The Effect of Glass Fiber Powder on the Properties of Waterborne Coatings with Thermochromic Ink on a Chinese Fir Surface
Abstract
:1. Introduction
2. Experiment Materials and Methods
2.1. Experimental Materials
2.2. Preparation of Ink
2.2.1. Preparation of the Wall Material Prepolymer
2.2.2. Preparation of Thermochromic Emulsion for Core Material
2.2.3. Preparation of Thermochromic Ink
2.3. Preparation of Coatings
2.4. Testing and Characterization
3. Results and Discussion
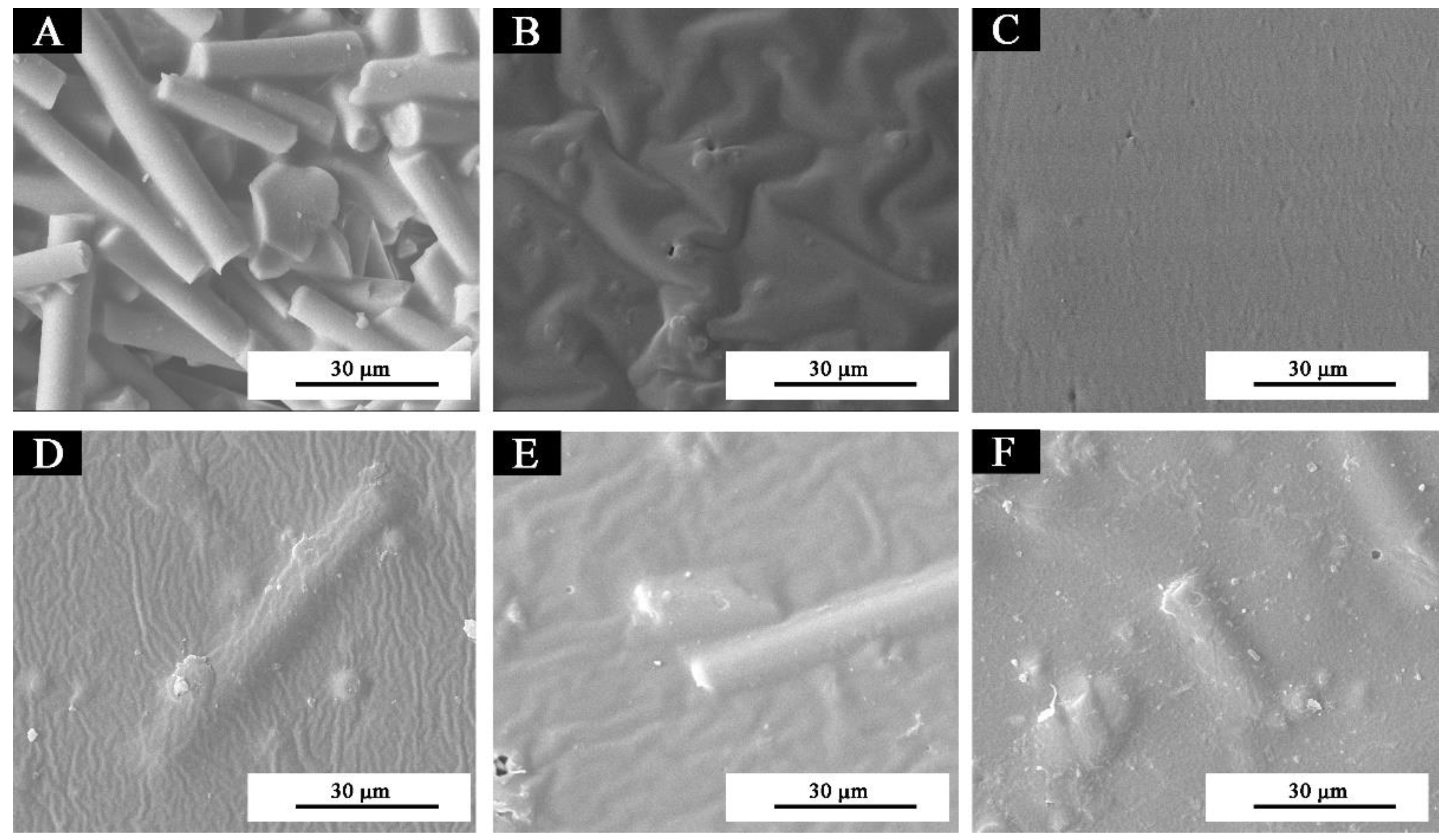
3.1. Microstructure Analysis
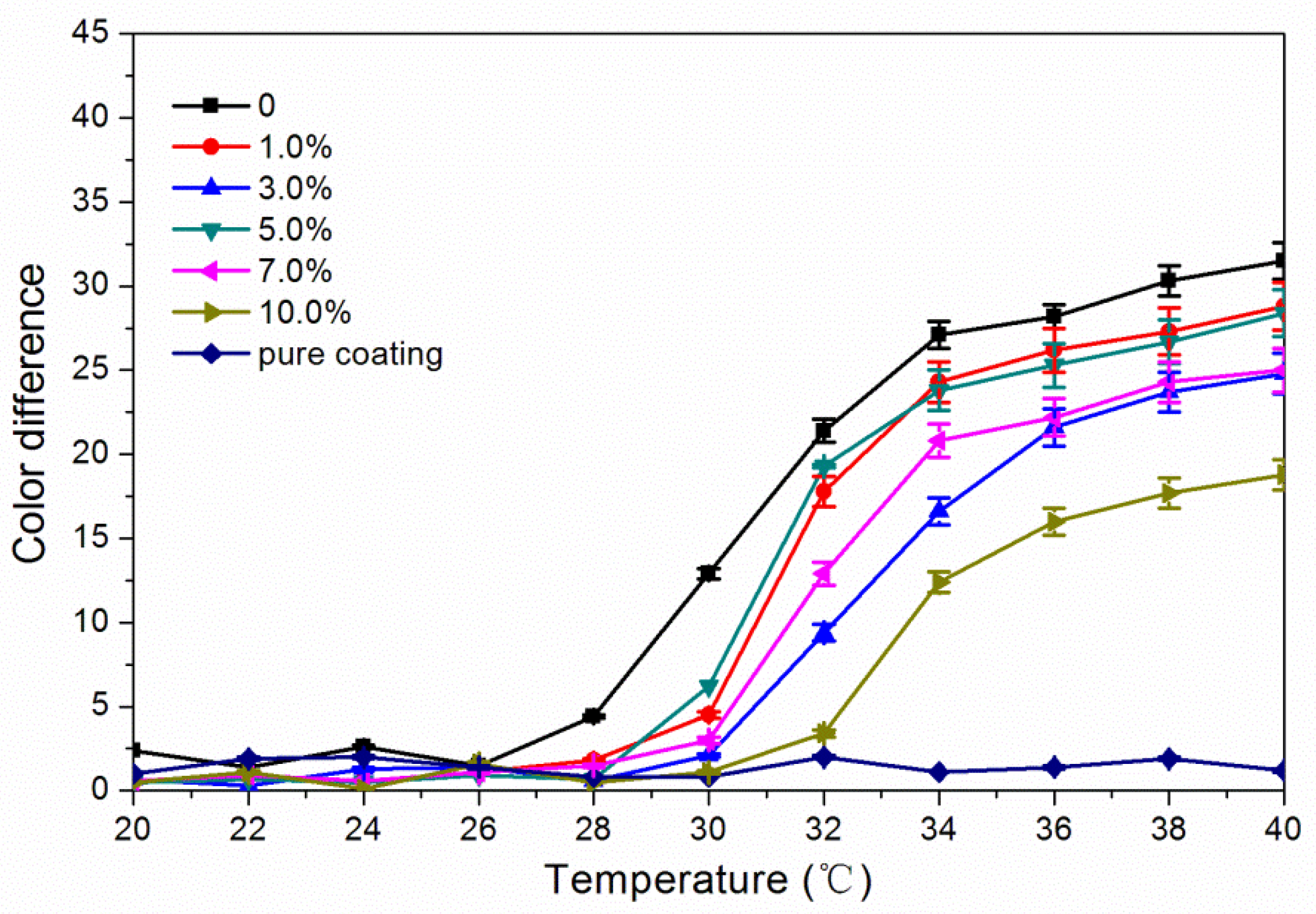
3.2. Effect of Glass FiberPpowder Concentration on OpticalPproperties
3.3. Effect of Glass Fiber Powder Concentration on Mechanical Properties
3.4. Effect of Glass Fiber Powder Concentration on Liquid Resistance
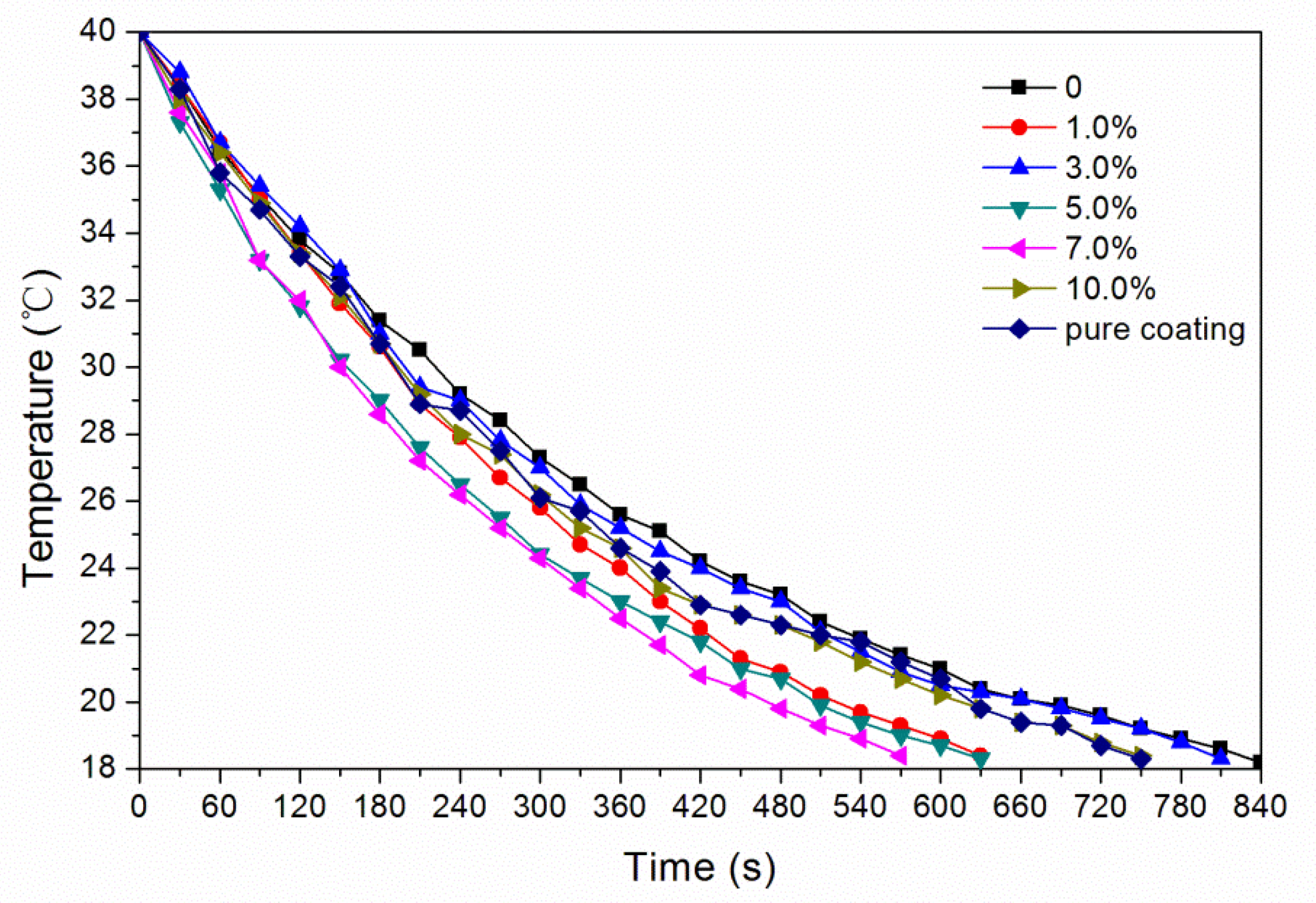
3.5. Effect of Glass Fiber Powder Concentration on Temperature Change of Waterborne Coatings
3.6. Infrared Spectrum Analysis
3.7. Stability of Thermochromic of Coatings
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zheng, S.-J.; Xu, Y.; Shen, Q.-H.; Yang, H. Preparation of thermochromic coatings and their energy saving analysis. Sol. Energy 2015, 112, 263–271. [Google Scholar] [CrossRef]
- De Souza, A.-V.; Valerio, A.; Buske, J.-L.-O.; Benedet, M.-E.; Pistor, V.; Machado, R.-A.-F. Influence of stabilizer additives on thermochromic coating for temperature monitoring. J. Coat. Technol. Res. 2016, 13, 1139–1144. [Google Scholar] [CrossRef]
- Wang, H.; Yue, L.-N.; Wang, X.-W.; Deng, M.-G.; Sun, Y.-J.; Gao, M. Imitation-mussel-based high-performance conductive coating on hydrophobic fabric for thermochromic application. J. Appl. Polym. Sci. 2019, 136, 47751. [Google Scholar] [CrossRef]
- Pedaballi, S.; Li, C.-C.; Song, Y.-J. Dispersion of microcapsules for the improved thermochromic performance of smart coatings. RSC Adv. 2019, 9, 24175–24183. [Google Scholar] [CrossRef] [Green Version]
- Kapovic, D.; Rozic, M.; Vukoje, M.; Lozo, B. Ink tack stability readings of the offset thermochromic inks. Pigm. Resin Technol. 2019, 48, 309–316. [Google Scholar] [CrossRef]
