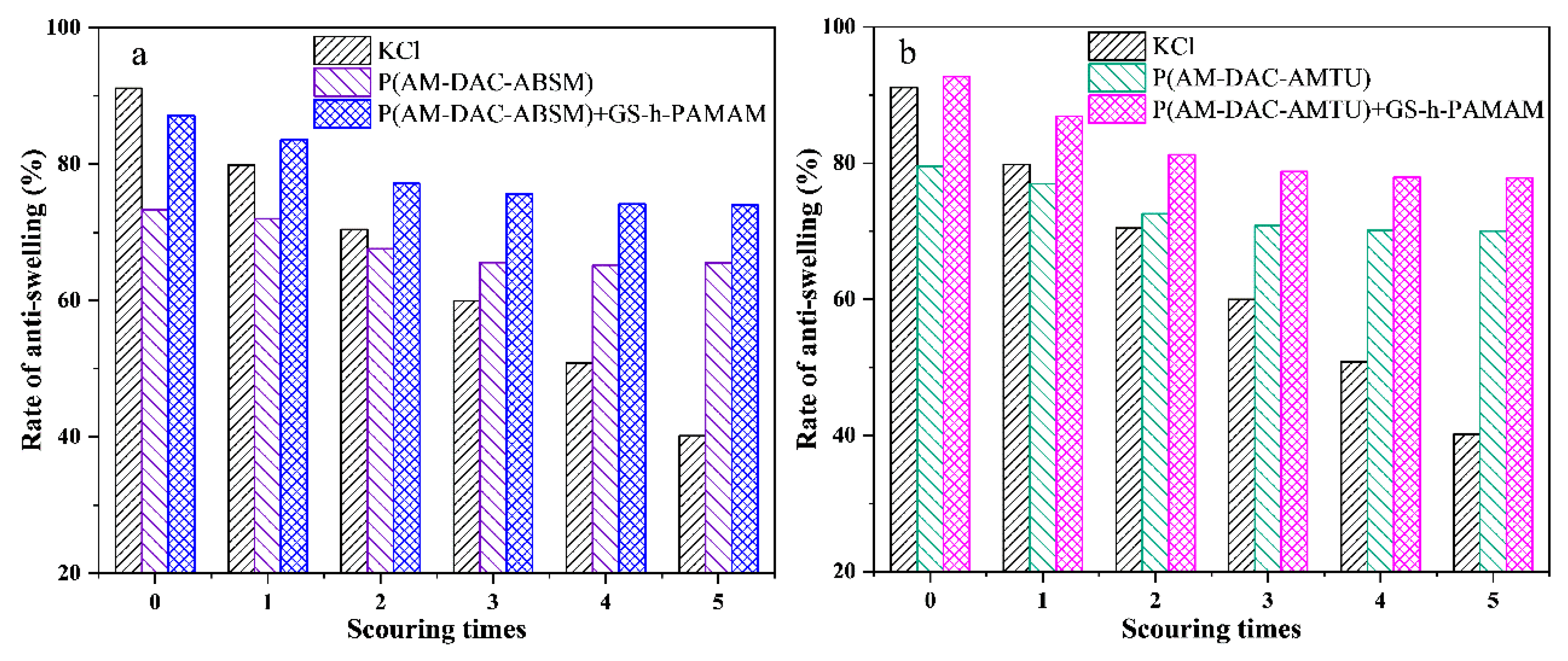
3.2.3. Effect of Scouring Times
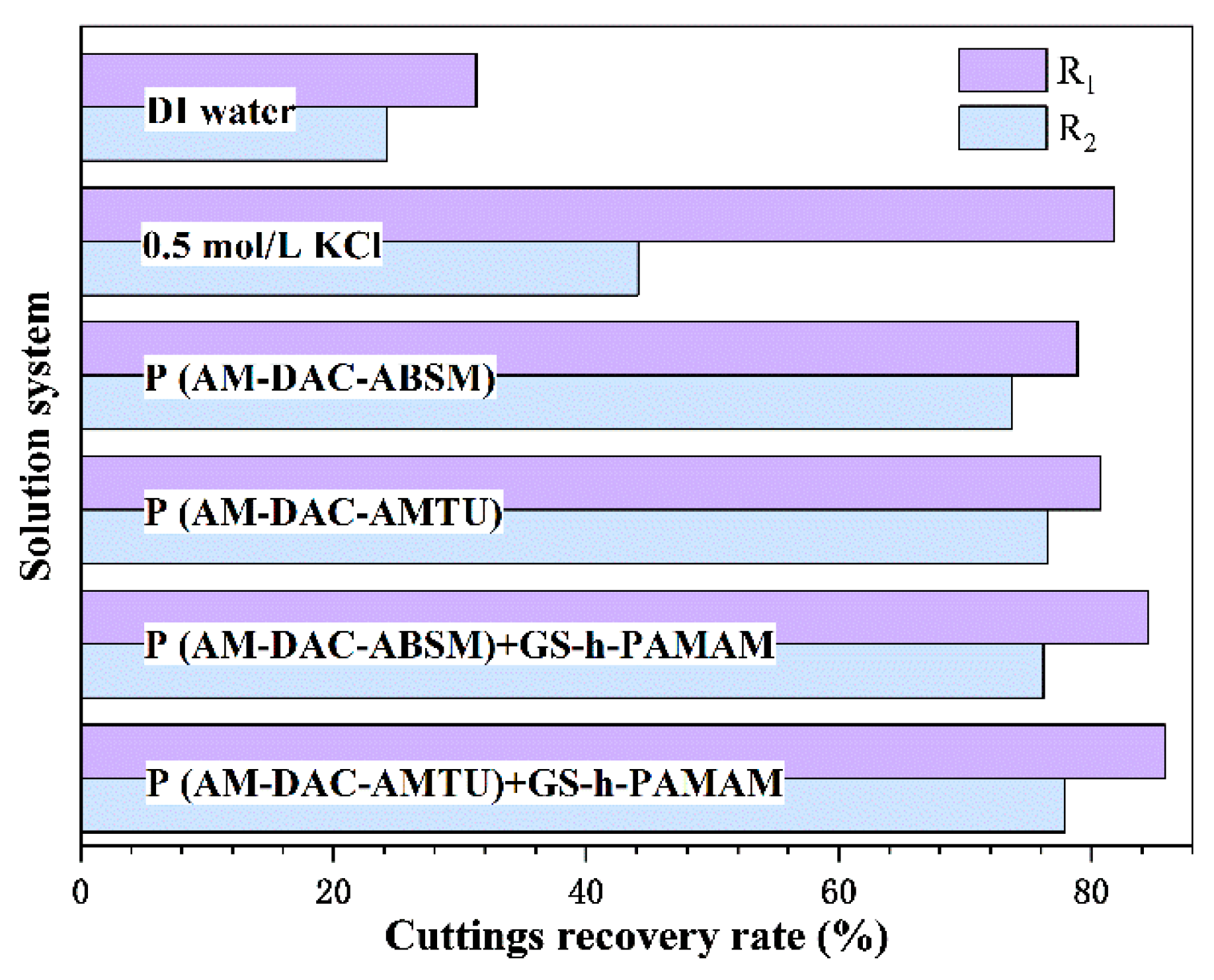
3.2.4. Effect on Cuttings Recovery Rate
3.3. Rheological Properties
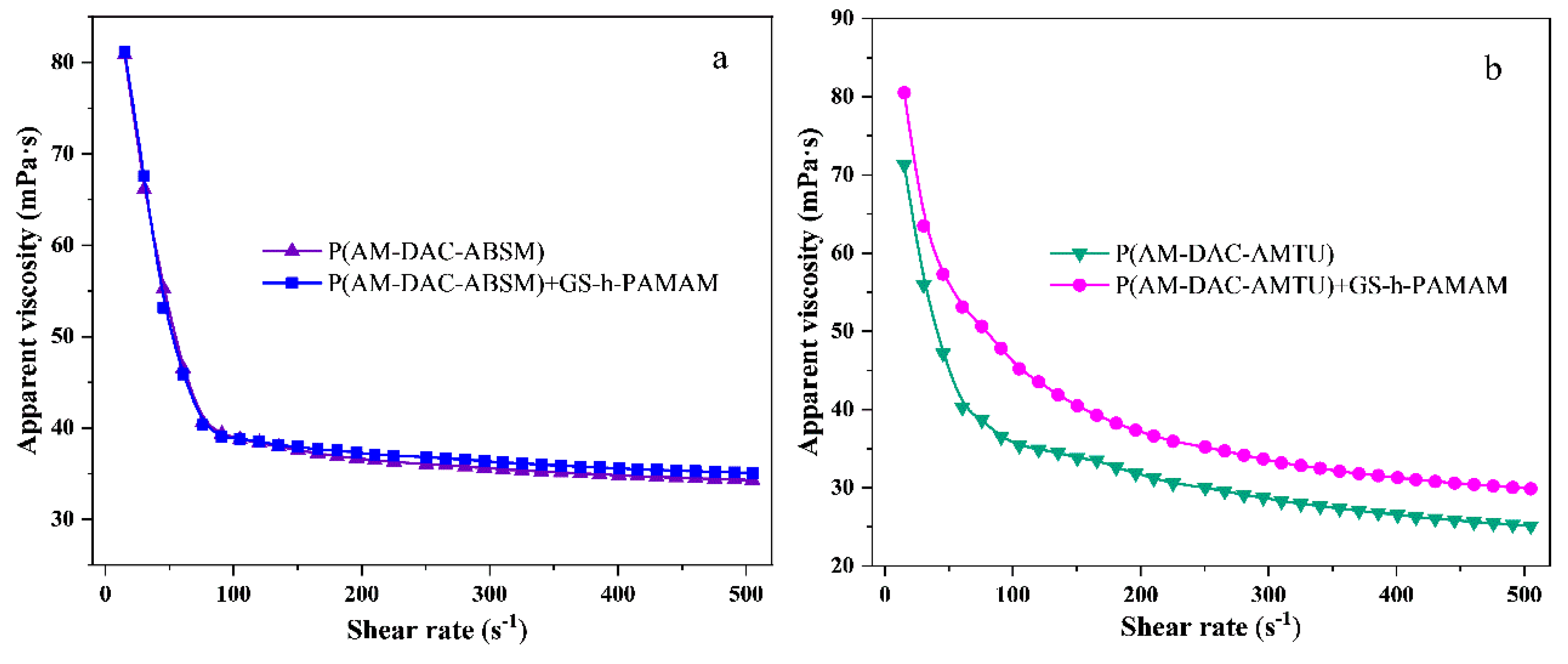
3.3.1. Shear Thinning
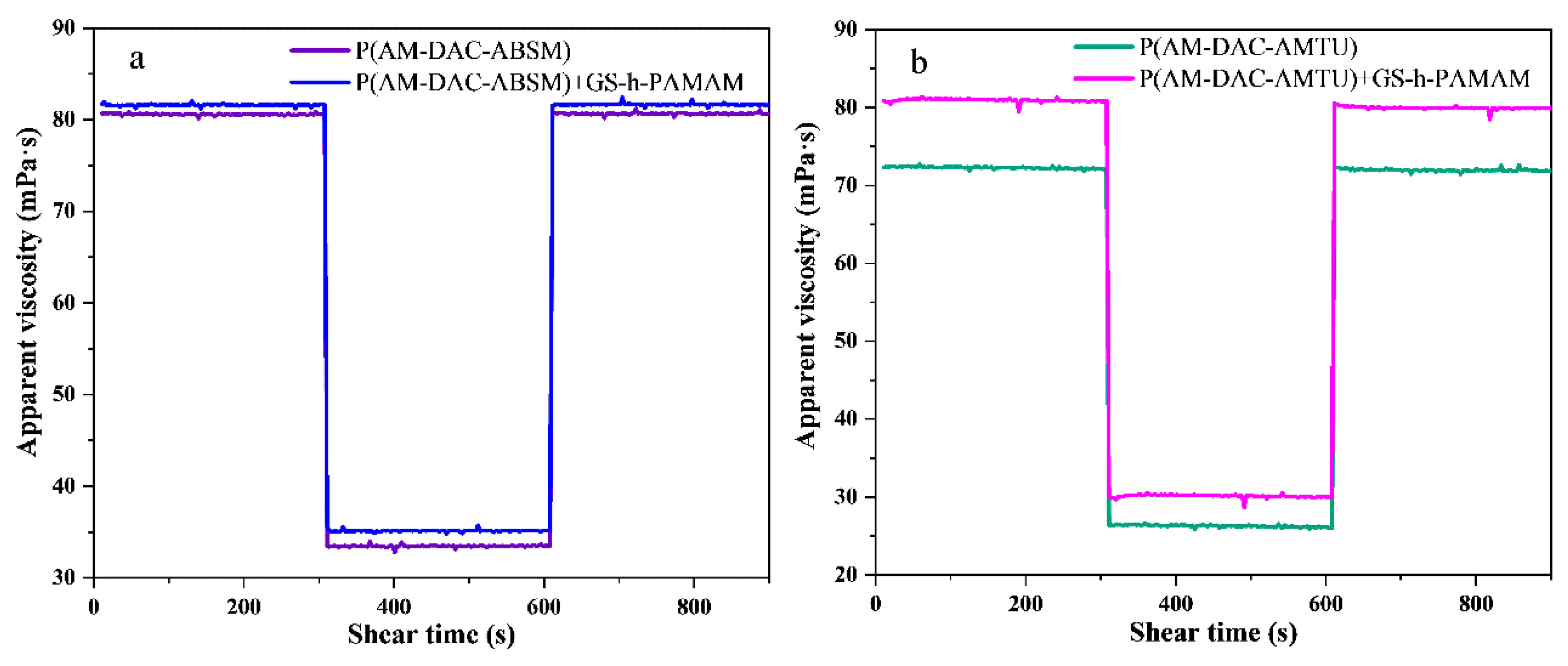
3.3.2. Shear Recovery
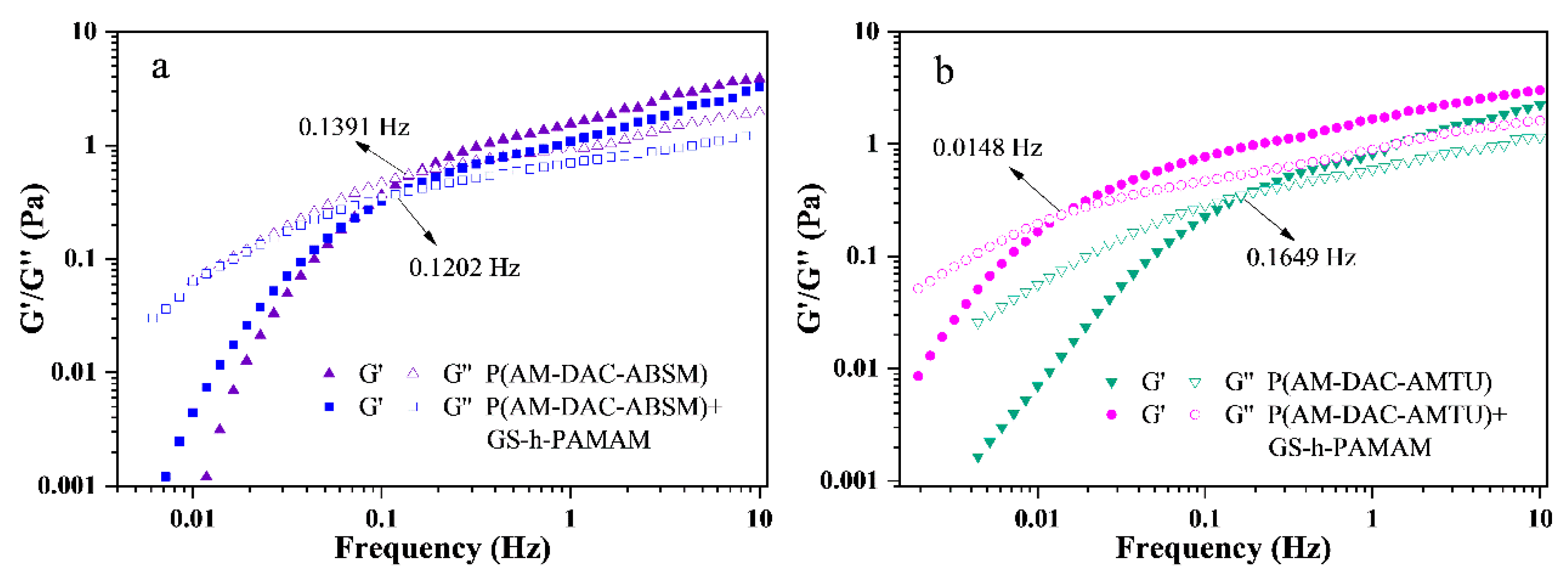
3.3.3. Viscoelasticity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sai, W.; Bin, W.; Junfei, D. Functional Supramolecular Gels Self-Assembled by Hydrogen Bonding Among Urea-Based Gelators. Prog. Chem. 2014, 1, 125–139. [Google Scholar]
- Hart, L.R.; Nguyen, N.A.; Harries, J.L. Perylene as an electron-rich moiety in healable, complementary π–π stacked, supramolecular polymer systems. Polymer 2015, 69, 293–300. [Google Scholar] [CrossRef]
- Schlosser, F.; Moos, M.; Lambert, C. Redox-switchable Intramolecular π− π-Stacking of Perylene Bisimide Dyes in a Cyclophane. Adv. Mater. 2013, 3, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Rybtchinski, B. Aqueous Supramolecular Polymers Based on Aromatic Amphiphiles: Rational Design, Complexity, and Functional Materials. In Hierarchical Macromolecular Structures: 60 Years after the Staudinger Nobel Prize II; Springer: Cham, Germany, 2013; Volume 1, pp. 363–387. [Google Scholar]
- Li, Y.; Wang, Y.; Huang, G. Chaotropic-anion-induced supramolecular self-assembly of ionic polymeric micelles. Angew. Chem. Int. Ed. 2014, 31, 8212–8216. [Google Scholar] [CrossRef]
