Electrochromic Self-Electrostabilized Polypyrrole Films Doped with Surfactant and Azo Dye
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.3. Characterization
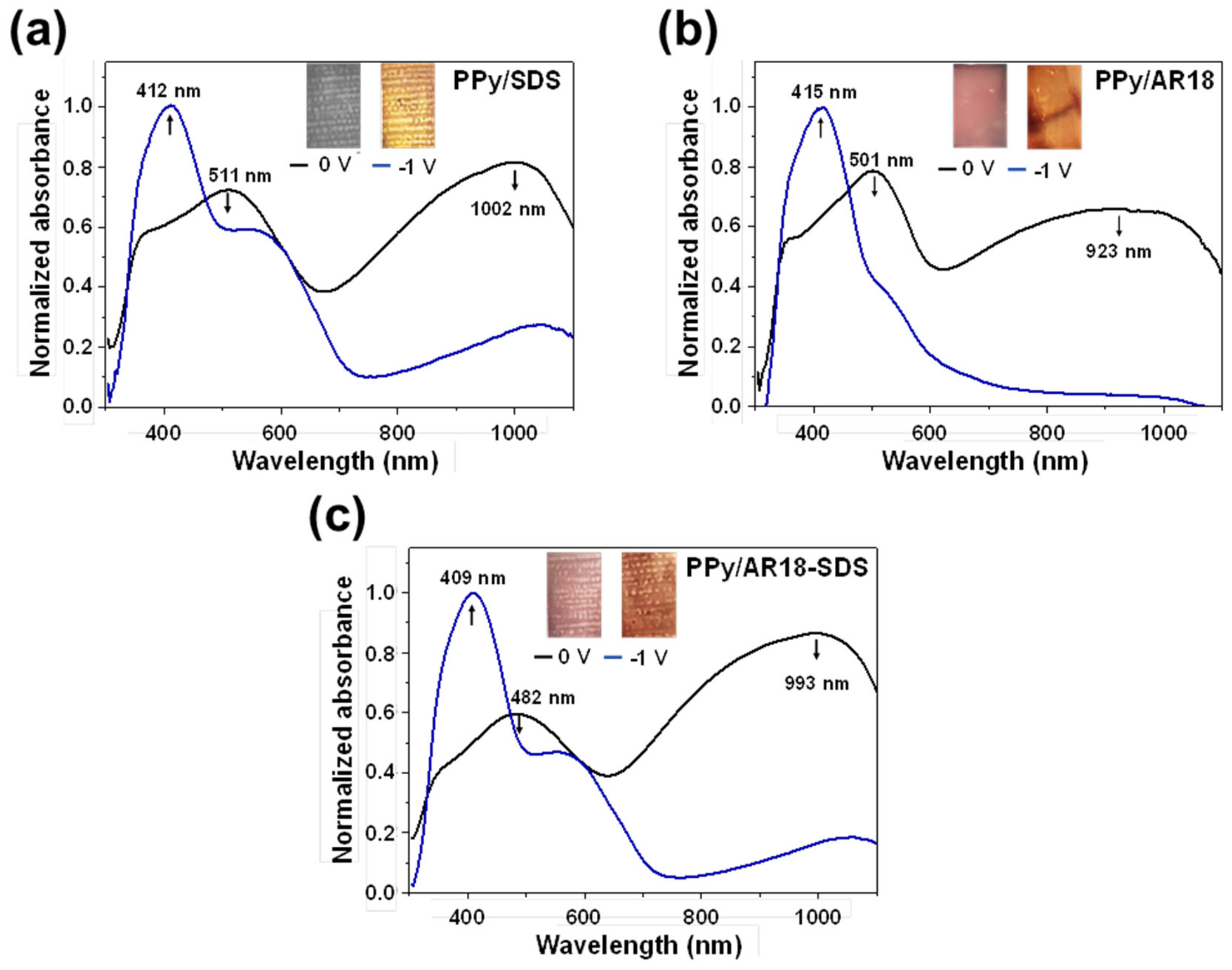
3. Results and Discussion
3.1. Electrochemical Behavior
3.2. Structural Characterization
3.3. Optical and Electrochromic Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Skotheim, T.A.; Reynolds, J. Handbook of Conjugated Polymers, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Le, T.H.; Kim, Y.; Yoon, H. Electrical and electrochemical properties of conducting polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive polymers: Opportunities and challenges in biomedical applications. Chem. Rev. 2018, 118, 6766–6843. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-X.; Li, X.-G.; Yang, Y.-L. Preparation, properties and applications of polypyrroles. React. Funct. Polym. 2001, 47, 125–139. [Google Scholar] [CrossRef]
- Ateh, D.D.; Navsaria, H.A.; Vadgama, P. Polypyrrole-based conducting polymers and interactions with biological tissues. J. R. Soc. Interface 2006, 3, 741–752. [Google Scholar] [CrossRef]
- Wei, W.L.; Du, P.C.; Liu, D.; Wang, Q.; Liu, P. Facile one-pot synthesis of well-defined coaxial sulfur/polypyrrole tubular nanocomposites as cathodes for long-cycling lithium–sulfur batteries. Nanoscale 2018, 10, 13037–13044. [Google Scholar] [CrossRef]
- Kim, J.-K.; Manuel, J.; Lee, M.-H.; Scheers, J.; Lim, D.-H.; Joahansson, P.; Ahn, J.H.; Matic, A.; Jacobsson, P. Towards flexible secondary lithium batteries: Polypyrrole-LiFePO4 thin electrodes with polymer electrolytes. J. Mater. Chem. 2012, 22, 15045–15049. [Google Scholar] [CrossRef]
- Luo, S.J.; Zhao, J.; Zou, J.; He, Z.; Xu, C.; Liu, F.; Huang, Y.; Dong, L.; Wang, L.; Zhang, H. Self-standing polypyrrole/black phosphorus laminated film: Promising electrode for flexible supercapacitor with enhanced capacitance and cycling stability. ACS Appl. Mater. Interfaces 2018, 10, 3538–3548. [Google Scholar] [CrossRef]
- Zhu, M.S.; Huang, Y.; Deng, Q.H.; Zhou, J.; Pei, Z.X.; Xue, Q.; Huang, Y.; Wang, Z.F.; Li, H.F.; Huang, Q.; et al. Highly flexible, freestanding supercapacitor electrode with enhanced performance obtained by hybridizing polypyrrole chains with MXene. Adv. Energy Mater. 2016, 6, 1600969. [Google Scholar] [CrossRef]
- Lee, J.-W.; Serna, F.; Nickels, J.; Schmidt, C.E. Carboxylic acid-functionalized conductive polypyrrole as a bioactive platform for cell adhesion. Biomacromolecules 2006, 7, 1692–1695. [Google Scholar] [CrossRef]