- Vukoje, M.; Miljanic, S.; Hrenovic, J.; Rozic, M. Thermochromic ink-paper interactions and their role in biodegradation of UV curable prints. Cellulose 2018, 25, 6121–6138. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, Y.-C.; Yang, F.; Zhang, H.-Q.; Wang, Y.-J. The implication of benzene–ethanol extractive on mechanical properties of waterborne coating and wood cell wall by nanoindentation. Coatings 2019, 9, 449. [Google Scholar] [CrossRef]
- Yan, X.-X.; Wang, L.; Qian, X.-Y. Effect of high-temperature calcined wheat straw powder after lignin removal on properties of waterborne wood coatings. Coatings 2019, 9, 444. [Google Scholar] [CrossRef]
- Mehta, L.; Wadgaonkar, K.; Suryawanshi, M.; Jagtap, R. Solvent-free microwave-assisted synthesis and characterization of polybenzoxazine as a thermochromic material for smart coatings. Colloid Ploym. Sci. 2019, 297, 795–798. [Google Scholar] [CrossRef]
- Nabuurs, T.; Soer, W.-J.; Peters, R. Isocyanate crosslinking in two-component waterborne coatings. Polym. Int. 2019, 68, 856–861. [Google Scholar] [CrossRef]
- Tanzadeh, R.; Tanzadeh, J.; Honarmand, M.; Tahami, S.-A. Experimental study on the effect of basalt and glass fibers on behavior of open-graded friction course asphalt modified with nano-silica. Constr. Build. Mater. 2019, 212, 467–475. [Google Scholar] [CrossRef]
- Ziari, H.; Moniri, A. Laboratory evaluation of the effect of synthetic Polyolefin-glass fibers on performance properties of hot mix asphalt. Constr. Build. Mater. 2019, 213, 459–468. [Google Scholar] [CrossRef]
- Schmidt, S.; Mahrholz, T.; Kuhn, A.; Wierach, P. Powder binders used for the manufacturing of wind turbine rotor blades. Part 2. Investigation of binder effects on the mechanical performance of glass fiber reinforced polymers. J. Compos. Mater. 2019, 53, 2261–2270. [Google Scholar] [CrossRef]
- Xue, B.; Xie, L.; Bao, Y.; Zhang, J.-H. Multilayered epoxy/glass fiber felt composites with excellently acoustical and thermal insulation properties. J. Appl. Polym. Sci. 2019, 136, 46935. [Google Scholar] [CrossRef]
