Targeted Drug Delivery Systems for the Treatment of Glaucoma: Most Advanced Systems Review
Abstract
:1. Introduction
2. Open-Angle Glaucoma
2.1. Basics and Prognosis
2.2. Antiglaucoma Drugs
3. Traditional Glaucoma Drug Forms and Mechanisms of Their Delivery—An Overview
4. Modern Ophthalmic Drug Release Systems
4.1. General Description, Goals, and Challenges
4.2. Drug Carriers for Internal Application in Glaucoma Treatment
4.3. Drug Carriers for External Application in Glaucoma Treatment
4.3.1. Drug-Loaded Contact Lenses and Contact Lenses with Drug-Loaded Coatings
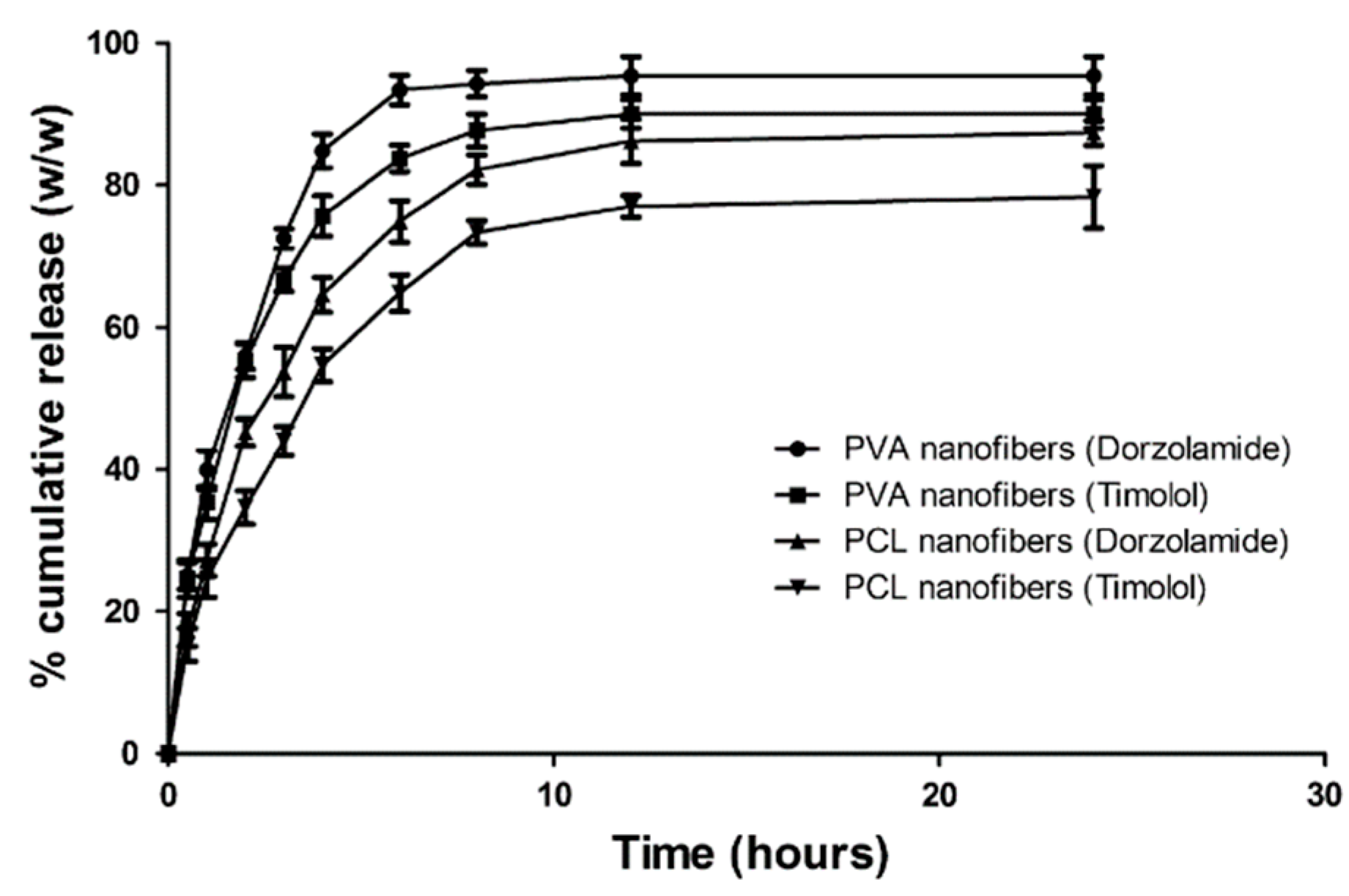
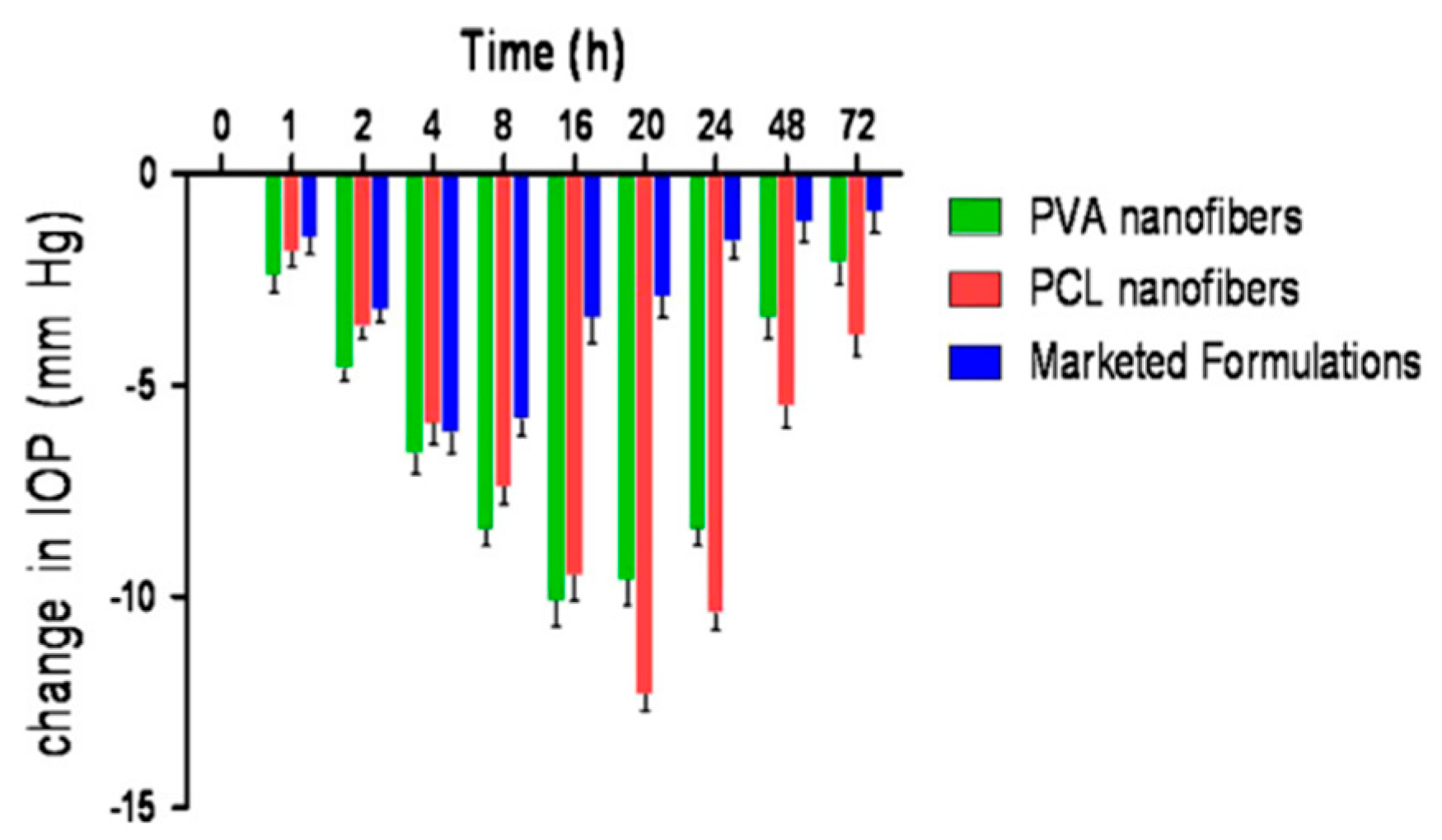
4.3.2. Nanofibers
5. Conclusions
Funding
Conflicts of Interest
References
- Varela-Garcia, A.; Concheiro, A.; Alvarez-Lorenzo, C. Soluplus micelles for acyclovir ocular delivery: Formulation and cornea and sclera permeability. Int. J. Pharm. 2018, 552, 39–47. [Google Scholar] [CrossRef]
- Martín-Biosca, Y.; Molero-Monfort, M.; Sagrado, S.; Villanueva-Camañas, R.M.; Medina-Hernández, M.J. Rapid in vitro test to predict ocular tissue permeability based on biopartitioning micellar chromatography. Eur. J. Pharm. Sci. 2003, 20, 209–216. [Google Scholar] [CrossRef]
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K. A comprehensive insight on ocular pharmacokinetics. Drug Deliv. Transl. Res. 2016, 6, 735–754. [Google Scholar] [CrossRef] [PubMed]
- Haghjou, N.; Abdekhodaie, M.J.; Cheng, Y. Retina-choroid-sclera permeability for ophthalmic drugs in the vitreous to blood direction: Quantitative assessment. Pharm. Res. 2013, 30, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.; Prausnitz, M.R. Predicted Permeability of the Cornea to Topical Drugs. Pharm. Res. 2001, 18, 1497–1508. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Khaw, P.T. Primary open-angle glaucoma. Lancet 2004, 363, 1711–1720. [Google Scholar] [CrossRef]
