Chemical, Thermo-Mechanical and Antimicrobial Properties of DBD Plasma Treated Disinfectant-Impregnated Wipes during Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
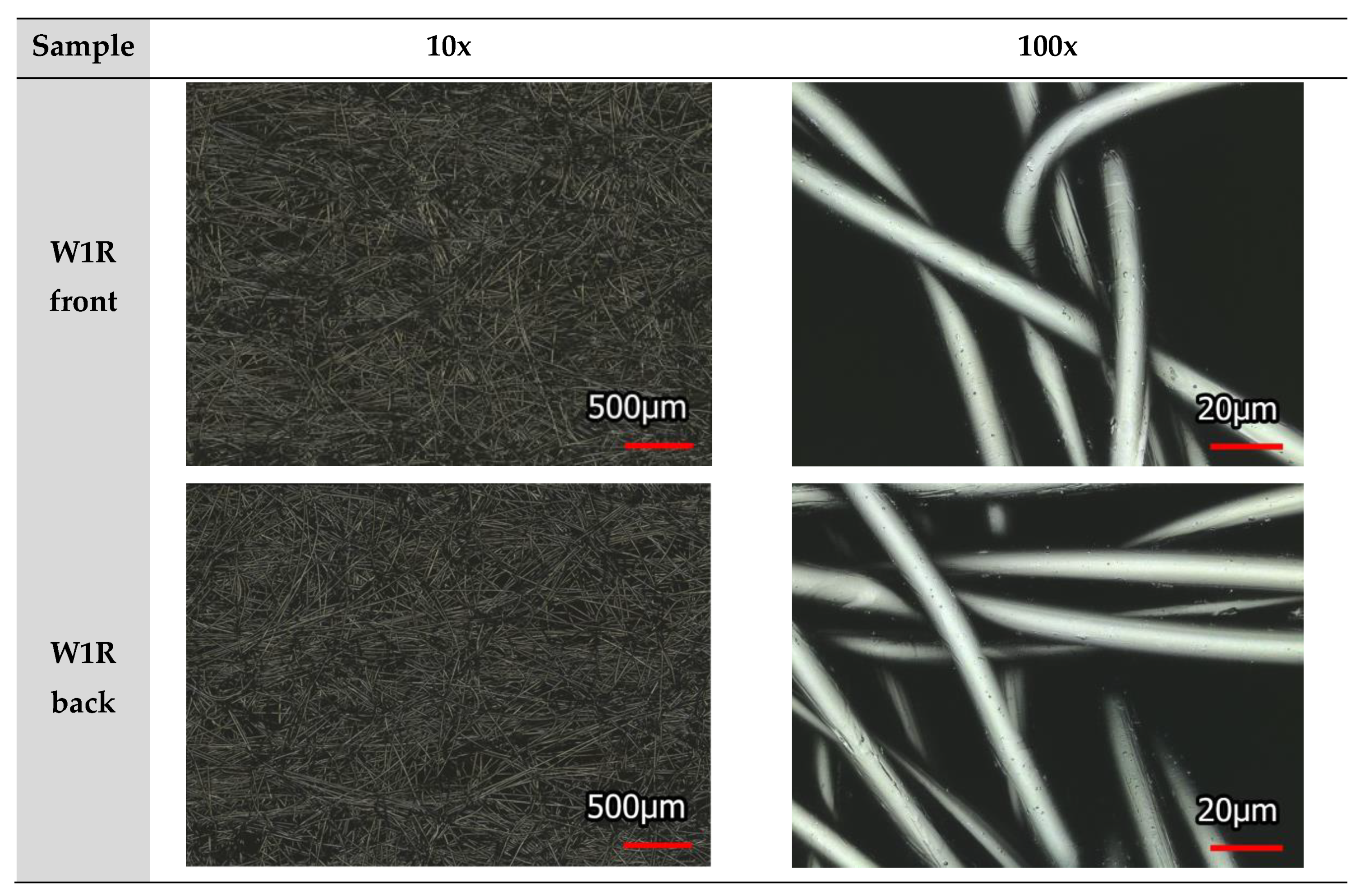
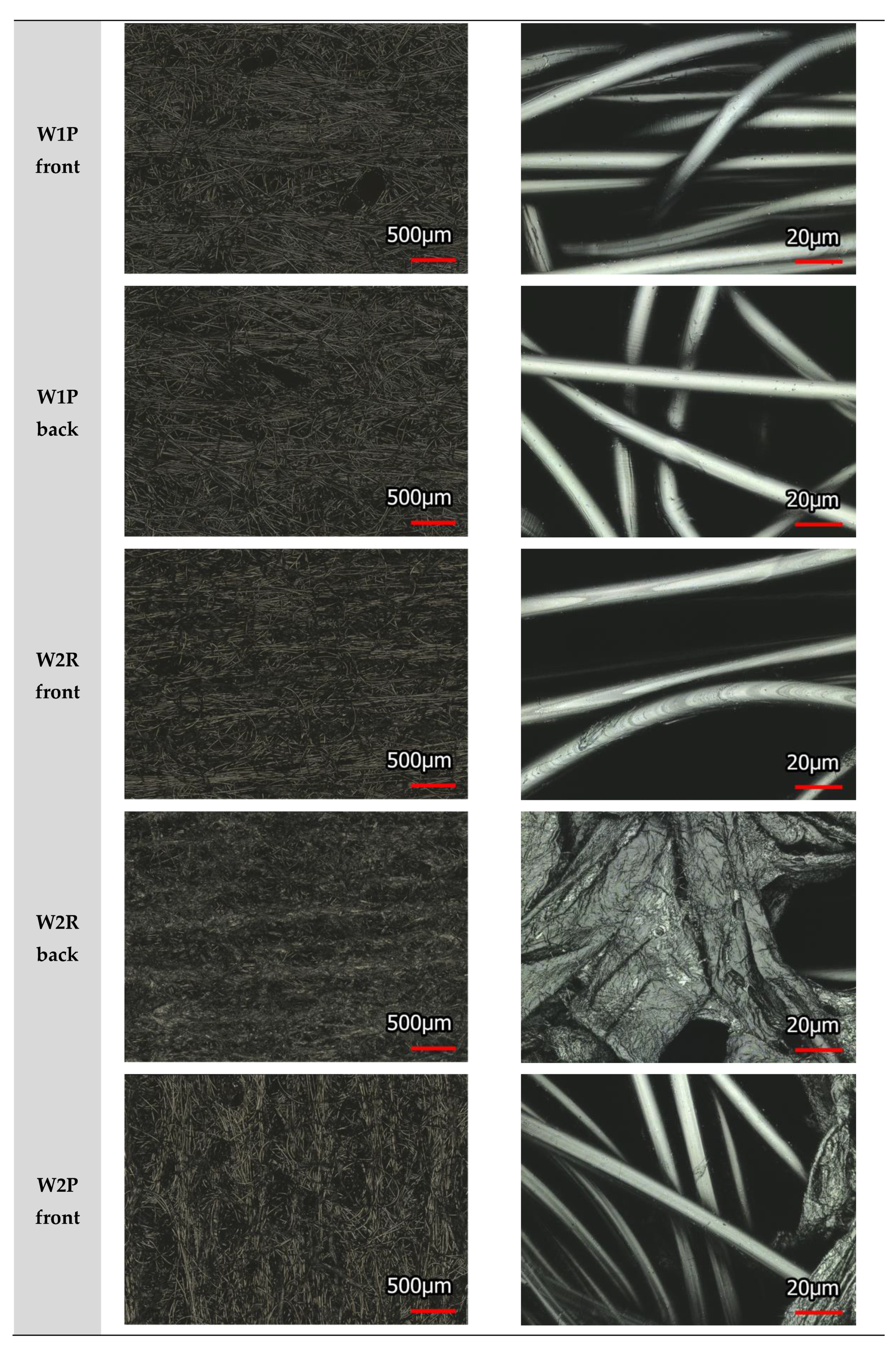
2.2. Sample Characterization
2.3. DBD Plasma Treatment of Wipe Samples
2.4. Contact Angle Measurement
2.5. Laser Scanning Microscope (LSM)
2.6. Storage of Wipe Samples in ADBAC Solution
2.7. X-Ray Photoelectron Spectroscopy (XPS)
2.8. Spectrophotometric Assessment of ADBAC Concentration
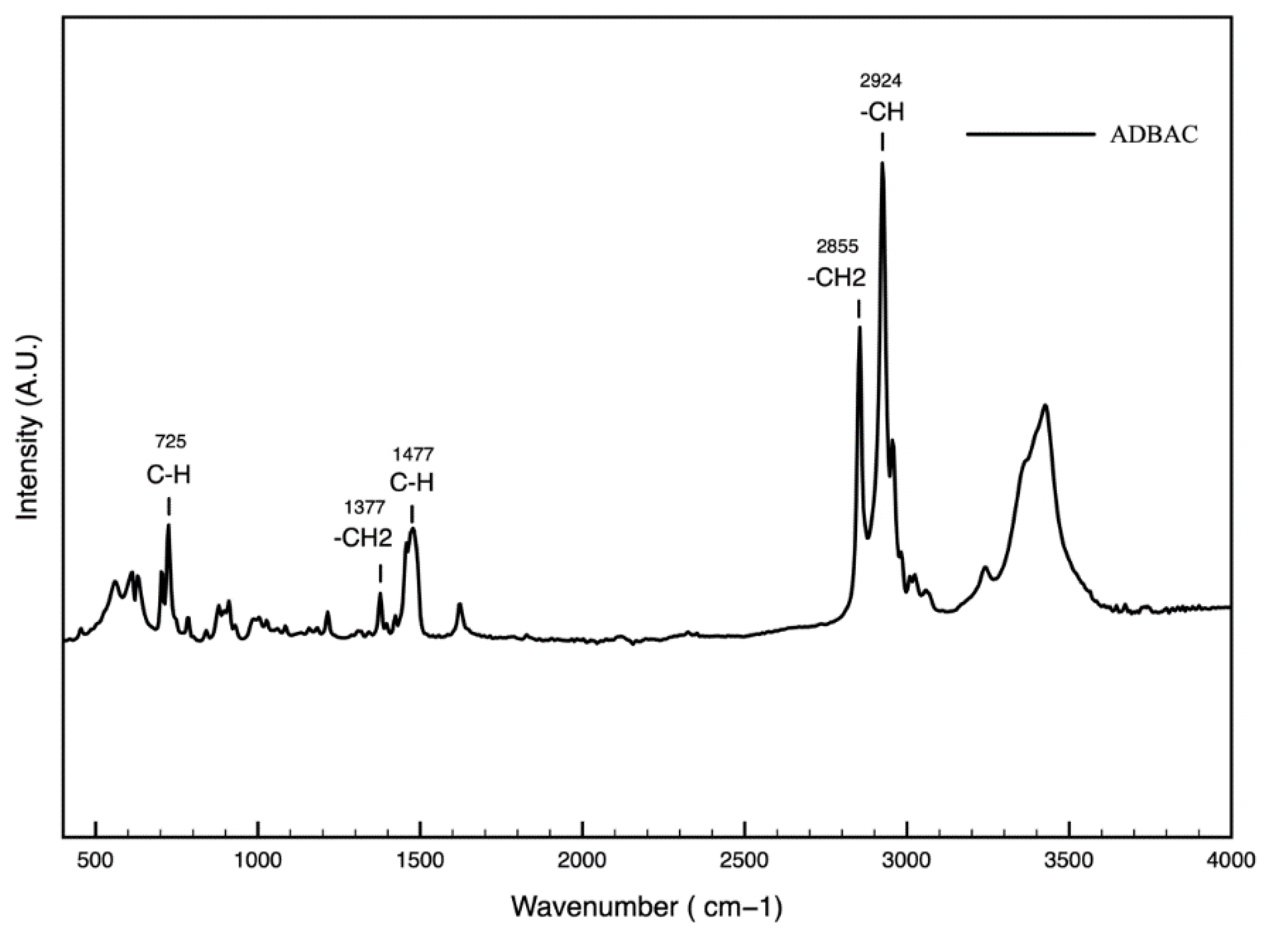
2.9. Fourier Transform Infrared Spectroscopy (FTIR)
2.10. Breaking Force and Elongation Measurement
2.11. Dynamic Mechanical Analysis (DMA)
2.12. Microbiology Test ASTM E 2149-13a
3. Results and Discussion
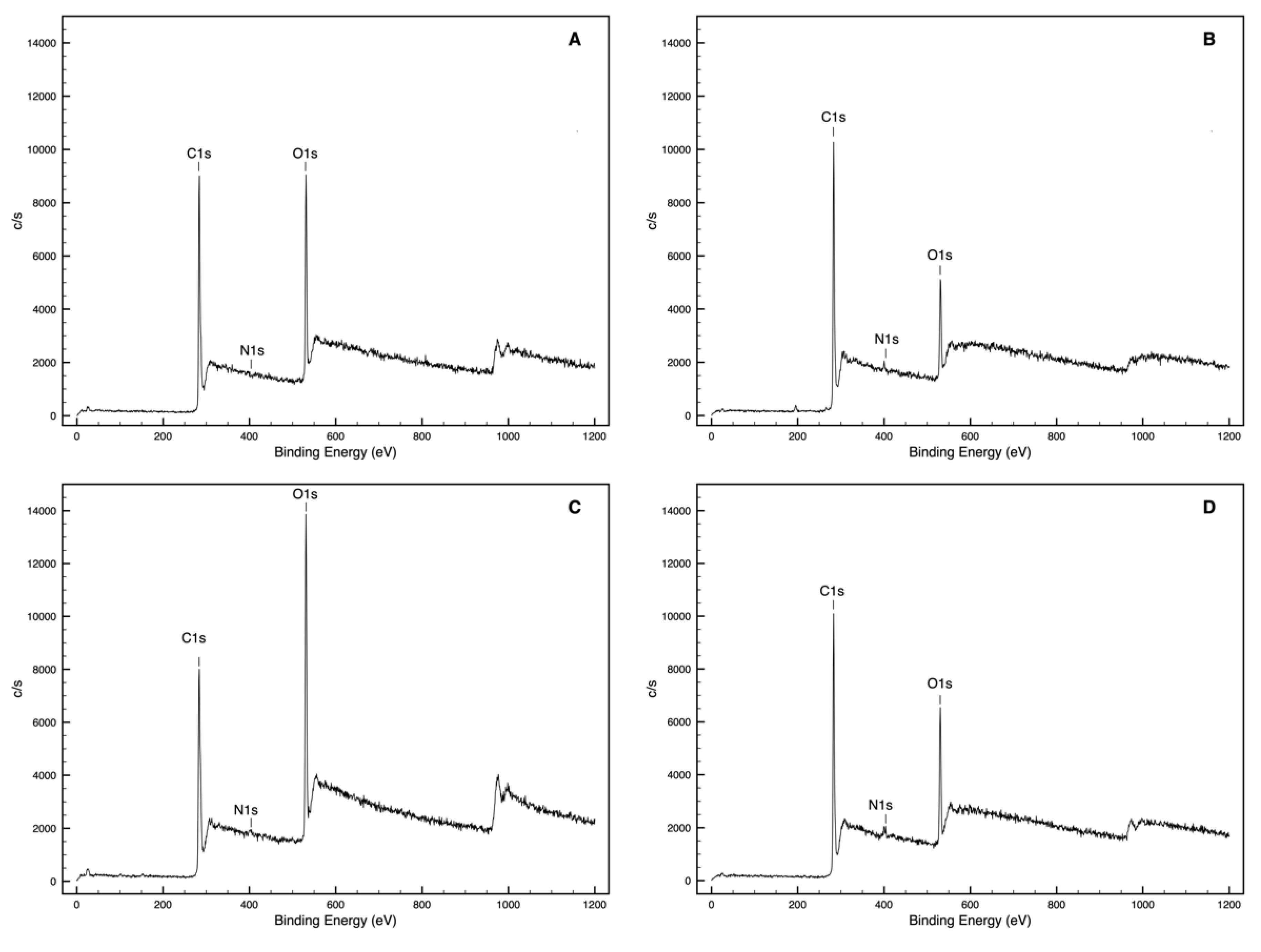
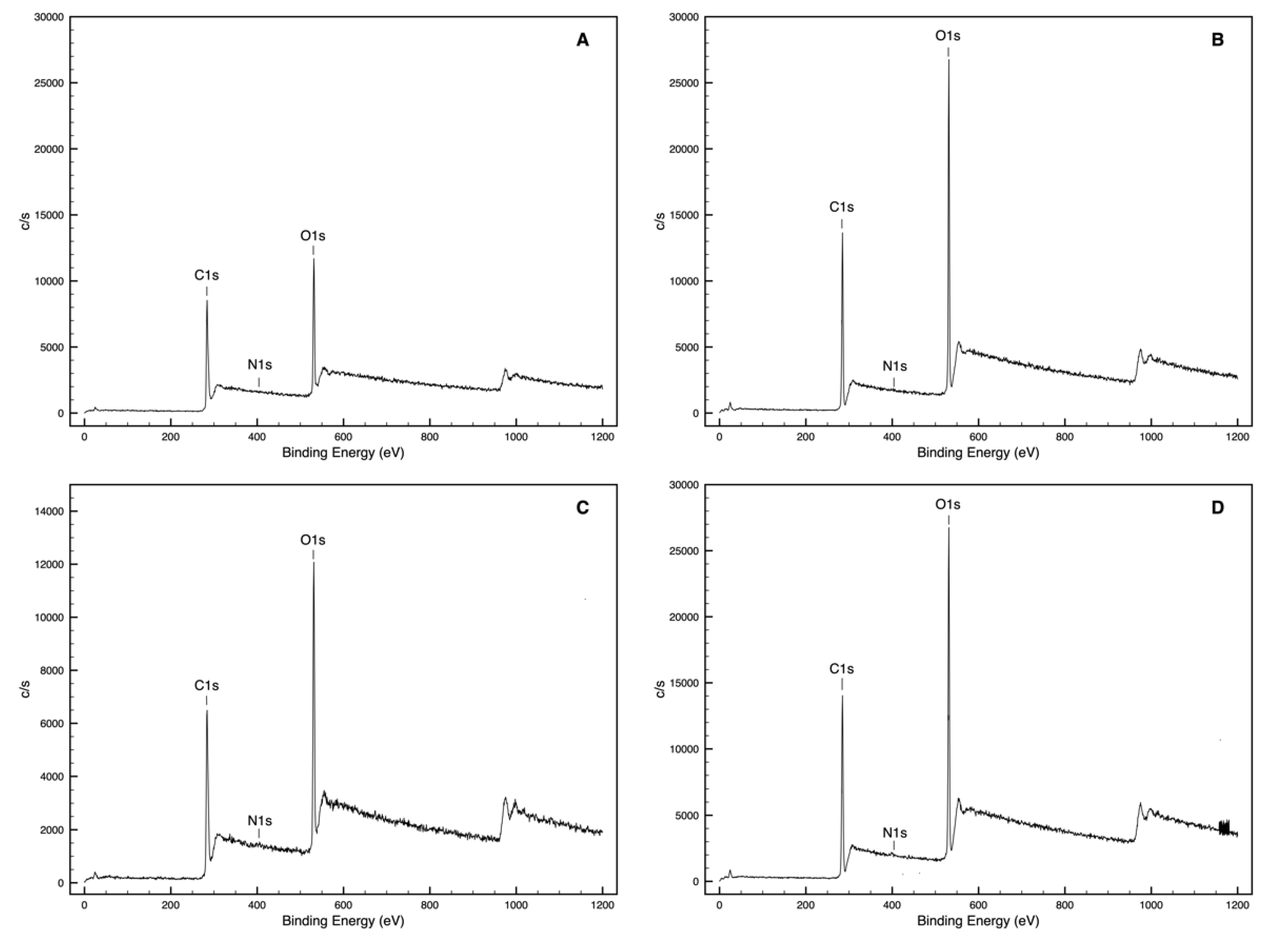
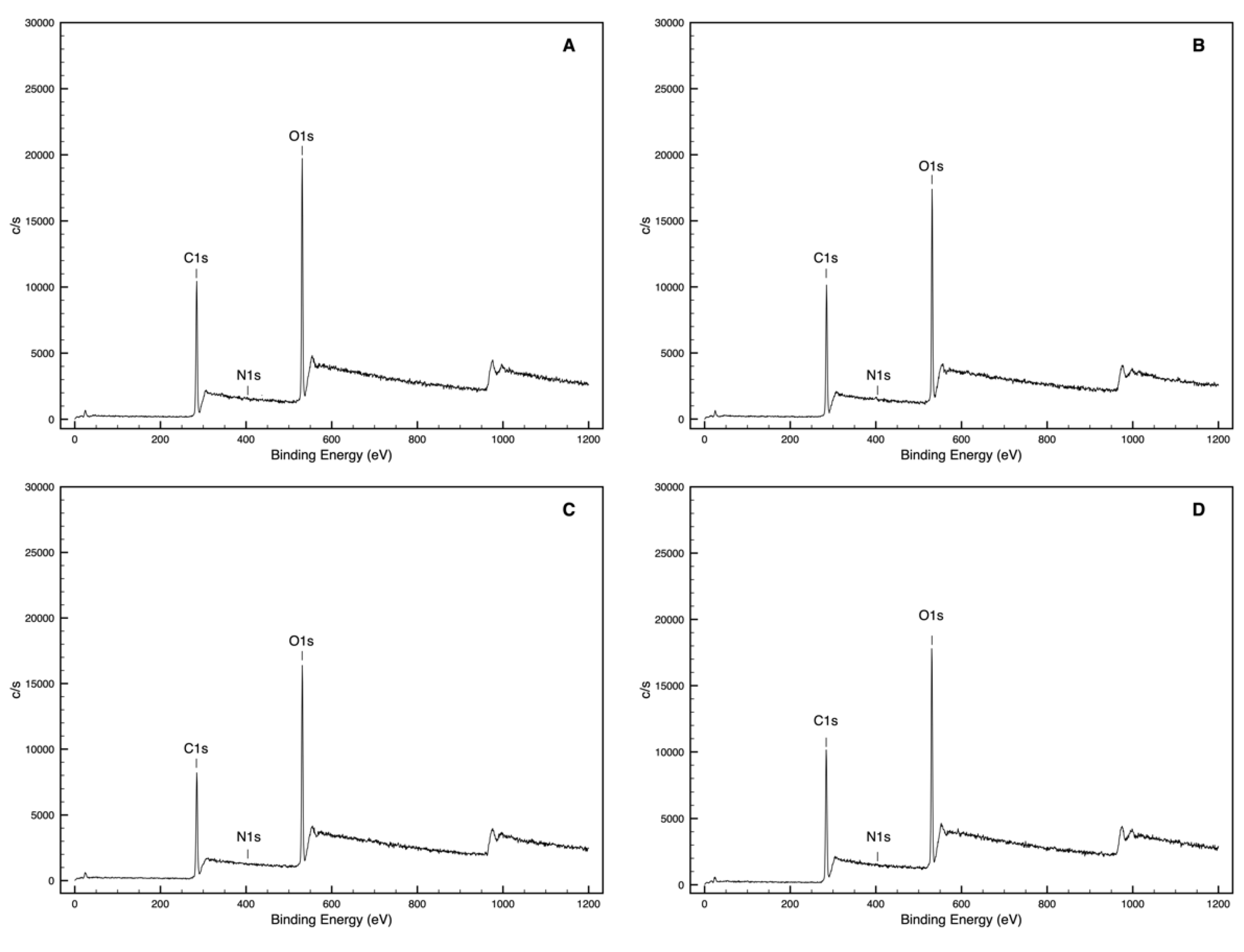
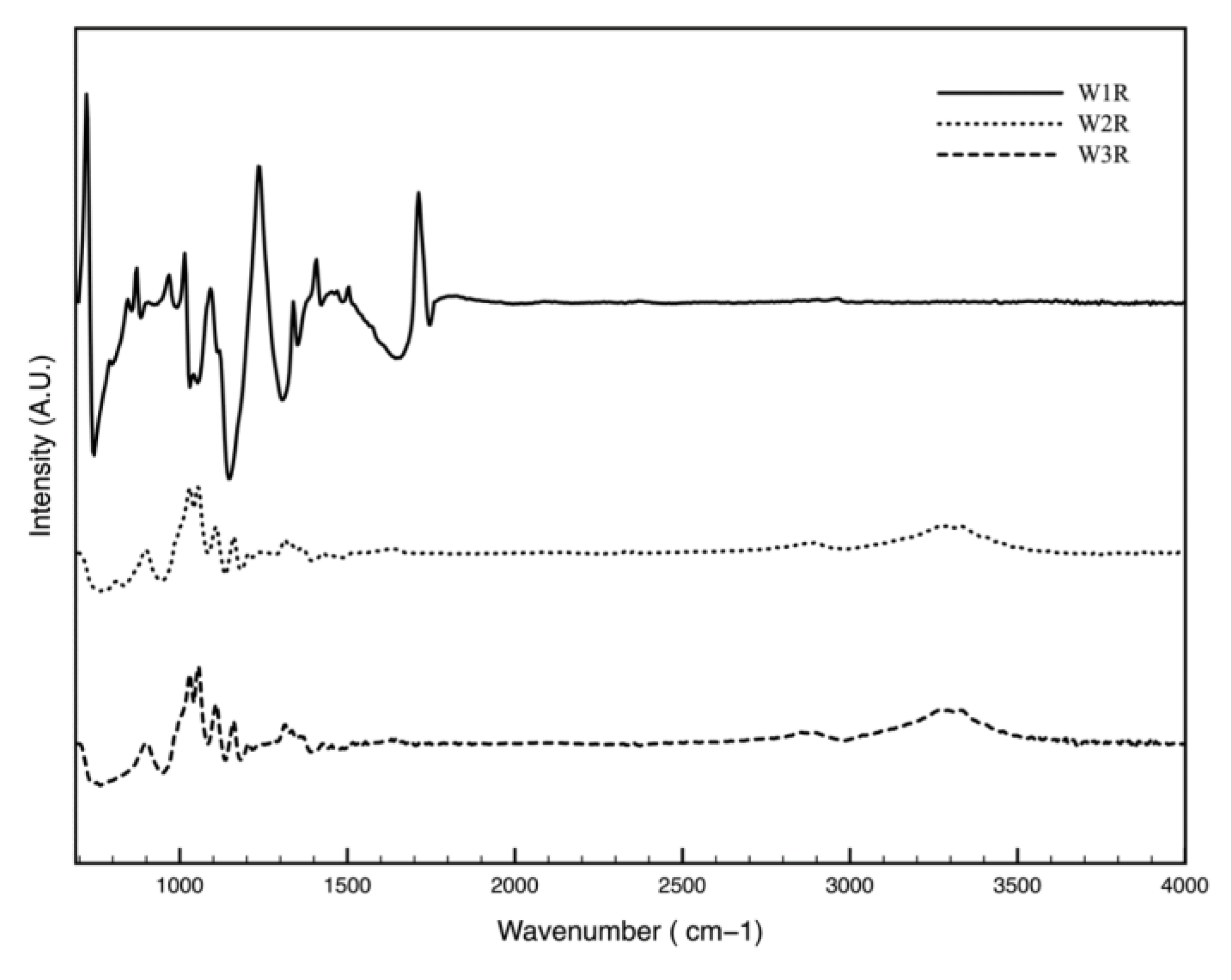
3.1. Chemical Interaction Assessment (XPS Analysis)
3.2. Adsorption of ADBAC During Storage Time
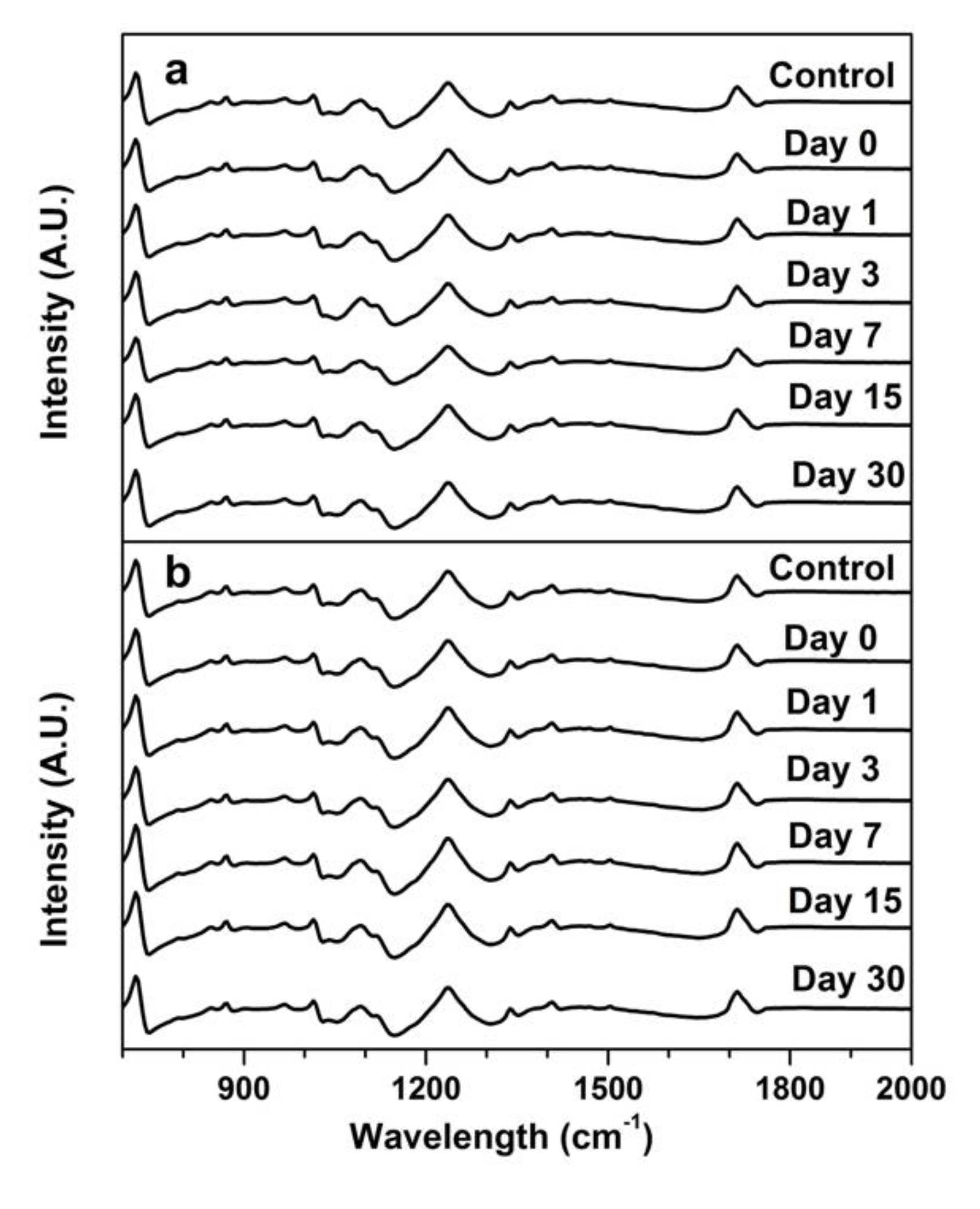
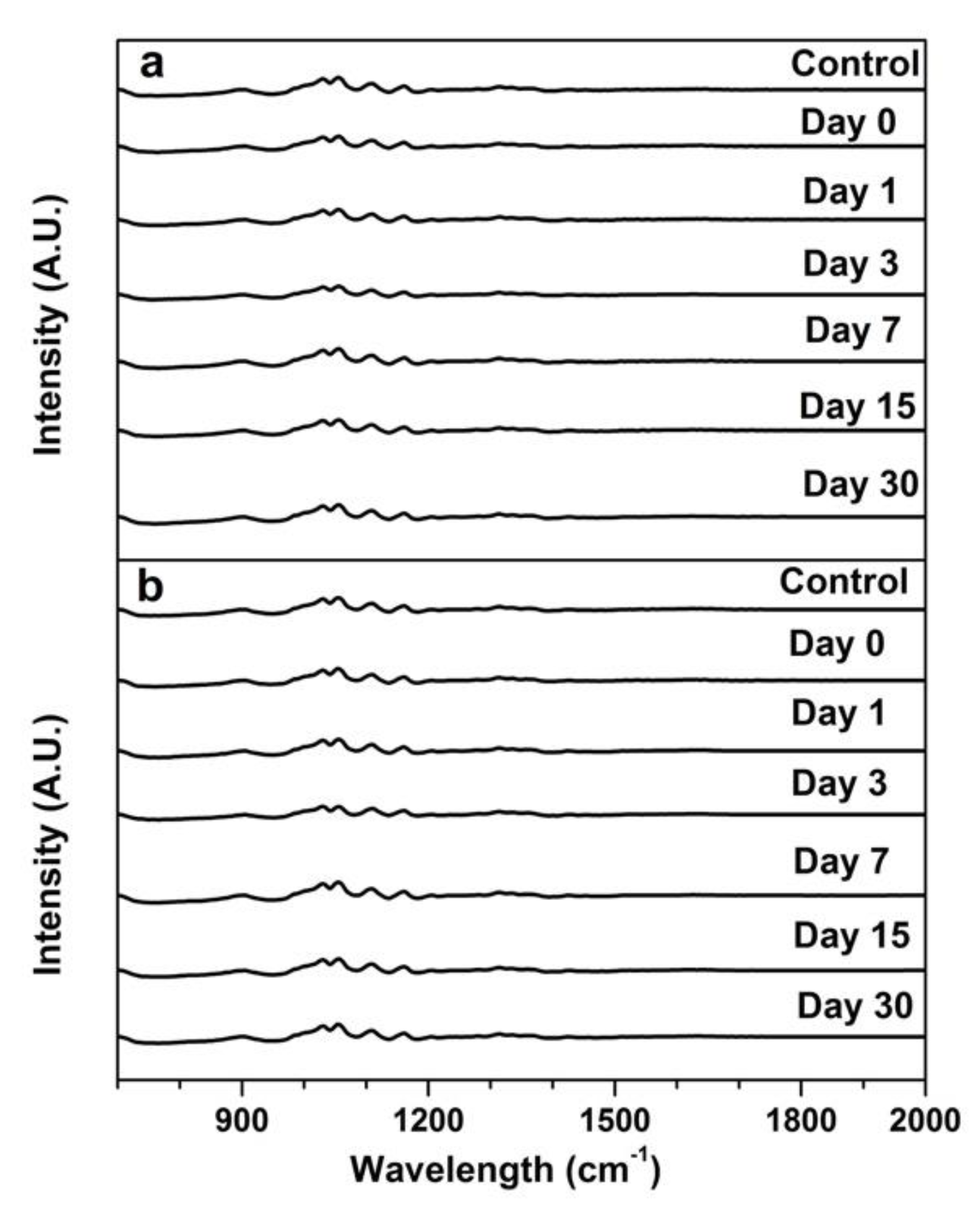
3.3. Chemical Change of the Wipe Surface Over Storage Time (FTIR)
3.4. Breaking Force and Elongation at Break Over Storage Time
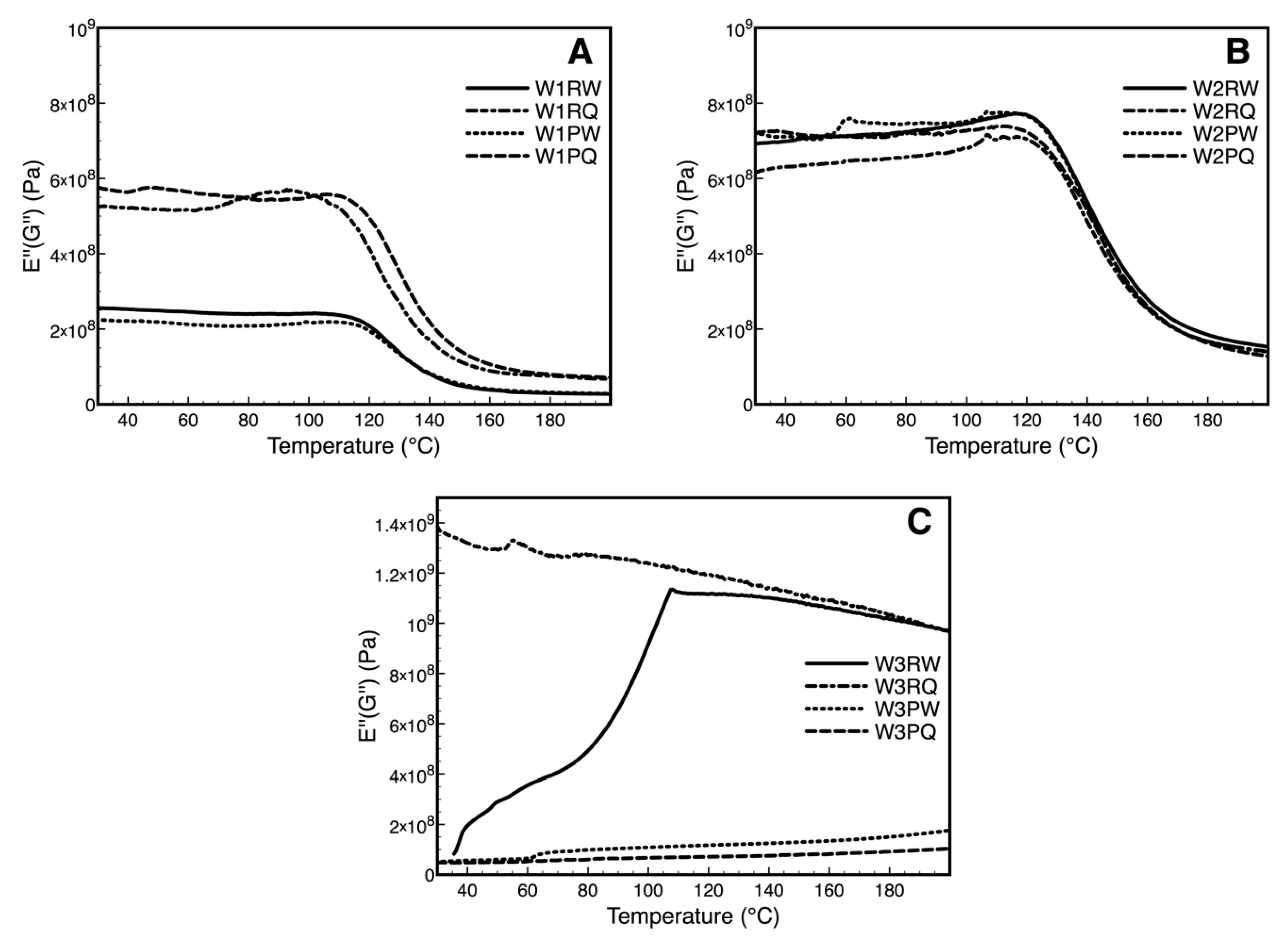
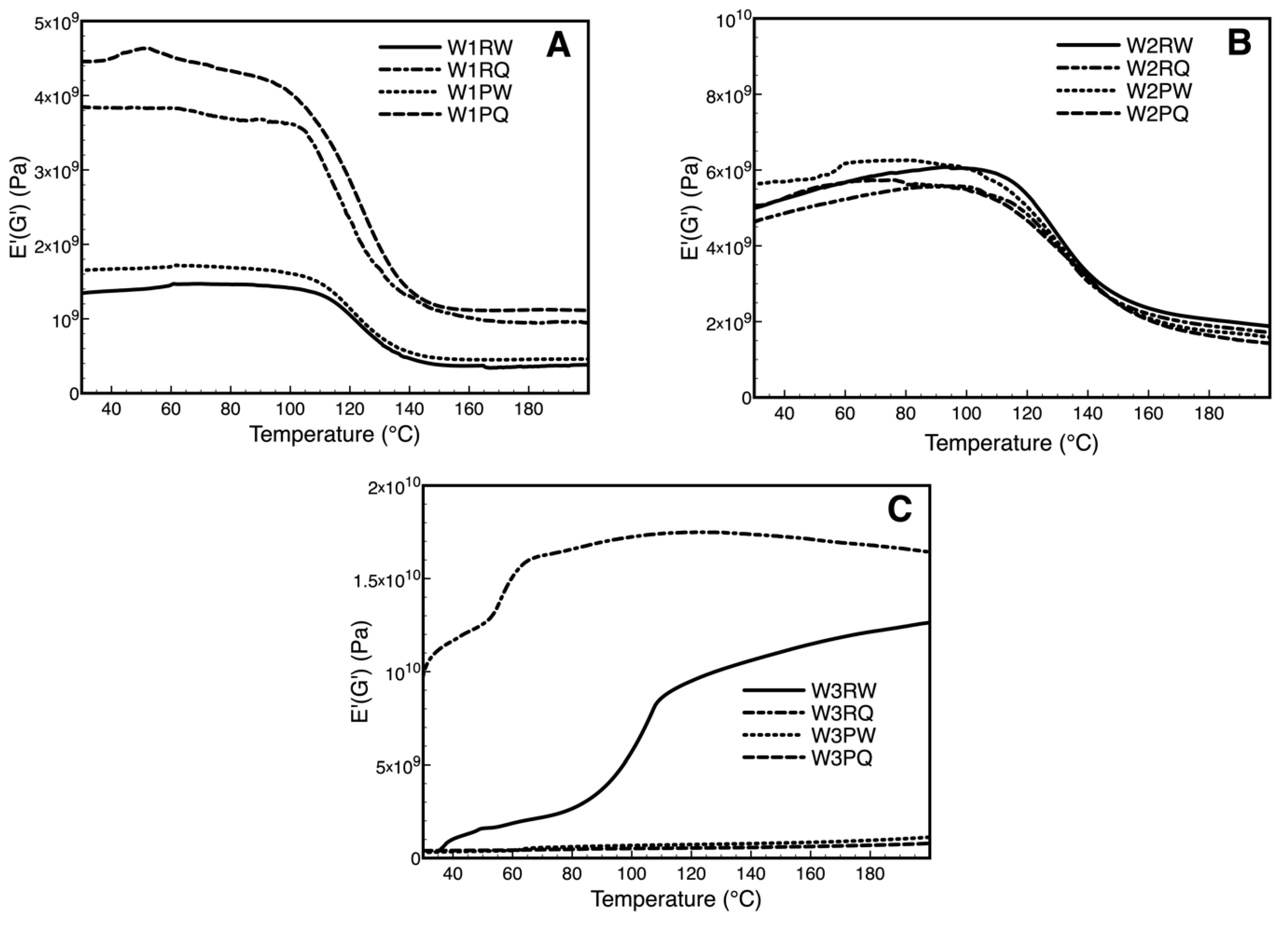
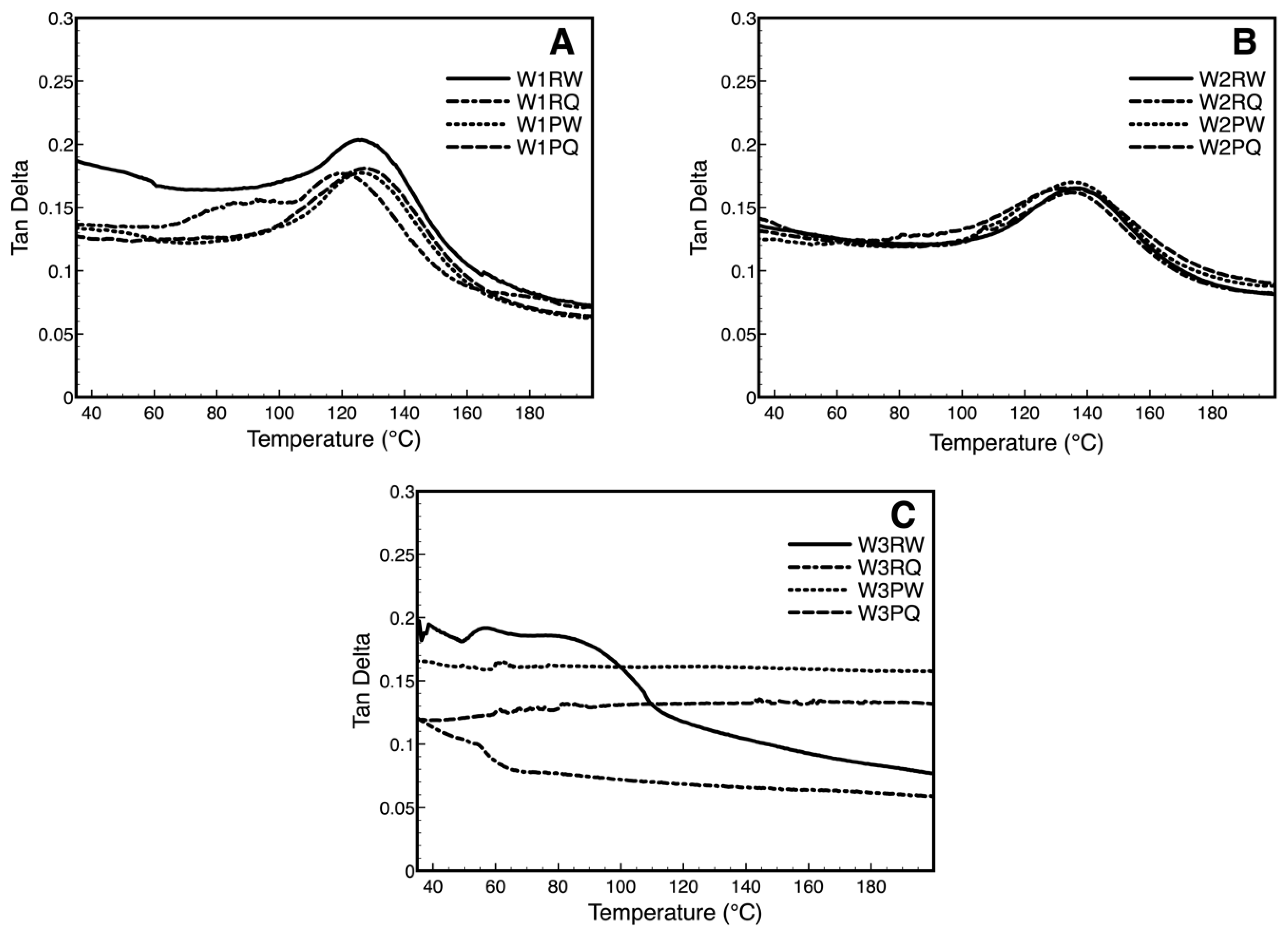
3.5. Dynamic Mechanical Analysis Over Storage Time (DMA)
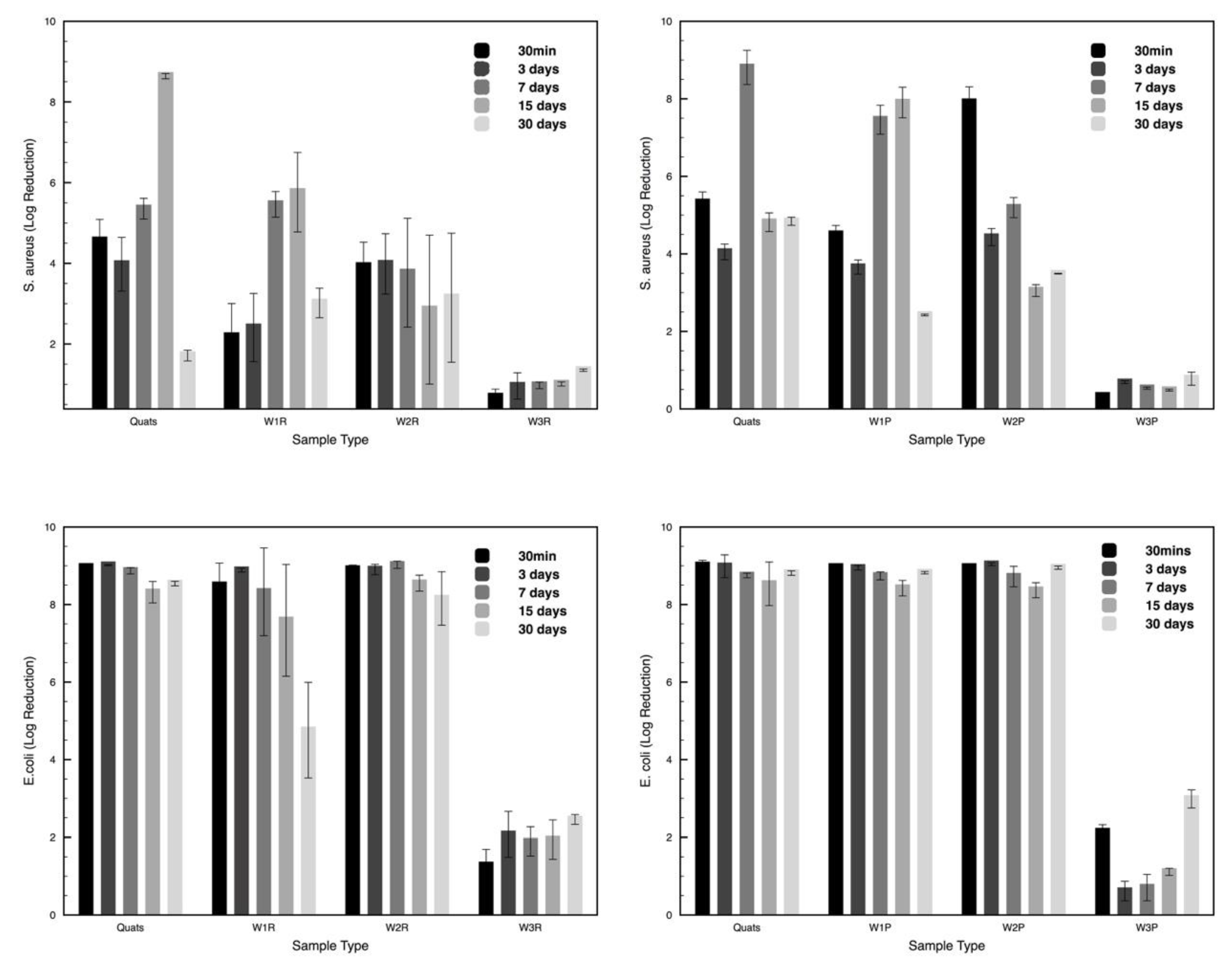
3.6. Antimicrbial Efficacy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A



| Source of Variation | SS | df | MS | F | P-value | F crit | |
|---|---|---|---|---|---|---|---|
| ANOVA result of S. aureus | Sample Type | 54.160191 | 3 | 18.053397 | 19.676001 | 6.30615 × 10−5 | 3.490295 |
| Storage Time | 6.8844590 | 4 | 1.7211148 | 1.8758052 | 0.179495939 | 3.259167 | |
| Error | 11.010406 | 12 | 0.9175339 | ||||
| Total | 72.055057 | 19 | |||||
| ANOVA result of E. coli | Sample Type | 149.61623 | 3 | 49.872078 | 68.638708 | 7.98187 × 10−8 | 3.490295 |
