Simple Route to Synthesize Fully Conjugated Ladder Isomer Copolymers with Carbazole Units
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. General Synthesis of Conjugated Ladder Polymers P3 and P6
3. Results
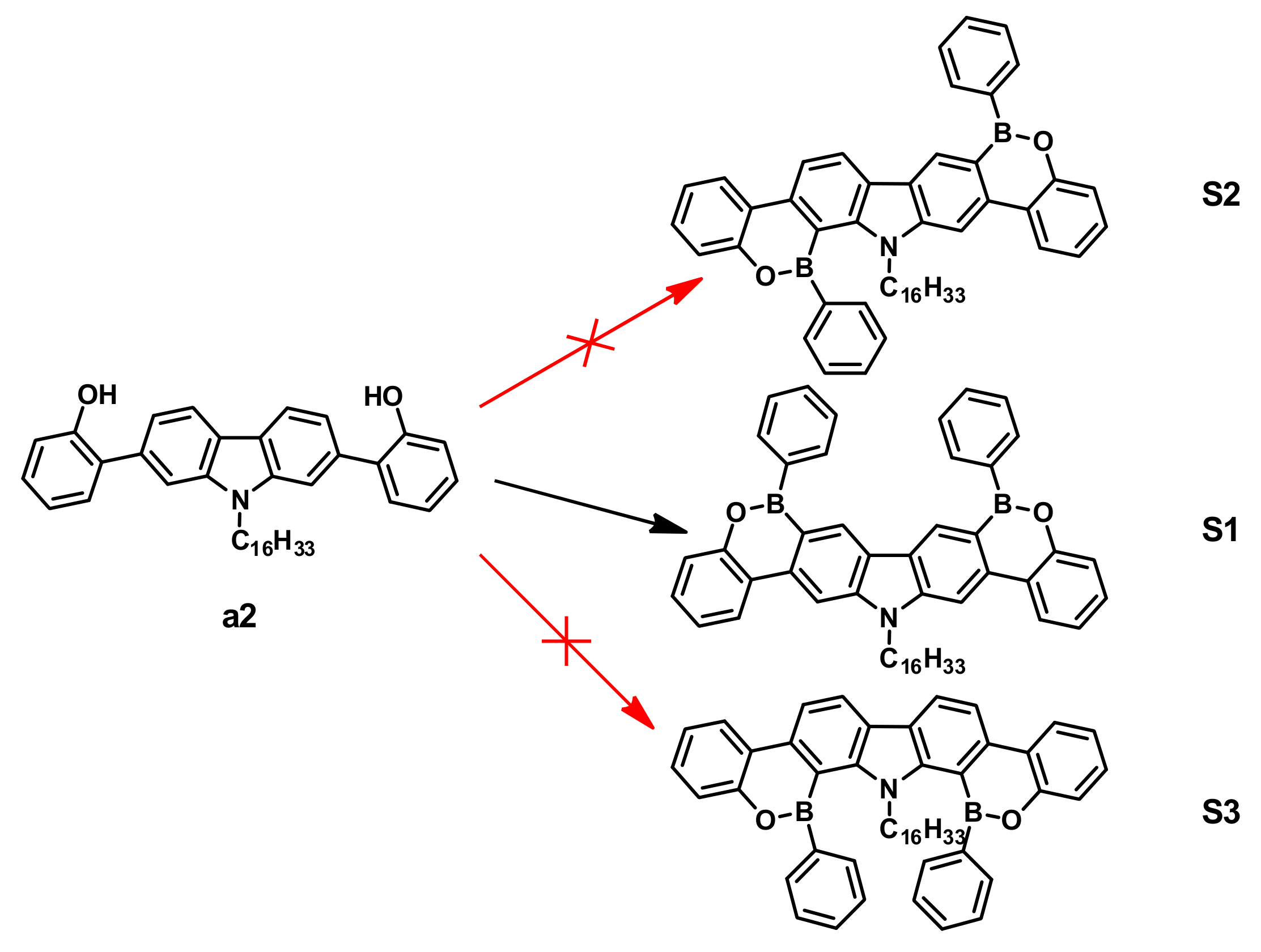
3.1. Synthetic Route Discussion
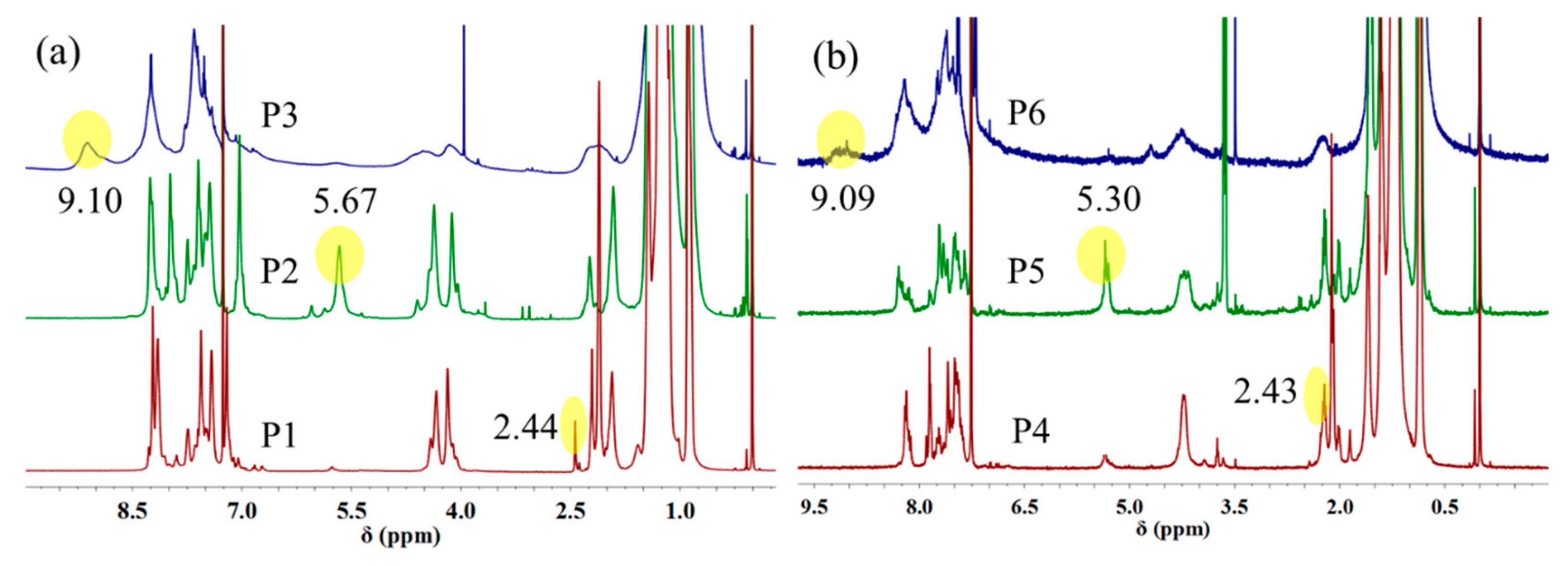
3.2. NMR Spectra of Polymers
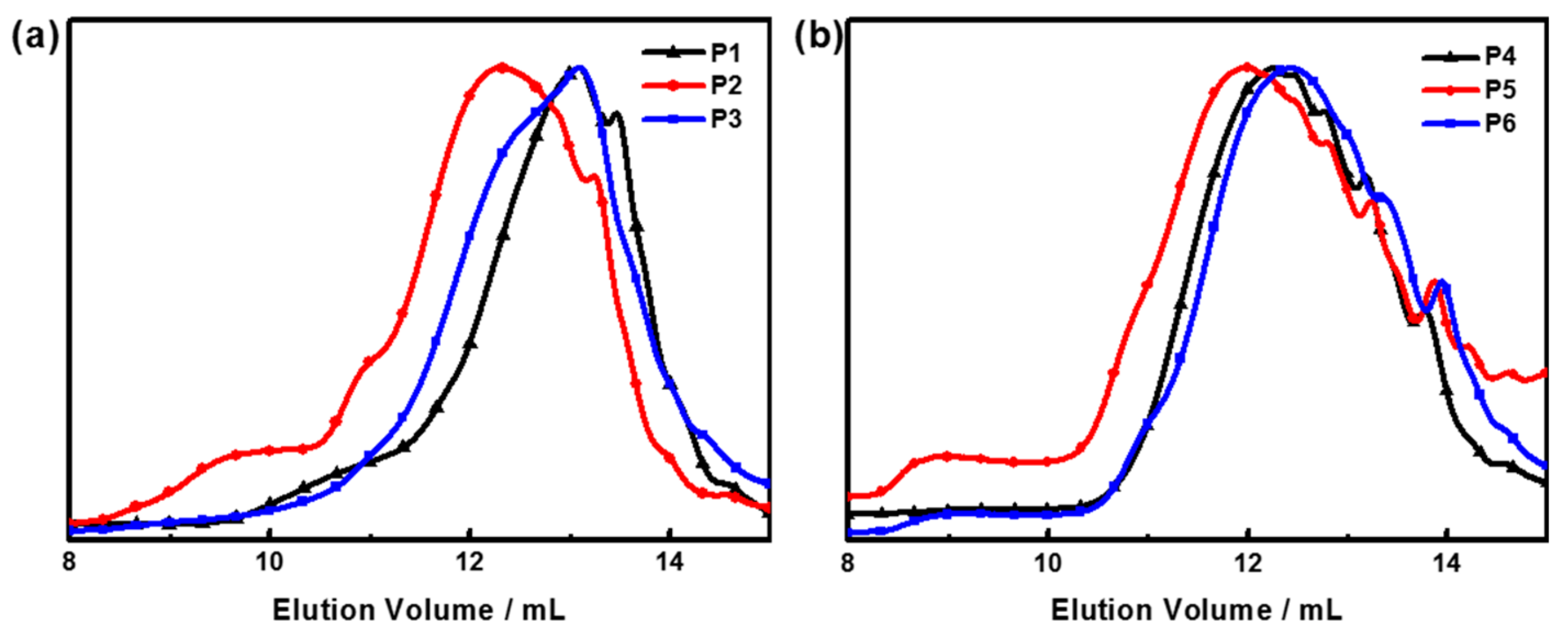
3.3. Size-Exclusion Chromatography (SEC) Trace for Polymers
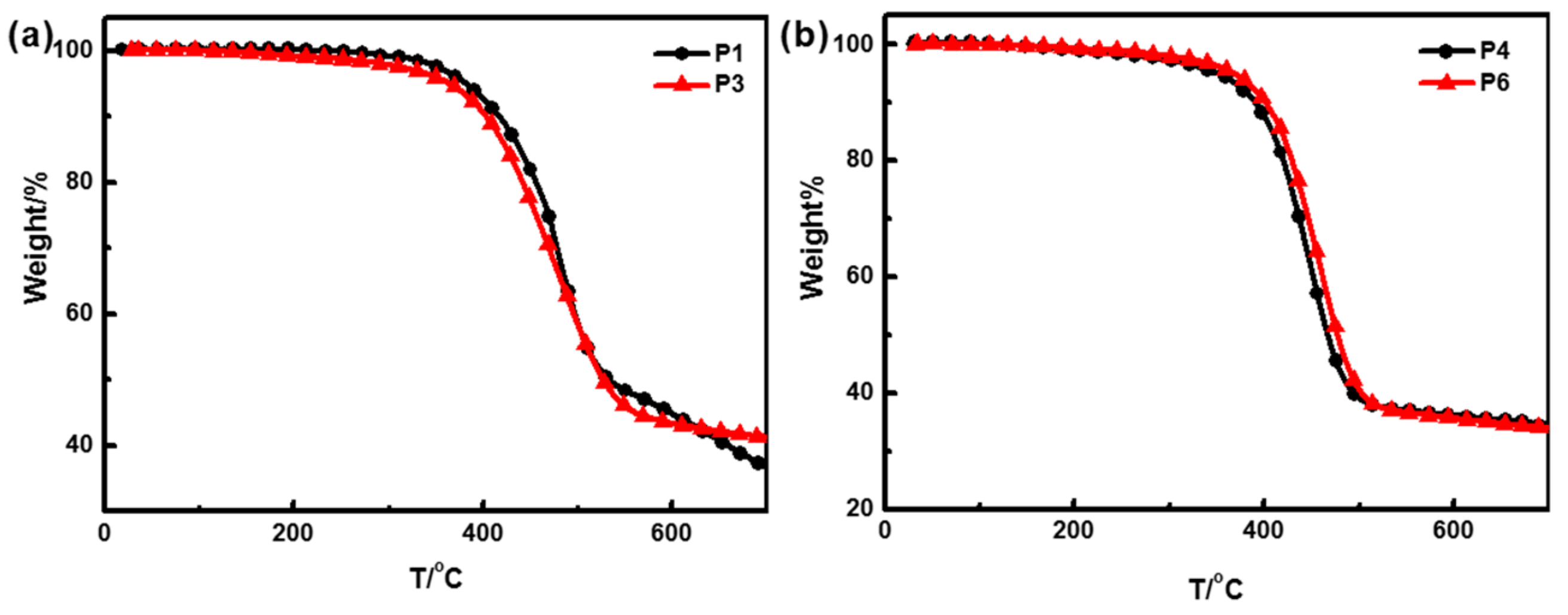
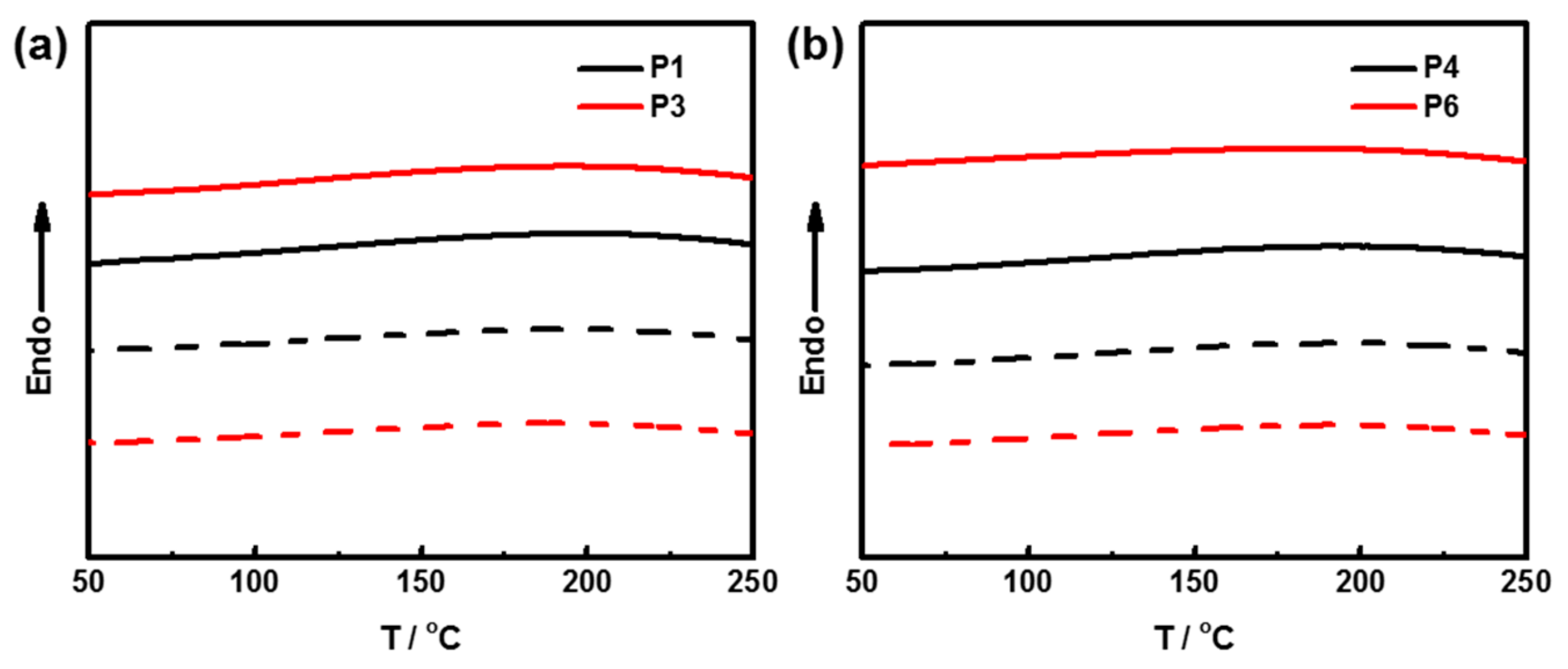
3.4. Thermal Properties of Polymers
3.5. FTIR Spectra of Polymers
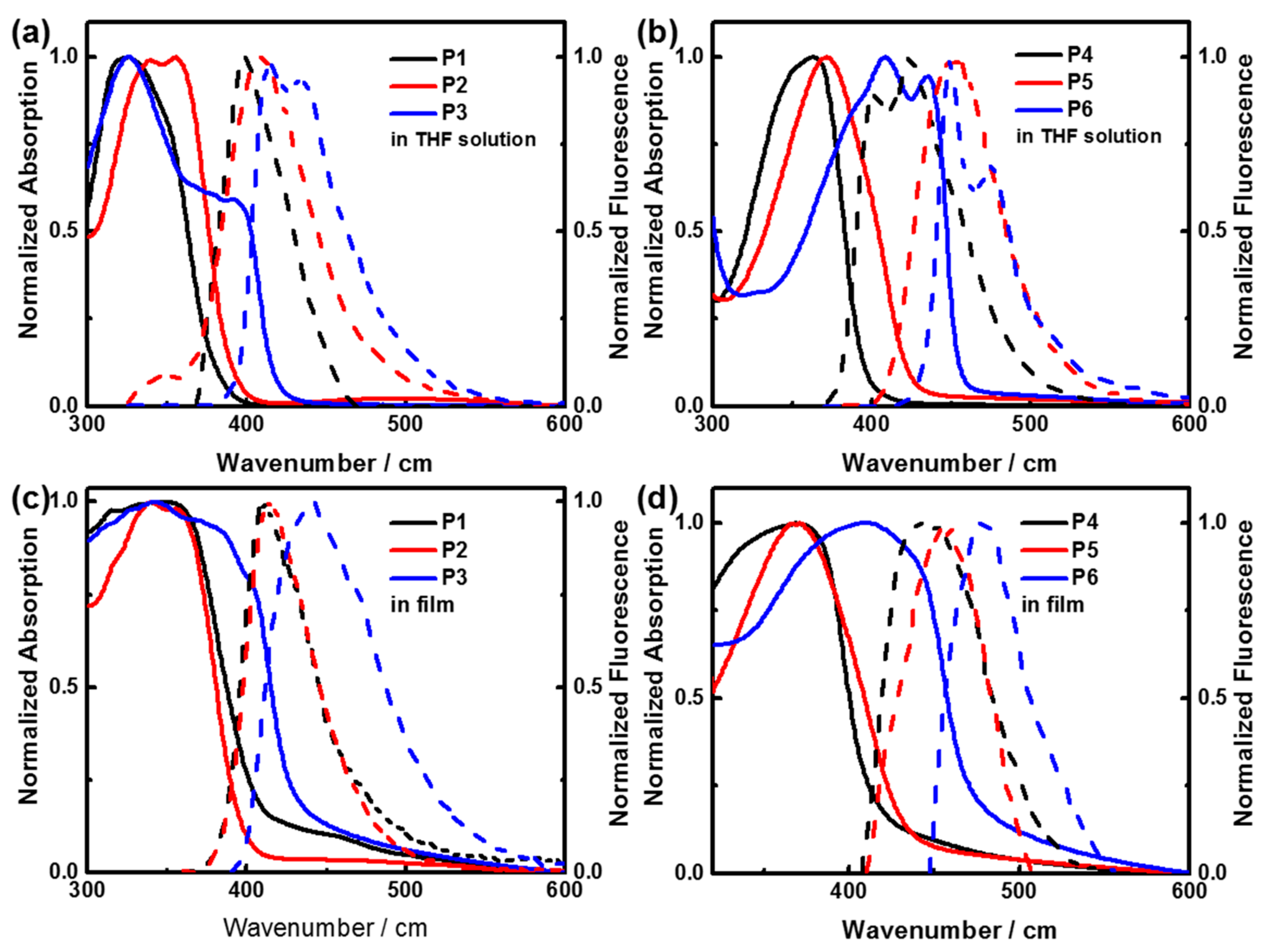
3.6. Photophysical Properties of Polymers
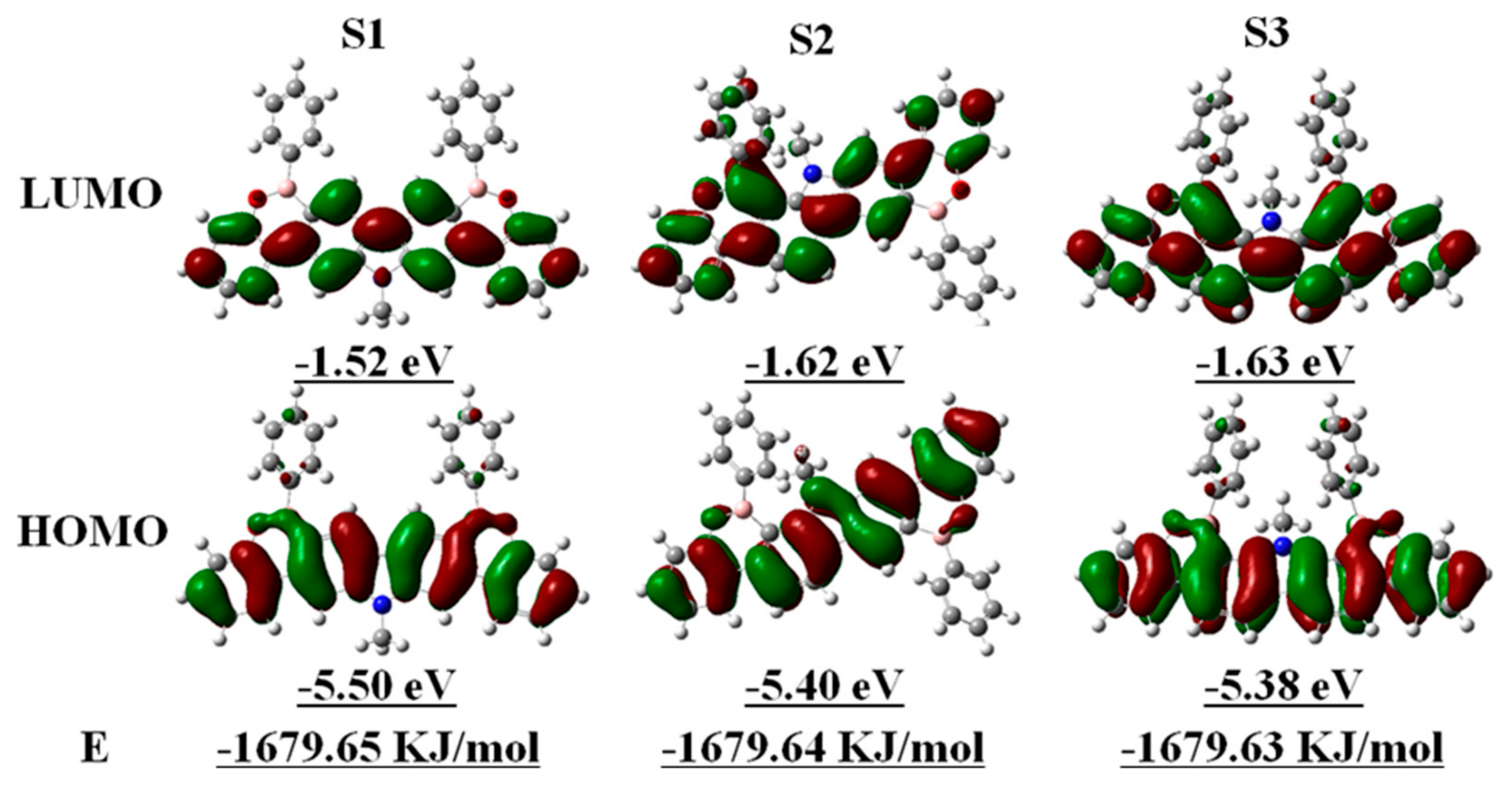
3.7. Electrochemical Properties of Polymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Trilling, F.; Ausländer, M.-K.; Scherf, U. Ladder-Type Polymers and Ladder-Type Polyelectrolytes with On-Chain Dibenz [a,h] anthracene Chromophores. Macromolecules 2019, 52, 3115–3122. [Google Scholar] [CrossRef]
- Takagi, K.; Tanaka, H.; Mikami, K. Ladderization of polystyrene derivatives by palladium-catalyzed polymer direct arylation. Polym. Chem. 2019, 10, 2647–2652. [Google Scholar] [CrossRef]
- Zhu, C.; Kalin, A.J.; Fang, L. Covalent and Noncovalent Approaches to Rigid Coplanar π-Conjugated Molecules and Macromolecules. Acc. Chem. Res. 2019, 52, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Chen, M.; Dalton, L.R. Ladder polymers: Recent developments in syntheses, characterization, and potential applications as electronic and optical materials. Chem. Mater. 1990, 2, 649–659. [Google Scholar] [CrossRef]