- Pillunat, L.E.; Erb, C.; Jünemann, A.G.M.; Kimmich, F. Micro-invasive glaucoma surgery (MIGS): A review of surgical procedures using stents. Clin. Ophthalmol. 2017, 11, 1583–1600. [Google Scholar] [CrossRef] [PubMed]
- Geroski, D.H.; Edelhauser, H.F. Drug delivery for posterior segment eye disease. Invest. Ophthalmol. Vis. Sci. 2000, 41, 961–964. [Google Scholar]
- Jünemann, A.G.M.; Chorągiewicz, T.; Ozimek, M.; Grieb, P.; Rejdak, R. Drug bioavailability from topically applied ocular drops. Does drop size matter? Ophthalmol. J. 2016, 1, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Rittenhouse, K.D.; Peiffer, R.L., Jr.; Pollack, G.M. Evaluation of microdialysis sampling of aqueous humor for in vivo models of ocular absorption and disposition. J. Pharm. Biomed. Anal. 1998, 16, 951–959. [Google Scholar] [CrossRef]
- Kanner, E.; Tsai, J.C. Glaucoma Medications Use and Safety in the Elderly Population. Drugs Aging 2006, 23, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Jani, R.; Rhone, E. Ion exchange resin technology for ophthalmic applications. In Modified-Release Drug Delivery Technology; Rathbone, M.J., Hadgraft, J., Roberts, M.S., Eds.; Marcel Dekker: New York, NY, USA, 2002; p. 316. [Google Scholar]
- Porth, C.M. Nervous System. Disorders of special sensory functions: Vision, hearing and vestibular function. In Essentials of Pathophysiology: Concepts of Altered Health States, 3rd ed.; Porth, C., Ed.; Wolters Kluwer Health: Philadelphia, PA, USA, 2011; p. 981. [Google Scholar]
- Ciolino, J.B.; Stefanescu, C.F.; Ross, A.E.; Salvador-Culla, B.; Cortez, P.; Ford, E.M.; Wymbs, K.A.; Sprague, S.L.; Mascoop, D.R.; Rudina, S.S.; et al. In vivo performance of a drug-eluting contact lens to treat glaucoma for a month. Biomaterials 2014, 35, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A Systematic Review and Meta-Analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, Y.H. Primary Open-Angle Glaucoma. N. Engl. J. Med. 2009, 360, 1113–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wistrand, P.J.; Schenholm, M.; Lönnerholm, G. Carbonic anhydrase isoenzymes CA I and CA II in the human eye. Invest Ophthalmol Vis Sci. 1986, 27, 419–428. [Google Scholar] [PubMed]