- Gao, H.-T.; Liu, X.-H.; Zhang, S.-J.; Qi, J.-L. Synergistic effect of glass fibre and Al powder on the mechanical properties of glass-ceramics. Ceram. Int. 2018, 44, 15167–15175. [Google Scholar] [CrossRef]
- Cavasin, M.; Giannis, S.; Salvo, M.; Casalegno, V.; Sangermano, M. Mechanical and thermal characterization of an epoxy foam as thermal layer insulation for a glass fiber reinforced polymer. J. Appl. Polym. Sci. 2018, 135, 46864. [Google Scholar] [CrossRef]
- GB/T 9754-2007 Paints and Varnishes—Determination of Specular Gloss of Non-Metallic Paint Films at 20°, 60° and 85°; Standardization Administration of the People’s Republic of China: Beijing, China, 2007; pp. 1–9. (In Chinese)
- Feng, H.; Xu, H.-S.; Zhang, F.-Z.; Wang, Z.-H. Color prediction of metallic coatings from measurements at common geometries in portable multiangle spectrophotometers. J. Coat. Technol. Res. 2018, 15, 957–966. [Google Scholar] [CrossRef]
- GB/T 1720-89 Determination of Adhesion of Film; Standardization Administration of the People’s Republic of China: Beijing, China, 1979; pp. 378–379. (In Chinese)
- GB/T 1732-93 Determination of Impact Resistance of Film; Standardization Administration of the People’s Republic of China: Beijing, China, 1993; pp. 418–420. (In Chinese)
- GB/T 1733-93 Determination of Resistance to Water of Films; Standardization Administration of the People’s Republic of China: Beijing, China, 1993; pp. 421–423. (In Chinese)
- Yan, X.-X.; Qian, X.-Y.; Lu, R.; Miyakoshi, T. Comparison and optimization of reactive dyes and coating performance on Fraxinus mandshurica veneer. Polymers 2018, 10, 1302. [Google Scholar] [CrossRef]
- Zhao, X.; Qi, Y.-H.; Zhang, Z.-P.; Li, K.-J.; Li, Z.-T. Electrochemical impedance spectroscopy investigation on the corrosive behaviour of waterborne silicate micaceous iron oxide coatings in seawater. Coatings 2019, 9, 415. [Google Scholar] [CrossRef]
- Yan, X.-X.; Wang, L.; Qian, X.-Y. Effect of urea-formaldehyde-coated epoxy microcapsule modification on gloss, toughness and chromatic distortion of acrylic copolymers waterborne coating. Coatings 2019, 9, 239. [Google Scholar] [CrossRef]