| Storage Time | 2.3886352 | 4 | 0.5971588 | 0.8218669 | 0.535746027 | 3.259167 | |
| Error | 8.7190589 | 12 | 0.7265882 | ||||
| Total | 160.723929 | 19 | |||||
| Source of Variation | SS | df | MS | F | P-value | F crit | |
|---|---|---|---|---|---|---|---|
| ANOVA result of S. aureus | Sample Type | 74.189835 | 3 | 24.729945 | 23.570227 | 2.55926 × 10−5 | 3.4902948 |
| Storage Time | 18.446129 | 4 | 4.6115324 | 4.3952731 | 0.02034882 | 3.2591667 | |
| Error | 12.590432 | 12 | 1.0492027 | ||||
| Total | 105.22640 | 19 | |||||
| ANOVA result of E. coli | Sample Type | 156.03905 | 3 | 52.013016 | 54,090543 | 3.03227 × 10−7 | 3.4902948 |
| Storage Time | 22.149016 | 4 | 5.5372540 | 5.7584253 | 0.00798589 | 3.2591667 | |
| Error | 11.539100 | 12 | 0.9615917 | ||||
| Total | 189.72717 | 19 | |||||
References
- Dancer, S.J. Controlling Hospital-Acquired Infection: Focus on the Role of the Environment and New Technologies for Decontamination. Clin. Microbiol. Rev. 2014, 27, 665–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckstein, B.C.; Adams, D.A.; Eckstein, E.C.; Rao, A.; Sethi, A.K.; Yadavalli, G.K.; Donskey, C.J. Reduction of Clostridium Difficile and vancomycin-resistant Enterococcus contamination of environmental surfaces after an intervention to improve cleaning methods. BMC Infect. Dis. 2007, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, E.A.; Nandy, P.; Lucas, A.D.; Hitchins, V.M. Ability of cleaning-disinfecting wipes to remove bacteria from medical device surfaces. Am. J. Infect. Control. 2015, 43, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
- Rutala, W.A.; Weber, D.J. Disinfectants used for environmental disinfection and new room decontamination technology. Am. J. Infect. Control. 2013, 41, S36–S41. [Google Scholar] [CrossRef] [PubMed]
- Amy, G.; Bull, R.; Craun, G.F.; Pegram, R.; Siddiqui, M. Disinfectants and disinfectant by-products; World Health Organization: Geneva, Swiss, 2000. [Google Scholar]