- Teo, Y.C.; Lai, H.W.; Xia, Y. Synthesis of Ladder Polymers: Developments, Challenges, and Opportunities. Chem. A Eur. J. 2017, 23, 14101–14112. [Google Scholar] [CrossRef]
- Congzhi, Z.; Lei, F. Locking the Coplanar Conformation of π-Conjugated Molecules and Macromolecules Using Dynamic Noncovalent Bonds. Macromol. Rapid Commun. 2018, 39, 1700241. [Google Scholar] [CrossRef]
- Ren, Y.; Baumgartner, T. Ladder-Type π-Conjugated 4-Hetero-1,4-dihydrophosphinines: A Structure–Property Study. Chem. A Eur. J. 2010, 5, 1918–1929. [Google Scholar] [CrossRef]
- Zheng, T.; Cai, Z.; Ho, W.R.; Yau, S.H.; Sharapov, V.; Goodson, I.T.; Yu, L. Synthesis of Ladder-Type Thienoacenes and Their Electronic and Optical Properties. J. Am. Chem. Soc. 2016, 138, 868–875. [Google Scholar] [CrossRef]
- Alahmadi, A.F.; Lalancette, R.A.; Jäkle, F. Highly Luminescent Ladderized Fluorene Copolymers Based on B–N Lewis Pair Functionalization. Macromol. Rapid Commun. 2018, 39, 1800456. [Google Scholar] [CrossRef]
- Liu, F.; Wu, Y.; Wang, C.; Ma, J.; Wu, F.; Zhang, Y.; Ba, X. Synthesis and Characterization of Fully Conjugated Ladder Naphthalene Bisimide Copolymers. Polymers 2018, 10, 790. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, J.; Fei, Z.; Bo, Z. Spiro-bridged ladder-type poly(p-phenylene)s: Towards structurally perfect light-emitting materials. J. Am. Chem. Soc. 2008, 130, 7192–7193. [Google Scholar] [CrossRef] [PubMed]