- Marani, S.-M.; Shahgholi, G.; Moinfar, A. Effect of nano coating materials on reduction of soil adhesion and external friction. Soil Till. Res. 2019, 193, 42–49. [Google Scholar] [CrossRef]
- Enriquez, E.; Fuertes, V.; Cabrera, M.-J.; Seores, J.; Munoz, D.; Fernandez, J.-F. Absence of surface flaking in hierarchical glass-ceramic coating: high impact resistant ceramic tiles. J. Eur. Ceram. Soc. 2019, 39, 4450–4456. [Google Scholar] [CrossRef]
- Wang, F.-F.; Feng, L.-J.; Lu, M. Mechanical properties of multi-walled carbon nanotube/waterborne polyurethane conductive coatings prepared by electrostatic spraying. Polymers 2019, 11, 714. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.-H.; Wei, X.-F.; Huang, B.-Q.; Zhang, W.; Hu, X.-W. Investigation on factors influencing fluorescence intensity of red fluorescent inkjet ink. Appl. Mech. Mater. 2013, 262, 518–522. [Google Scholar] [CrossRef]
- Zhou, Y.-C.; Chen, Z.-Z.; Gong, H.-J.; Chen, L.; Yu, H.-Q.; Wu, W.L. Characteristics of dehydration during rice husk pyrolysis and catalytic mechanism of dehydration reaction with NiO/gamma-Al2O3 as catalyst. Fuel 2019, 245, 131–138. [Google Scholar] [CrossRef]
- Melinte, G.; Baia, L.; Simon, V.; Simon, S. Hydrogen peroxide versus water synthesis of bioglass-nanocrystalline hydroxyapatite composites. J. Mater. Sci. 2011, 46, 7393–7400. [Google Scholar] [CrossRef]





| Concentration of Glass Fiber Powder (%) | Weight of Glass Fiber Powder (g) | Weight of Thermochromic Ink (g) | Weight of Waterborne Primer (g) | Weight of Waterborne Topcoat (g) | Weight of Waterborne Thermochromic Coating (g) |
|---|---|---|---|---|---|
| 0 | 0 | 15.0 | 100.0 | 85.0 | 200.0 |
| 1.0 | 1.0 | 15.0 | 100.0 | 84.0 | 200.0 |
| 3.0 | 3.0 | 15.0 | 100.0 | 82.0 | 200.0 |
| 5.0 | 5.0 | 15.0 | 100.0 | 80.0 | 200.0 |
| 7.0 | 7.0 | 15.0 | 100.0 | 78.0 | 200.0 |
| 10.0 | 10.0 | 15.0 | 100.0 | 75.0 | 200.0 |
| Concentration of Glass Fiber Powder (%) | Chromatic Value | 18 °C | 20 °C | 22 °C | 24 °C | 26° C | 28 °C | 30 °C | 32 °C | 34 °C | 36 °C | 38 °C | 40 °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pure coating | L | 84.0 | 84.8 | 85.3 | 85.3 | 84.3 | 84.7 | 83.8 | 83.8 | 83.8 | 83.9 | 85.6 | 84.6 |
