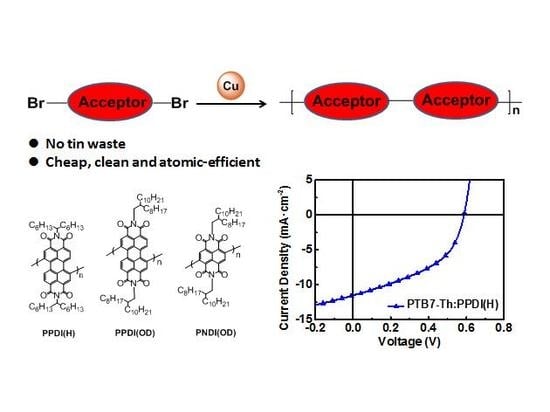
Microwave-Assisted Classic Ullmann C–C Coupling Polymerization for Acceptor-Acceptor Homopolymers
Abstract
1. Introduction
2. Results and Discussion
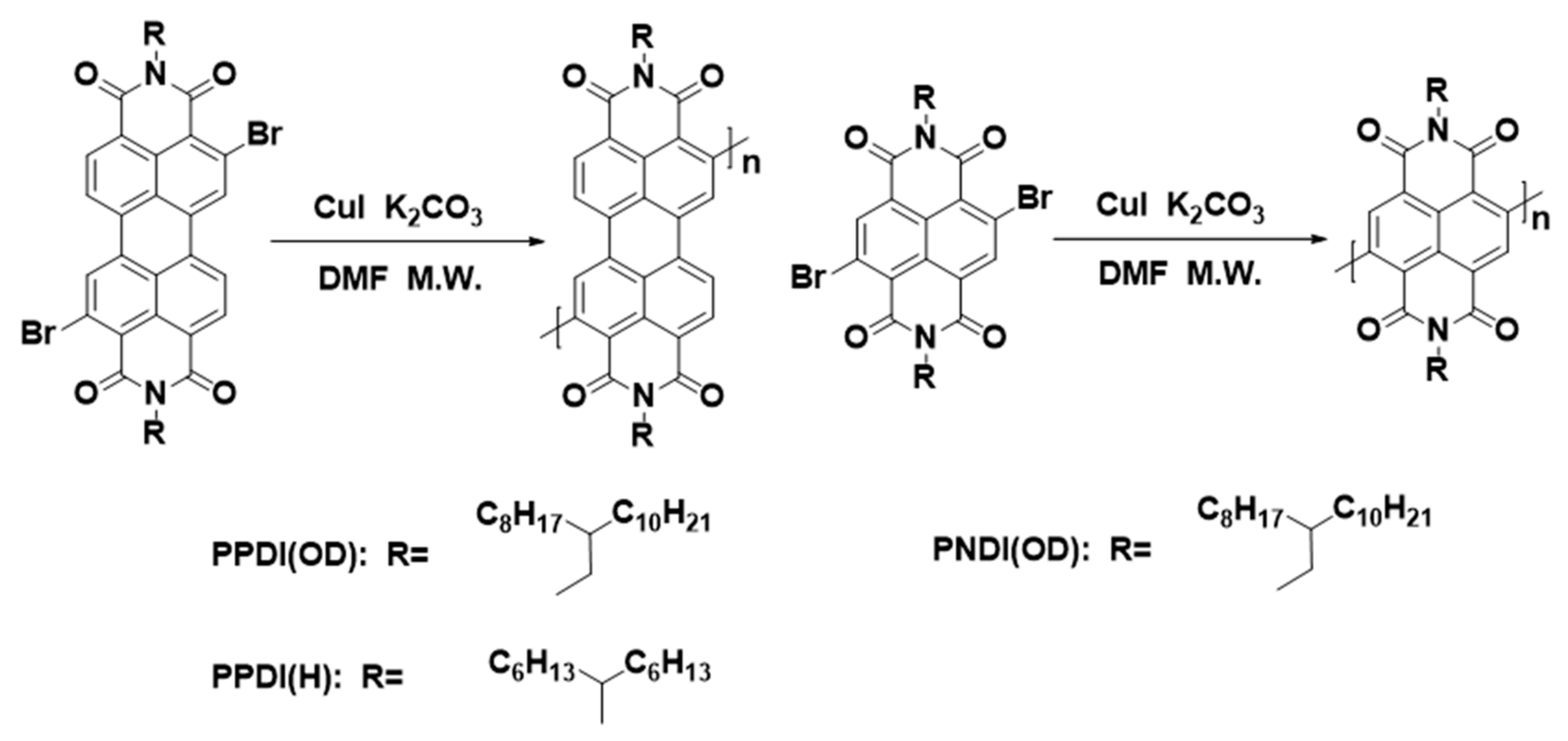
2.1. Ullmann Polymerization
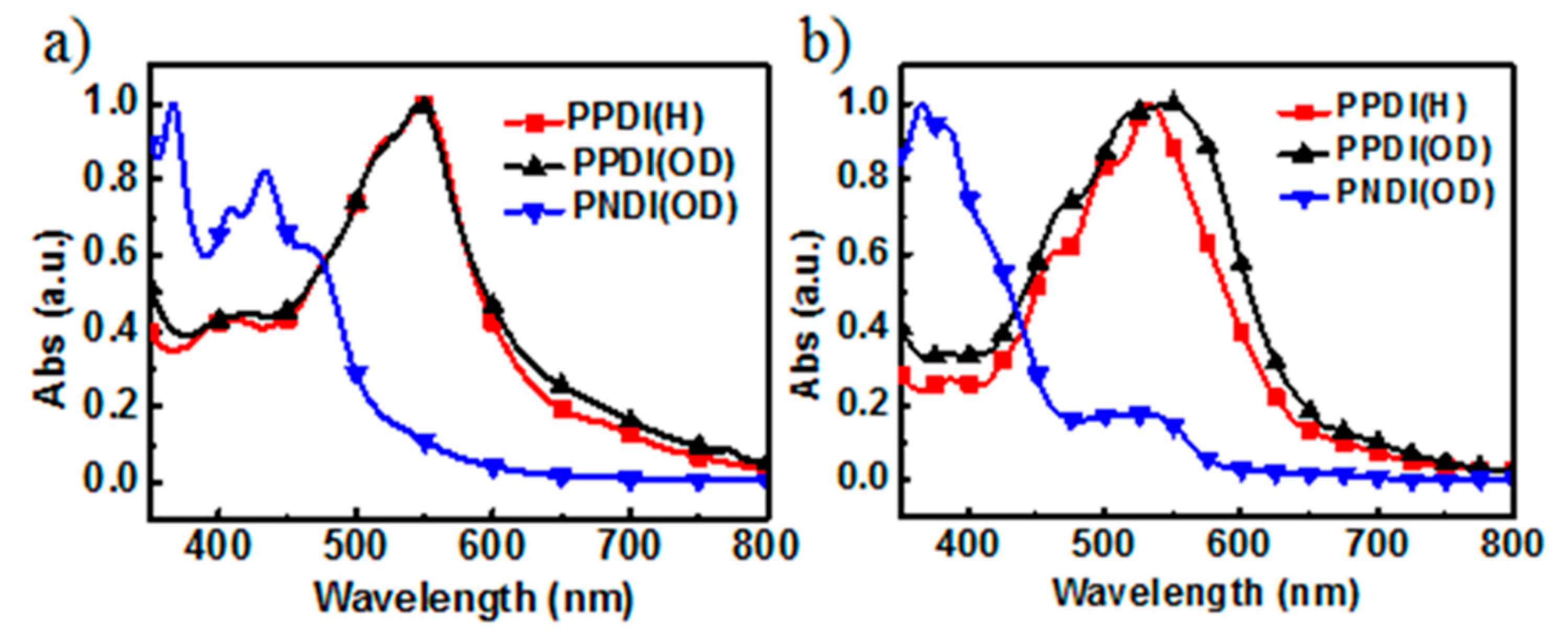
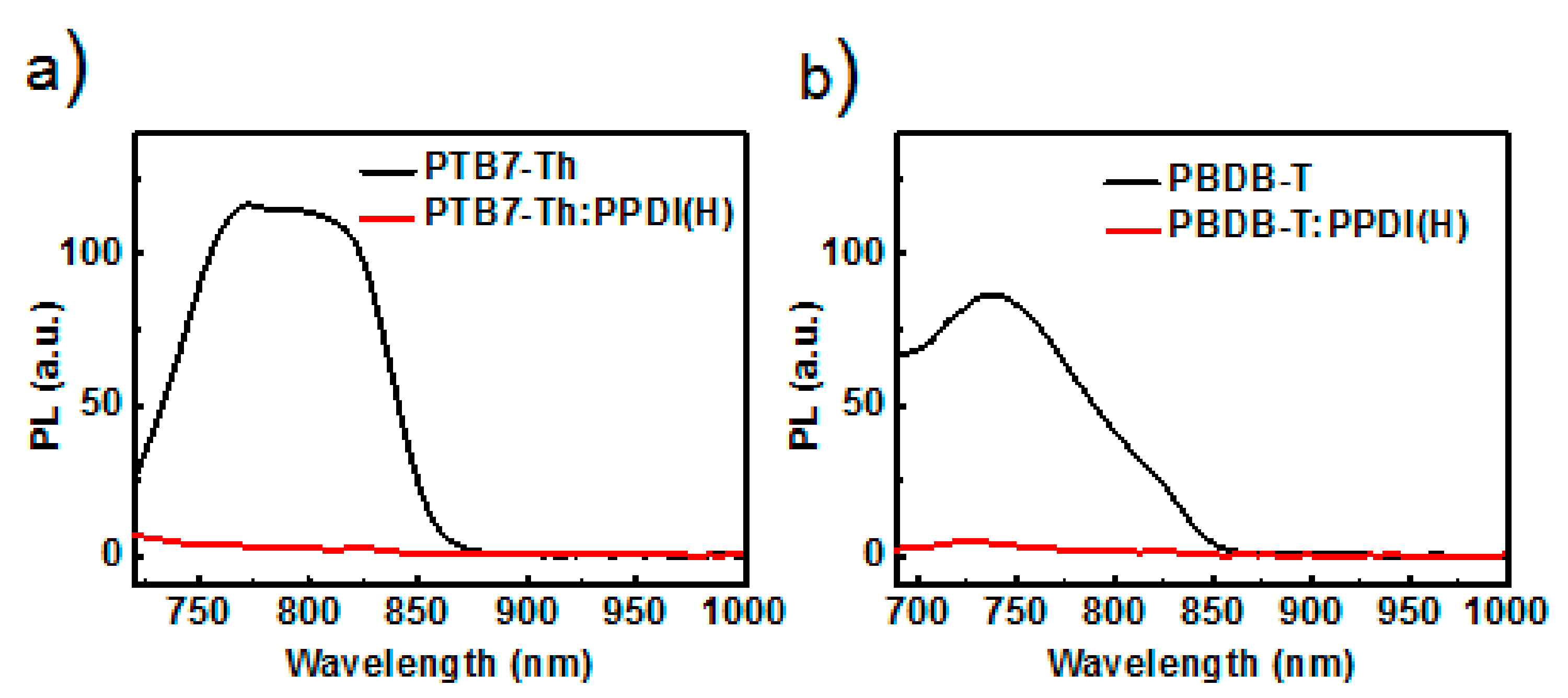
2.2. Optical and Electrochemical Properties
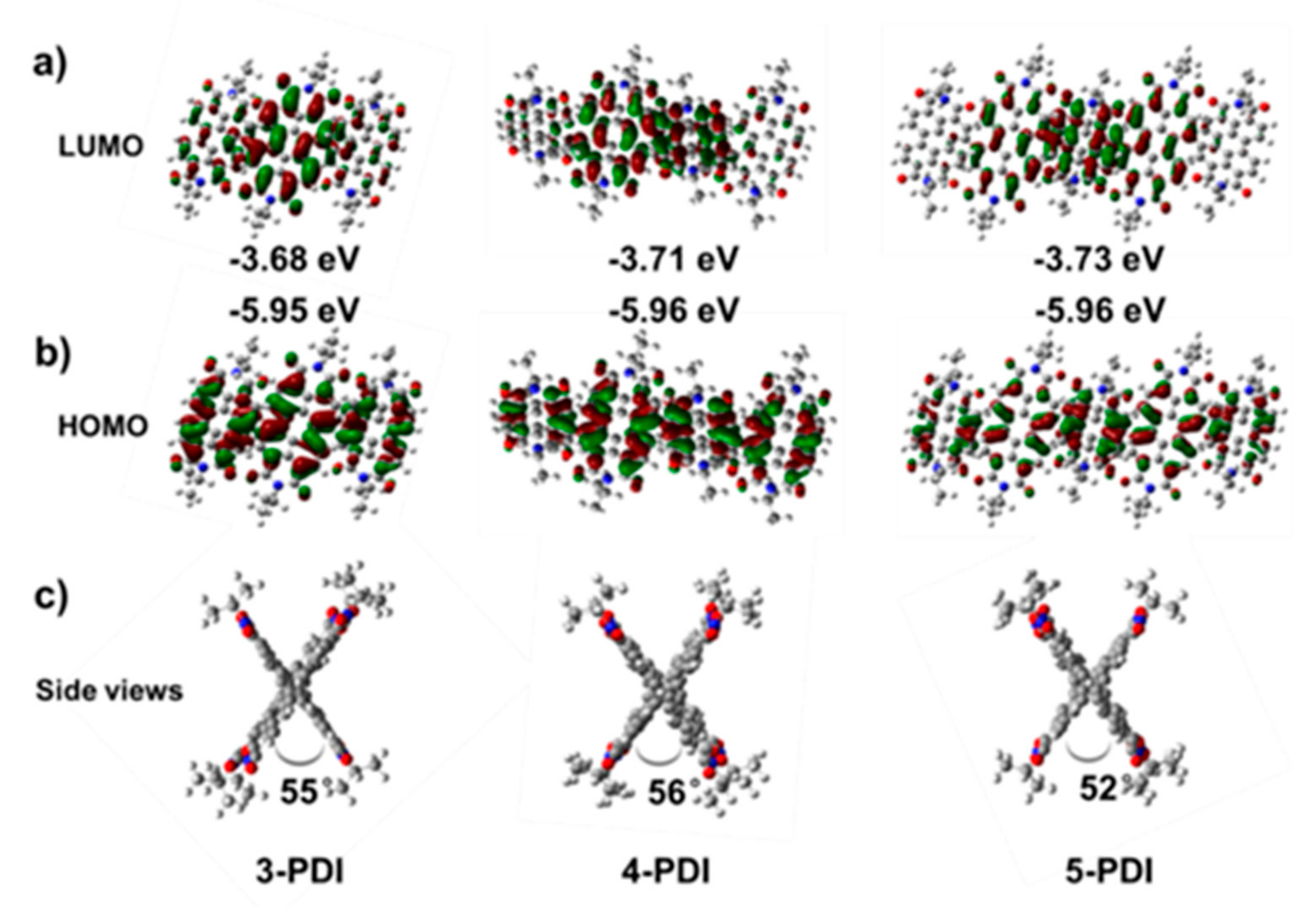
2.3. DFT Calculation
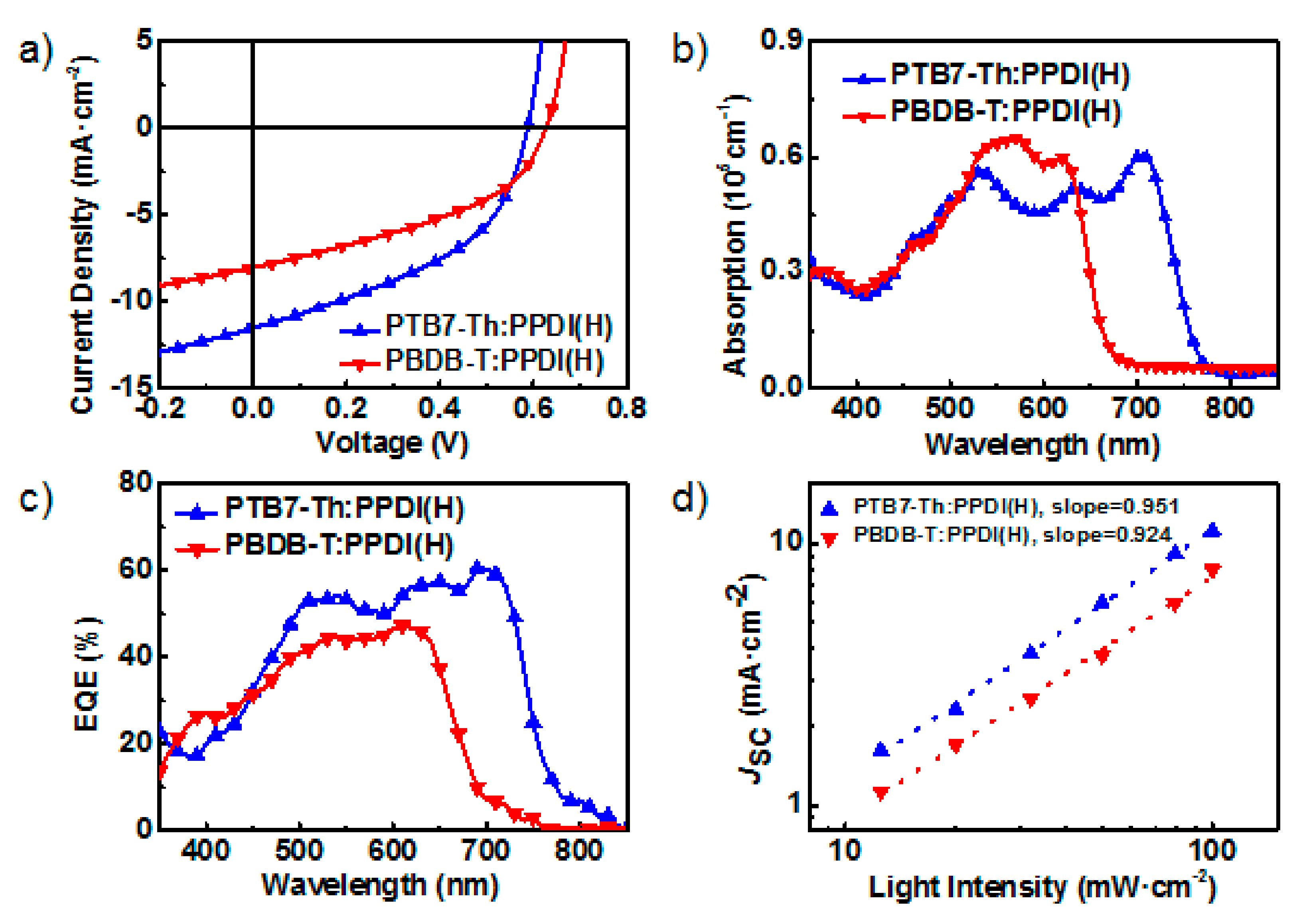
2.4. Fabrication and Characterization of All-PSCs
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hwang, Y.J.; CourtrightZhou, N.; Dudnik, A.S.; Li, T.I.N.G.; Manley, E.F.; Aldrich, T.J.; Guo, P.; Liao, H.C.; Chen, Z.; Chen, L.X.; et al. All-Polymer Solar Cell Performance Optimized via Systematic Molecular Weight Tuning of both Donor and Acceptor Polymers. J. Am. Chem. Soc. 2016, 138, 1240–1251. [Google Scholar]
- Kim, T.; Kim, J.H.; Kang, T.E.; Lee, C.; Kang, H.; Shin, M.; Wang, C.; Ma, B.; Jeong, U.; Kim, T.S. Flexible, Highly Efficient All-Polymer Solar Cells. Nat. Commun. 2015, 6, 8547. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.J.; Courtright, B.A.E.; Ferreira, A.S.; Tolbert, S.H.; Jenekhe, S.A. 7.7% Efficient All-Polymer Solar Cells. Adv. Mater. 2015, 27, 4578–4584. [Google Scholar]
- Zhou, Y.; Gu, K.L.; Gu, X.; Kurosawa, T.; Yan, H.; Guo, Y.; Koleilat, G.I.; Zhao, D.; Toney, M.F.; Bao, Z. All-Polymer Solar Cells Employing Non-Halogenated Solvent and Additive. Chem. Mater. 2016, 28, 5037–5042. [Google Scholar] [CrossRef]
