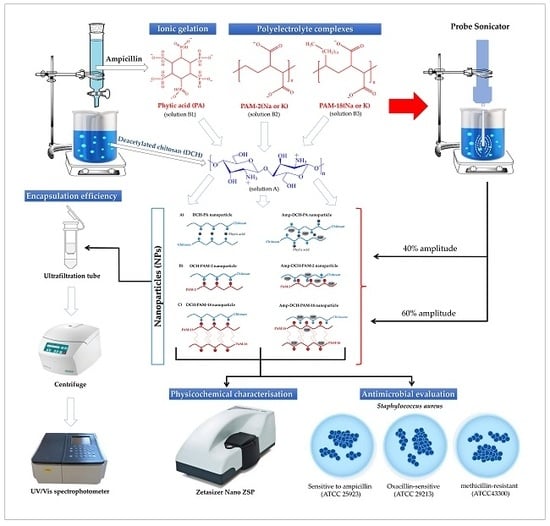
Synthesis, Characterisation and Biological Evaluation of Ampicillin–Chitosan–Polyanion Nanoparticles Produced by Ionic Gelation and Polyelectrolyte Complexation Assisted by High-Intensity Sonication
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production and Characterisation of Highly Deacetylated Chitosan
2.3. Preparation of Nanoparticulate Systems
2.4. Physicochemical Characterisation of the Nanoparticles
2.4.1. Particle Size, Polydispersity Index (PDI) and Zeta Potential Analyses
2.4.2. Encapsulation Efficiency (EE)
2.5. Stability of the Nanoparticle Systems
2.6. Antimicrobial Effect of the Nanoparticles
2.7. Statistical Analysis
3. Results and Discussion
3.1. Production and Characterisation of a Highly Deacetylated Chitosan
3.2. Production and Characterisation of Nanoparticulate Systems
3.2.1. Particle Size
3.2.2. Polydispersity
3.2.3. Zeta Potential
3.2.4. Encapsulation Efficiency
3.3. Stability of the Nanoparticulate Systems
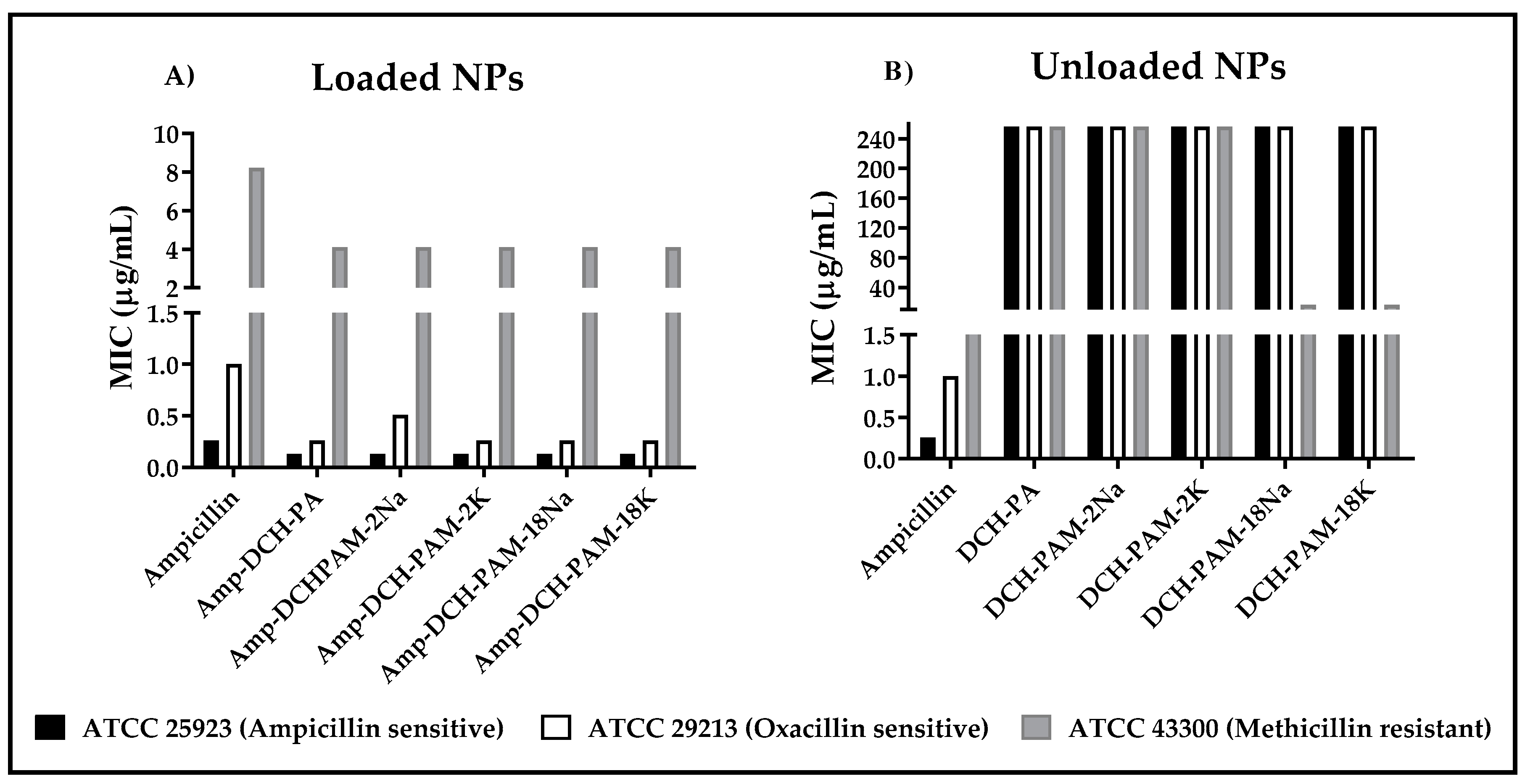