- Babel, A.; Jenekhe, S.A. High Electron Mobility in Ladder Polymer Field-Effect Transistors. J. Am. Chem. Soc. 2003, 125, 13656–13657. [Google Scholar] [CrossRef] [PubMed]
- Durban, M.M.; Kazarinoff, P.D.; Segawa, Y.; Luscombe, C.K. Synthesis and Characterization of Solution-Processable Ladderized n-Type Naphthalene Bisimide Copolymers for OFET Applications. Macromolecules 2011, 44, 4721–4728. [Google Scholar] [CrossRef]
- Cai, Z.; Awais, M.A.; Zhang, N.; Yu, L. Exploration of Syntheses and Functions of Higher Ladder-type π-Conjugated Heteroacenes. Chem 2018, 4, 2538–2570. [Google Scholar] [CrossRef]
- Sun, H.; Vagin, M.; Wang, S.; Crispin, X.; Forchheimer, R.; Berggren, M.; Fabiano, S. Complementary Logic Circuits Based on High-Performance n-Type Organic Electrochemical Transistors. Adv. Mater. 2018, 30, 1704916. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, H.; Harbuzaru, A.; Uddin, M.A.; Arrechea-Marcos, I.; Ling, S.; Yu, J.; Tang, Y.; Sun, H.; López Navarrete, J.T.; et al. (Semi)ladder-Type Bithiophene Imide-Based All-Acceptor Semiconductors: Synthesis, Structure–Property Correlations, and Unipolar n-Type Transistor Performance. J. Am. Chem. Soc. 2018, 140, 6095–6108. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Rajeeva, B.B.; Yuan, T.; Guo, Z.-H.; Lin, Y.-H.; Al-Hashimi, M.; Zheng, Y.; Fang, L. Thermodynamic synthesis of solution processable ladder polymers. Chem. Sci. 2016, 7, 881–889. [Google Scholar] [CrossRef]