- Yuan, C.; Guo, J.; Tan, M. Multistimuli responsive and electroactive supramolecular gels based on ionic liquid gemini guest. ACS Macro Lett. 2014, 3, 271–275. [Google Scholar] [CrossRef]
- Evans, N.H.; Beer, P.D. Advances in anion supramolecular chemistry: From recognition to chemical applications. Angew. Chem. Int. Ed. 2014, 44, 11716–11754. [Google Scholar] [CrossRef]
- Miyauchi, M.; Kawaguchi, Y.; Harada, A. Formation of supramolecular polymers constructed by cyclodextrins with cinnamamide. J. Incl. Phenom. Macrocycl. 2004, 50, 57–62. [Google Scholar]
- Castellano, R.K.; Clark, R.; Craig, S.L. Emergent mechanical properties of self-assembled polymeric capsules. Proc. Natl. Acad. Sci. USA 2000, 97, 12418–12421. [Google Scholar] [CrossRef] [Green Version]
- Versteegen, R.M.; Van Beek, D.J.M.; Sijbesma, R.P. Dendrimer-Based Transient Supramolecular Networks. J. Am. Chem. Soc. 2005, 40, 13862–13868. [Google Scholar] [CrossRef]
- Liu, C.; Gao, C.; Yan, D. Honeycomb-patterned photoluminescent films fabricated by self-assembly of hyperbranched polymers. Angew. Chem. Int. Ed. 2007, 46, 4128–4131. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Zhang, J. Two unprecedented aromatic guanidines supramolecular chains self-assembled by hydrogen bonding interaction. J. Mol. Struct. 2015, 1097, 145–150. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, W.; Zhou, Y. Complex self-assembly of hyperbranched polyamidoamine/linear polyacrylic acid in water and their functionalization. J. Phys. Chem. B 2009, 22, 7729–7736. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhou, Y.; Yan, D. Hyperbranched polymer vesicles: From self-assembly, characterization, mechanisms, and properties to applications. Chem. Soc. Rev. 2015, 12, 3874–3889. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Pei, F.; Sun, X. Fabrication of supramolecular hyperbranched polyamidoamine–dextran conjugates and their self-assembly in the presence of EGCG. New J. Chem. 2018, 24, 19600–19607. [Google Scholar] [CrossRef]
- Yang, W.; Pan, C.Y. Synthesis and fluorescent properties of biodegradable hyperbranched poly (amido amine) s. Macromol. Rapid Commun. 2009, 24, 2096–2101. [Google Scholar] [CrossRef]
- Jiang, G.; Wang, Y.; Sun, X. Facile one-pot approach for preparing fluorescent and biodegradable hyperbranched poly (amidoamine) s. Polym. Chem. 2010, 5, 618–620. [Google Scholar] [CrossRef]
- -Yang, W.; Pan, C.Y.; Liu, X.Q. Multiple functional hyperbranched poly (amido amine) nanoparticles: Synthesis and application in cell imaging. Biomacromolecules 2011, 5, 1523–1531. [Google Scholar] [CrossRef]
- Chen, K.J.; Wolahan, S.M.; Wang, H. A small MRI contrast agent library of gadolinium (III)-encapsulated supramolecular nanoparticles for improved relaxivity and sensitivity. Biomaterials 2011, 8, 2160–2165. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, L.; Pang, Y. Photoluminescent hyperbranched poly (amido amine) containing β-cyclodextrin as a nonviral gene delivery vector. Bioconjugate Chem. 2011, 6, 1162–1170. [Google Scholar] [CrossRef]
- Pedziwiatr-Werbicka, E.; Milowska, K.; Dzmitruk, V. Dendrimers and hyperbranched structures for biomedical applications. Eur. Polym. J. 2019, 119, 61–73. [Google Scholar] [CrossRef]