- Ye, L.; Jiao, X.; Zhang, H.; Li, S.; Yao, H.; Ade, H.; Hou, J. 2D-Conjugated Benzodithiophene-Based Polymer Acceptor: Design, Synthesis, Nanomorphology, and Photovoltaic Performance. Macromolecules 2015, 48, 7156–7163. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, L.; Yang, M.; Zhan, C.; Xie, Z.; Verpoort, F.; Xiao, S. Taking the Place of Perylene Diimide: Perylene Tetracarboxylic Tetraester as A Building Block for Polymeric Acceptors to Achieve Higher Open Circuit Voltage in All-Polymer Bulk Heterojunction Solar Cells. Polym. Chem. 2013, 4, 5612–5620. [Google Scholar] [CrossRef]
- Liu, S.; Kan, Z.; Thomas, S.; Cruciani, F.; Brédas, J.L.; Beaujuge, P.M. Thieno[3,4-c]Pyrrole-4,6-Dione-3,4-Difluorothiophene Polymer Acceptors for Efficient All-Polymer Bulk Heterojunction Solar Cells. Angew. Chem. Int. Ed. 2016, 55, 12996–13000. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Bao, X.; Qiu, M.; Liu, Z.; Wang, N.; Zhang, G.; Yang, R.; Zhang, D. New π-Conjugated Polymers as Acceptors Designed for All-Polymer Solar Cells Based on Imide/amide-derivatives. J. Mater. Chem. C. 2016, 4, 185–192. [Google Scholar] [CrossRef]
- Liu, S.; Song, X.; Thomas, S.; Kan, Z.; Cruciani, F.; Laquai, F.; Bredas, J.L.; Beaujuge, P.M. Thieno[3,4-c]Pyrrole-4,6-Dione-Based Polymer Acceptors for High Open-Circuit Voltage All-Polymer Solar Cells. Adv. Energy Mater. 2017, 7, 1602574. [Google Scholar] [CrossRef]
- Fang, K.; Huang, Y.; Chang, G.; Yang, J.; Shen, Y.; Ye, X. Conjugated Polymers Based on Modified Benzo[1,2-b:4,5-b′]dithiophene and Perylene Diimide Derivatives: Good Optical Properties and Tunable Electrochemical Performance. Macromol. Res. 2015, 23, 545–551. [Google Scholar] [CrossRef]