- Zeng, W.; Gopalakrishna, T.Y.; Phan, H.; Tanaka, T.; Herng, T.S.; Ding, J.; Osuka, A.; Wu, J. Superoctazethrene: An Open-Shell Graphene-like Molecule Possessing Large Diradical Character but Still with Reasonable Stability. J. Am. Chem. Soc. 2018, 140, 14054–14058. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, W.; Ito, H.; Itami, K. Rapid Access to Nanographenes and Fused Heteroaromatics by Palladium-Catalyzed Annulative π-Extension Reaction of Unfunctionalized Aromatics with Diiodobiaryls. Angew. Chem. Int. Ed. 2017, 129, 12392–12396. [Google Scholar] [CrossRef]
- Koga, Y.; Kaneda, T.; Saito, Y.; Murakami, K.; Itami, K. Synthesis of partially and fully fused polyaromatics by annulative chlorophenylene dimerization. Science 2018, 359, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhang, H.; Song, M.; Cheng, C.; Zhao, H.; Wu, Y. One-Step Route to Ladder-Type C–N Linked Conjugated Polymers. Macromol. Chem. Phys. 2019, 220, 1900044. [Google Scholar] [CrossRef]
- Curiel, D.; Más-Montoya, M.; Usea, L.; Espinosa, A.; Orenes, R.A.; Molina, P. Indolocarbazole-based ligands for ladder-type four-coordinate boron complexes. Org. Lett. 2012, 14, 3360–3363. [Google Scholar] [CrossRef] [PubMed]