3.4. Antimicrobial Effect of the Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Valaperta, R.; Tejada, M.; Frigerio, M. Staphylococcus aureus nosocomial infections: The role of a rapid and low-cost characterization for the establishment of a surveillance system. New Microbiol. 2010, 33, 223–232. [Google Scholar] [PubMed]
- Naber, C.K. Staphylococcus aureus bacteremia: Epidemiology, pathophysiology, and management strategies. Clin. Infect. Dis. 2009, 48, S231–S237. [Google Scholar] [CrossRef] [PubMed]
- Vandenbergh, M.F.Q.; Verbrugh, H.A. Carriage of Staphylococcus aureus: Epidemiology and clinical relevance. J. Lab. Clin. Med. 1999, 133, 525–534. [Google Scholar] [CrossRef]
- Niaz, T.; Shabbir, S.; Noor, T.; Abbasi, R.; Raza, Z.A.; Imran, M. Polyelectrolyte multicomponent colloidosomes loaded with nisin Z for enhanced antimicrobial activity against foodborne resistant pathogens. Front. Microbiol. 2018, 8, 2700. [Google Scholar] [CrossRef] [PubMed]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-strategies to fight multidrug resistant bacteria-”A battle of the titans”. Front Microbiol. 2018, 9, 1–26. [Google Scholar] [CrossRef]
- Tacconelli, E. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- World Health Organization. Antibiotic Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 12 September 2018).
