Albumin-Enriched Fibrin Hydrogel Embedded in Active Ferromagnetic Networks Improves Osteoblast Differentiation and Vascular Self-Organisation
Abstract
1. Introduction
2. Materials and Methods
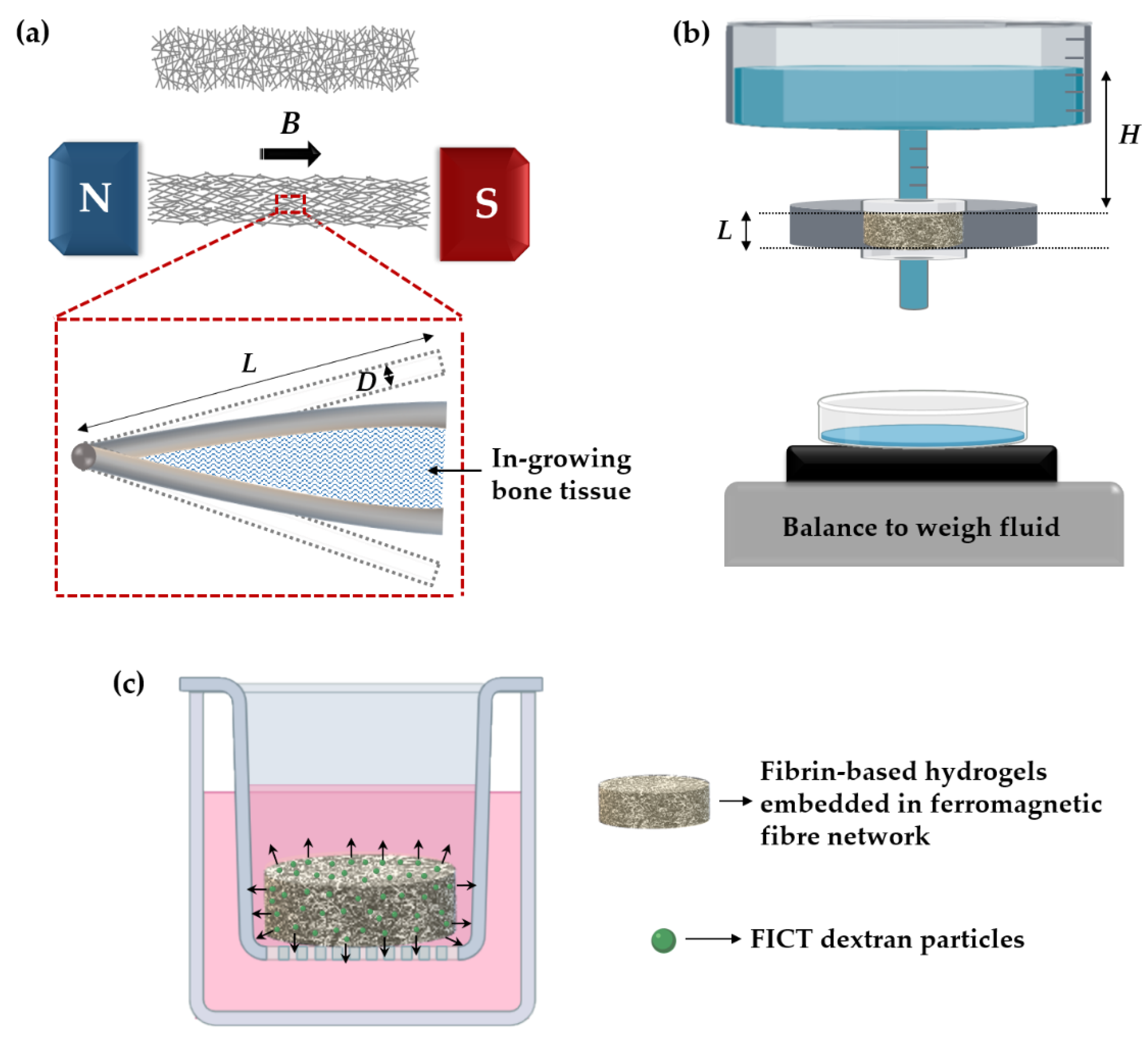
2.1. Ferromagnetic Fibre Networks
2.2. Cell Co-Culture
2.3. Fabrication of Hydrogels and Hydrogel-Impregnated Fibre Networks
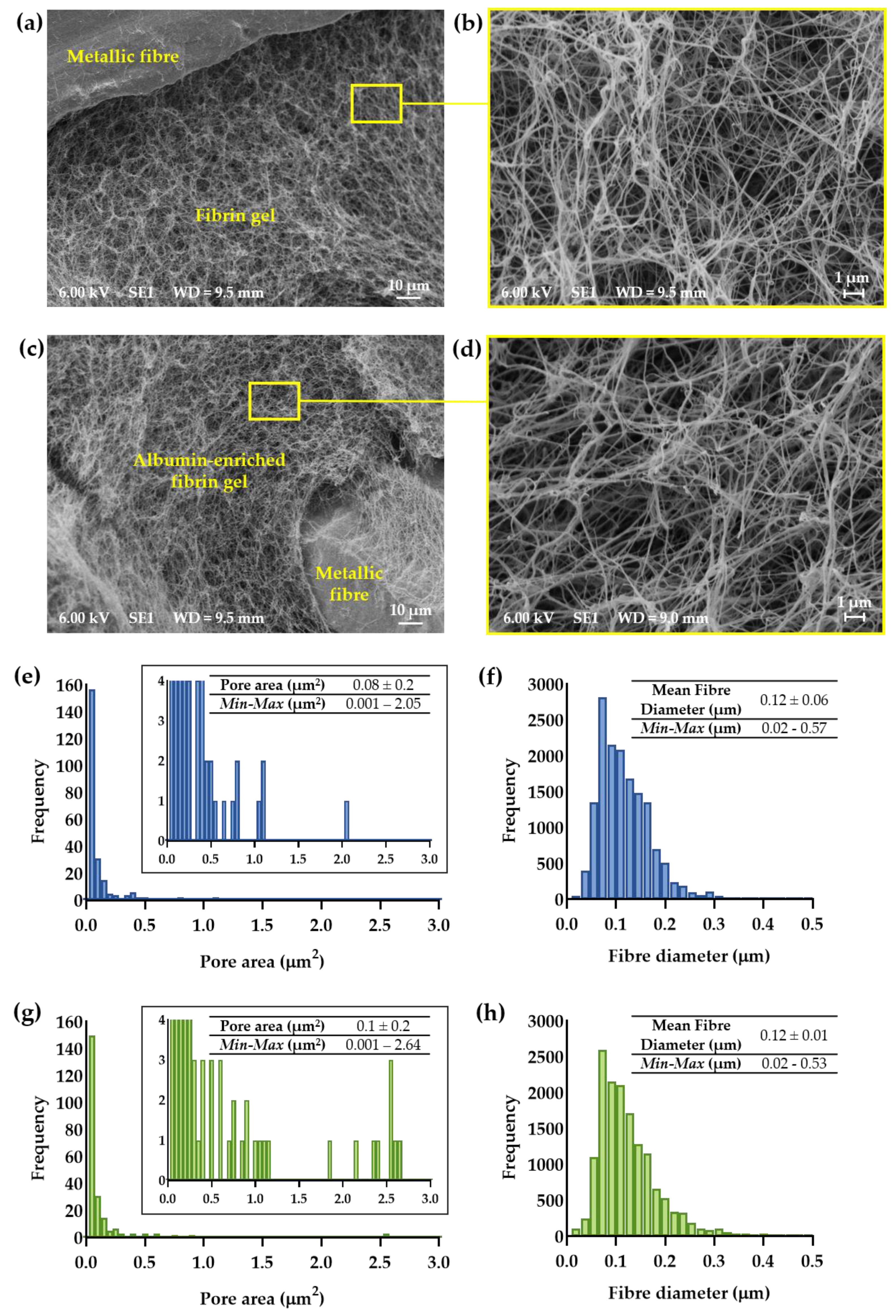
2.4. Scanning Electron Microscopy and Morphometric Analysis of Hydrogel-Impregnated Network Structure
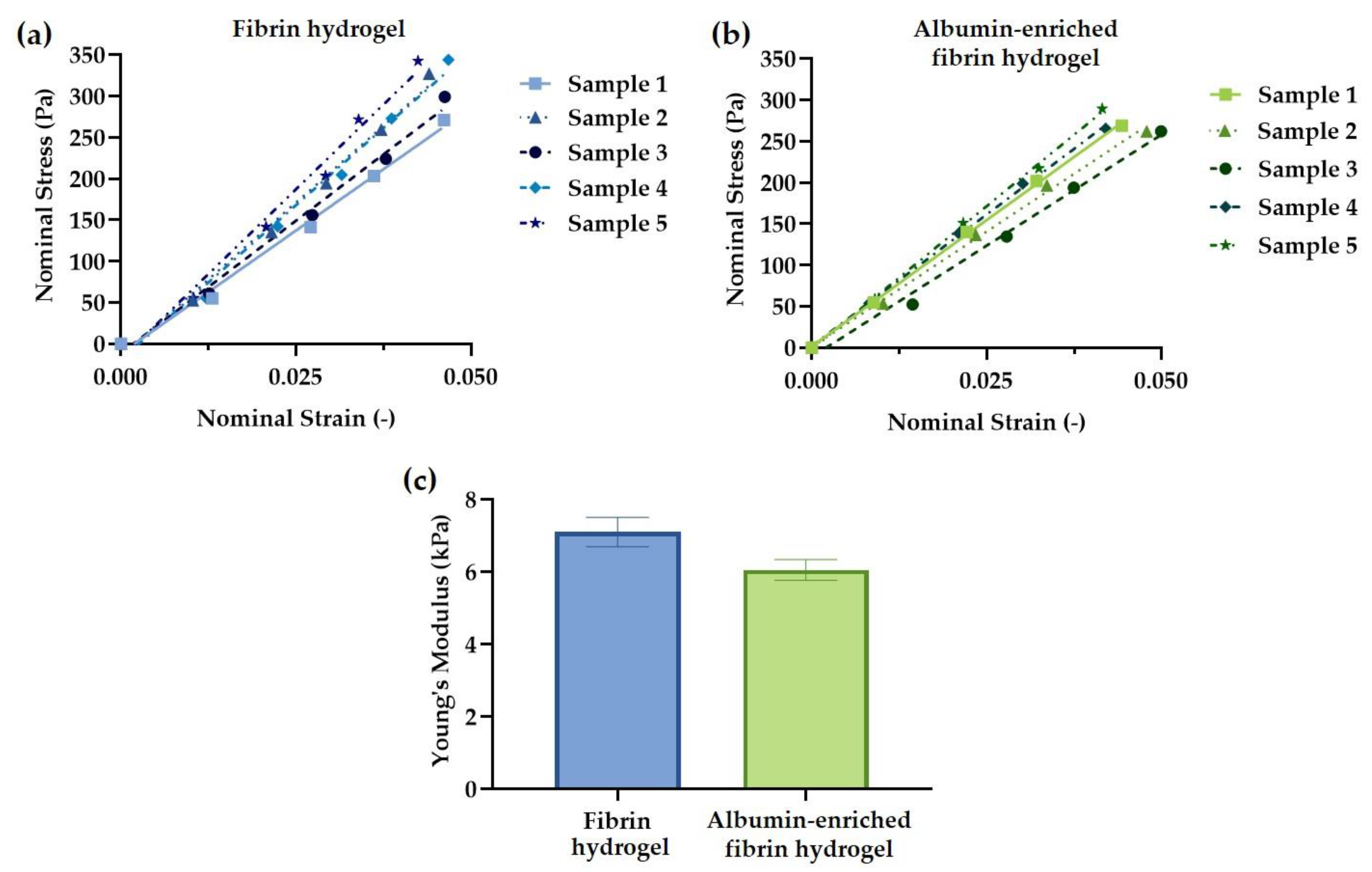
2.5. Hydrogel Mechanical Testing
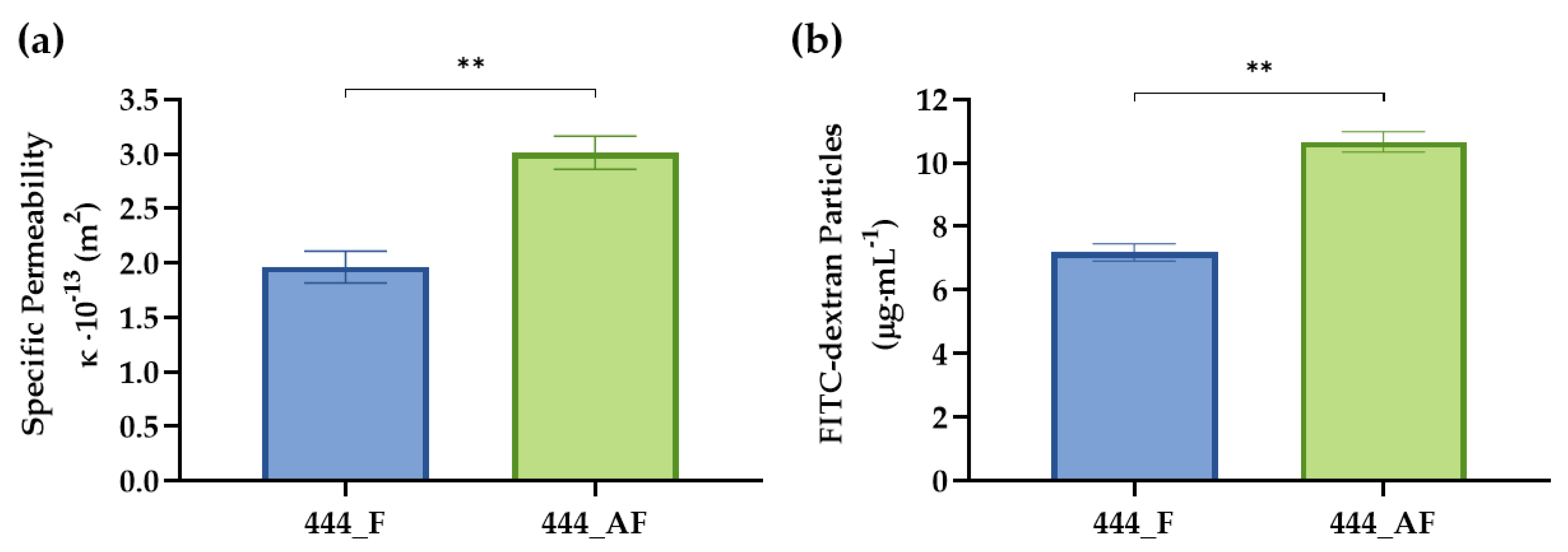
2.6. Specific Permeability of Hydrogel-Impregnated Networks
2.7. FITC-Dextran Diffusion
2.8. Vascular Analysis Using AngioTool
2.9. Cell Mineralisation
2.10. Real-Time Polymerase Chain Reaction (RT-PCR)
2.11. Statistical Analysis
3. Results and Discussion
3.1. Morphometric Analysis of Hydrogel-Impregnated Network Structure
3.2. Hydrogel Mechanical Testing
3.3. Specific Permeability and Diffusion of Hydrogel-Impregnated Networks
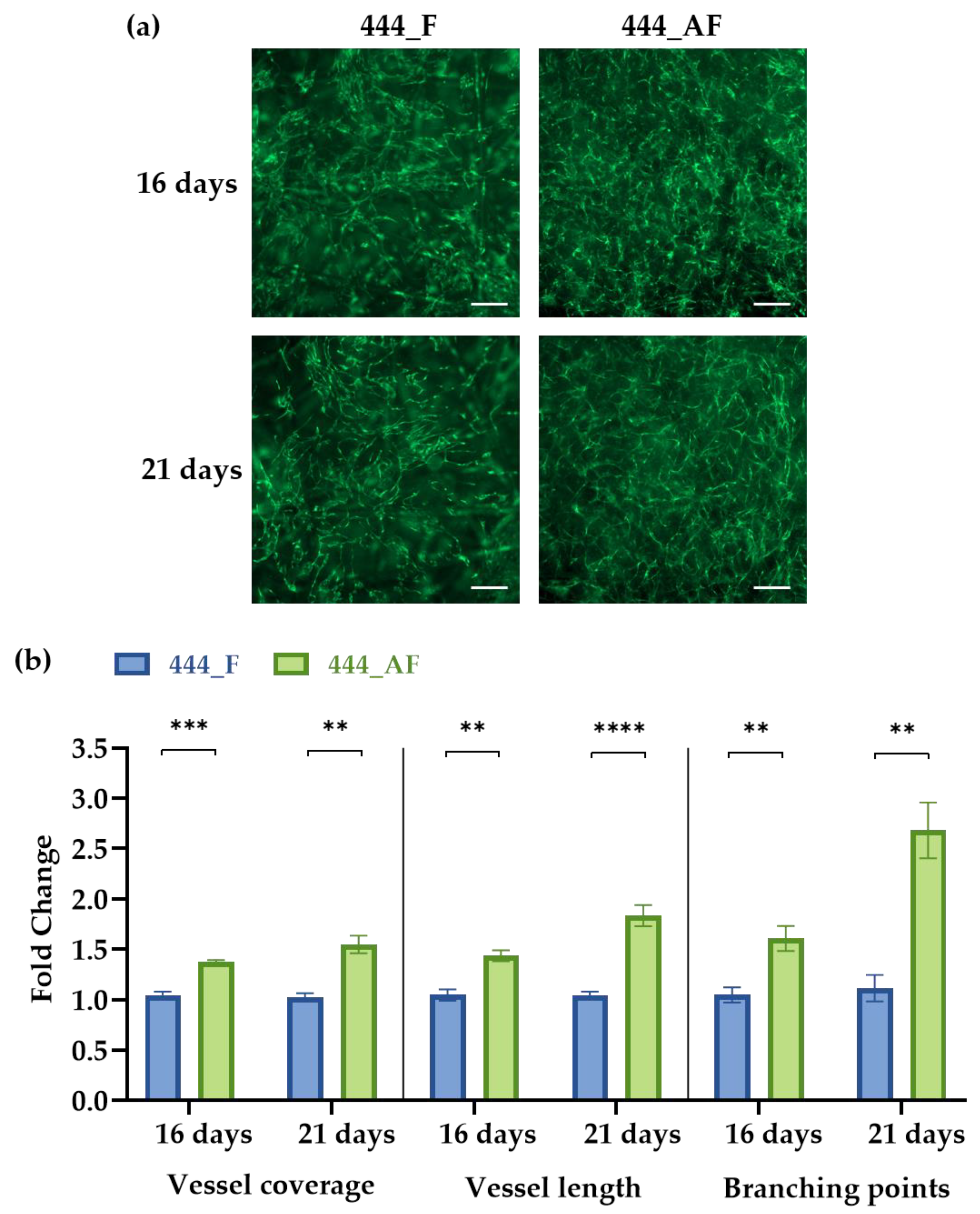
3.4. Vascular Analysis Using AngioTool
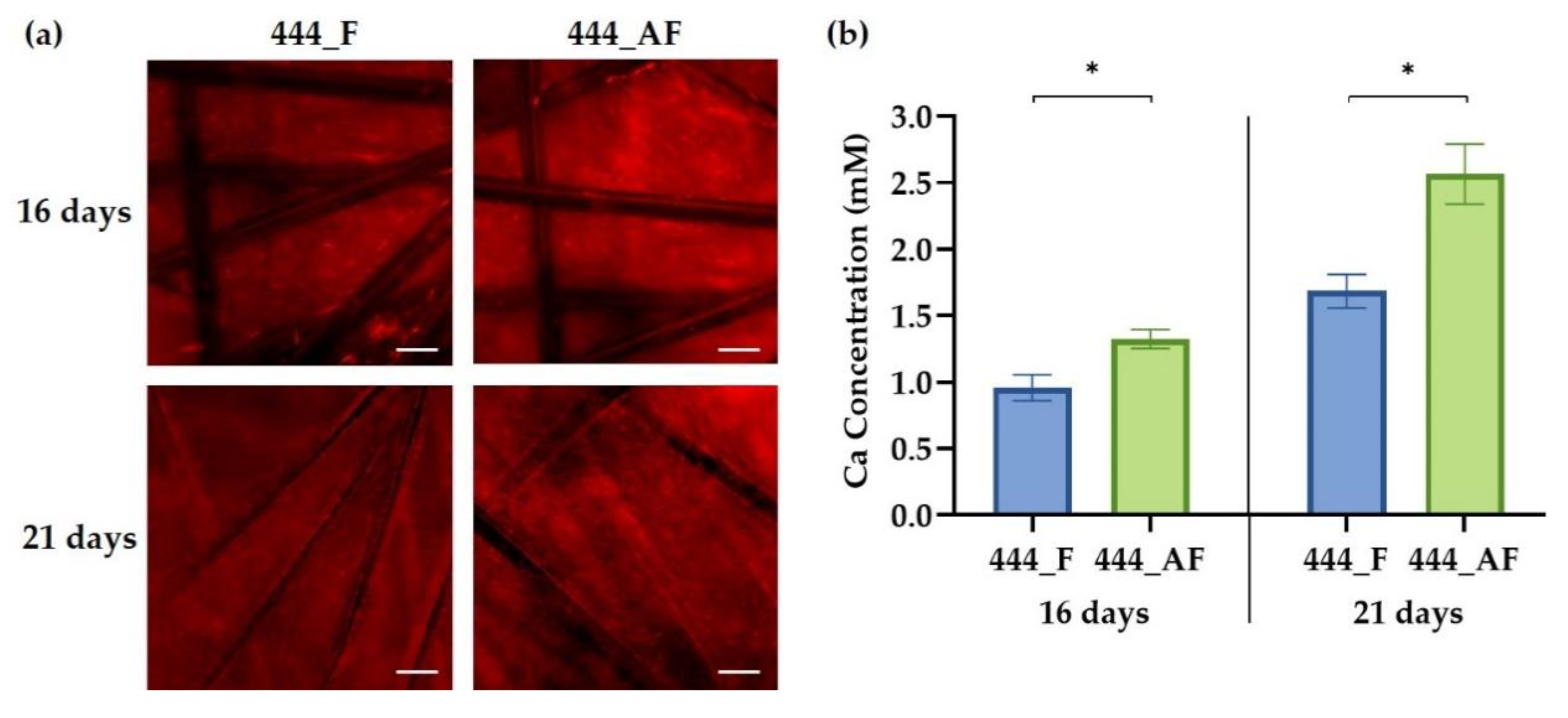
3.5. Cell Mineralisation
3.6. Quantification of Gene Expression Levels
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Isaac, G.; Wilcox, R.; Jones, A.; Thompson, J. Finite element analysis of polyethylene wear in total hip replacement: A literature review. Proc. Inst. Mech. Eng. H 2019, 233, 1067–1088. [Google Scholar] [CrossRef] [PubMed]
- Merola, M.; Affatato, S. Materials for Hip Prostheses: A Review of Wear and Loading Considerations. Materials 2019, 12, 495. [Google Scholar] [CrossRef] [PubMed]
- Bayliss, L.E.; Culliford, D.; Monk, A.P.; Glyn-Jones, S.; Prieto-Alhambra, D.; Judge, A.; Cooper, C.; Carr, A.J.; Arden, N.K.; Beard, D.J. The effect of patient age at intervention on risk of implant revision after total replacement of the hip or knee: A population-based cohort study. Lancet 2017, 389, 1424–1430. [Google Scholar] [CrossRef]