- Deng, Z.; Wu, F.; Chen, L.; Chen, Y. Novel Photovoltaic Donor 1-Acceptor-Donor 2-Acceptor Terpolymers with Tunable Energy Levels Based on Difluorinated Benzothiadiazole Acceptor. RSC Adv. 2015, 5, 12087–12093. [Google Scholar] [CrossRef]
- Sharma, S.; Kolhe, N.B.; Gupta, V.; Bharti, V.; Sharma, A.; Datt, R.; Chand, S.; Asha, S.K. Improved All-Polymer Solar Cell Performance of n-Type Naphthalene Diimide–Bithiophene P(NDI2OD-T2) Copolymer by Incorporation of Perylene Diimide as Coacceptor. Macromolecules 2016, 49, 8113–8125. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, J.B.; Yoon, S.C.; Jung, I.H.; Hwang, D.H. Enhanced and Controllable Open-Circuit Voltage Using 2D-Conjugated Benzodithiophene (BDT) Homopolymers by Alkylthio Substitution. J. Mater. Chem. C. 2016, 4, 2170–2177. [Google Scholar] [CrossRef]
- Po, R.; Bernardi, A.; Calabrese, A.; Carbonera, C.; Corso, G.; Pellegrino, A. From Lab to Fab: How Must the Polymer Solar Cell Materials Design Change? An Industrial Perspective. Energy Environ. Sci. 2014, 7, 925–943. [Google Scholar] [CrossRef]
- Gwinner, M.C.; Brenner, T.J.K.; Lee, J.K.; Newby, C.; Ober, C.K.; Mcneill, C.R.; Sirringhaus, H. Organic Field-Effect Transistors and Solar Cells Using Novel High Electron-Affinity Conjugated Copolymers Based on Alkylbenzotriazole and Benzothiadiazole. J. Mater. Chem. 2012, 22, 4436–4439. [Google Scholar] [CrossRef]