- Katz, M.L.; Mueller, L.V.; Polyakov, M.; Weinstock, S.F. Where have all the antibiotic patents gone? Nat. Biotechnol. 2006, 24, 1529–1531. [Google Scholar] [CrossRef]
- Xiong, M.H.; Bao, Y.; Yang, X.Z.; Zhu, Y.H.; Wang, J. Delivery of antibiotics with polymeric particles. Adv. Drug Deliv. Rev. 2014, 78, 63–76. [Google Scholar] [CrossRef]
- Kalhapure, R.S.; Suleman, N.; Mocktar, C.; Seedat, N.; Govender, T. Nanoengineered drug delivery systems for enhancing antibiotic therapy. J. Pharm. Sci. 2015, 104, 872–905. [Google Scholar] [CrossRef]
- Kumar, M.; Curtis, A.; Hoskins, C. Application of nanoparticle technologies in the combat against anti-microbial resistance. Pharmaceutics 2018, 10, 11. [Google Scholar] [CrossRef]
- Arenas, T.; Mora, C.; Salamanca, C.H.; Jaramillo, M.C. Activity of (2E)-3-(2, 3-dimetoxifenil)-1-(4-metilfenil) prop-2-en-1-ona in the presence of poli(maleic acid-co-2-vinyl-pyrrolidone) on a β-lactamase producing clinical isolate of Staphylococcus aureus. Iatreia 2012, 25, 12–19. [Google Scholar]
- Salamanca, C.H.; Yarce, C.J.; Roman, Y.; Davalos, A.F.; Rivera, G.R. Application of nanoparticle technology to reduce the anti-microbial resistance through β-lactam antibiotic-polymer inclusion nano-complex. Pharmaceutics 2018, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Arévalo, L.M.; Yarce, C.J.; Oñate-Garzón, J.; Salamanca, C.H. Decrease of antimicrobial resistance through polyelectrolyte-coated nanoliposomes loaded with β-lactam drug. Pharmaceuticals 2019, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Cantor, S.; Vargas, L.; Rojas, O.E.A.; Yarce, C.J.; Salamanca, C.H.; Oñate-Garzón, J. Evaluation of the antimicrobial activity of cationic peptides loaded in surface-modified nanoliposomes against foodborne bacteria. Int. J. Mol. Sci. 2019, 20, 680. [Google Scholar] [CrossRef] [PubMed]
- Ngan, L.T.K.; Wang, S.L.; Hiep, D.M.; Luong, P.M.; Vui, N.T.; Dinh, T.M.; Dzung, N.A. Preparation of chitosan nanoparticles by spray drying, and their antibacterial activity. Res. Chem. Intermed. 2014, 40, 2165–2175. [Google Scholar] [CrossRef]
- Yang, J.; Lu, H.; Li, M.; Liu, J.; Zhang, S.; Xiong, L.; Sun, Q. Development of chitosan-sodium phytate nanoparticles as a potent antibacterial agent. Carbohydr. Polym. 2017, 178, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.M.; El-Bisi, M.K.; Taha, G.M.; El-Alfy, E.A. Chitosan nanoparticles loaded antibiotics as drug delivery biomaterial. J. Appl. Pharm. Sci. 2015, 5, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef]
- Katas, H.; Alpar, H.O. Development and characterisation of chitosan nanoparticles for siRNA delivery. J. Control. Release 2006, 115, 216–225. [Google Scholar] [CrossRef]
- Bugnicourt, L.; Ladavière, C. Interests of chitosan nanoparticles ionically cross-linked with tripolyphosphate for biomedical applications. Prog. Polym. Sci. 2016, 60, 1–17. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, W.; Wang, C.; Hu, J.; Fu, S. Chitosan nanoparticles as a novel delivery system for ammonium glycyrrhizinate. Int. J. Pharm. 2005, 295, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Janes, K.A.; Fresneau, M.P.; Marazuela, A.; Fabra, A.; Alonso, M.J. Chitosan nanoparticles as delivery systems for doxorubicin. J. Control. Release 2001, 73, 255–267. [Google Scholar] [CrossRef]
- Du, W.L.; Niu, S.S.; Xu, Y.L.; Xu, Z.R.; Fan, C.L. Antibacterial activity of chitosan tripolyphosphate nanoparticles loaded with various metal ions. Carbohydr. Polym. 2009, 75, 385–389. [Google Scholar] [CrossRef]
- Mohammadpour Dounighi, N.; Eskandari, R.; Avadi, M.R.; Zolfagharian, H.; Mir Mohammad Sadeghi, A.; Rezayat, M. Preparation and in vitro characterization of chitosan nanoparticles containing Mesobuthus eupeus scorpion venom as an antigen delivery system. J. Venom. Anim. Toxins Incl. Trop. Dis. 2012, 18, 44–52. [Google Scholar] [Green Version]