- Wyatt, M.; Hooper, G.; Frampton, C.; Rothwell, A. Survival outcomes of cemented compared to uncemented stems in primary total hip replacement. World J. Orthop. 2014, 5, 591–596. [Google Scholar] [CrossRef]
- Abu-Amer, Y.; Darwech, I.; Clohisy, J.C. Aseptic loosening of total joint replacements: Mechanisms underlying osteolysis and potential therapies. Arthritis Res. Ther. 2007, 9, S6. [Google Scholar] [CrossRef]
- Delaunay, C.; Hamadouche, M.; Girard, J.; Duhamel, A. What are the causes for failures of primary hip arthroplasties in France? Clin. Orthop. Relat. Res. 2013, 471, 3863–3869. [Google Scholar] [CrossRef]
- Phedy, P.; Ismail, H.D.; Hoo, C.; Djaja, Y.P. Total hip replacement: A meta-analysis to evaluate survival of cemented, cementless and hybrid implants. World J. Orthop. 2017, 8, 192. [Google Scholar] [CrossRef]
- Lovett, M.; Lee, K.; Edwards, A.; Kaplan, D.L. Vascularization strategies for tissue engineering. Tissue Eng. Part B Rev. 2009, 15, 353–370. [Google Scholar] [CrossRef]
- Markaki, A.E.; Clyne, T.W. Magneto-mechanical actuation of bonded ferromagnetic fibre arrays. Acta Mater. 2005, 53, 877–889. [Google Scholar] [CrossRef]
- Markaki, A.E.; Clyne, T.W. Magneto-mechanical stimulation of bone growth in a bonded array of ferromagnetic fibres. Biomaterials 2004, 25, 4805–4815. [Google Scholar] [CrossRef]
- Malheiro, V.N.; Skepper, J.N.; Brooks, R.A.; Markaki, A.E. In vitro osteoblast response to ferritic stainless steel fiber networks for magneto-active layers on implants. J. Biomed. Mater. Res. A 2013, 101, 1588–1598. [Google Scholar] [CrossRef] [PubMed]
- Katarivas Levy, G.; Birch, M.A.; Brooks, R.A.; Neelakantan, S.; Markaki, A.E. Stimulation of Human Osteoblast Differentiation in Magneto-Mechanically Actuated Ferromagnetic Fiber Networks. J. Clin. Med. 2019, 8, 1522. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.T.; Yoo, J.J. Implant design in cementless hip arthroplasty. Hip Pelvis 2016, 28, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, X.; Hou, W.; Nune, K.C.; Misra, R.D.K.; Correa-Rodriguez, V.L.; Guo, Z.; Hao, Y.; Yang, R.; Murr, L.E. Fabrication of open-cellular (porous) titanium alloy implants: Osseointegration, vascularization and preliminary human trials. Sci. China Mater. 2018, 61, 525–536. [Google Scholar] [CrossRef]