- Rengasamy, R.S. Composite Nonwovens in Wipes, in Composite Non-Woven Materials. 2014; 89–119. [Google Scholar]
- Soukupova, V.; Boguslavsky, L.; AnandJiwala, R.D. Studies on the Properties of Biodegradable Wipes made by the Hydroentanglement Bonding Technique. Text. Res. J. 2016, 77, 301–311. [Google Scholar] [CrossRef]
- Engelbrecht, K.; Ambrose, D.; Sifuentes, L.; Gerba, C.; Weart, I.; Koenig, D. Decreased activity of commercially available disinfectants containing quaternary ammonium compounds when exposed to cotton towels. Am. J. Infect. Control. 2013, 41, 908–911. [Google Scholar] [CrossRef] [PubMed]
- Bloß, R.; Meyer, S.; Kampf, G. Adsorption of active ingredients of surface disinfectants depends on the type of fabric used for surface treatment. J. Hosp. Infect. 2010, 75, 56–61. [Google Scholar] [CrossRef]
- Boyce, J.M.; Sullivan, L.; Booker, A.; Baker, J. Quaternary Ammonium Disinfectant Issues Encountered in an Environmental Services Department. Infect. Control. Hosp. Epidemiol. 2015, 37, 340–342. [Google Scholar] [CrossRef] [Green Version]
- Siani, H.; Cooper, C.; Maillard, J.-Y. Efficacy of “sporicidal” wipes against Clostridium difficile. Am. J. Infect. Control. 2011, 39, 212–218. [Google Scholar] [CrossRef]
- Kenters, N.; Huijskens, E.G.; De Wit, S.C.; Van Rosmalen, J.; Voss, A. Effectiveness of cleaning-disinfection wipes and sprays against multidrug-resistant outbreak strains. Am. J. Infect. Control. 2017, 45, e69–e73. [Google Scholar] [CrossRef]
- Bresee, R.R. General Effects of Ageing on Textiles. J. Am. Inst. Conserv. 2013, 25, 39–48. [Google Scholar] [CrossRef]
- Huang, C.; Chen, Y.; Sun, G.; Yan, K. Disinfectant Performance of a Chlorine Regenerable Antibacterial Microfiber Fabric as a Reusable Wiper. Materials 2019, 12, 127. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, A.; Deng, X.; Xiong, Q.; Cvelbar, U.; Degeyter, N.; Morent, R.; Leys, C. Non-thermal plasma technology for the development of antimicrobial surfaces: A review. J. Phys. D Appl. Phys. 2016, 49, 204002. [Google Scholar] [CrossRef]