- Rodrigues, S.; Da Costa, A.M.R.; Grenha, A. Chitosan/carrageenan nanoparticles: Effect of cross-linking with tripolyphosphate and charge ratios. Carbohydr. Polym. 2012, 89, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Dai, Y.N.; Zhang, J.P.; Wang, A.Q.; Wei, Q. Chitosan-alginate nanoparticles as a novel drug delivery system for nifedipine. Int. J. Biomed. Sci. 2008, 4, 221–228. [Google Scholar]
- Sarmento, B.; Ferreira, D.; Veiga, F.; Ribeiro, A. Characterization of insulin-loaded alginate nanoparticles produced by ionotropic pre-gelation through DSC and FTIR studies. Carbohydr. Polym. 2006, 66, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.; Xie, J.; Zhang, X.; Cai, J.; Xia, S. Polysaccharide-based nanoparticles by chitosan and gum arabic polyelectrolyte complexation as carriers for curcumin. Food Hydrocoll. 2016, 57, 236–245. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Chakraborty, S.; Bhattacharya, S.; Mishra, R.; Kundu, P.P. PH-sensitive chitosan/alginate core-shell nanoparticles for efficient and safe oral insulin delivery. Int. J. Biol. Macromol. 2015, 72, 640–648. [Google Scholar] [CrossRef]
- Yarce, C.J.; Echeverri, J.D.; Palacio, M.A.; Rivera, C.A.; Salamanca, C.H. Relationship between surface properties and in vitro drug release from compressed matrix containing polymeric materials with different hydrophobicity degrees. Pharmaceuticals 2017, 10, 15. [Google Scholar] [CrossRef]
- Yarce, C.J.; Pineda, D.; Correa, C.E.; Salamanca, C.H. Relationship between surface properties and in vitro drug release from a compressed matrix containing an amphiphilic polymer material. Pharmaceuticals 2016, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Kasaai, M.R. Calculation of Mark–Houwink–Sakurada (MHS) equation viscometric constants for chitosan in any solvent–temperature system using experimental reported viscometric constants data. Carbohydr. Polym. 2007, 68, 477–488. [Google Scholar] [CrossRef]
- Baxter, A.; Dillon, M.; Anthony Taylor, K.D.; Roberts, G.A.F. Improved method for ir determination of the degree of N-acetylation of chitosan. Int. J. Biol. Macromol. 1992, 14, 166–169. [Google Scholar] [CrossRef]
- Kasaai, M.R. A review of several reported procedures to determine the degree of N-acetylation for chitin and chitosan using infrared spectroscopy. Carbohydr. Polym. 2008, 71, 497–508. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. MO7-A10: Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved Standard, 10th ed.; Clinical and laboratory Standards Institute: Wayne, PA, USA, 2015; pp. 15–47. [Google Scholar]
- Rojas, J.Y.; Ciro, Y.; Salamanca, C. Effect of the degree of acetylation on the physical and tableting properties of chitin. In Chitin: Properties, Applications and Research; NOVA Science Publishers Inc.: Hauppauge, NY, USA, 2017; in press. [Google Scholar]
- Yuan, Y.; Chesnutt, B.; Haggard, W.O.; Bumgardner, J.D. Deacetylation of chitosan: Material characterization and in vitro evaluation via albumin adsorption and pre-osteoblastic cell cultures. Materials 2011, 4, 1399–1416. [Google Scholar] [CrossRef]
- Sacco, P.; Paoletti, S.; Cok, M.; Asaro, F.; Abrami, M.; Grassi, M.; Donati, I. Insight into the ionotropic gelation of chitosan using tripolyphosphate and pyrophosphate as cross-linkers. Int. J. Biol. Macromol. 2016, 92, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Usmiati, S.; Richana, N.; Mangunwidjaja, D.; Noor, E.; Prangdimurti, E. The using of ionic gelation method based on polysaccharides for encapsulating the macromolecules–a review. Encapsulation Prot. Bioact. Compd. 2014, 67, 79–84. [Google Scholar]
- Koukaras, E.N.; Papadimitriou, S.A.; Bikiaris, D.N.; Froudakis, G.E. Insight on the formation of chitosan nanoparticles through ionotropic gelation with tripolyphosphate. Mol. Pharm. 2012, 9, 2856–2862. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Ampicillin. Compendium of food additive specifications. In Proceedings of the joint FAO/WHO Expert Committee on Food Additives Eighty-Fifth Meeting (Residues of Veterinary Drugs); Geneva, Switzerland, 17 October 2017.
