Highly Efficient Polydopamine-coated Poly(methyl methacrylate) Nanofiber Supported Platinum–Nickel Bimetallic Catalyst for Formaldehyde Oxidation at Room Temperature
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Apparatus
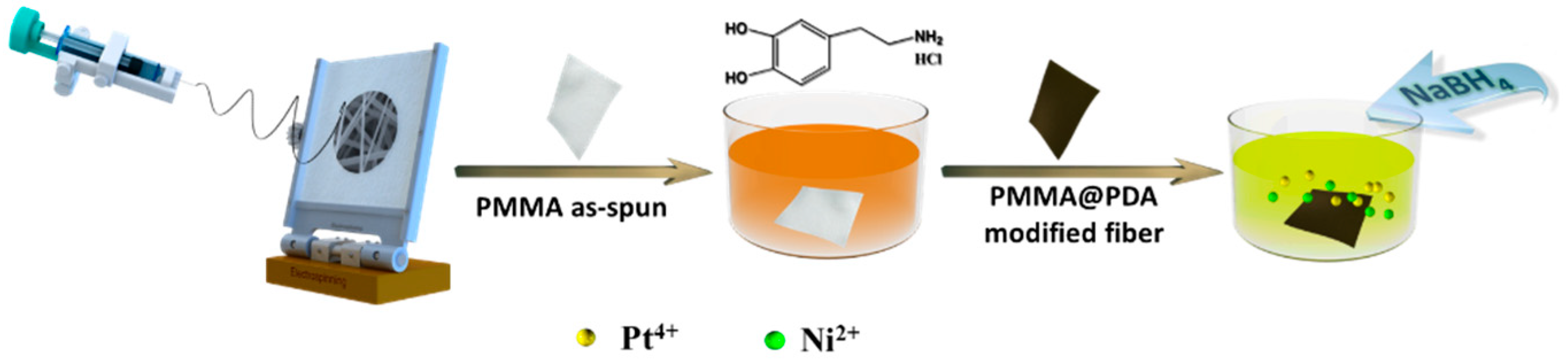
2.2. Preparation of PMMA@PDA Nanofibers
2.3. Preparation of PtNi/PMMA@PDA Membrane Catalyst
2.4. Characterization
2.5. Test of the Catalytic Performance
3. Result and Discussion
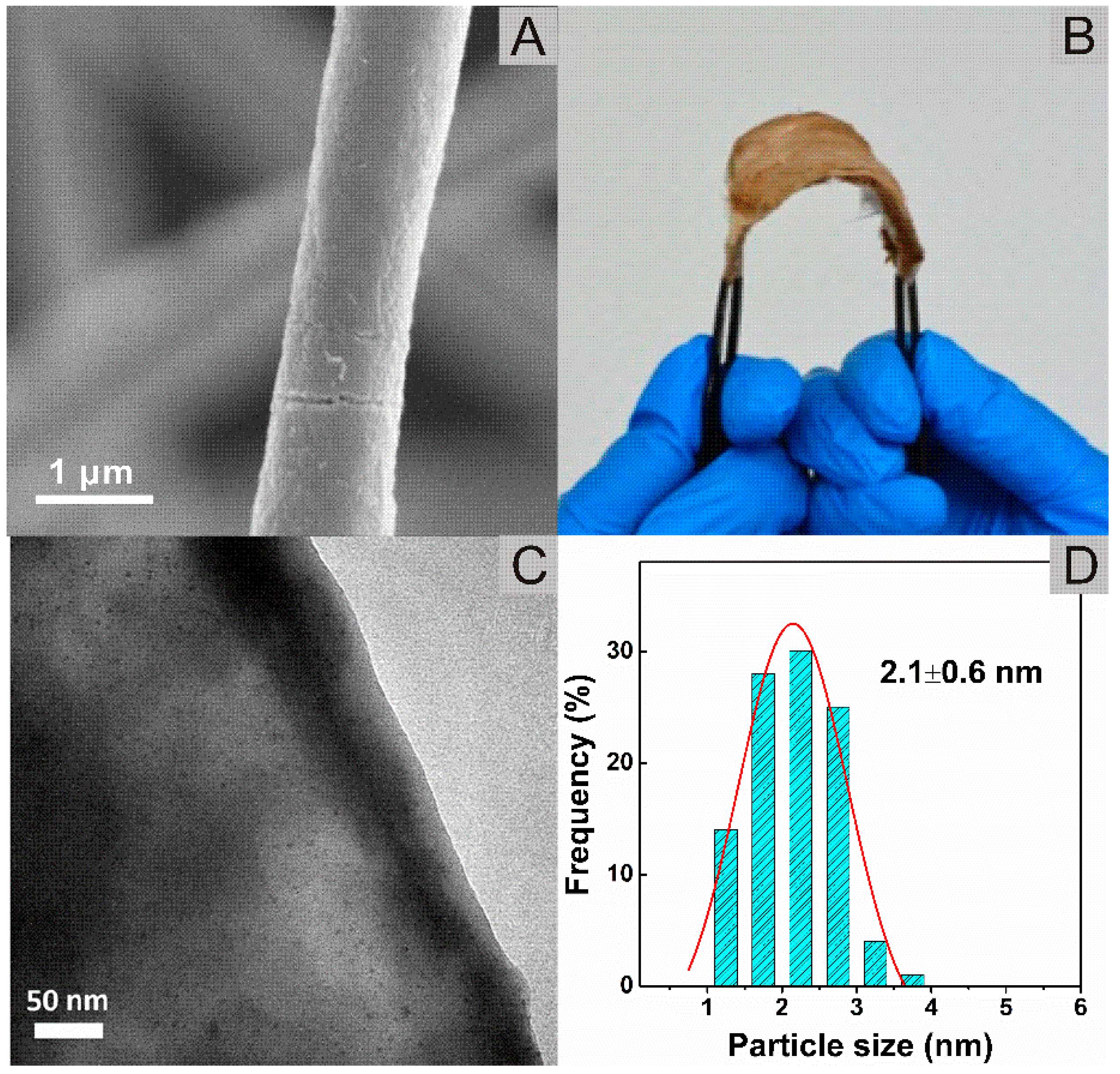
3.1. Morphology of PMMA@PDA Support
3.2. Deposition of PtNi NPs on PMMA@PDA and Morphology of PtNi/PMMA@PDA Nanofibrous Membrane
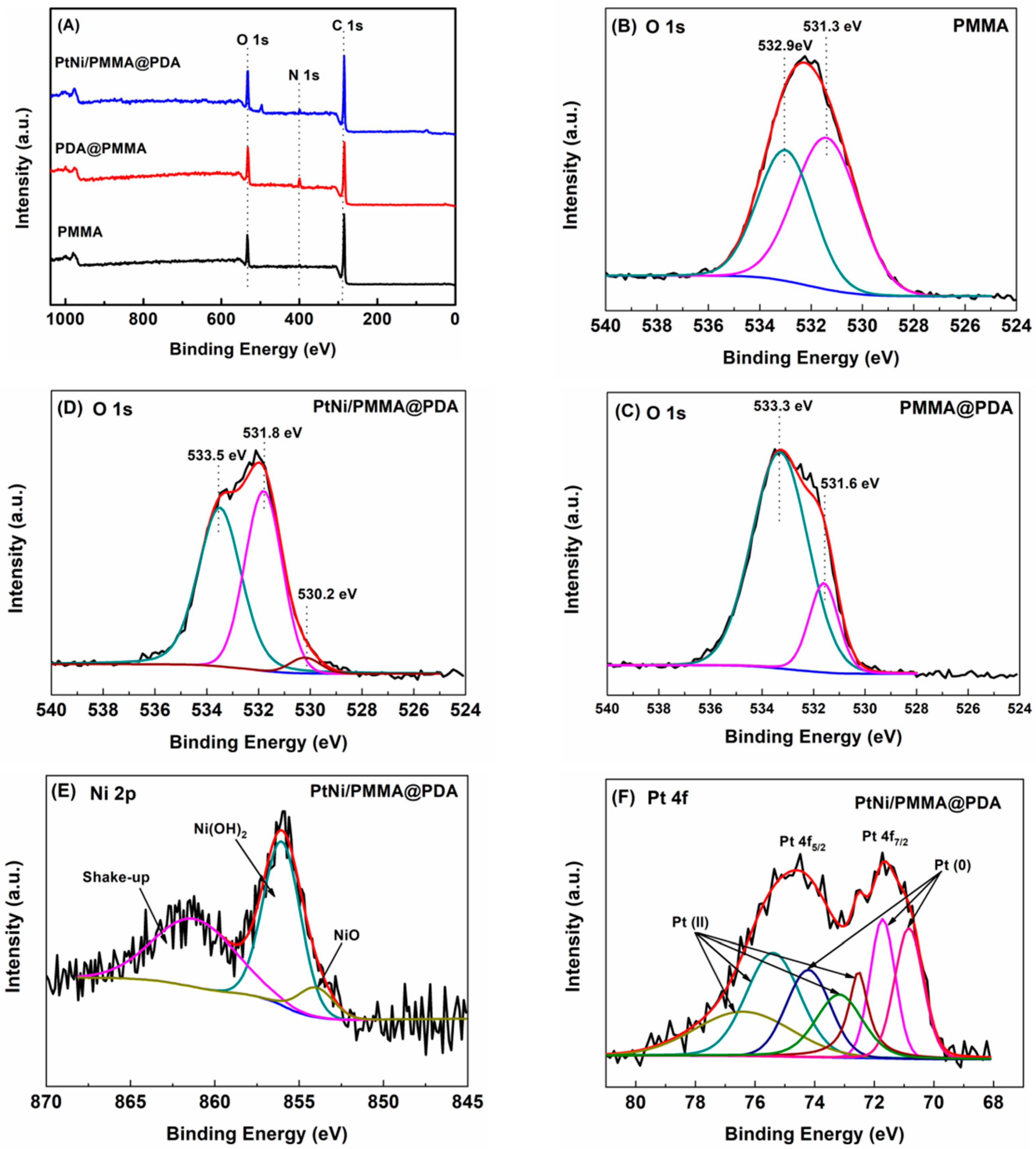
3.3. XPS of PtNi/PMMA@PDA Nanofibrous Membrane
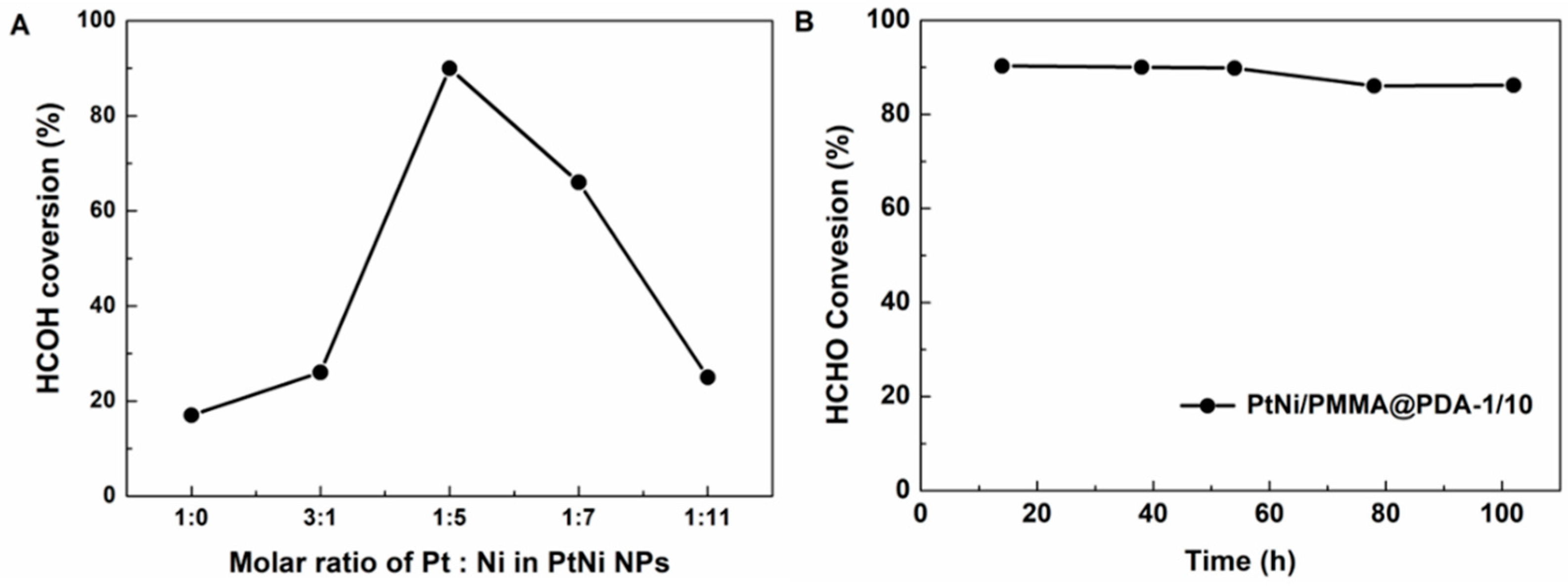
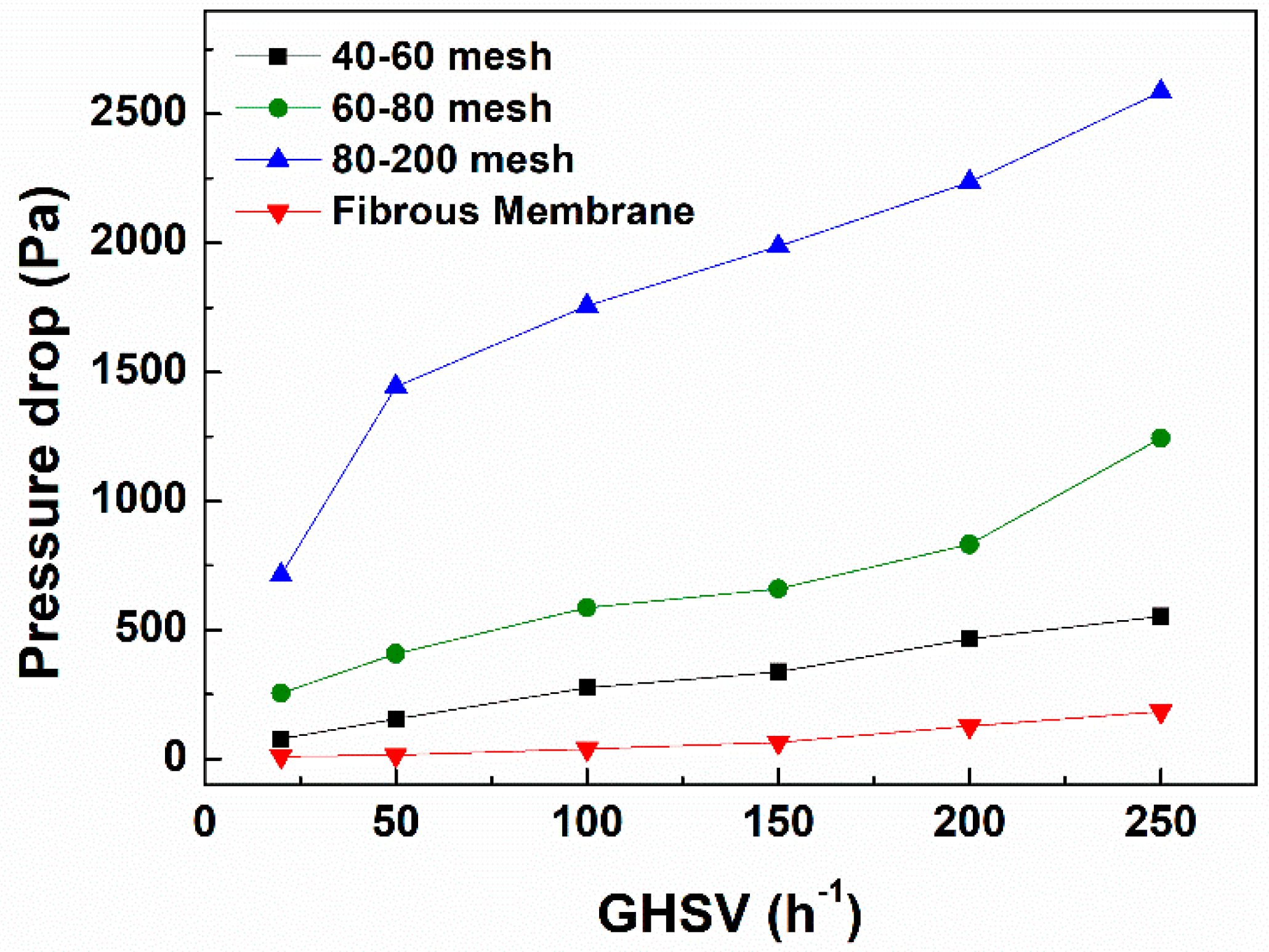
3.4. Catalytic Properties of PtNi/PMMA@PDA