- Tendero, C.; Tixier, C.; Tristant, P.; Desmaison, J.; Leprince, P. Atmospheric pressure plasmas: A review. Spectrochim. Acta Part B At. Spectrosc. 2006, 61, 2–30. [Google Scholar] [CrossRef]
- Zille, A.; Oliveira, F.R.; Souto, A.P. Plasma Treatment in Textile Industry. Plasma Process. Polym. 2015, 12, 98–131. [Google Scholar] [CrossRef]
- Leroux, F.; Perwuelz, A.; Campagne, C.; Behary, N. Atmospheric air-plasma treatments of polyester textile structures. J. Adhes. Sci. Technol. 2006, 20, 939–957. [Google Scholar] [CrossRef]
- Kolářová, K.; Vosmanská, V.; Rimpelová, S.; Svorcik, V. Effect of plasma treatment on cellulose fiber. Cellulose 2013, 20, 953–961. [Google Scholar] [CrossRef]
- Kramar, A.D.; Obradović, B.M.; Vesel, A.; Kuraica, M.M.; Kostic, M.M. Surface cleaning of raw cotton fibers with atmospheric pressure air plasma. Cellulose 2018, 25, 4199–4209. [Google Scholar] [CrossRef]
- Kusano, Y.; Madsen, B.; Berglund, L.; Aitomäki, Y.; Oksman, K. Dielectric barrier discharge plasma treatment of cellulose nanofibre surfaces. Surf. Eng. 2017, 34, 825–831. [Google Scholar] [CrossRef]
- ASTM International. ASTM E2149-13a Standard Test Method for Determining the Antimicrobial Activity of Antimicrobial Agents Under Dynamic Contact Conditions; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- ASTM International. Standard Test Method for Thickness of Textile Materials; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Zille, A.; Fernandes, M.M.; Francesko, A.; Tzanov, T.; Fernandes, M.; Oliveira, F.R.; Almeida, L.; Amorim, T.; Carneiro, N.; Esteves, M.F.; et al. Size and Aging Effects on Antimicrobial Efficiency of Silver Nanoparticles Coated on Polyamide Fabrics Activated by Atmospheric DBD Plasma. ACS Appl. Mater. Interfaces 2015, 7, 13731–13744. [Google Scholar] [CrossRef]