- Sivaraman, K.M.; Ozkale, B.; Ergeneman, O.; Luhmann, T.; Fortunato, G.; Zeeshan, M.A.; Nelson, B.J.; Pane, S. Redox cycling for passive modification of polypyrrole surface properties: Effects on cell adhesion and proliferation. Adv. Healthc. Mater. 2013, 2, 591–598. [Google Scholar] [CrossRef]
- Nowicka, A.M.; Fau, M.; Rapecki, T.; Doten, M. Polypyrrole-Au nanoparticles composite as suitable platform for DNA biosensor with electrochemical impedance spectroscopy detection. Electrochim. Acta 2014, 140, 65–71. [Google Scholar] [CrossRef]
- Fabregat, G.; Córdova-Mateo, E.; Armelin, E.; Bertran, O.; Alemán, C. Ultrathin films of polypyrrole derivatives for dopamine detection. J. Phys. Chem. C 2011, 115, 14933–14941. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, L.; Soeller, C.; Travas-Sejdic, J. Conducting polymers for electrochemical DNA sensing. Biomaterials 2009, 30, 2132–2148. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.; Borgens, R.B.; Cho, Y.N. Well-ordered porous conductive polypyrrole as a new platform for neural interfaces. Langmuir 2011, 27, 6179–6184. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, M.; Xiao, Y.; Che, J. A feasible way for the fabrication of single walled carbon nanotube/polypyrrole composite film with controlled pore size for neural interface. Colloids Surf. B Biointerfaces 2015, 126, 138–145. [Google Scholar] [CrossRef]
- Takagi, S.; Makuta, S.; Veamatahau, A.; Otsuka, Y.; Tachibana, Y. Organic/inorganic hybrid electrochromic devices based on photoelectrochemically formed polypyrrole/TiO2 nanohybrid films. J. Mater. Chem. 2012, 22, 22181–22189. [Google Scholar] [CrossRef]
- Camurlu, P. Polypyrrole derivatives for electrochromic applications. RSC Adv. 2014, 4, 55832–55845. [Google Scholar] [CrossRef]
- Loguercio, L.; Alves, C.; Thesing, A.; Ferreira, J. Enhanced electrochromic properties of a polypyrrole–indigo carmine–gold nanoparticles nanocomposite. Phys. Chem. Chem. Phys. 2015, 17, 1234–1240. [Google Scholar] [CrossRef]
- Ferreira, J.; Brolo, A.G.; Girotto, E.M. Probing speciation inside a conducting polymer matrix by in situ spectroelectrochemistry. Electrochim. Acta 2011, 56, 3101–3107. [Google Scholar] [CrossRef]
- Girotto, E.M.; De Paoli, M.-A. Polypyrrole color modulation and electrochromic contrast enhancement by doping with a dye. Adv. Mater. 1998, 10, 790–793. [Google Scholar] [CrossRef]
- Mosayebzadeh, Z.; Ansari, R.; Mohammad-khah, A.; Arvand, M. Electrochemical preparation of a copper ion selective electrode based on polypyrrole conducting polymer doped with Ponceau 4R azo dye. Anal. Bioanal. Electrochem. 2013, 5, 109–129. [Google Scholar]