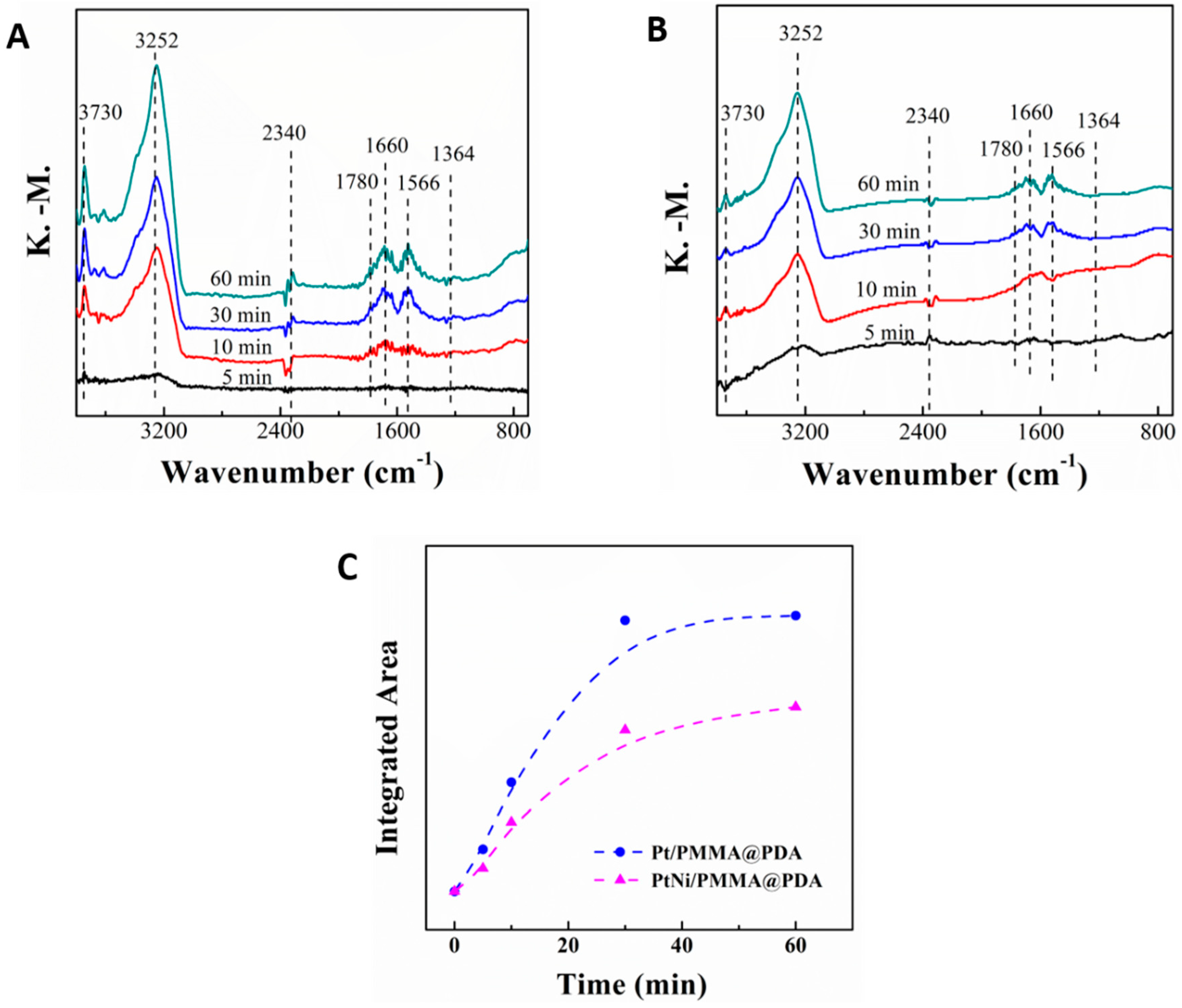
3.5. In Situ DRIFT
4. Conclusions
5. Patent
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Collins, J.J.; Ness, R.; Tyl, R.W.; Krivanek, N.; Esmen, N.A.; Hall, T.A. A Review of Adverse Pregnancy Outcomes and Formaldehyde Exposure in Human and Animal Studies. Regul. Toxicol. Pharmacol. 2001, 34, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Raub, J.A.; Mathieu-Nolf, M.; Hampson, N.B.; Thom, S.R. Carbon monoxide poisoning—A public health perspective. Toxicology 2000, 145, 1–14. [Google Scholar] [CrossRef]
- Salthammer, T.; Mentese, S.; Marutzky, R. Formaldehyde in the Indoor Environment. Chem. Rev. 2010, 110, 2536–2572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; He, H.; Tanaka, K.-I. Perfect catalytic oxidation of formaldehyde over a Pt/TiO2 catalyst at room temperature. Catal. Commun. 