- Mishra, B.; Mishra, M.; Yadav, S.K. Antibacterial loaded spray dried chitosan polyelectrolyte complexes as dry powder aerosol for the treatment of lung infections. Iran. J. Pharm. Res. 2017, 16, 74–92. [Google Scholar]
- Patil, P.; Chavanke, D.; Wagh, M. A review on ionotropic gelation method: Novel approach for controlled gastroretentive gelispheres. Int. J. Pharm. Pharm. Sci. 2012, 4, 27–32. [Google Scholar]
- Moustafine, R.I.; Margulis, E.B.; Sibgatullina, L.F.; Kemenova, V.A.; Van der Mooter, G. Comparative evaluation of interpolyelectrolyte complexes of chitosan with Eudragit® L100 and Eudragit® L100-55 as potential carriers for oral controlled drug delivery. Eur. J. Pharm. Biopharm. 2008, 70, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Patil, J.S.; Marapur, S.C.; Gurav, P.B.; Banagar, A.V. Ionotropic gelation and polyelectrolyte complexation technique: Novel approach to drug encapsulation. In Handbook of Encapsulation and Controlled Releas, 1st ed.; Mishra, M., Ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2016; pp. 273–296. [Google Scholar]
- Badawy, M.E.I.; Taktak, N.E.M.; Awad, O.M.; Elfiki, S.A.; Abou El_Ela, N.E. Preparation and characterization of biopolymers chitosan/alginate/gelatin gel spheres crosslinked by glutaraldehyde. J. Macromol. Sci. Part B Phys. 2017, 56, 359–372. [Google Scholar] [CrossRef]
- van der Vegt, N.F.; Nayar, D. The hydrophobic effect and the role of cosolvents. J. Phys. Chem. 2017, 121, 9986–9998. [Google Scholar] [CrossRef] [PubMed]
- Akagi, T.; Baba, M.; Akashi, M. Preparation of nanoparticles by the self-organization of polymers consisting of hydrophobic and hydrophilic segments: Potential applications. Polymer 2007, 48, 6729–6747. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.A.; Walker, R.C.; Levit, S.L.; Tang, C. Single-step self-assembly and physical crosslinking of PEGylated chitosan nanoparticles by tannic acid. Polymers 2019, 11, 749. [Google Scholar] [CrossRef]
- Arya, N.; Chakraborty, S.; Dube, N.; Katti, D.S. Electrospraying: A facile technique for synthesis of chitosan-based micro/nanospheres for drug delivery applications. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 88, 17–31. [Google Scholar] [CrossRef]
- Wang, C.Y.; Yang, C.H.; Huang, K.S.; Yeh, C.S.; Wang, A.H.J.; Chen, C.H. Electrostatic droplets assisted in situ synthesis of superparamagnetic chitosan microparticles for magnetic-responsive controlled drug release and copper ion removal. J. Mater. Chem. B 2013, 1, 2205–2212. [Google Scholar] [CrossRef]
- Ankerfors, C.; Ondaral, S.; Wagberg, L.; Ödberg, L. Using jet mixing to prepare polyelectrolyte complexes: Complex properties and their interaction with silicon oxide surfaces. J. Colloid Interface Sci. 2010, 351, 88–95. [Google Scholar] [CrossRef]
- Raafat, D.; Von Bargen, K.; Haas, A.; Sahl, H.G. Insights into the mode of action of chitosan as an antibacterial compound. Appl. Environ. Microbiol. 2008, 74, 3764–3773. [Google Scholar] [CrossRef]
- Fischer, W. Lipoteichoic acid and lipids in the membrane of Staphylococcus aureus. Med. Microbiol. Inmunol. 1994, 183, 61–67. [Google Scholar] [CrossRef]
- Halder, S.; Yadav, K.K.; Sarkar, R.; Mukherjee, S.; Saha, P.; Haldar, S.; Karmakar, S.; Sen, T. Alteration of Zeta potential and membrane permeability in bacteria: A study with cationic agents. Springerplus 2015, 4, 672. [Google Scholar] [CrossRef] [PubMed]