- Lee, J.K.; Gwinner, M.C.; Berger, R.; Newby, C.; Zentel, R.; Friend, R.H.; Sirringhaus, H.; Ober, C.K. High-Performance Electron-Transporting Polymers Derived from A Heteroaryl Bis(trifluoroborate). J. Am. Chem. Soc. 2011, 133, 9949–9951. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lv, L.; Li, L.; Chen, Y.; Zhang, K.; Chen, H.; Dong, H.; Huang, J.; Shen, G.; Yang, Z. High-Performance All-Polymer Photoresponse Devices Based on Acceptor–Acceptor Conjugated Polymers. Adv. Funct. Mater. 2016, 26, 6306–6315. [Google Scholar] [CrossRef]
- Ge, C.W.; Mei, C.Y.; Ling, J.; Wang, J.T.; Zhao, F.G.; Liang, L.; Li, H.J.; Xie, Y.S.; Li, W.S.J. Acceptor–Acceptor Conjugated Copolymers Based on Perylenediimide and Benzothiadiazole for All-Polymer Solar Cells. Polym. Sci. Part A Polym. Chem. 2014, 52, 1200–1215. [Google Scholar] [CrossRef]
- Banal, J.L.; Subbiah, J.; Graham, H.; Lee, J.K.; Ghiggino, K.P.; Wong, W.W.H. Electron Deficient Conjugated Polymers Based on Benzotriazole. Polym. Chem. 2013, 4, 1077–1083. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, H.; Qin, M.; Zhao, J.; Wang, Y.; Wang, H.; Wang, Y.; Facchetti, A.; Lu, X.; Guo, X. Thiazole Imide-Based All-Acceptor Homopolymer: Achieving High-Performance Unipolar Electron Transport in Organic Thin-Film Transistors. Adv. Mater. 2018, 30, 1705745. [Google Scholar] [CrossRef]
- Stalder, R.; Mei, J.; Subbiah, J.; Grand, C.; Estrada, L.A.; So, F.; Reynolds, J.R. n-Type Conjugated Polyisoindigos. Macromolecules 2011, 44, 6303–6310. [Google Scholar] [CrossRef]
- Matthew, M.D.; Peter, D.K.; Christine, K.L. Synthesis and Characterization of Thiophene-Containing Naphthalene Diimide n-Type Copolymers for OFET Applications. Macromolecules 2010, 43, 6348–6352. [Google Scholar]