- ASTM International. ASTM D 5035-11—Standard Test Method for Breaking Force and Elongation of Textile Fabrics (Strip Method); ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- Leroux, F.; Campagne, C.; Perwuelz, A.; Gengembre, L. Atmospheric air plasma treatment of polyester textile materials. Textile structure influence on surface oxidation and silicon resin adhesion. Surf. Coat. Technol. 2009, 203, 3178–3183. [Google Scholar] [CrossRef]
- Molina, J.; Fernández, J.; Fernandes, M.; Souto, A.; Esteves, M.; Bonastre, J.; Cases, F.; Souto, A. Plasma treatment of polyester fabrics to increase the adhesion of reduced graphene oxide. Synth. Met. 2015, 202, 110–122. [Google Scholar] [CrossRef]
- Wang, Q.; Fan, X.-R.; Cui, L.; Wang, P.; Wu, J.; Chen, J. Plasma-Aided Cotton Bioscouring: Dielectric Barrier Discharge Versus Low-Pressure Oxygen Plasma. Plasma Chem. Plasma Process. 2009, 29, 399–409. [Google Scholar] [CrossRef]
- De Geyter, N.; Morent, R.; Leys, C. Surface modification of a polyester non-woven with a dielectric barrier discharge in air at medium pressure. Surf. Coat. Technol. 2006, 201, 2460–2466. [Google Scholar] [CrossRef]
- Zhang, C.; Fang, K. Surface modification of polyester fabrics for inkjet printing with atmospheric-pressure air/Ar plasma. Surf. Coat. Technol. 2009, 203, 2058–2063. [Google Scholar] [CrossRef]
- Reischl, M.; Ribitsch, V.; Stana-Kleinschek, K.; Stana-Kleinschek, K. Electrokinetic Investigations of Oriented Cellulose Polymers. Macromol. Symp. 2006, 244, 31–47. [Google Scholar] [CrossRef]
- Kan, C.-W.; Man, W.-S. Surface Characterisation of Atmospheric Pressure Plasma Treated Cotton Fabric-Effect of Operation Parameters. Polymers 2018, 10, 250. [Google Scholar] [CrossRef]
- Alila, S.; Boufi, S.; Belgacem, M.N.; Beneventi, D. Adsorption of a Cationic Surfactant onto Cellulosic Fibers I. Surface Charge Effects. Langmuir 2005, 21, 8106–8113. [Google Scholar] [CrossRef]
- Palaskar, S.; Kale, K.H.; Nadiger, G.S.; Desai, A.N. Dielectric barrier discharge plasma induced surface modification of polyester/cotton blended fabrics to impart water repellency using HMDSO. J. Appl. Polym. Sci. 2011, 122, 1092–1100. [Google Scholar] [CrossRef]