- Wakim, S.; Bouchard, J.; Blouin, N.; Michaud, A.; Leclerc, M. Synthesis of Diindolocarbazoles by Ullmann Reaction: A Rapid Route to Ladder Oligo(p-aniline)s. Org. Lett. 2004, 6, 3413–3416. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, A.; Yamada, H.; Yamaguchi, S. Phosphonium- and borate-bridged zwitterionic ladder stilbene and its extended analogues. Angew. Chem. Int. Ed. 2008, 47, 5582–5585. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Kadowaki, S.; Goya, T.; Murakami, M. Synthesis of Silafluorenes by Iridium-Catalyzed [2 + 2 + 2] Cycloaddition of Silicon-Bridged Diynes with Alkynes. Org. Lett. 2007, 9, 133–136. [Google Scholar] [CrossRef]
- Zhu, C.; Ji, X.; You, D.; Chen, T.L.; Mu, A.U.; Barker, K.P.; Klivansky, L.M.; Liu, Y.; Fang, L. Extraordinary Redox Activities in Ladder-Type Conjugated Molecules Enabled by B←N Coordination-Promoted Delocalization and Hyperconjugation. J. Am. Chem. Soc. 2018, 140, 18173–18182. [Google Scholar] [CrossRef] [PubMed]
- Tasior, M.; Gryko, D.T. Synthesis and Properties of Ladder-Type BN-Heteroacenes and Diazabenzoindoles Built on a Pyrrolopyrrole Scaffold. J. Org. Chem. 2016, 81, 6580–6586. [Google Scholar] [CrossRef]
- Pais, V.F.; Alcaide, M.M.; López-Rodríguez, R.; Collado, D.; Nájera, F.; Pérez-Inestrosa, E.; Álvarez, E.; Lassaletta, J.M.; Fernández, R.; Ros, A. Strongly Emissive and Photostable Four-Coordinate Organoboron N,C Chelates and Their Use in Fluorescence Microscopy. Chem. A Eur. J. 2015, 21, 15369–15376. [Google Scholar] [CrossRef]