| a | 5.9 | 5.3 | 5.0 | 4.8 | 4.8 | 5.5 | 5.2 | 4.6 | 5.2 | 5.5 | 4.9 | 4.9 | |
| b | 28.5 | 28.7 | 29.6 | 29.5 | 27.8 | 28.4 | 28.1 | 27.0 | 27.7 | 27.2 | 28.5 | 28.3 | |
| C | 29.1 | 28.2 | 30.1 | 29.9 | 28.2 | 28.9 | 28.6 | 27.4 | 28.2 | 27.8 | 28.9 | 28.7 | |
| h | 78.2 | 79.3 | 80.3 | 80.6 | 80.1 | 79.0 | 79.3 | 80.3 | 79.2 | 78.5 | 80.1 | 80.1 | |
| 0 | L | 65.8 | 64.3 | 66.6 | 66.7 | 66.2 | 68.0 | 72.5 | 76.6 | 79.4 | 79.9 | 81.2 | 81.6 |
| a | 40.3 | 38.5 | 39.4 | 37.9 | 38.9 | 36.5 | 29.3 | 21.8 | 16.9 | 15.9 | 14.3 | 13.2 | |
| b | 24.9 | 24.5 | 25.6 | 25.4 | 25.1 | 24.9 | 24.5 | 25.1 | 26.3 | 26.4 | 27.4 | 27.8 | |
| C | 47.4 | 45.7 | 47.0 | 45.6 | 46.3 | 44.2 | 38.3 | 33.2 | 31.3 | 30.8 | 30.9 | 30.8 | |
| h | 31.7 | 32.4 | 33.0 | 33.8 | 32.8 | 34.3 | 39.9 | 49.0 | 57.2 | 58.8 | 62.4 | 64.5 | |
| 1.0 | L | 65.5 | 65.5 | 65.8 | 65.1 | 65.8 | 66.1 | 67.4 | 74.1 | 77.3 | 78.1 | 78.4 | 79.1 |
| a | 40.8 | 41.1 | 40.2 | 40.8 | 40.0 | 39.2 | 37.0 | 25.4 | 19.6 | 17.9 | 16.8 | 15.4 | |
| b | 26.0 | 25.6 | 25.4 | 25.6 | 25.3 | 25.3 | 24.4 | 23.5 | 24.5 | 24.8 | 24.6 | 25.6 | |
| C | 48.4 | 48.4 | 47.5 | 48.2 | 47.4 | 46.7 | 44.4 | 34.6 | 31.4 | 30.6 | 29.8 | 29.9 | |
| h | 32.5 | 31.9 | 32.3 | 32.1 | 32.3 | 32.8 | 33.4 | 42.7 | 51.3 | 54.2 | 55.6 | 58.9 | |
| 3.0 | L | 68.5 | 68.6 | 68.7 | 69.4 | 69.2 | 68.7 | 69.5 | 73.9 | 79.0 | 79.5 | 80.8 | 81.1 |
| a | 34.3 | 35.0 | 34.3 | 33.6 | 33.3 | 33.7 | 32.6 | 26.7 | 17.7 | 15.7 | 14.1 | 13.1 | |
| b | 27.5 | 27.7 | 27.9 | 28.1 | 28.2 | 27.6 | 28.1 | 26.7 | 27.9 | 28.8 | 29.0 | 29.6 | |
| C | 44.0 | 44.7 | 44.2 | 43.8 | 43.7 | 43.6 | 43.1 | 37.8 | 33.1 | 32.8 | 32.3 | 32.4 | |
| h | 38.7 | 38.4 | 39.1 | 39.9 | 40.2 | 39.3 | 40.7 | 45.0 | 57.5 | 61.3 | 63.9 | 66.0 | |
| 5.0 | L | 65.5 | 65.6 | 65.7 | 65.6 | 66.0 | 65.9 | 68.4 | 74.7 | 77.0 | 77.9 | 78.6 | 79.0 |
| a | 38.2 | 37.8 | 37.5 | 37.8 | 37.5 | 37.6 | 32.7 | 21.2 | 17.4 | 16.1 | 14.9 | 13.2 | |