- Mozafari, M.; Chauhan, N.P.S. Advanced Functional Polymers for Biomedical Applications; Elsevier Science: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Pérignon, N.; Mingotaud, A.F.; Marty, J.D. Formation and stabilization in water of metal nanoparticles by a hyperbranched polymer chemically analogous to PAMAM dendrimers. Chem. Mater. 2004, 24, 4856–4858. [Google Scholar] [CrossRef]
- Liu, C.; Gao, C.; Yan, D. Synergistic supramolecular encapsulation of amphiphilic hyperbranched polymer to dyes. Macromolecules 2006, 23, 8102–8111. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, X.W.; Lu, R. Magnetic nanoparticles modified with hyperbranched polyamidoamine for the extraction of benzoylurea insecticides prior to their quantitation by HPLC. Microchim. Acta 2019, 186, 351. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, Z.; Weng, X. Preparation of Highly Fluorescent and pH Responsive CdTe Quantum Dots Within Dynamic Covalent Hyperbranched Polymers and Their In Vitro Application as Fluorescence Probe. Sci. Adv. Mater. 2015, 4, 615–622. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.; Yang, L. Tetraphenylethene decorated hyperbranched poly (amido amine)s as metal/organic-solvent-free turn-on AIE probe for specific pyrophosphate detection. Sens. Actuators B Chem. 2019, 291, 25–33. [Google Scholar] [CrossRef]