- Bosdet, M.J.D.; Jaska, C.A.; Piers, W.E.; And, T.S.S.; Parvez, M. Blue Fluorescent 4a-Aza-4b-boraphenanthrenes. Chem. A Eur. J. 2007, 9, 1395–1398. [Google Scholar] [CrossRef]
- Long, X.; Ding, Z.; Dou, C.; Zhang, J.; Liu, J.; Wang, L. Polymer Acceptor Based on Double B←N Bridged Bipyridine (BNBP) Unit for High-Efficiency All-Polymer Solar Cells. Adv. Mater. 2016, 28, 6504–6508. [Google Scholar] [CrossRef]
- Zhu, C.; Guo, Z.-H.; Mu, A.U.; Liu, Y.; Wheeler, S.E.; Fang, L. Low Band Gap Coplanar Conjugated Molecules Featuring Dynamic Intramolecular Lewis Acid–Base Coordination. J. Org. Chem. 2016, 81, 4347–4352. [Google Scholar] [CrossRef] [PubMed]
- Pais, V.F.; Ramírezlópez, P.; Romeroarenas, A.; Collado, D.; Nájera, F.; Pérezinestrosa, E.; Fernández, R.; Lassaletta, J.M.; Ros, A.; Pischel, U. Red-Emitting Tetracoordinate Organoboron Chelates: Synthesis, Photophysical Properties, and Fluorescence Microscopy. J. Org. Chem. 2016, 81, 9605–9611. [Google Scholar] [CrossRef] [PubMed]
- Shimogawa, H.; Yoshikawa, O.; Aramaki, Y.; Murata, M.; Wakamiya, A.; Murata, Y. 4,7-Bis [3-(dimesitylboryl)thien-2-yl] benzothiadiazole: Solvato-, Thermo-, and Mechanochromism Based on the Reversible Formation of an Intramolecular B-N Bond. Chem. A Eur. J. 2017, 23, 3784–3791. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, F.; Liu, J.; Tang, R.; Fu, Y.; Wu, D.; Xu, Q.; Zhuang, X.; He, G.; Feng, X. Ladder-Type BN-Embedded Heteroacenes with Blue Emission. Org. Lett. 2013, 15, 5714–5717. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, H.; Dong, J.; Wu, Y.; Li, W. Highly Efficient Synthesis of a Ladder-Type BN-Heteroacene and Polyheteroacene. Asian J. Org. Chem. 2018, 7, 465–470. [Google Scholar] [CrossRef]
- Shen, X.; Wu, Y.; Bai, L.; Zhao, H.; Ba, X. Microwave-assisted synthesis of 4,9-linked pyrene-based ladder conjugated polymers. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1285–1288. [Google Scholar] [CrossRef]
- Cheng, J.; Li, B.; Ren, X.; Liu, F.; Zhao, H.; Wang, H.; Wu, Y.; Chen, W.; Ba, X. Highly twisted ladder-type backbone bearing perylene diimides for non-fullerene acceptors in organic solar cells. Dye. Pigment. 2019, 161, 221–226. [Google Scholar] [CrossRef]
- Kakkar, R.; Pathak, M.; Radhika, N.P. A DFT study of the structures of pyruvic acid isomers and their decarboxylation. Org. Biomol. Chem. 2006, 4, 886–895. [Google Scholar] [CrossRef]
- Ghiasi, R.; Ara, T.J.; Hakimyoun, A.H. Spectroscopic studies and molecular orbital analysis on platinanaphthalenes and ring-fused B-N platinanaphthalenes. Russ. J. Phys. Chem. A 2014, 88, 616–624. [Google Scholar] [CrossRef]
- Türker, L. A DFT study on TNGU isomers and aluminized cis-TNGU composites. Def. Technol. 2018, 14, 109–118. [Google Scholar] [CrossRef]