- Brooks, A.M.; Gillies, W.E. Ocular beta-blockers in glaucoma management. Clinical pharmacological aspects. Drugs Aging. 1992, 2, 208–221. [Google Scholar] [CrossRef]
- Nietgen, G.W.; Schmidt, J.; Hesse, L.; Honemann, C.W.; Durieux, M.E. Muscarinic receptor functioning and distribution in the eye: Molecular basis and implications for clinical diagnosis and therapy. Eye 1999, 13, 285–300. [Google Scholar] [CrossRef] [Green Version]
- Abrams, P.; Andersson, K.-E.; Buccafusco, J.J.; Chapple, C.; Chet de Groat, W.; Fryer, A.D.; Kay, G.; Laties, A.; Nathanson, N.M.; Pasricha, P.J.; et al. Muscarinic receptors: Their distribution and function in body systems, and the implications for treating overactive bladder. Br. J. Pharmacol. 2006, 148, 565–578. [Google Scholar] [CrossRef]
- Mcauliffe-Curtin, D.; Buckley, C. Review of Alpha Adrenoceptor Function in the Eye. Eye 1989, 3, 472–476. [Google Scholar] [CrossRef]
- Schlötzer-Schrehardt, U.; Zenkel, M.; Nüsing, R.M. Expression and localization of FP and EP prostanoid receptor subtypes in human ocular tissues. Invest Ophthalmol Vis Sci. 2002, 43, 1475–1487. [Google Scholar] [PubMed]
- Plazonnet, B. Ophthalmic drug delivery. In Modified-Release Drug Delivery Technology; Rathbone, M.J., Hadgraft, J., Roberts, M.S., Eds.; Marcel Dekker: New York, NY, USA, 2002; pp. 291–292. [Google Scholar]
- Bustein, N.L. Fundamental concepts in ocular pharmacology. Basic Science of Ocular Pharmacology. In Clinical Ocular Pharmacology, 2nd ed.; Bartlett, J.D., Jaanus, S.D., Eds.; Butterworth-Heinemann: Stoneham, MA, USA, 1989; p. 20. [Google Scholar]