- Katas, H.; Mohamad, A.; Zin, N.M. Physicochemical effects of chitosan-tripolyphosphate nanoparticles on antibacterial activity against Gram-positive and Gram-negative bacteria. J. Med. Sci. 2011, 11, 192–197. [Google Scholar] [CrossRef]
- Fuda, C.; Suvorov, M.; Vakulenko, S.B.; Mobashery, S. The basis for resistance to β-lactam antibiotics by penicillin-binding protein 2a of methicillin-resistant Staphylococcus aureus. J. Biol. Chem. 2004, 279, 40802–40806. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Woo, J.Y.; Ahn, C.B.; Je, J.Y. Chitosan-hydroxycinnamic acid conjugates: Preparation, antioxidant and antimicrobial activity. Food Chem. 2014, 148, 97–104. [Google Scholar] [CrossRef]
- Eom, S.; Kang, S.K.; Lee, D.S.; Myeong, J.I.; Lee, J.; Kim, H.W.; Kim, K.; Je, J.Y.; Jung, W.K.; Kim, Y.M. Synergistic antibacterial effect and antibacterial action mode of chitosan-ferulic acid conjugate against methicillin-resistant Staphylococcus aureus. J. Microbiol. Biotechnol. 2016, 26, 784–789. [Google Scholar] [CrossRef]
- Epand, R.F.; Savage, P.B.; Epand, R.M. Bacterial lipid composition and the antimicrobial efficacy of cationic steroid compounds (Ceragenins). Biochim. Biophys. Acta Biomembr. 2007, 1768, 2500–2509. [Google Scholar] [CrossRef] [Green Version]
- Oñate-Garzón, J.; Ausili, A.; Manrique-Moreno, M.; Torrecillas, A.; Aranda, F.J.; Patiño, E.; Gomez-Fernández, J.C. The increase in positively charged residues in cecropin D-like Galleria mellonella favors its interaction with membrane models that imitate bacterial membranes. Arch. Biochem. Biophys. 2017, 629, 54–62. [Google Scholar] [CrossRef]
- García-Fernández, E.; Koch, G.; Wagner, R.M.; Fekete, A.; Stengel, S.T.; Schneider, J.; Mielich-Süss, B.; Geibel, S.; Markert, S.M.; Stigloher, C.; et al. Membrane microdomain disassembly inhibits MRSA antibiotic resistance. Cell 2017, 171, 1354–1367. [Google Scholar] [CrossRef]
- Vollmer, W.; Höltje, J.V. The architecture of the murein (peptidoglycan) in gram-negative bacteria: Vertical scaffold or horizontal layer(s)? J. Bacteriol. 2004, 186, 5978–5987. [Google Scholar] [CrossRef]
- Liu, H.; Du, Y.; Wang, X.; Sun, L. Chitosan kills bacteria through cell membrane damage. Int. J. Food Microbiol. 2004, 95, 147–155. [Google Scholar] [CrossRef]
- Fuda, C.; Hesek, D.; Lee, M.; Morio, K.I.; Nowak, T.; Mobashery, S. Activation for catalysis of penicillin-binding protein 2a from methicillin-resistant Staphylococcus aureus by bacterial cell wall. J. Am. Chem. Soc. 2005, 127, 2056–2057. [Google Scholar] [CrossRef] [PubMed]
- Fuda, C.; Hesek, D.; Lee, M.; Heilmayer, W.; Novak, R.; Vakulenko, S.B.; Mobashery, S. Mechanistic basis for the action of new cephalosporin antibiotics effective against methicillin- and vancomycin-resistant Staphylococcus aureus. J. Biol. Chem. 2006, 281, 10035–10041. [Google Scholar] [CrossRef] [PubMed]



| Factor | Ampicillin-Unloaded Nanoparticles | Ampicillin-Loaded Nanoparticles | ||||||||||
| Particle Size (nm) | Particle Size (nm) | |||||||||||
| Level | Average | Group | Level | Average | Group | |||||||
| Sonication Amplitude | 40 | 172.6 | A | 40 | 179.8 | A | ||||||
| 60 | 162.1 | B | 60 | 162.0 | B | |||||||