- Almeida, A.K.A.; Dias, J.M.M.; Silva, A.J.C.; Santos, D.P.; Navarro, M.; Tonholo, J.; Goulart, M.O.F. Conjugated and fluorescent polymer based on dansyl-substituted pyrrole prepared by electrochemical polymerization in acetonitrile containing boron trifluoride diethyl etherate. Electrochim. Acta 2014, 122, 50–56. [Google Scholar] [CrossRef]
- Girotto, E.M.; Gazotti, W.A.; Tormena, C.F.; De Paoli, M.-A. Photoelectronic and transport properties of polypyrrole doped with a dianionic dye. Electrochim. Acta 2002, 47, 1351–1357. [Google Scholar] [CrossRef]
- Ferreira, J.; Santos, M.J.L.; Matos, R.; Ferreira, O.P.; Rubira, A.F.; Girotto, E.M. Structural and electrochromic study of polypyrrole synthesized with azo and anthraquinone dyes. J. Electroanal. Chem. 2006, 591, 27–32. [Google Scholar] [CrossRef]
- Cihaner, A.; Algi, F. Electrochemical and optical properties of new soluble dithienylpyrroles based on azo dyes. Electrochim. Acta 2009, 54, 1702–1709. [Google Scholar] [CrossRef]
- Almeida, A.K.A.; Dias, J.M.M.; Santos, D.P.; Nogueira, F.A.R.; Navarro, M.; Tonholo, J.; Lima, D.J.P.; Ribeiro, A.S. A magenta polypyrrole derivatised with Methyl Red azo dye: Synthesis and spectroelectrochemical characterisation. Electrochim. Acta 2017, 240, 239–249. [Google Scholar] [CrossRef]
- Kwon, W.J.; Suh, D.H.; Chin, B.D.; Yu, J.W. Preparation of polypyrrole nanoparticles in mixed surfactants system. J. Appl. Polym. Sci. 2008, 110, 1324–1329. [Google Scholar] [CrossRef]
- Omastova, M.; Trchova, M.; Kovarova, J.; Stejskal, J. Synthesis and structural study of polypyrroles prepared in the presence of surfactants. Synth. Met. 2003, 138, 447–455. [Google Scholar] [CrossRef]
- Mei, J.; Bao, Z. Side chain engineering in solution-processable conjugated polymers. Chem. Mater. 2013, 26, 604–615. [Google Scholar] [CrossRef]
- Stejskal, J.; Omastova, M.; Fedorova, S.; Prokes, J.; Trchová, M. Polyaniline and polypyrrole prepared in the presence of surfactants: A comparative conductivity study. Polymer 2003, 44, 1353–1358. [Google Scholar] [CrossRef]
- Rai, A.R.U.; Jun, A.P.; Walaiporn, P.-O. Synthesis of highly conductive polypyrrole nanoparticles via microemulsion polymerization. J. Met. Mater. Mineral. 2008, 18, 27–31. [Google Scholar]
- Rawal, I.; Kaur, A. Effect of anionic surfactant concentration on the variable range hopping conduction in polypyrrole nanoparticles. J. Appl. Phys. 2014, 115, 043717. [Google Scholar] [CrossRef]