- Osuagwu, U.L.; Alanazi, S.A. Eye rubbing-induced changes in intraocular pressure and corneal thickness measured at five locations, in subjects with ocular allergy. Int. J. Ophthalmol. 2015, 8, 81–88. [Google Scholar] [PubMed] [Green Version]
- Coroi, M.C.; Bungau, S.; Tit, M. Preservatives from the eye drops and the ocular surface. Rom. J. Ophthalmol. 2015, 59, 2–5. [Google Scholar] [PubMed]
- Hegde, R.R.; Verma, A.; Ghosh, A. Microemulsion: New Insights into the Ocular Drug Delivery. ISRN Pharmaceutics 2013. [Google Scholar] [CrossRef] [PubMed]
- Palacz, O. Anatomia topograficzna z elementami anatomii chirurgicznej i klinicznej. In Okulistyka współczesna, 2nd ed.; Orłowski, W., Ed.; Państwowy Zakład Wydawnictw Lekarskich: Warsaw, Poland, 1986; pp. 20–22. [Google Scholar]
- Tasman, W. Duane’s Foundations of Clinical Ophthalmology; Lippincott-Raven: Philadelphia, PA, USA, 1995. [Google Scholar]
- Rausnitz, M.R.P.; Noonan, J.S. Permeability of Cornea, Sclera, and Conjunctiva: A Literature Analysis for Drug Delivery to the Eye. J. Pharm. Sci. 1998, 87, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R. Measurement and Prediction of Transient Transport across Sclera for Drug Delivery to the Eye. Ind. Eng. Chem. Res. 1998, 37, 2903–2907. [Google Scholar] [CrossRef]
- Nakielski, P. Systemy uwalniania leków oparte na nanowłóknach. Doctoral thesis, Institute of Fundamental Technological Research Polish Academy of Sciences, Warsaw, Poland, 2014. [Google Scholar]
- Fazeli, M.; Nejad, H.B.; Mehrgan, H.; Elahian, L. Microbial contamination of preserved ophthalmic drops in outpatient departments: Possibility of an extended period of use. Daru 2004, 12, 151–155. [Google Scholar]
- Lux, A.; Maier, S.; Dinslage, S.; Süverkrüp, R.; Diestelhorst, M. A comparative bioavailability study of three conventional eye drops versus a single lyophilisate. Br. J. Ophthalmol. 2003, 87, 436–440. [Google Scholar] [CrossRef]