- Sambiagio, C.; Marsden, S.P.; Blacker, A.J.; McGowan, P.C. Copper Catalysed Ullmann Type Chemistry: From Mechanistic Aspects to Modern Development. Chem. Soc. Rev 2014, 43, 3525–3550. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, S.; Pawar, G.G.; Kumar, S.V.; Jiang, Y.; Ma, D. Selected Copper-Based Reactions for C-N, C-O, C-S, and C-C Bond Formation. Angew. Chem. Int. Ed. 2017, 56, 16136–16179. [Google Scholar] [CrossRef]
- Gorgun, K.; Caglar, Y.; Yakuphanoglu, F. Synthesis and Photodiode Characterization of Novel Twisted Carbazole Derivatives with 1,3,5-Benzene Core. Silicon 2017, 10, 693–702. [Google Scholar] [CrossRef]
- Gorgun, K.; Caglar, Y. Synthesis of Novel Carbazole Derived Substances Using Some Organoboron Compounds by Palladium Catalyzed and Investigation of Its Semiconductor Device Characteristics. J. Mol. Struct. 2018, 1157, 106–111. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.Q.; Wang, X.S. An Efficient Synthesis of 16H-Dibenzo[2,3:6,7][1,4]Oxazepino[5,4-b]Quinazolin-16-Ones via An Ullmann Reaction Catalyzed by CuI. Org. Biomol. Chem. 2018, 16, 1679–1685. [Google Scholar] [CrossRef]
- Khan, F.; Dlugosch, M.; Liu, X.; Banwell, M.G. The Palladium-Catalyzed Ullmann Cross-Coupling Reaction: A Modern Variant on A Time-Honored Process. Acc. Chem. Res. 2018, 51, 1784–1795. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Qi, L.; Sun, J.P.; Long, J.J. Synthesis of An Anthraquinonoid Disperse Reactive Dye Based on A Ligand-Free Ullmann Reaction. Color. Technol. 2017, 133, 283–292. [Google Scholar] [CrossRef]