| b | 24.0 | 23.8 | 24.1 | 23.8 | 23.9 | 24.1 | 23.4 | 23.6 | 24.3 | 24.3 | 24.8 | 25.2 | |
| C | 45.2 | 44.7 | 44.6 | 44.7 | 44.5 | 44.7 | 40.2 | 31.8 | 29.9 | 29.1 | 28.2 | 28.4 | |
| h | 32.1 | 32.1 | 32.7 | 32.1 | 32.5 | 32.6 | 35.5 | 48.0 | 54.3 | 56.3 | 59.4 | 62.3 | |
| 7.0 | L | 67.4 | 67.4 | 67.3 | 67.5 | 67.6 | 68.1 | 68.6 | 73.5 | 77.3 | 77.8 | 78.8 | 79.1 |
| a | 34.5 | 34.0 | 33.7 | 33.9 | 33.5 | 33.2 | 31.8 | 23.2 | 16.2 | 14.9 | 13.1 | 12.4 | |
| b | 25.0 | 25.4 | 25.3 | 25.2 | 25.5 | 25.0 | 24.5 | 24.0 | 25.3 | 25.2 | 26.1 | 26.1 | |
| C | 42.6 | 42.4 | 42.1 | 42.3 | 42.1 | 41.6 | 40.2 | 33.4 | 30.0 | 29.3 | 29.3 | 28.9 | |
| h | 35.9 | 36.7 | 36.9 | 36.6 | 37.3 | 36.9 | 37.6 | 46.0 | 57.4 | 59.3 | 63.3 | 64.4 | |
| 10.0 | L | 67.6 | 67.5 | 67.1 | 67.5 | 67.0 | 67.5 | 68.0 | 68.9 | 73.3 | 74.9 | 75.6 | 76.3 |
| a | 29.9 | 30.4 | 30.8 | 29.8 | 31.3 | 29.9 | 29.1 | 27.5 | 18.9 | 15.7 | 14.1 | 13.2 | |
| b | 24.4 | 24.2 | 24.1 | 24.4 | 23.8 | 23.9 | 23.7 | 22.3 | 23.8 | 24.0 | 24.6 | 24.9 | |
| C | 38.7 | 38.8 | 39.1 | 38.5 | 39.4 | 38.3 | 37.6 | 35.4 | 30.4 | 28.7 | 28.4 | 28.2 | |
| h | 39.2 | 38.5 | 38.0 | 39.3 | 37.2 | 38.6 | 39.1 | 39.0 | 51.5 | 56.8 | 60.1 | 62.1 |
| Concentration of Glass Fiber Powder (%) | 20° Gloss (%) | 60° Gloss (%) | 85° Gloss (%) |
|---|---|---|---|
| Pure coating | 14.1 | 43.1 | 54.7 |
| 0 | 26.0 | 55.6 | 79.1 |
| 1.0 | 19.0 | 44.1 | 56.5 |
| 3.0 | 16.8 | 37.6 | 41.0 |
| 5.0 | 12.4 | 35.1 | 39.4 |
| 7.0 | 9.3 | 29.8 | 35.2 |
| 10.0 | 6.3 | 21.5 | 26.0 |
| Concentration of Glass Fiber Powder (%) | Damage Area (%) | Adhesion Level (level) |
|---|---|---|
| Pure coating | 0 | 0 |
| 0 | 0 | 0 |
| 1.0 | 0 | 0 |
| 3.0 | 0 | 0 |
| 5.0 | 0 | 0 |
| 7.0 | 0 | 0 |
| 10.0 | 0 | 0 |
| Concentration of Glass Fiber Powder (%) | Impact Resistance (kg·cm) | Hardness (H) |
|---|---|---|
| Pure coating | 4.0 | HB |
| 0 | 5.0 | H |
| 1.0 | 5.0 | H |
| 3.0 | 5.0 | 2H |
| 5.0 | 5.0 | 2H |
| 7.0 | 5.0 | 2H |
| 10.0 | 5.0 | 3H |
| Concentration of Glass Fiber Powder (%) | Chromatic Value | Original | NaCl | Detergent | Ethanol | Red Ink |
|---|---|---|---|---|---|---|