- Kumari, A.; Sharma, P.K.; Garg, V.K.; Garg, G. Ocular inserts - Advancement in therapy of eye diseases. J. Adv. Pharm. Technol. Res. 2010, 1, 291–296. [Google Scholar] [CrossRef]
- Nguyen, T.; Latkany, R. Review of hydroxypropyl cellulose ophthalmic inserts for treatment of dry eye. Clin. Ophthalmol. 2011, 5, 587–591. [Google Scholar]
- Reddy, I.K.; Aziz, W. Artificial tear formulations, irrigating solutions and contact lens products. In Ocular therapeutics and drug delivery; Reddy, I.K., Ed.; Technomic Publishing Company: Pennsylvania, PA, USA, 1996; p. 184. [Google Scholar]
- Souied, E.H.; Dugel, P.U.; Ferreira, A.; Hashmonay, R.; Lu, J.; Kelly, S.P. Severe Ocular Inflammation Following Ranibizumab or Aflibercept Injections for Age-Related Macular Degeneration: A Retrospective Claims Database Analysis. Ophthalmic Epidemiol. 2016, 23, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laude, A.; Lim, J.W.K.; Srinagesh, V.; Tong, L. The effect of intravitreal injections on dry eye, and proposed management strategies. Clin. Ophthalmol. 2017, 11, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Meyer, L.M.; Schonfeld, C.-L. Secondary Glaucoma After Intravitreal Dexamethasone 0.7 mg Implant in Patients with Retinal Vein Occlusion: A One-Year Follow-Up. J Ocul. Pharmacol. Ther. 2013, 29, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, M.L. Modification of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Bruschi, M.L., Ed.; Elsevier: Amsterdam, Netherlands, 2015; pp. 15–28. [Google Scholar]
- Dhaliwal, K.; Dosanjh, P. Biodegradable Polymers and their Role in Drug Delivery Systems. BJSTR 2018, 11, 8315–8320. [Google Scholar] [CrossRef]
- Gao, S.; Tang, G.; Hua, D.; Xiong, R.; Han, J.; Jiang, S.; Zhang, Q.; Huang, C. Stimuli-responsive bio-based polymeric systems and their applications. J. Mater. Chem. B. 2019, 7, 709–729. [Google Scholar] [CrossRef]