- Buranawat, B.; Kalia, P.; Di Silvio, L. Vascularisation of tissue-engineered constructs. In Standardisation in Cell and Tissue Engineering: Methods and Protocols; Salih, V., Ed.; Elsevier: Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 77–103. [Google Scholar]
- Chen, W.; Thein-Han, W.; Weir, M.D.; Chen, Q.; Xu, H.H. Prevascularization of biofunctional calcium phosphate cement for dental and craniofacial repairs. Dent. Mater. 2014, 30, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.; Miller, S.; Case, E.; Leach, J.K. Supplementation of fibrin gels with sodium chloride enhances physical properties and ensuing osteogenic response. Acta Biomater. 2011, 7, 691–699. [Google Scholar] [CrossRef]
- Nseir, N.; Regev, O.; Kaully, T.; Blumenthal, J.; Levenberg, S.; Zussman, E. Biodegradable scaffold fabricated of electrospun albumin fibers: Mechanical and biological characterization. Tissue Eng. Part C Methods 2013, 19, 257–264. [Google Scholar] [CrossRef]
- Horváthy, D.B.; Simon, M.; Schwarz, C.M.; Masteling, M.; Vácz, G.; Hornyák, I.; Lacza, Z. Serum albumin as a local therapeutic agent in cell therapy and tissue engineering. Biofactors 2017, 43, 315–330. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Igarashi, A.; Misawa, H.; Tsurusaki, Y. Enhancement of albumin expression in bone tissues with healing rat fractures. J. Cell Biochem. 2003, 89, 356–363. [Google Scholar] [CrossRef]
- Ishida, K.; Sawada, N.; Yamaguchi, M. Expression of albumin in bone tissues and osteoblastic cells: Involvement of hormonal regulation. Int. J. Mol. Med. 2004, 14, 891–895. [Google Scholar] [CrossRef]
- Ishida, K.; Yamaguchi, M. Role of albumin in osteoblastic cells: Enhancement of cell proliferation and suppression of alkaline phosphatase activity. Int. J. Mol. Med. 2004, 14, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- Bernards, M.T.; Qin, C.; Jiang, S. MC3T3-E1 cell adhesion to hydroxyapatite with adsorbed bone sialoprotein, bone osteopontin, and