- Barbey, R.; Perrier, S. A Facile Route to Functional Hyperbranched Polymers by Combining Reversible Addition–Fragmentation Chain Transfer Polymerization, Thiol–Yne Chemistry, and Postpolymerization Modification Strategies. ACS Macro Lett. 2013, 5, 366–370. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, L.; Zhang, F. Multicarboxylic hyperbranched polyglycerol modified SBA-15 for the adsorption of cationic dyes and copper ions from aqueous media. Appl. Surf. Sci. 2012, 13, 5291–5299. [Google Scholar] [CrossRef]
- Cao, L. Synthesis and Functional Study of Hyperbranched Polyamidoamine Polymers. Ph.D. Thesis, FuDan University, Shanghai, China, 2005. [Google Scholar]
- Tekin, N.; Demirbas, O.; Alkan, M. Adsorption of cationic polyacrylamide onto kaolinite. Microporous Mesoporous Mater. 2005, 3, 340–350. [Google Scholar] [CrossRef]
- Zhang, S.; Qiu, Z.; Huang, W. Characterization of a novel aluminum-based shale stabilizer. J. Pet. Sci. Eng. 2013, 3, 36–40. [Google Scholar] [CrossRef]
- Hou, J.; Liu, Y.; Song, G. Synthesis and Application of a New High Temperature High Performance Salt Resistant Shale Inhibitor. Drill. Fluid Complet. Fluid 2016, 1, 22–27. [Google Scholar]
- Livney, Y.D.; Portnaya, I.; Faupin, B. Interactions between inorganic salts and polyacrylamide in aqueous solutions and gels. J. Polym. Sci. Polym. Phys. 2003, 5, 508–519. [Google Scholar] [CrossRef]
- Balaban, R.D.C.; Vidal, E.L.F.; Borges, M.R. Design of experiments to evaluate clay swelling inhibition by different combinations of organic compounds and inorganic salts for application in water base drilling fluids. Appl. Clay Sci. 2015, 105, 124–130. [Google Scholar] [CrossRef]