- Basuki, J.S.; Qie, F.; Mulet, X.; Suryadinata, R.; Vashi, A.V.; Peng, Y.Y.; Li, L.; Hao, X.; Tan, T.; Hughes, T.C. Photo-Modulated Therapeutic Protein Release from a Hydrogel Depot Using Visible Light. Anew. Chem. Int. Ed. 2017, 56, 966–971. [Google Scholar] [CrossRef]
- Patel, S.R.; Lin, A.S.P.; Edelhauser, H.F.; Prausnitz, M.R. Suprachoroidal Drug Delivery to the Back of the Eye Using Hollow Microneedles. Pharm Res. 2011, 28, 166–176. [Google Scholar] [CrossRef]
- Durairaj, C. Ocular Pharmacokinetics. Part I General Principles. In Handbook of Experimental Pharmacology; Barrett, J.E., Ed.; Springer: Los Angeles, CA, USA, 2017; p. 41. [Google Scholar]
- Kim, Y.C.; Edelhauser, H.F.; Prausnitz, M.R. Targeted Delivery of Antiglaucoma Drugs to the Supraciliary Space Using Microneedles. Invest. Ophthalmol. Vis. Sci. 2014, 55, 7387–7397. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.H.; Desit, P.; Prausnitz, M.R. Targeted Drug Delivery in the Suprachoroidal Space by Swollen Hydrogel Pushing. Invest. Ophthalmol. Vis. Sci. 2018, 59, 2069–2079. [Google Scholar] [CrossRef]
- Zou, L.; Nair, A.; Weng, H.; Tsai, Y.-T.; Hu, Z.; Tang, L. Intraocular Pressure Changes: An Important Determinant of the Biocompatibility of Intravitreous Implants. Plos One 2011, 6, 1–9. [Google Scholar] [CrossRef]
- Lai, J.Y.; Hsieh, A.C. A gelatin-g-poly(N-isopropylacrylamide) biodegradable in situ gelling delivery system for the intracameral administration of pilocarpine. Biomaterials 2012, 33, 2372–2387. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Kima, J.; Kudisch, M.; Konichi da Silva, N.R.; Asada, H.; Aya-Shibuya, E.; Bloomer, M.M.; Mudumba, S.; Bhisitkul, R.B.; Desai, T.A. Long-term intraocular pressure reduction with intracameral polycaprolactone glaucoma devices that deliver a novel anti-glaucoma agent. J. Control. Release 2018, 269, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Hughes, P.M.; Robinson, M.R.; Burke, J.A. Intraocular pressure reduction with intracameral bimatoprost implants. Continuation-in-part of application No. US 8,673,341 B2, 18 March 2004. [Google Scholar]