bovine serum albumin. Colloids Surf. B Biointerfaces 2008, 64, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Weszl, M.; Skaliczki, G.; Cselenyák, A.; Kiss, L.; Major, T.; Schandl, K.; Bognár, E.; Stadler, G.; Peterbauer, A.; Csönge, L. Freeze-dried human serum albumin improves the adherence and proliferation of mesenchymal stem cells on mineralized human bone allografts. J. Orthop. Res. 2012, 30, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Horvathy, D.B.; Vacz, G.; Szabó, T.; Szigyarto, I.C.; Toro, I.; Vamos, B.; Hornyák, I.; Renner, K.; Klára, T.; Szabo, B.T. Serum albumin coating of demineralized bone matrix results in stronger new bone formation. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Skaliczki, G.; Schandl, K.; Weszl, M.; Major, T.; Kovács, M.; Skaliczki, J.; Szendrői, M.; Dobó-Nagy, C.; Lacza, Z. Serum albumin enhances bone healing in a nonunion femoral defect model in rats: A computer tomography micromorphometry study. Int. Orthop. 2013, 37, 741–745. [Google Scholar] [CrossRef]
- Klára, T.; Csönge, L.; Janositz, G.; Csernátony, Z.; Lacza, Z. Albumin-coated structural lyophilized bone allografts: A clinical report of 10 cases. Cell Tissue Bank. 2014, 15, 89–97. [Google Scholar] [CrossRef]
- Schandl, K.; Horváthy, D.B.; Doros, A.; Majzik, E.; Schwarz, C.M.; Csönge, L.; Abkarovits, G.; Bucsi, L.; Lacza, Z. Bone-Albumin filling decreases donor site morbidity and enhances bone formation after anterior cruciate ligament reconstruction with bone-patellar tendon-bone autografts. Int. Orthop. 2016, 40, 2097–2104. [Google Scholar] [CrossRef]
- Ong, J.; Zhao, J.; Justin, A.W.; Markaki, A.E. Albumin-based hydrogels for regenerative engineering and cell transplantation. Biotechnol. Bioeng. 2019. [Google Scholar] [CrossRef]
- Noori, A.; Ashrafi, S.J.; Vaez-Ghaemi, R.; Hatamian-Zaremi, A.; Webster, T.J. A review of fibrin and fibrin composites for bone tissue engineering. Int. J. Nanomed. 2017, 12, 4937. [Google Scholar] [CrossRef]
- Jockenhoevel, S.; Flanagan, T.C. Cardiovascular tissue engineering based on fibrin-gel-scaffolds. In Tissue Engineering for Tissue and Organ Regeneration; Eberli, D., Ed.; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar]