- Bao, X.; Qiao, X.; Bao, C.; Liu, Y.; Zhao, X.; Lu, Y.; Chen, G. Synthesis of Tamibarotene via Ullmann-Type Coupling. Org. Process Res. Dev. 2017, 21, 748–753. [Google Scholar] [CrossRef]
- Audisio, D.; Messaoudi, S.; Peyrat, J.F.; Brion, J.D.; Alami, M. A General Copper Powder-Catalyzed Ullmann-Type Reaction of 3-Halo-4(1H)-Quinolones with Various Nitrogen-Containing Nucleophiles. J. Org. Chem. 2011, 76, 4995–5005. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.V.; Thomas, A.W. Modern Synthetic Methods for Copper-Mediated C(aryl)[bond]O, C(aryl)[bond]N, and C(aryl)[bond]S Bond Formation. Angew. Chem. Int. Ed. 2003, 42, 5400–5449. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Wu, Q.; Nam, C.Y.; Grubbs, R.B. Polymerization of Tellurophene Derivatives by Microwave-Assisted Palladium-Catalyzed Ipso-Arylative Polymerization. Angew. Chem. Int. Ed. 2014, 53, 10691–10695. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Zhou, Y.; Zheng, Y.Q.; Pei, J.; Zhao, D. Towards Rational Design of Organic Electron Acceptors for Photovoltaics: A Study Based on Perylenediimide Derivatives. Chem. Sci. 2013, 4, 4389–4394. [Google Scholar] [CrossRef]
- Singh, R.; Aluicio-Sarduy, E.; Kan, Z.; Ye, T.; Mackenzie, R.C.I.; Keivanidis, P.E. Fullerene-Free Organic Solar Cells with An Efficiency of 3.7% Based on A Low-Cost Geometrically Planar Perylene Diimide Monomer. J. Mater. Chem. A 2014, 2, 14348–14353. [Google Scholar] [CrossRef]