- Li, S.-M.; Fu, L.-H.; Ma, M.-G.; Zhu, J.-F.; Sun, R.-C.; Xu, F. Simultaneous microwave-assisted synthesis, characterization, thermal stability, and antimicrobial activity of cellulose/AgCl nanocomposites. Biomass Bioenergy 2012, 47, 516–521. [Google Scholar] [CrossRef]
- Algar, I.; Fernandes, S.C.M.; Mondragon, G.; Castro, C.; Garcia-Astrain, C.; Gabilondo, N.; Retegi, A.; Eceiza, A. Pineapple agroindustrial residues for the production of high value bacterial cellulose with different morphologies. J. Appl. Polym. Sci. 2015, 132, 41237. [Google Scholar] [CrossRef]
- Schwanninger, M.; Rodrigues, J.; Pereira, H.; Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- Široký, J.; Blackburn, R.S.; Bechtold, T.; Taylor, J.; White, P. Attenuated total reflectance Fourier-transform Infrared spectroscopy analysis of crystallinity changes in lyocell following continuous treatment with sodium hydroxide. Cellulose 2009, 17, 103–115. [Google Scholar] [CrossRef]
- Cheng, S.; Yuen, C.; Kan, C.-W.; Cheuk, K.; Daoud, W.; Lam, P.; Tsoi, W. Influence of atmospheric pressure plasma treatment on various fibrous materials: Performance properties and surface adhesion analysis. Vacuum 2010, 84, 1466–1470. [Google Scholar] [CrossRef]
- Ceria, A.; Rombaldoni, F.; Rovero, G.; Mazzuchetti, G.; Sicardi, S. The effect of an innovative atmospheric plasma jet treatment on physical and mechanical properties of wool fabrics. J. Mater. Process. Technol. 2010, 210, 720–726. [Google Scholar] [CrossRef]
- Varan, N.Y.; Eryuruk, S. The Effects of Quat-Silane Antimicrobials on the Physical and Mechanical Properties of Cotton and Cotton/Elastane Fabrics Used for Clothing. IOP Conf. Series: Mater. Sci. Eng. 2018, 460, 460. [Google Scholar]
- Rahimi, M.H.; Parvinzadeh, M.; Navid, M.Y.; Ahmadi, S. Thermal Characterization and Flammability of Polyester Fiber Coated with Nonionic and Cationic Softeners. J. Surfactants Deterg. 2011, 14, 595–603. [Google Scholar] [CrossRef]