- Navratil, T.; Das, S.; Garcia, A.; Tully, J. Glaucoma treatment via Intracameral Ocular Implants. International Application Number PCT/US2016/04395 1, 25 July 2016. [Google Scholar]
- Taniguchi, E.V.; Kalout, P.; Pasquale, L.R.; Kohane, D.S.; Ciolino, J.B. Clinicians’ perspectives on the use of drug-eluting contact lenses for the treatment of glaucoma. Ther. Deliv. 2014, 5, 1077–1083. [Google Scholar] [CrossRef]
- Peng, C.C.; Burke, M.T.; Carbia, B.E.; Plummer, C.; Chauhan, A. Extended drug delivery by contact lenses for glaucoma therapy. J. Control. Release 2012, 162, 152–158. [Google Scholar] [CrossRef]
- Schultz, C.L.; Poling, T.R.; Mint, J.O. A medical device/drug delivery system for treatment of glaucoma. Clin. Exp. Optom. 2009, 92, 343–348. [Google Scholar] [CrossRef]
- Kim, J.; Peng, C.-C.; Chauhan, A. Extended release of dexamethasone from silicone-hydrogel contact lenses containing vitamin E. J. Control. Release 2010, 148, 110–116. [Google Scholar] [CrossRef]
- Hsu, K.H.; Carbia, B.E.; Plummer, C.; Chauhan, A. Dual drug delivery from vitamin E loaded contact lenses for glaucoma therapy. Eur. J. Pharm. Biopharm. 2015, 94, 312–321. [Google Scholar] [CrossRef]
- Mohammadi, S.; Jones, L.; Gorbet, M. Extended Latanoprost Release from Commercial Contact Lenses: In Vitro Studies Using Corneal Models. Plos One 2014, 9, 1–10. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Nair, A.S.; Parvathy, J. Extended wear therapeutic contact lens fabricated from timolol imprinted carboxymethyl chitosan-g-hydroxy ethyl methacrylate-g-poly acrylamide as a onetime medication for glaucoma. Eur. J. Pharm. Biopharm. 2016, 109, 61–71. [Google Scholar] [CrossRef]