- Liu, Y.; Mu, C.; Jiang, K.; Zhao, J.; Li, Y.; Zhang, L.; Li, Z.; Lai, J.Y.L.; Hu, H.; Ma, T. A Tetraphenylethylene Core-Based 3D Structure Small Molecular Acceptor Enabling Efficient Non-Fullerene Organic Solar Cells. Adv. Mater. 2015, 27, 1015–1020. [Google Scholar] [CrossRef]
- Rajaram, S.; Shivanna, R.; Kandappa, S.K.; Narayan, K.S. Nonplanar Perylene Diimides as Potential Alternatives to Fullerenes in Organic Solar Cells. J. Phys. Chem. Lett. 2012, 3, 2405–2408. [Google Scholar] [CrossRef]
- Zhang, S.; Ye, L.; Zhao, W.; Liu, D.; Yao, H.; Hou, J. Side Chain Selection for Designing Highly Efficient Photovoltaic Polymers with 2D-Conjugated Structure. Macromolecules 2014, 47, 4653–4659. [Google Scholar] [CrossRef]
- Qian, D.; Ye, L.; Zhang, M.; Liang, Y.; Li, L.; Huang, Y.; Guo, X.; Zhang, S.; Tan, Z.; Hou, J. Design, Application, and Morphology Study of a New Photovoltaic Polymer with Strong Aggregation in Solution State. Macromolecules 2012, 45, 9611–9617. [Google Scholar] [CrossRef]
- Brabec, C.J.; Winder, C.; Sariciftci, N.S.; Hummelen, J.C.; Dhanabalan, A.; Van Hal, P.A.; Janssen, R.A.J. A Low-Bandgap Semiconducting Polymer for Photovoltaic Devices and Infrared Emitting Diodes. Adv. Funct. Mater. 2002, 12, 709–712. [Google Scholar] [CrossRef]

| Entry a | Solvent | T (°C) | Time (h) | Heat | Yield (%) | Repeat Unit b |
|---|---|---|---|---|---|---|
| 1 | DMSO | 80 | 12 | Oil Bath | 0 | No |
| 2 | Toluene | 80 | 12 | Oil Bath | 0 | No |
| 3 | DMF | 80 | 12 | Oil Bath | 0 | No |
| 4 | DMF | 150 | 12 | Oil Bath | 30 | 5 |
| 5 | DMF | 150 | 24 | Oil Bath | 32 | 6 |