- Naoi, K.; Oura, Y.; Maeda, M.; Nakamura, S. Electrochemistry of surfactant-doped polypyrrole film(I): Formation of columnar structure by electropolymerization. J. Electrochem. Soc. 1995, 142, 417–422. [Google Scholar] [CrossRef]
- Chen, G.; Wang, Z.; Xia, D.; Zhang, L.; Hui, R.; Zhang, J. Whelk-like helixes of polypyrrole synthesized by electropolymerization. Adv. Funct. Mater. 2007, 17, 1844–1848. [Google Scholar] [CrossRef]
- Nikoofard, H.; Masdarolomoor, F.; Falahatkar, M.; Amin, A.H. Electro-chemical preparation and characterization of poly(1-amino-9,10-anthraquinone) films in a micelle solution of sodium dodecyl sulfate. Synth. Met. 2015, 209, 212–219. [Google Scholar] [CrossRef]
- Tsakova, V.; Ilieva, G.; Filjova, D. Role of the anionic dopant of poly(3,4-ethylenedioxythiophene) for the electroanalytical performance: Electrooxidation of acetaminophen. Electrochim. Acta 2015, 179, 343–349. [Google Scholar] [CrossRef]
- Tao, Y.-J.; Cheng, H.-F.; Zheng, W.-W.; Zhang, Z.-Y.; Liu, D.-Q. Electrosynthesises and characterizations of copolymers based on pyrrole and 3,4-ethylenedioxythiophene in aqueous micellar solution. Synth. Met. 2012, 162, 728–734. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Tsakova, V.; Winkels, S.; Schultze, J.W. Anodic polymerization of 3,4-ethylenedioxythiophene from aqueous microemulsions. Electrochim. Acta 2000, 46, 759–768. [Google Scholar] [CrossRef]
- Carabias-Martínez, R.; Becerro-Domínguez, F.; Martín-González, F.; Hernández-Méndez, J.; Córdova-Orellana, R. Polypyrrole-dodecyl sulphate electrode as a microsensor for electroinactive cations in flow-injection analysis and ion chromatography. Anal. Chim. Acta 1993, 279, 299–307. [Google Scholar]
- Loguercio, L.F.; de Matos, C.F.; de Oliveira, M.C.; Marin, G.; Khan, S.; Balzaretti, N.M.; Dupont, J.; Santos, M.J.L.; Santos, J.F.L. Synergistic interplay of ionic liquid and dodecyl sulphate driving the oxidation state of polypyrrole based electrodes. New J. Chem. 2018, 42, 13828–13835. [Google Scholar] [CrossRef]
- Aradilla, D.; Estrany, F.; Alemán, C. Symmetric supercapacitors based on multilayers of conducting polymers. J. Phys. Chem. C 2011, 115, 8430–8438. [Google Scholar] [CrossRef]
- Careem, M.A.; Velmurugu, Y.; Skaarup, S.; West, K. A voltammetry study on the diffusion of counter ions in polypyrrole films. J. Power Sourc. 2006, 159, 210–214. [Google Scholar] [CrossRef]
- Bendrea, A.-D.; Fabregat, G.; Cianga, L.; Estrany, F.; del Valle, L.J.; Cianga, I.; Alemán, C. Hybrid materials consisting of an all-conjugated polythiophene backbone and grafted hydrophilic poly(ethylene glycol) chains. Polym. Chem. 2013, 4, 2709–2723. [Google Scholar] [CrossRef]