- Babu, K.F.; Narayanan, T.N.; Kulandainathan, M.A. Molecular Motions Aided Thermally Responsive Biocompatible Textile Pads. Adv. Mater. Interfaces 2014, 1, 1300139. [Google Scholar] [CrossRef]
- Kafi, A.A.; Magniez, K.; Fox, B.L. A surface-property relationship of atmospheric plasma treated jute composites. Compos. Sci. Technol. 2011, 71, 1692–1698. [Google Scholar] [CrossRef]
- Nagalakshmaiah, M.; El Kissi, N.; Dufresne, A. Ionic Compatibilization of Cellulose Nanocrystals with Quaternary Ammonium Salt and Their Melt Extrusion with Polypropylene. ACS Appl. Mater. Interfaces 2016, 8, 8755–8764. [Google Scholar] [CrossRef]
- Stana-Kleinschek, K.; Strnad, S.; Ribitsch, V.; Stana-Kleinschek, K. Surface characterization and adsorption abilities of cellulose fibers. Polym. Eng. Sci. 1999, 39, 1412–1424. [Google Scholar] [CrossRef]
- Corradini, E.; Imam, S.H.; Agnelli, J.A.M.; Mattoso, L.H.C. Effect of Coconut, Sisal and Jute Fibers on the Properties of Starch/Gluten/Glycerol Matrix. J. Polym. Environ. 2009, 17, 1–9. [Google Scholar] [CrossRef]
- Lavorgna, M.; Piscitelli, F.; Mangiacapra, P.; Buonocore, G.G. Study of the combined effect of both clay and glycerol plasticizer on the properties of chitosan films. Carbohydr. Polym. 2010, 82, 291–298. [Google Scholar] [CrossRef]
- Luo, S.P.; Cao, J.Z.; Peng, Y. Properties of Glycerin-Thermally Modified Wood Flour/Polypropylene Composites. Polym. Compos. 2014, 35, 201–207. [Google Scholar] [CrossRef]
- Hinchliffe, D.J.; Condon, B.D.; Slopek, R.P.; Reynolds, M. The adsorption of alkyl-dimethyl-benzyl-ammonium chloride onto cotton nonwoven hydroentangled substrates at the solid–liquid interface is minimized by additive chemistries. Text. Res. J. 2016, 87, 70–80. [Google Scholar] [CrossRef]
- Morais, D.S.; Guedes, R.M.; Lopes, M.A. Antimicrobial Approaches for Textiles: From Research to Market. Materials 2016, 9, 498. [Google Scholar] [CrossRef]



| Sample | Components | Structure | Dimension (cm) of a 0.05 g Sample | Fabric Thickness (mm ± CV%) | Areal Density (g m−2 ± CV%) |
|---|---|---|---|---|---|