| 6 | DMF | 150 | 5 | M. W. | 38 | 11 |
| Material | λpeak a (nm) | λonset (eV) | Egb (eV) | LUMO (eV) | HOMO c (eV) |
|---|---|---|---|---|---|
| PPDI(H) | 532 | 638 | 1.94 | −4.00 | −5.94 |
| PPDI(OD) | 545 | 667 | 1.87 | −4.05 | −5.92 |
| PNDI(OD) | 531 | 575 | 2.16 | −3.84 | -6.00 |
| Blend Film | PCE (%) | VOC (V) | JSC (mA·cm-2) | FF (%) |
|---|---|---|---|---|
| PTB7-Th:PPDI(H) | 2.87 ± 0.22 | 0.58 ± 0.01 | 11.24 ± 0.28 | 45.0 ± 0.5 |
| PBDB-T:PPDI(H) | 1.95 ± 0.13 | 0.61 ± 0.01 | 7.88 ± 0.24 | 41.9 ± 0.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Chen, Y.; Ye, P.; Jia, X.; Wu, X.; Wu, J.; Shi, Q.; Peng, A.; Huang, H. Microwave-Assisted Classic Ullmann C–C Coupling Polymerization for Acceptor-Acceptor Homopolymers. Polymers 2019, 11, 1741. https://doi.org/10.3390/polym11111741
Li Z, Chen Y, Ye P, Jia X, Wu X, Wu J, Shi Q, Peng A, Huang H. Microwave-Assisted Classic Ullmann C–C Coupling Polymerization for Acceptor-Acceptor Homopolymers. Polymers. 2019; 11(11):1741. https://doi.org/10.3390/polym11111741
Chicago/Turabian StyleLi, Zijie, Yusheng Chen, Pan Ye, Xiangli Jia, Xiaoxi Wu, Jianfei Wu, Qinqin Shi, Aidong Peng, and Hui Huang. 2019. "Microwave-Assisted Classic Ullmann C–C Coupling Polymerization for Acceptor-Acceptor Homopolymers" Polymers 11, no. 11: 1741. https://doi.org/10.3390/polym11111741
APA StyleLi, Z., Chen, Y., Ye, P., Jia, X., Wu, X., Wu, J., Shi, Q., Peng, A., & Huang, H. (2019). Microwave-Assisted Classic Ullmann C–C Coupling Polymerization for Acceptor-Acceptor Homopolymers. Polymers, 11(11), 1741. https://doi.org/10.3390/polym11111741