- Fabregat, G.; Alemán, C.; Casas, M.T.; Armelin, E. Controlling the morphology of poly(N-cyanoethylpyrrole). J. Phys. Chem. B 2012, 116, 5064–5070. [Google Scholar] [CrossRef]
- Alemán, C.; Casanovas, J.; Torras, J.; Bertran, O.; Armelin, E.; Oliver, R.; Estrany, F. Cross-linking in polypyrrole and poly(N-methylpyrrole): Comparative experimental and theoretical studies. Polymer 2008, 49, 1066–1075. [Google Scholar] [CrossRef]
- Summerton, E.; Zimbitas, G.; Britton, M.; Bakali, S. Crystallisation of sodium dodecyl sulfate and the corresponding effect of 1-dodecanol addition. J. Cryst. Growth 2016, 455, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Chantraine, F.; Viana, M.; Cazalbou, S.; Brielles, N.; Mondain-Monval, O.; Pouget, C.; Branlard, P.; Rubinstenn, G.; Chulia, D. From compressibility to structural investigation of sodium dodecyl sulphate—Part 2: A singular behavior under pressure. Powder Technol. 2007, 177, 41–50. [Google Scholar] [CrossRef]
- Rodríguez-Ropero, F.; Casanovas, J.; Alemán, C. Ab initio calculations on π-stacked thiophene dimer, trimer, and tetramer: Structure, interaction energy, cooperative effects, and intermolecular electronic parameters. J. Comput. Chem. 2008, 29, 69–78. [Google Scholar] [CrossRef]
- Beaujuge, P.M.; Reynolds, J.R. Color control in π-conjugated organic polymers for use in eectrochromic devices. Chem. Rev. 2010, 110, 268–320. [Google Scholar] [CrossRef]
- Aradilla, D.; Casanovas, J.; Estrany, F.; Iribarren, J.I.; Alemán, C. New insights into the characterization of poly(3-chlorothiophene) for electrochromic devices. Polym. Chem. 2012, 3, 436–449. [Google Scholar] [CrossRef]
- Casanovas, J.; Aradilla, D.; Poater, J.; Solà, M.; Estrany, F.; Alemán, C. Properties of poly(3-halidethiophene)s. Phys. Chem. Chem. Phys. 2012, 14, 10050–11062. [Google Scholar] [CrossRef] [PubMed]
- Somani, P.R.; Radhakrishnan, S. Electrochromic materials and devices: Present and future. Mater. Chem. Phys. 2002, 77, 117–133. [Google Scholar] [CrossRef]
- Genies, E.M.; Bidan, G.; Diaz, A.F. Spectroelectrochemical study of polypyrrole films. J. Electroanal. Chem. 1983, 149, 101–113. [Google Scholar] [CrossRef]
- Lakshmanan, R.; Raja, P.P.; Shivaprakash, N.C. Fabrication of fast switching electrochromic window based on poly (3, 4-(2, 2-dimethylpropylenedioxy) thiophene) thin film. J. Mater. Sci. Mater. Electron. 2016, 27, 6035–6042. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, G.; He, Y.; Fu, R.; Gu, Y.; Chen, S. Preparation, Characterization, and Electrochromic Properties of Nanocellulose-Based Polyaniline Nanocomposite Films. ACS Appl. Mater. Interfaces 2017, 9, 16426–16434. [Google Scholar] [CrossRef]