- Sarsenbekuly, B.; Kang, W.; Fan, H. Study of salt tolerance and temperature resistance of a hydrophobically modified polyacrylamide based novel functional polymer for EOR. Colloids Surf. A 2017, 514, 91–97. [Google Scholar] [CrossRef]
- Jia, H.; Chen, H. Using DSC technique to investigate the non-isothermal gelation kinetics of the multi-crosslinked Chromium acetate (Cr3+)-Polyethyleneimine (PEI)-Polymer gel sealant. J. Pet. Sci. Eng. 2018, 165, 105–113. [Google Scholar] [CrossRef]
- Xiao, X.; Kong, D.; Qiu, X. Shape-memory polymers with adjustable high glass transition temperatures. Macromolecules 2015, 11, 3582–3589. [Google Scholar] [CrossRef]
- Tan, B.; Thomas, N.L. A review of the water barrier properties of polymer/clay and polymer/graphene nanocomposites. J. Membr. Sci. 2016, 514, 595–612. [Google Scholar] [CrossRef] [Green Version]
- Ayirala, S.; Yousef, A. A state-of-the-art review to develop injection-water-chemistry requirement guidelines for IOR/EOR projects. SPE Prod. Oper. 2015, 1, 26–42. [Google Scholar] [CrossRef]
- Boek, E.S.; Coveney, P.V.; Skipper, N.T. Monte Carlo molecular modeling studies of hydrated Li-, Na-, and K-smectites: Understanding the role of potassium as a clay swelling inhibitor. J. Am. Chem. Soc. 1995, 50, 12608–12617. [Google Scholar] [CrossRef]
- Lin, L.; Luo, Y.; Li, X. Synthesis of Diblock Polyampholyte PAMPS-b-PMAPTAC and Its Adsorption on Bentonite. Polymers 2019, 1, 49. [Google Scholar] [CrossRef]
- Ma, J.; Cui, P.; Zhao, L. Synthesis and solution behavior of hydrophobic association water-soluble polymers containing arylalkyl group. Eur. Polym. J. 2002, 8, 1627–1633. [Google Scholar] [CrossRef]