- Lesman, A.; Koffler, J.; Atlas, R.; Blinder, Y.J.; Kam, Z.; Levenberg, S. Engineering vessel-like networks within multicellular fibrin-based constructs. Biomaterials 2011, 32, 7856–7869. [Google Scholar] [CrossRef]
- Janmey, P.A.; Winer, J.P.; Weisel, J.W. Fibrin gels and their clinical and bioengineering applications. J. R. Soc. Interface 2009, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Weisel, J.W. Fibrinogen and fibrin. In Advances in Protein Chemistry; Academic Press: New York, NY, USA, 2005; Volume 70, pp. 247–299. [Google Scholar]
- Spear, R.L.; Brooks, R.A.; Markaki, A.E. Short-term in vitro responses of human peripheral blood monocytes to ferritic stainless steel fiber networks. J. Biomed. Mater. Res. A 2013, 101, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Neelakantan, S.; Bosbach, W.; Woodhouse, J.; Markaki, A.E. Characterization and deformation response of orthotropic fibre networks with auxetic out-of-plane behaviour. Acta Mater. 2014, 66, 326–339. [Google Scholar] [CrossRef]
- Tsarouchas, D.; Markaki, A.E. Extraction of fibre network architecture by X-ray tomography and prediction of elastic properties using an affine analytical model. Acta Mater. 2011, 59, 6989–7002. [Google Scholar] [CrossRef]
- Torbet, J. Fibrin assembly in human plasma and fibrinogen/albumin mixtures. Biochemistry 1986, 25, 5309–5314. [Google Scholar] [CrossRef]
- Langenbach, F.; Handschel, J. Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res. Ther. 2013, 4, 117. [Google Scholar] [CrossRef]
- Hotaling, N.A.; Bharti, K.; Kriel, H.; Simon, C.G., Jr. DiameterJ: A validated open source nanofiber diameter measurement tool. Biomaterials 2015, 61, 327–338. [Google Scholar] [CrossRef]
- Varley, M.C.; Neelakantan, S.; Clyne, T.W.; Dean, J.; Brooks, R.A.; Markaki, A.E. Cell structure, stiffness and permeability of freeze-dried collagen scaffolds in dry and hydrated states. Acta Biomater. 2016, 33, 166–175. [Google Scholar] [CrossRef]