- Liu, D.; Yu, B.; Su, X.; Wang, X.; Zhang, Y.-M.; Li, M.; Zhang, S.X.-A. Photo-/Baso-Chromisms and the Application of a Dual-Addressable Molecular Switch. Chem. Asian J. 2019, 14, 2838–2845. [Google Scholar] [CrossRef] [PubMed]


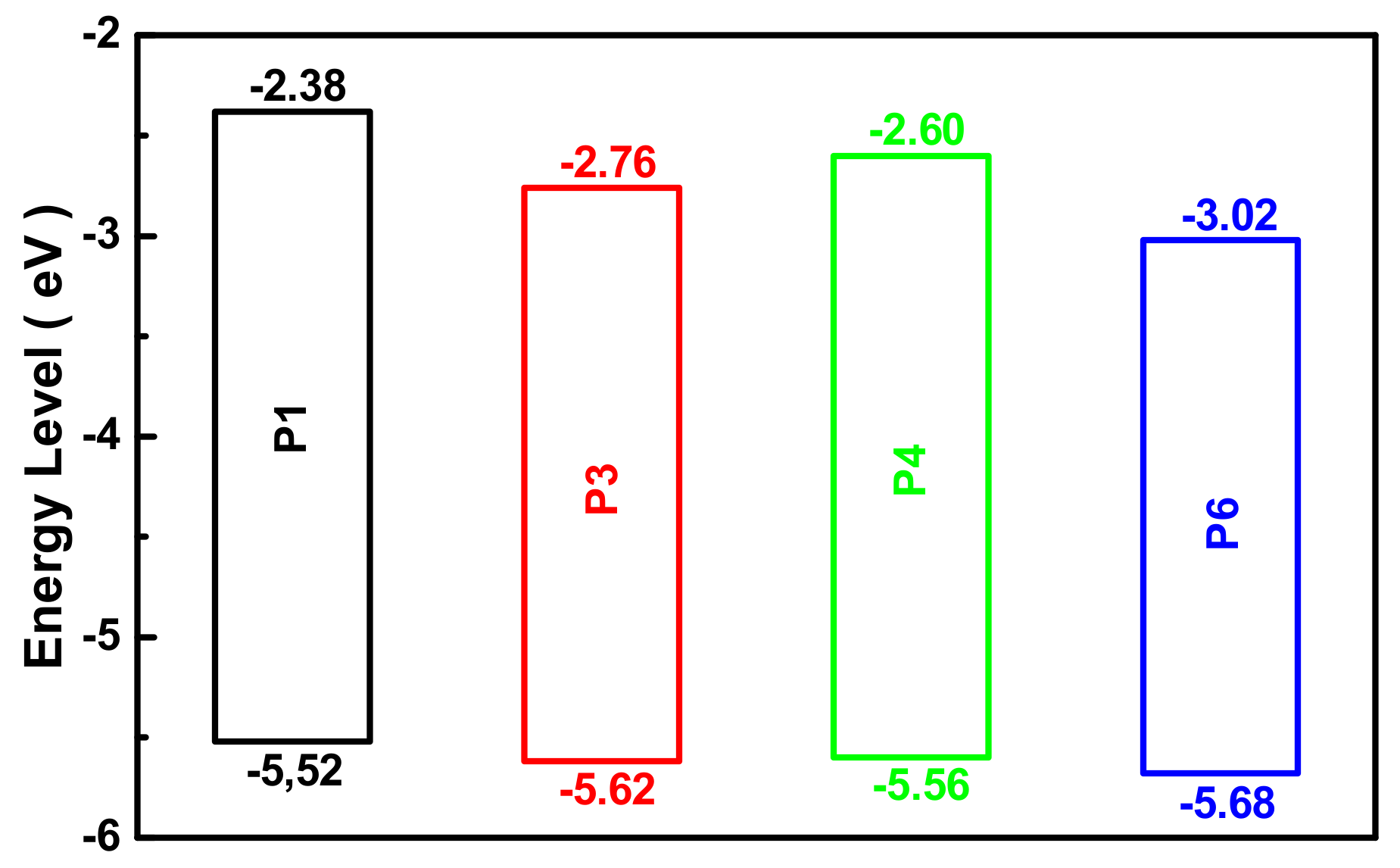
| Mn × 103 g/mol a | Mw × 103 g/mol a | Ða | T5%/°C b | HOMO/eV c | LUMO/eV d | Egap/eV e | |
|---|---|---|---|---|---|---|---|
| P1 | 7.1 | 10.6 | 1.49 | 381 | −5.52 | −2.38 | 3.14 |
| P3 | 7.8 | 11.4 | 1.46 | 363 | −5.62 | −2.76 | 2.86 |
| P4 | 9.8 | 15.6 | 1.59 | 351 | −5.56 | −2.60 | 3.00 |
| P6 | 10.8 | 15.1 | 1.40 | 368 | −5.68 | −3.02 | 2.66 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Liu, F.; Wang, C.; Shen, J.; Wu, Y. Simple Route to Synthesize Fully Conjugated Ladder Isomer Copolymers with Carbazole Units. Polymers 2019, 11, 1619. https://doi.org/10.3390/polym11101619
Chen S, Liu F, Wang C, Shen J, Wu Y. Simple Route to Synthesize Fully Conjugated Ladder Isomer Copolymers with Carbazole Units. Polymers. 2019; 11(10):1619. https://doi.org/10.3390/polym11101619
Chicago/Turabian StyleChen, Shuang, Feng Liu, Chao Wang, Jinghui Shen, and Yonggang Wu. 2019. "Simple Route to Synthesize Fully Conjugated Ladder Isomer Copolymers with Carbazole Units" Polymers 11, no. 10: 1619. https://doi.org/10.3390/polym11101619
APA StyleChen, S., Liu, F., Wang, C., Shen, J., & Wu, Y. (2019). Simple Route to Synthesize Fully Conjugated Ladder Isomer Copolymers with Carbazole Units. Polymers, 11(10), 1619. https://doi.org/10.3390/polym11101619