| Type of polyanion | PAM-18K | 228.1 | A | PAM-18K | 249.2 | A | ||||||
| Phytic Acid | 181.2 | B | Phytic Acid | 167.1 | B | |||||||
| PAM-18Na | 153.0 | C | PAM-18Na | 161.1 | B | |||||||
| PAM-2Na | 140.7 | D | PAM-2Na | 146.5 | C | |||||||
| PAM-2K | 133.8 | D | PAM-2K | 130.7 | D | |||||||
| Polydispersity | Polydispersity | |||||||||||
| Factor | Level | Average | Group | Level | Average | Group | ||||||
| Sonication Amplitude | 40 | 0.276 | A | 40 | 0.321 | A | ||||||
| 60 | 0.271 | A | 60 | 0.269 | B | |||||||
| Type of Polyanion | PAM-18K | 0.382 | A | PAM-18K | 0.412 | A | ||||||
| PAM-18Na | 0.331 | B | PAM-18Na | 0.323 | B | |||||||
| PAM-2K | 0.240 | C | PAM-2Na | 0.303 | B | C | ||||||
| Phytic Acid | 0.208 | D | PAM-2K | 0.239 | C | D | ||||||
| PAM-2Na | 0.206 | D | Phytic Acid | 0.199 | D | |||||||
| Zeta Potential (mV) | Zeta Potential (mV) | |||||||||||
| Factor | Level | Average | Group | Level | Average | Group | ||||||
| Sonication Amplitude | 40 | +43.6 | A | 60 | +44.5 | A | ||||||
| 60 | +43.1 | A | 40 | +42.8 | A | |||||||
| Type of Polyanion | PAM-18K | +49.9 | A | PAM-18Na | +49.2 | A | ||||||
| PAM-18Na | +44.8 | B | Phytic Acid | +44.7 | B | |||||||
| PAM-2Na | +42.4 | B | PAM-18K | +43.3 | B | C | ||||||
| PAM-2K | +41.7 | B | C | PAM-2K | +41.6 | B | C | |||||
| Phytic Acid | +38.0 | C | PAM-2Na | +39.5 | C | |||||||
| Encapsulation Efficiency | Encapsulation Efficiency (%) | |||||||||||
| Factor | Level | Average | Group | Level | Average | Group | ||||||
| Sonication Amplitude | - | - | - | - | - | - | 60 | 69.1 | A | |||
| - | - | - | - | - | - | 40 | 66.7 | A | ||||
| Type of Polyanion | - | - | - | - | - | PAM-18K | 70.8 | A | ||||
| - | - | - | - | - | PAM-18Na | 70.6 | A | |||||
| - | - | - | - | - | Phytic Acid | 67.3 | A | |||||
| - | - | - | - | - | PAM-2Na | 67.2 | A | |||||
| - | - | - | - | - | PAM-2K | 63.7 | A | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciro, Y.; Rojas, J.; Oñate-Garzon, J.; Salamanca, C.H. Synthesis, Characterisation and Biological Evaluation of Ampicillin–Chitosan–Polyanion Nanoparticles Produced by Ionic Gelation and Polyelectrolyte Complexation Assisted by High-Intensity Sonication. Polymers 2019, 11, 1758. https://doi.org/10.3390/polym11111758
Ciro Y, Rojas J, Oñate-Garzon J, Salamanca CH. Synthesis, Characterisation and Biological Evaluation of Ampicillin–Chitosan–Polyanion Nanoparticles Produced by Ionic Gelation and Polyelectrolyte Complexation Assisted by High-Intensity Sonication. Polymers. 2019; 11(11):1758. https://doi.org/10.3390/polym11111758
Chicago/Turabian StyleCiro, Yhors, John Rojas, Jose Oñate-Garzon, and Constain H. Salamanca. 2019. "Synthesis, Characterisation and Biological Evaluation of Ampicillin–Chitosan–Polyanion Nanoparticles Produced by Ionic Gelation and Polyelectrolyte Complexation Assisted by High-Intensity Sonication" Polymers 11, no. 11: 1758. https://doi.org/10.3390/polym11111758
APA StyleCiro, Y., Rojas, J., Oñate-Garzon, J., & Salamanca, C. H. (2019). Synthesis, Characterisation and Biological Evaluation of Ampicillin–Chitosan–Polyanion Nanoparticles Produced by Ionic Gelation and Polyelectrolyte Complexation Assisted by High-Intensity Sonication. Polymers, 11(11), 1758. https://doi.org/10.3390/polym11111758