- Wu, Y.; Al-Ameen, M.A.; Ghosh, G. Integrated effects of matrix mechanics and vascular endothelial growth factor (VEGF) on capillary sprouting. Ann. Biomed. Eng. 2014, 42, 1024–1036. [Google Scholar] [CrossRef]
- Zudaire, E.; Gambardella, L.; Kurcz, C.; Vermeren, S. A computational tool for quantitative analysis of vascular networks. PLoS ONE 2011, 6, e27385. [Google Scholar] [CrossRef]
- Gregory, C.A.; Gunn, W.G.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Nair, C.; Dhall, D. Studies on fibrin network structure: The effect of some plasma proteins. Thromb. Res. 1991, 61, 315–325. [Google Scholar] [CrossRef]
- Galanakis, D.K.; Lane, B.P.; Simon, S.R. Albumin modulates lateral assembly of fibrin polymers: Evidence of enhanced fine fibril formation and of unique synergism with fibrinogen. Biochemistry 1987, 26, 2389–2400. [Google Scholar] [CrossRef]
- Marx, G.; Harari, N. Albumin indirectly modulates fibrin and protofibrin ultrastructure. Biochemistry 1989, 28, 8242–8248. [Google Scholar] [CrossRef]
- Chiu, C.L.; Hecht, V.; Duong, H.; Wu, B.; Tawil, B. Permeability of three-dimensional fibrin constructs corresponds to fibrinogen and thrombin concentrations. Biores. Open Access 2012, 1, 34–40. [Google Scholar] [CrossRef]
- Kim, O.V.; Xu, Z.; Rosen, E.D.; Alber, M.S. Fibrin networks regulate protein transport during thrombus development. PLoS Comput. Biol. 2013, 9, e1003095. [Google Scholar] [CrossRef]
- Tan, A.W.; Liau, L.L.; Chua, K.H.; Ahmad, R.; Akbar, S.A.; Pingguan-Murphy, B. Enhanced in vitro angiogenic behaviour of human umbilical vein endothelial cells on thermally oxidized TiO2 nanofibrous surfaces. Sci. Rep. 2016, 6, 21828. [Google Scholar] [CrossRef]
- Kaijzel, E.; Koolwijk, P.; Van Erck, M.; Van Hinsbergh, V.; De Maat, M. Molecular weight fibrinogen variants determine angiogenesis rate in a fibrin matrix in vitro and in vivo. J. Thromb. Haemost. 2006, 4, 1975–1981. [Google Scholar] [CrossRef]
- Mitsak, A.G.; Kemppainen, J.M.; Harris, M.T.; Hollister, S.J. Effect of polycaprolactone scaffold permeability on bone regeneration in vivo. Tissue Eng. Part A 2011, 17, 1831–1839. [Google Scholar] [CrossRef]