| W1 | 100% polyester | Hydroentangled nonwoven | 2.5 × 4.5 | 0.36 ± 5.93 | 40.92 ± 1.59 |
| W2 | 55% cellulose 45% polyester | Hydroentangled nonwoven | 2.5 × 2.5 | 0.54 ± 3.02 | 69.58 ± 0.96 |
| W3 | 100% bleached cotton | 1/1 plain weave | 2.5 × 1.5 | 0.98 ± 5.09 | 118.72 ± 0.41 |
| Untreated | Plasma Treated | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Chemical Composition (%) | Atomic Ratio | Chemical Composition (%) | Atomic Ratio | |||||||
| C | O | N | O/C | N/C | C | O | N | O/C | N/C | |
| W1 | 73.3 | 26.2 | 0.5 | 0.36 | 0.01 | 65.2 | 33.2 | 0.7 | 0.51 | 0.01 |
| W2 | 69.1 | 30.5 | 0.4 | 0.44 | 0.01 | 65.2 | 33.8 | 1.1 | 0.52 | 0.02 |
| W3 | 61.9 | 37.7 | 0.4 | 0.61 | 0.01 | 60.7 | 38.7 | 0.6 | 0.64 | 0.01 |
| W1Q | 82.2 | 15.1 | 1.7 | 0.18 | 0.02 | 79.2 | 18.5 | 2.2 | 0.23 | 0.03 |
| W2Q | 60.8 | 38.6 | 0.6 | 0.63 | 0.01 | 61.3 | 38.2 | 0.5 | 0.62 | 0.01 |
| W3Q | 62.9 | 36.1 | 1.0 | 0.57 | 0.02 | 62.3 | 37.0 | 0.7 | 0.59 | 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Cvelbar, U.; Strazar, P.; Vossebein, L.; Zille, A. Chemical, Thermo-Mechanical and Antimicrobial Properties of DBD Plasma Treated Disinfectant-Impregnated Wipes during Storage. Polymers 2019, 11, 1769. https://doi.org/10.3390/polym11111769
Song X, Cvelbar U, Strazar P, Vossebein L, Zille A. Chemical, Thermo-Mechanical and Antimicrobial Properties of DBD Plasma Treated Disinfectant-Impregnated Wipes during Storage. Polymers. 2019; 11(11):1769. https://doi.org/10.3390/polym11111769
Chicago/Turabian StyleSong, Xinyu, Uros Cvelbar, Petra Strazar, Lutz Vossebein, and Andrea Zille. 2019. "Chemical, Thermo-Mechanical and Antimicrobial Properties of DBD Plasma Treated Disinfectant-Impregnated Wipes during Storage" Polymers 11, no. 11: 1769. https://doi.org/10.3390/polym11111769
APA StyleSong, X., Cvelbar, U., Strazar, P., Vossebein, L., & Zille, A. (2019). Chemical, Thermo-Mechanical and Antimicrobial Properties of DBD Plasma Treated Disinfectant-Impregnated Wipes during Storage. Polymers, 11(11), 1769. https://doi.org/10.3390/polym11111769