| Pure coating | L | 84.0 | 84.5 | 84.7 | 84.3 | 85.6 |
| a | 5.9 | 6.1 | 6.2 | 6.6 | 6.9 | |
| b | 28.5 | 29.1 | 28.7 | 27.9 | 28.6 | |
| C | 29.1 | 30.2 | 30.8 | 27.8 | 29.9 | |
| h | 78.2 | 77.7 | 78.2 | 76.0 | 76.6 | |
| 0 | L | 65.8 | 66.5 | 65.9 | 65.9 | 54.8 |
| a | 40.3 | 39.3 | 39.0 | 39.5 | 70.2 | |
| b | 24.9 | 24.9 | 24.9 | 24.6 | 21.6 | |
| C | 47.4 | 45.7 | 45.5 | 42.8 | 73.5 | |
| h | 31.7 | 33.0 | 33.2 | 35.8 | 17.1 | |
| 1.0 | L | 65.5 | 65.1 | 66.0 | 66.3 | 42.4 |
| a | 40.8 | 40.1 | 39.9 | 40.1 | 73.0 | |
| b | 26.0 | 26.7 | 26.0 | 26.7 | 37.4 | |
| c | 48.4 | 48.2 | 47.7 | 48.3 | 82.0 | |
| H | 32.5 | 33.6 | 33.6 | 33.1 | 27.1 | |
| 3.0 | L | 68.5 | 69.3 | 68.1 | 68.7 | 47.0 |
| a | 34.3 | 33.8 | 35.1 | 33.9 | 74.6 | |
| b | 27.5 | 27.0 | 27.2 | 26.8 | 37.5 | |
| C | 44.0 | 43.2 | 44.8 | 43.3 | 83.5 | |
| h | 38.7 | 38.0 | 37.4 | 39.1 | 26.7 | |
| 5.0 | L | 65.5 | 66.1 | 65.3 | 66.2 | 44.8 |
| a | 38.2 | 37.6 | 38.6 | 37.8 | 73.8 | |
| b | 24.0 | 24.4 | 23.5 | 24.5 | 37.4 | |
| C | 45.2 | 44.0 | 45.9 | 44.7 | 82.8 | |
| h | 32.1 | 32.6 | 31.4 | 32.6 | 26.9 | |
| 7.0 | L | 67.4 | 68.1 | 68.1 | 67.8 | 45.0 |
| a | 34.5 | 34.1 | 33.7 | 33.8 | 72.4 | |
| b | 25.0 | 25.6 | 25.0 | 25.8 | 35.6 | |
| C | 42.6 | 41.2 | 41.6 | 41.1 | 80.7 | |
| h | 35.9 | 36.7 | 36.0 | 36.9 | 26.2 | |
| 10.0 | L | 67.6 | 67.6 | 67.9 | 66.9 | 47.6 |
| a | 29.9 | 30.4 | 29.4 | 30.3 | 73.0 | |
| b | 24.4 | 25.2 | 23.8 | 24.8 | 33.7 | |
| C | 38.7 | 39.5 | 38.4 | 39.2 | 80.4 | |
| h | 39.2 | 39.2 | 38.2 | 39.3 | 24.8 |
| Concentration of Glass Fiber Powder (%) | NaCl | Detergent | Ethanol | Red Ink |
|---|---|---|---|---|
| Pure coating | 0.8 | 0.8 | 1.0 | 1.9 |
| 0 | 1.2 | 1.3 | 0.9 | 32.0 |
| 1.0 | 1.1 | 1.0 | 1.3 | 41.2 |
| 3.0 | 1.1 | 0.9 | 0.8 | 46.8 |
| 5.0 | 0.9 | 0.7 | 0.9 | 43.3 |
| 7.0 | 1.0 | 1.1 | 1.1 | 45.3 |
| 10.0 | 0.9 | 0.8 | 0.9 | 48.4 |
| Concentration of Glass Fiber Powder (%) | NaCl (Level) | Detergent (Level) | Ethanol (Level) | Red Ink (Level) |
|---|---|---|---|---|
| Pure coating | 1 | 1 | 1 | 1 |
| 0 | 1 | 1 | 1 | 3 |
| 1.0 | 1 | 1 | 1 | 3 |
| 3.0 | 1 | 1 | 1 | 3 |