- Chen, W.; Liu, X.; Chen, Q.; Bao, C.; Zhao, L.; Zhu, Z.; Xu, H.H. Angiogenic and osteogenic regeneration in rats via calcium phosphate scaffold and endothelial cell co-culture with human bone marrow mesenchymal stem cells (MSCs), human umbilical cord MSCs, human induced pluripotent stem cell-derived MSCs and human embryonic stem cell-derived MSCs. J. Tissue Eng. Regen. Med. 2018, 12, 191–203. [Google Scholar] [PubMed]
- Blair, H.C.; Larrouture, Q.C.; Li, Y.; Lin, H.; Beer-Stoltz, D.; Liu, L.; Tuan, R.S.; Robinson, L.J.; Schlesinger, P.H.; Nelson, D.J. Osteoblast differentiation and bone matrix formation in vivo and in vitro. Tissue Eng. Part B Rev. 2017, 23, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Rutkovskiy, A.; Stensløkken, K.-O.; Vaage, I.J. Osteoblast differentiation at a glance. Med. Sci. Monit. Basic Res. 2016, 22, 95. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Suzawa, M.; Kikuchi, T.; Matsumoto, T. Interaction of matrix collagen with osteoblastic cells enhances stimulatory effects of bone morphogenetic protein (BMP)-2 on the differentiation of osteoblasts. Bone 1995, 6, 568. [Google Scholar] [CrossRef]
- Samee, M.; Kasugai, S.; Kondo, H.; Ohya, K.; Shimokawa, H.; Kuroda, S. Bone morphogenetic protein-2 (BMP-2) and vascular endothelial growth factor (VEGF) transfection to human periosteal cells enhances osteoblast differentiation and bone formation. J. Pharm. Sci. 2008, 108, 18–31. [Google Scholar] [CrossRef]
- Landau, S.; Ben-Shaul, S.; Levenberg, S. Oscillatory Strain Promotes Vessel Stabilization and Alignment through Fibroblast YAP-Mediated Mechanosensitivity. Adv. Sci. 2018, 5, 1800506. [Google Scholar] [CrossRef]
- Landau, S.; Szklanny, A.A.; Yeo, G.C.; Shandalov, Y.; Kosobrodova, E.; Weiss, A.S.; Levenberg, S. Tropoelastin coated PLLA-PLGA scaffolds promote vascular network formation. Biomaterials 2017, 122, 72–82. [Google Scholar] [CrossRef]