- Zhong, H.; Qiu, Z.; Zhang, D. Inhibiting shale hydration and dispersion with amine-terminated polyamidoamine dendrimers. J. Nat. Gas Sci. Eng. 2016, 28, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Khodja, M.; Canselier, J.P.; Bergaya, F. Shale problems and water-based drilling fluid optimisation in the Hassi Messaoud Algerian oil field. Appl. Clay Sci. 2010, 4, 383–393. [Google Scholar] [CrossRef]
- Nojiri, S.; Yamada, H.; Kimata, S. Supramolecular polypropylene with self-complementary hydrogen bonding system. Polymer 2016, 87, 308–315. [Google Scholar] [CrossRef]
- Stahl, G.A.; Schulz, D.N. Water-Soluble Polymers for Petroleum Recovery; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Miao, L.; Guo, H.; Zuckermann, M.J. Conformation of polymer brushes under shear: Chain tilting and stretching. Macromolecules 1996, 6, 2289–2297. [Google Scholar] [CrossRef]
- Chen, W.; Chen, J.; Liu, L. Effects of chain stiffness on conformational and dynamical properties of individual ring polymers in shear flow. Macromolecules 2013, 18, 7542–7549. [Google Scholar] [CrossRef]
- Sato, K.; Nakajima, T.; Hisamatsu, T. Phase-separation-induced anomalous stiffening, toughening, and self-healing of polyacrylamide gels. Adv. Mater. 2015, 43, 6990–6998. [Google Scholar] [CrossRef]
- Ferry, J.D. Viscoelastic Properties of Polymers; John Wiley & Sons: Hoboken, NJ, USA, 1980. [Google Scholar]
- Farajzadeh, R.; Andrianov, A.; Zitha, P.L.J. Investigation of immiscible and miscible foam for enhancing oil recovery. Ind. Eng. Chem. Res. 2009, 4, 1910–1919. [Google Scholar] [CrossRef]