2005, 6, 211–214. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, F.; Zhai, Y.; Ariga, H.; Yi, N.; Liu, Y.; Asakura, K.; Flytzani-Stephanopoulos, M.; He, H. Alkali-Metal-Promoted Pt/TiO2 Opens a More Efficient Pathway to Formaldehyde Oxidation at Ambient Temperatures. Angew. Chem. Int. Ed. 2012, 51, 9628–9632. [Google Scholar] [CrossRef]
- Nie, L.; Yu, J.; Jaroniec, M.; Tao, F.F. Room-temperature catalytic oxidation of formaldehyde on catalysts. Catal. Sci. Technol. 2016, 6, 3649–3669. [Google Scholar] [CrossRef]
- Yang, T.; Huo, Y.; Liu, Y.; Rui, Z.; Ji, H. Efficient formaldehyde oxidation over nickel hydroxide promoted Pt/γ-Al2O3 with a low Pt content. Appl. Catal. B Environ. 2017, 200, 543–551. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Prakash Dwivedi, R.; Alothman, Z.A.; Mola, G.T. Novel development of nanoparticles to bimetallic nanoparticles and their composites: A review. J. King Saud Univ. Sci. 2019, 31, 257–269. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Naushad, M.; Kumar, A.; Al-Muhtaseb, A.H.; Dhiman, P.; Ghfar, A.A.; Stadler, F.J.; Khan, M.R. Photoremediation of toxic dye from aqueous environment using monometallic and bimetallic quantum dots based nanocomposites. J. Clean. Prod. 2018, 172, 2919–2930. [Google Scholar] [CrossRef]
- Nie, L.; Zheng, Y.; Yu, J. Efficient decomposition of formaldehyde at room temperature over Pt/honeycomb ceramics with ultra-low Pt content. Dalton Trans. 2014, 43, 12935–12942. [Google Scholar] [CrossRef]
- Yu, J.; Li, X.; Xu, Z.; Xiao, W. NaOH-Modified Ceramic Honeycomb with Enhanced Formaldehyde Adsorption and Removal Performance. Environ. Sci. Technol. 2013, 47, 9928–9933. [Google Scholar] [CrossRef]