| System | [ClO4−] (mM) | [SDS] (mM) | [AR18] (mM) | [AR1] (mM) | Method | L (µm) |
|---|---|---|---|---|---|---|
| PPy/ClO4− | 100 | - | - | - | CP | 5.0 ± 0.3 |
| PPy/SDS | - | 100 | - | - | CP | 4.2 ± 0.2 5.0 ± 0.3 |
| PPy/AR18-SDS | - | 100 | 0.5 | - | CP, CV, CA | 5.1 ± 0.2 |
| PPy/AR18 | - | - | 0.5 | - | CP | 6.4 ± 0.2 |
| PPy/AR1-SDS | - | 100 | - | 0.5 | CP | 4.9 ± 0.2 |
| PPy/AR1 | - | - | - | 0.5 | CP | 6.2 ± 0.2 |
| System | Deposition Charge (C) | Number of Cycles | Coulombic Efficiency | ||
|---|---|---|---|---|---|
| 25 | 100 | 25 Cycles | 100 Cycles | ||
| PPy/ClO4− | 0.3 | 16% | - | 0.23 | - |
| PPy/SDS | 0.3 | −4% | - | 0.27 | - |
| PPy/SDS | 0.2 | −6% | 10% | 0.22 | 0.19 |
| PPy/AR18 | 0.2 | 16% | 27% | 0.26 | 0.23 |
| PPy/AR18-SDS | 0.2 | −29% | −59% | 0.33 | 0.41 |
| PPy/AR1 | 0.2 | 3% | - | 0.26 | - |
| PPy/AR1-SDS | 0.2 | −16% | - | 0.30 | - |
| System | L (µm) | Rq (nm) | λmax (nm) | εg (eV) |
|---|---|---|---|---|
| PPy/SDS | 4.0 | 215 | 465 | 1.93 |
| PPy/AR18-SDS | 5.1 | 358 | 474 | 1.92 |
| PPy/AR18 | 6.4 | 45 | 503 | 2.07 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayat, M.; Izadan, H.; Molina, B.G.; Sánchez, M.; Santiago, S.; Semnani, D.; Dinari, M.; Guirado, G.; Estrany, F.; Alemán, C. Electrochromic Self-Electrostabilized Polypyrrole Films Doped with Surfactant and Azo Dye. Polymers 2019, 11, 1757. https://doi.org/10.3390/polym11111757
Bayat M, Izadan H, Molina BG, Sánchez M, Santiago S, Semnani D, Dinari M, Guirado G, Estrany F, Alemán C. Electrochromic Self-Electrostabilized Polypyrrole Films Doped with Surfactant and Azo Dye. Polymers. 2019; 11(11):1757. https://doi.org/10.3390/polym11111757
Chicago/Turabian StyleBayat, Maryam, Hossein Izadan, Brenda G. Molina, Margarita Sánchez, Sara Santiago, Dariush Semnani, Mohammad Dinari, Gonzalo Guirado, Francesc Estrany, and Carlos Alemán. 2019. "Electrochromic Self-Electrostabilized Polypyrrole Films Doped with Surfactant and Azo Dye" Polymers 11, no. 11: 1757. https://doi.org/10.3390/polym11111757
APA StyleBayat, M., Izadan, H., Molina, B. G., Sánchez, M., Santiago, S., Semnani, D., Dinari, M., Guirado, G., Estrany, F., & Alemán, C. (2019). Electrochromic Self-Electrostabilized Polypyrrole Films Doped with Surfactant and Azo Dye. Polymers, 11(11), 1757. https://doi.org/10.3390/polym11111757