| P(AM-DAC-ABSM) | P(AM-DAC-AMTU) | ||||||
|---|---|---|---|---|---|---|---|
| AM:DAC | ABSM | V50 | Tem. (°C) | AM:DAC | AMTU | V50 | Tem. (°C) |
| 6:4 | 8‰ | 0.3‰ | 65 | 7:3 | 10‰ | 0.3‰ | 55 |
| h-PAMAM | GS-h-PAMAM | |||||
|---|---|---|---|---|---|---|
| MA:DETA | Tem. (°C) | Time (h) | h-PAMAM:DCDA | pH | Tem. (°C) | Time (h) |
| 1.2:1 | 140 | 3 | 1:1.5 | 3 | 80 | 4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Gou, S.; Fei, Y.; Yang, X.; Liu, M.; Huang, J. Self-Assembly Supramolecular Systems Based on Guanidinium Salts Modified Hyperbranched Polyamidoamine and Cationic Acrylamide Copolymers. Polymers 2019, 11, 1781. https://doi.org/10.3390/polym11111781
Wu Q, Gou S, Fei Y, Yang X, Liu M, Huang J. Self-Assembly Supramolecular Systems Based on Guanidinium Salts Modified Hyperbranched Polyamidoamine and Cationic Acrylamide Copolymers. Polymers. 2019; 11(11):1781. https://doi.org/10.3390/polym11111781
Chicago/Turabian StyleWu, Qi, Shaohua Gou, Yumei Fei, Xiaoyan Yang, Mengyu Liu, and Jinglun Huang. 2019. "Self-Assembly Supramolecular Systems Based on Guanidinium Salts Modified Hyperbranched Polyamidoamine and Cationic Acrylamide Copolymers" Polymers 11, no. 11: 1781. https://doi.org/10.3390/polym11111781
APA StyleWu, Q., Gou, S., Fei, Y., Yang, X., Liu, M., & Huang, J. (2019). Self-Assembly Supramolecular Systems Based on Guanidinium Salts Modified Hyperbranched Polyamidoamine and Cationic Acrylamide Copolymers. Polymers, 11(11), 1781. https://doi.org/10.3390/polym11111781