| 5.0 | 1 | 1 | 1 | 3 |
| 7.0 | 1 | 1 | 1 | 3 |
| 10.0 | 1 | 1 | 1 | 3 |
| Concentration of Glass Fiber Powder (%) | Color Value | 18 °C | Three Months at 18 °C | 30 °C | 30 °C Heating 24 h | 30 °C Heating 48 h | 30 °C Heating 72 h |
|---|---|---|---|---|---|---|---|
| 3.0 | L | 68.5 | 69.5 | 69.5 | 69.0 | 68.4 | 70.3 |
| a | 34.3 | 33.6 | 32.6 | 33.2 | 33.5 | 31.5 | |
| b | 27.5 | 28.0 | 28.1 | 29.2 | 27.6 | 27.2 | |
| C | 44.0 | 43.8 | 43.1 | 42.7 | 42.9 | 42.3 | |
| h | 38.7 | 39.8 | 40.7 | 38.9 | 39.7 | 41.5 |
| Concentration of Glass Fiber Powder (%) | Color Difference after Three Months at 18 °C | Color Difference 30 °C Heating 24 h | Color Difference 30 °C Heating 48 h | Color Difference 30 °C Heating 72 h |
|---|---|---|---|---|
| 3.0 | 1.4 | 1.3 | 1.5 | 1.6 |
| Sample | L | a | b | C | h | ΔE | Gloss (%) |
|---|---|---|---|---|---|---|---|
| Before aging | 72.0 ± 0 | 19.3 ± 0 | 35.3 ± 0 | 40.3 ± 0 | 61.2 ± 0 | – | 35.9 ± 0 |
| After aging | 71.4 ± 0 | 22.1 ± 0 | 33.4 ± 0 | 40.2 ± 0 | 58.1 ± 0 | 3.4 ± 0 | 34.8 ± 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Qian, X.; Chang, Y.; Lu, R.; Miyakoshi, T. The Effect of Glass Fiber Powder on the Properties of Waterborne Coatings with Thermochromic Ink on a Chinese Fir Surface. Polymers 2019, 11, 1733. https://doi.org/10.3390/polym11111733
Yan X, Qian X, Chang Y, Lu R, Miyakoshi T. The Effect of Glass Fiber Powder on the Properties of Waterborne Coatings with Thermochromic Ink on a Chinese Fir Surface. Polymers. 2019; 11(11):1733. https://doi.org/10.3390/polym11111733
Chicago/Turabian StyleYan, Xiaoxing, Xingyu Qian, Yijuan Chang, Rong Lu, and Tetsuo Miyakoshi. 2019. "The Effect of Glass Fiber Powder on the Properties of Waterborne Coatings with Thermochromic Ink on a Chinese Fir Surface" Polymers 11, no. 11: 1733. https://doi.org/10.3390/polym11111733
APA StyleYan, X., Qian, X., Chang, Y., Lu, R., & Miyakoshi, T. (2019). The Effect of Glass Fiber Powder on the Properties of Waterborne Coatings with Thermochromic Ink on a Chinese Fir Surface. Polymers, 11(11), 1733. https://doi.org/10.3390/polym11111733