- Pedersen, T.O.; Blois, A.L.; Xue, Y.; Xing, Z.; Cottler-Fox, M.; Fristad, I.; Leknes, K.N.; Lorens, J.B.; Mustafa, K. Osteogenic stimulatory conditions enhance growth and maturation of endothelial cell microvascular networks in culture with mesenchymal stem cells. J. Tissue Eng. 2012, 3, 2041731412443236. [Google Scholar] [CrossRef]

| Gene | Oligo Name | Primer Sequence |
|---|---|---|
| GAPDH | hum_GAPDH_5/3 | 5: CTCTGCTCCTCCTGTTCGACA 3: ACGACCAAATCCGTTGACTC |
| ALP | hum_ALP_5/3 | 5: CCCAAAGGCTTCTTCTTG 3: CTGGTAGTTGTTGTGAGCAT |
| OCN | hum_OCN_5/3 | 5: GACTGTGACGAGTTGGCTGA 3: CTGGAGAGGAGCAGAACTGG |
| COL1A1 | hum_COL1A1_5/3 | 5: ATGCCTGGTGAACGTGGT 3: AGGAGAGCCATCAGCACCT |
| vWF | hum_vWF_5/3 | 5: CGGCTTGCACCATTCAGCTA 3: TGCAGAAGTGAGTATCACAGCCATC |
| VEGFA | HM_VEGFA_SLFW_Fwd/Rev | F: GAGCCTTGCCTTGCTGCTCTAC R: CACCAGGGTCTCGATTGGATG |
| ANGPT1 | HM_ANGPT1_SLFW_Fwd/Rev | F: CCTGATCTTACACGGTGCTGATT R: GTCCCGCAGTATAGAACATTCCA |
| ANGPT2 | HM_ANGPT2_SLFW_Fwd/Rev | F: AAGAGATCAAGGCCTACTGTGACA R: TCCTCACGTCGCTGAATAATTG |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katarivas Levy, G.; Ong, J.; Birch, M.A.; Justin, A.W.; Markaki, A.E. Albumin-Enriched Fibrin Hydrogel Embedded in Active Ferromagnetic Networks Improves Osteoblast Differentiation and Vascular Self-Organisation. Polymers 2019, 11, 1743. https://doi.org/10.3390/polym11111743
Katarivas Levy G, Ong J, Birch MA, Justin AW, Markaki AE. Albumin-Enriched Fibrin Hydrogel Embedded in Active Ferromagnetic Networks Improves Osteoblast Differentiation and Vascular Self-Organisation. Polymers. 2019; 11(11):1743. https://doi.org/10.3390/polym11111743
Chicago/Turabian StyleKatarivas Levy, Galit, John Ong, Mark A. Birch, Alexander W. Justin, and Athina E. Markaki. 2019. "Albumin-Enriched Fibrin Hydrogel Embedded in Active Ferromagnetic Networks Improves Osteoblast Differentiation and Vascular Self-Organisation" Polymers 11, no. 11: 1743. https://doi.org/10.3390/polym11111743
APA StyleKatarivas Levy, G., Ong, J., Birch, M. A., Justin, A. W., & Markaki, A. E. (2019). Albumin-Enriched Fibrin Hydrogel Embedded in Active Ferromagnetic Networks Improves Osteoblast Differentiation and Vascular Self-Organisation. Polymers, 11(11), 1743. https://doi.org/10.3390/polym11111743