- Mehta, P.; Al-Kinani, A.A.; Arshad, M.S.; Chang, M.-W.; Alany, R.G.; Ahmad, Z. Development and characterisation of electrospun timolol maleate-loaded polymeric contact lens coatings containing various permeation enhancers. Int. J. Pharm. 2017, 532, 408–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakkar, S.; Misra, M. Electrospun polymeric nanofibers: New horizons in drug delivery. Eur. J. Pharm. Sci. 2017, 107, 148–167. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Chaudhary, S.; Rani, S.; Sharma, A.K.; Gupta, L.; Gupta, U. Dendrimer-drug Conjugates in Drug Delivery and Targeting. Pharm. Nanotechnol. 2015, 3, 239–260. [Google Scholar] [CrossRef]
- Lancina, M.G.; Singh, S.; Kompella, U.B.; Husain, S.; Yang, H. Fast Dissolving Dendrimer Nanofiber Mats as Alternative to Eye Drops for More Efficient Antiglaucoma Drug Delivery. ACS Biomater. Sci. Eng. 2017, 3, 1861–1868. [Google Scholar] [CrossRef]
- Gagandeep; Garg, T.; Malik, B.; Rath, G.; Goyal, A.K. Development and characterization of nano-fiber patch for the treatment of glaucoma. Eur. J. Pharm. Sci. 2014, 53, 10–16. [Google Scholar] [CrossRef]

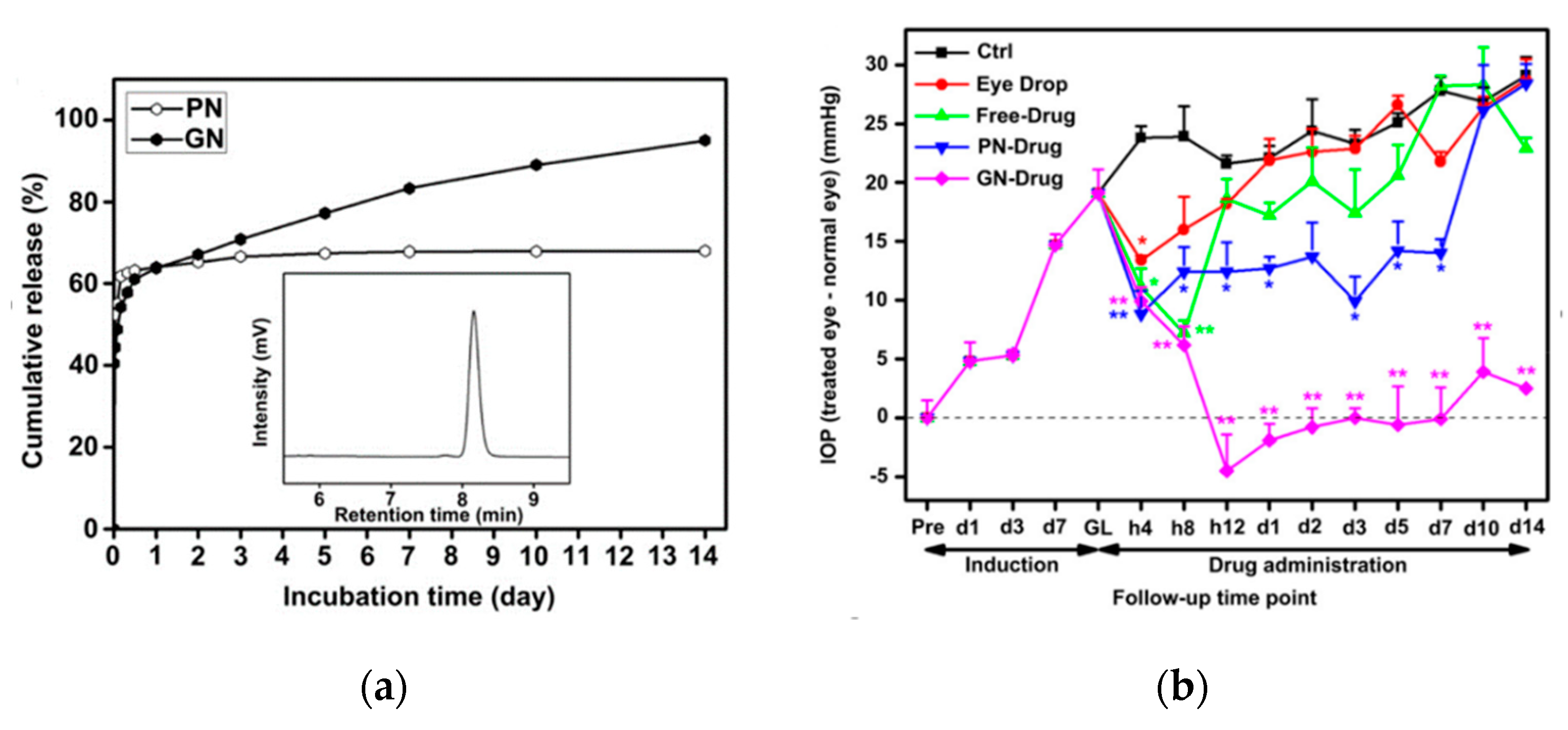


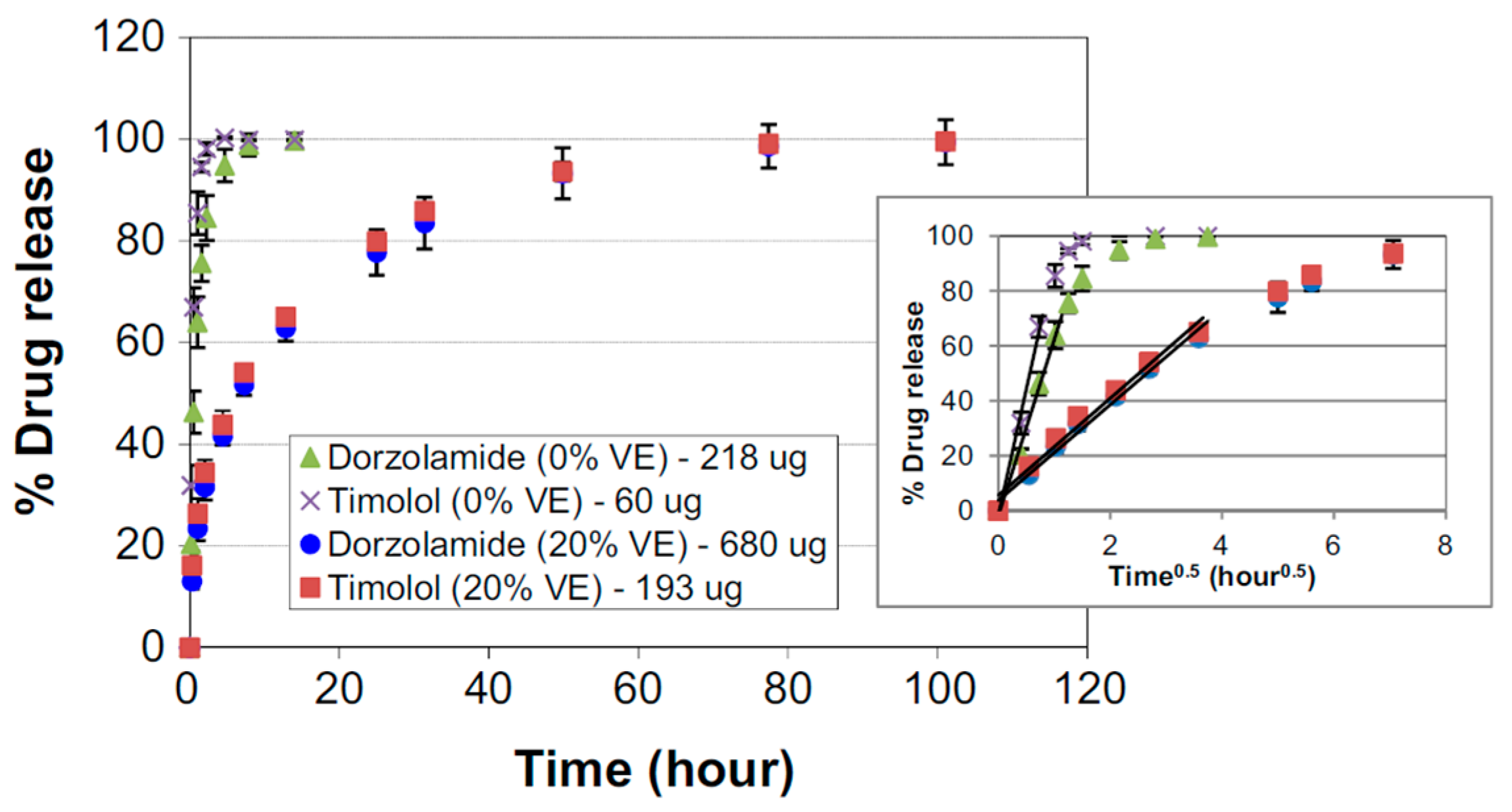
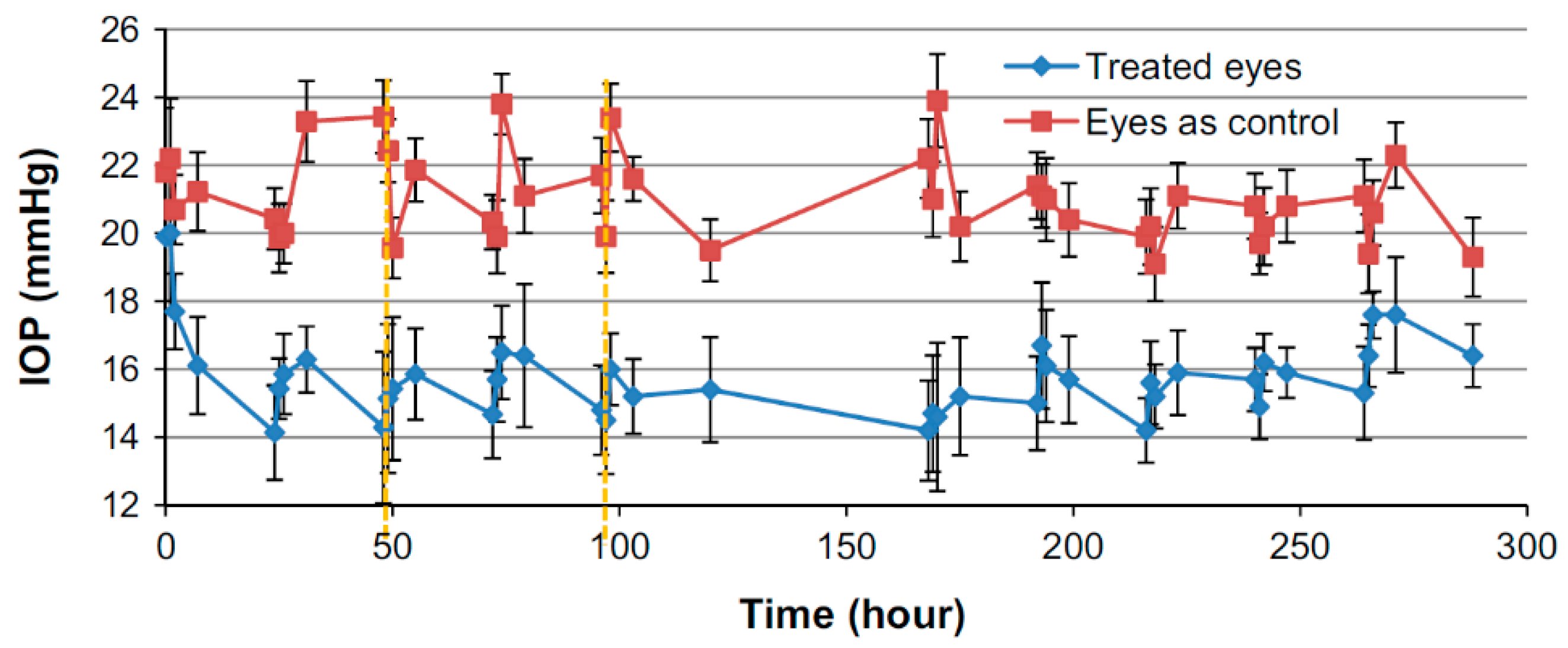
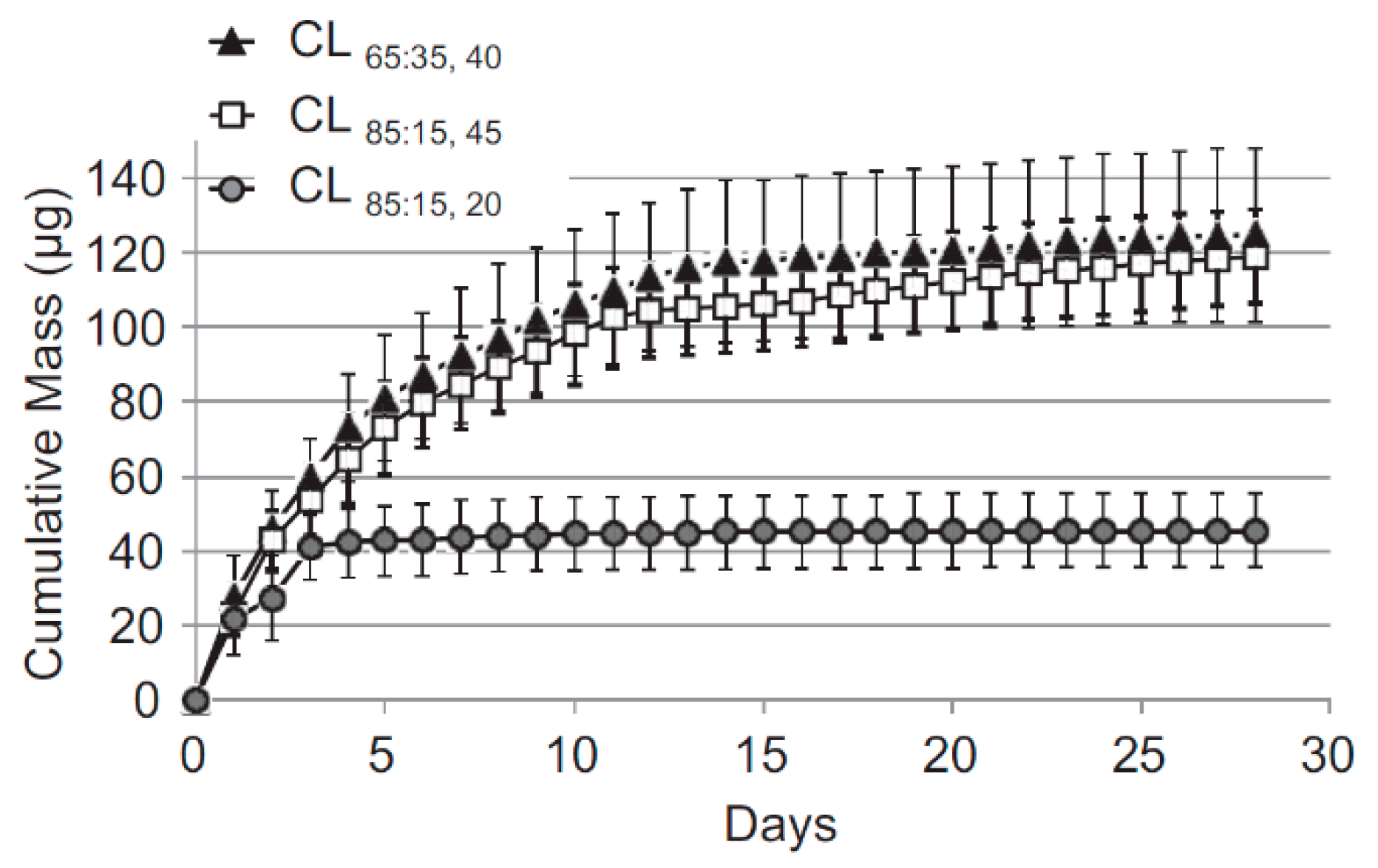
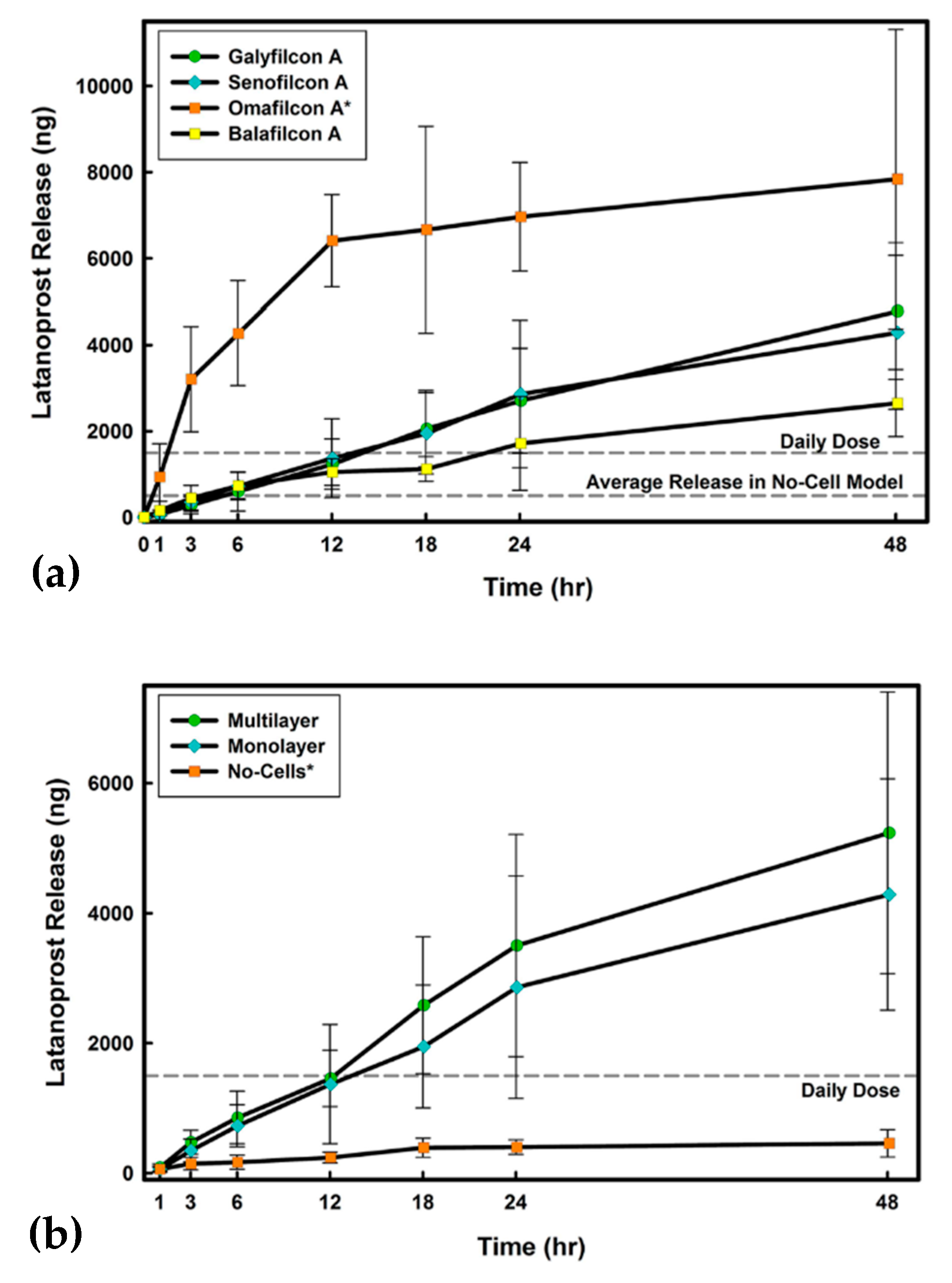
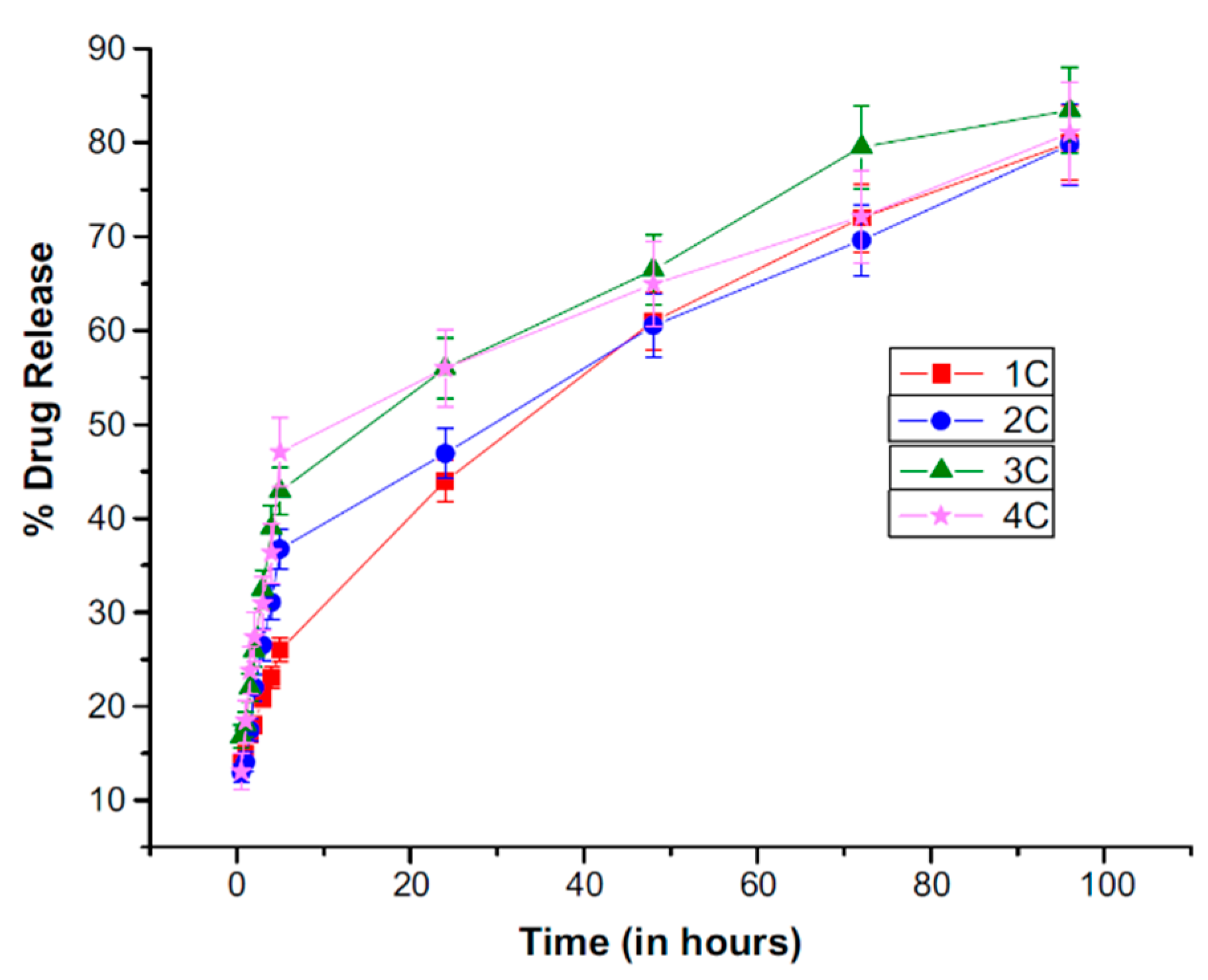
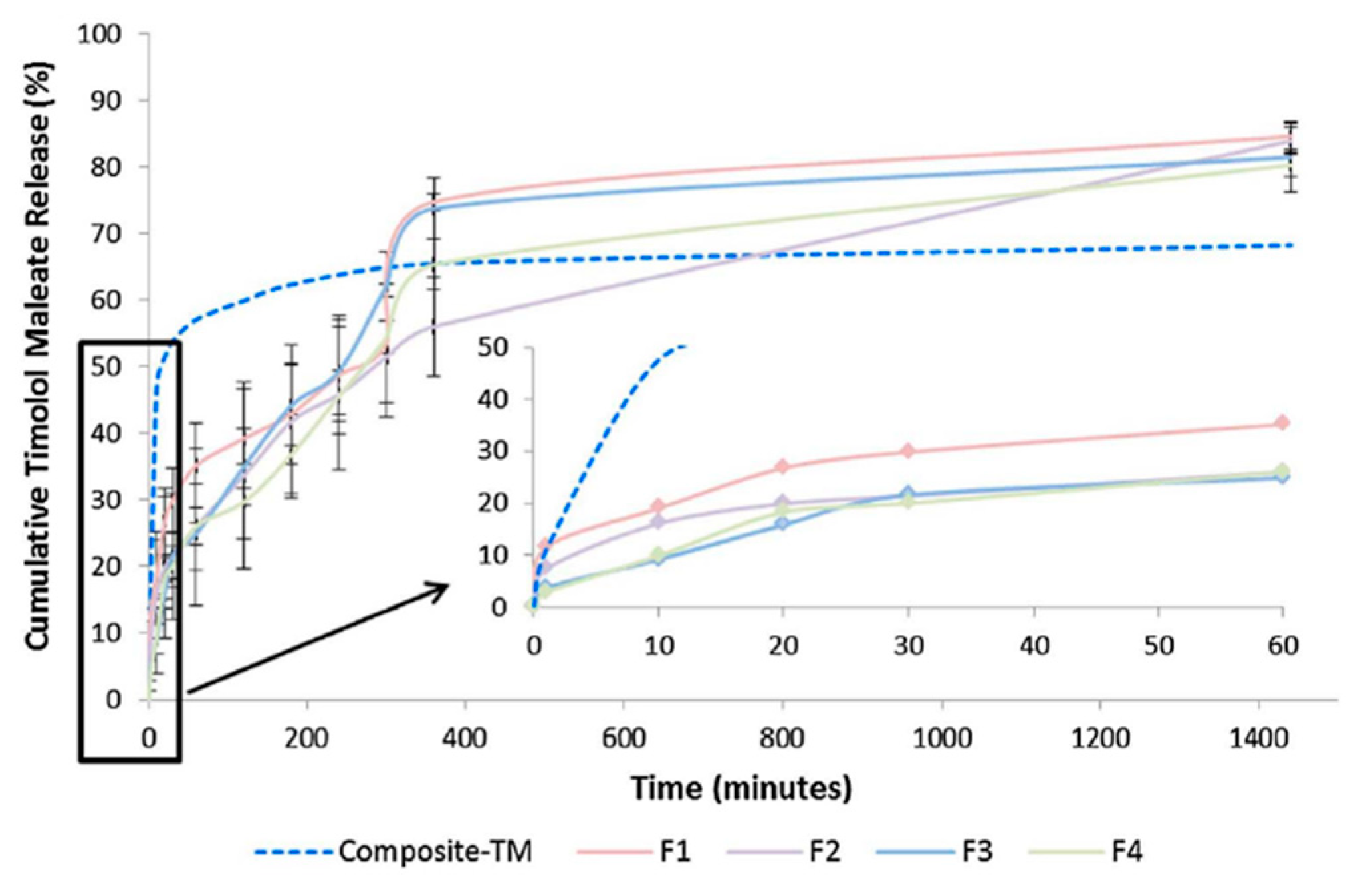
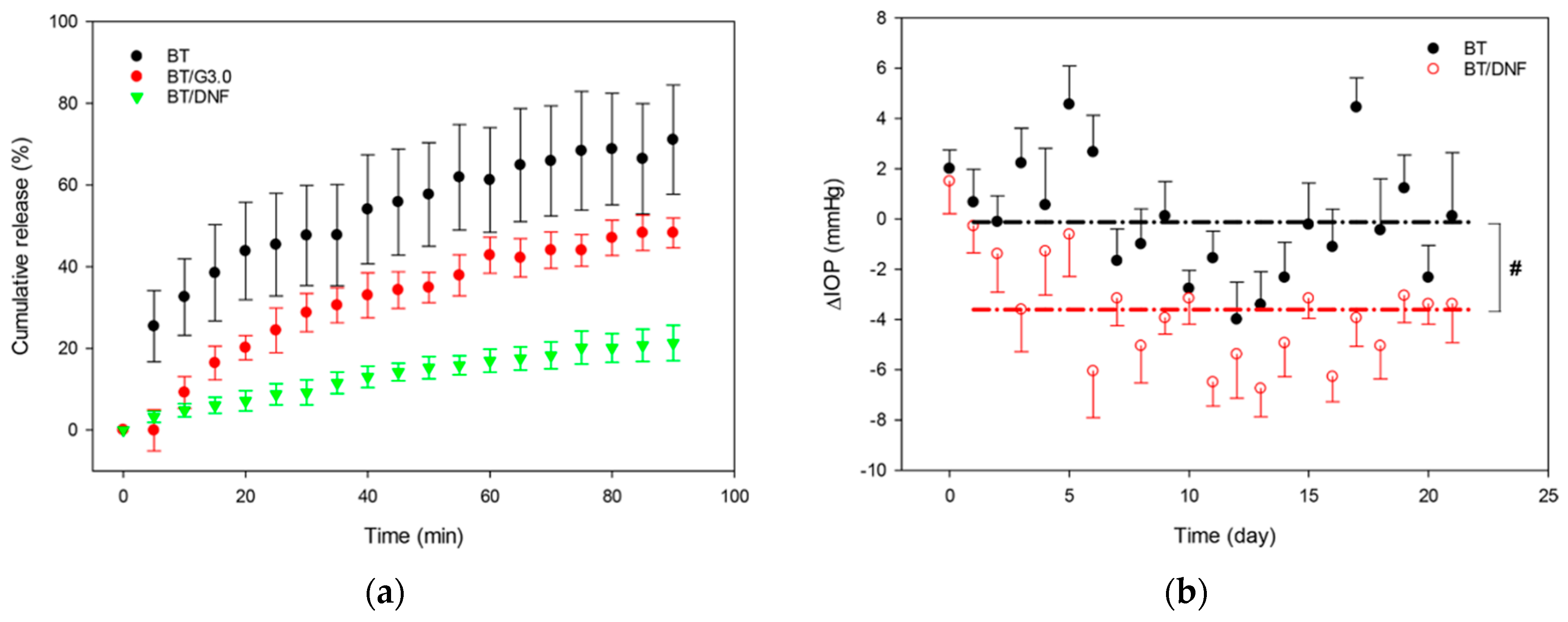
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cegielska, O.; Sajkiewicz, P. Targeted Drug Delivery Systems for the Treatment of Glaucoma: Most Advanced Systems Review. Polymers 2019, 11, 1742. https://doi.org/10.3390/polym11111742
Cegielska O, Sajkiewicz P. Targeted Drug Delivery Systems for the Treatment of Glaucoma: Most Advanced Systems Review. Polymers. 2019; 11(11):1742. https://doi.org/10.3390/polym11111742
Chicago/Turabian StyleCegielska, Olga, and Paweł Sajkiewicz. 2019. "Targeted Drug Delivery Systems for the Treatment of Glaucoma: Most Advanced Systems Review" Polymers 11, no. 11: 1742. https://doi.org/10.3390/polym11111742