- Ikegami, M.; Matsumoto, T.; Kobayashi, Y.; Jikihara, Y.; Nakayama, T.; Ohashi, H.; Honma, T.; Takei, T.; Haruta, M. Air purification by gold catalysts supported on PET nonwoven fabric. Appl. Catal. B Environ. 2013, 134–135, 130–135. [Google Scholar] [CrossRef]
- Wang, J.; Yunus, R.; Li, J.; Li, P.; Zhang, P.; Kim, J. In situ synthesis of manganese oxides on polyester fiber for formaldehyde decomposition at room temperature. Appl. Surf. Sci. 2015, 357, 787–794. [Google Scholar] [CrossRef]
- Pardieu, E.; Chau, N.T.T.; Dintzer, T.; Romero, T.; Favier, D.; Roland, T.; Edouard, D.; Jierry, L.; Ritleng, V. Polydopamine-coated open cell polyurethane foams as an inexpensive, flexible yet robust catalyst support: A proof of concept. Chem. Commun. 2016, 52, 4691–4693. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, S.; Fan, W.; Tian, Y.; Zhao, X. The synthesis of novel Co–Al2O3 nanofibrous membranes with efficient activation of peroxymonosulfate for bisphenol A degradation. Environ. Sci. Nano 2018, 5, 1933–1942. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, Y.; Fu, G.; Duchesne, P.N.; Gu, L.; Zheng, Y.; Weng, X.; Chen, M.; Zhang, P.; Pao, C.-W.; et al. Interfacial Effects in Iron-Nickel Hydroxide–Platinum Nanoparticles Enhance Catalytic Oxidation. Science 2014, 344, 495–499. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, J.; Jaroniec, M. Efficient catalytic removal of formaldehyde at room temperature using AlOOH nanoflakes with deposited Pt. Appl. Catal. B Environ. 2015, 163, 306–312. [Google Scholar] [CrossRef]
- Chen, B.-B.; Shi, C.; Crocker, M.; Wang, Y.; Zhu, A.-M. Catalytic removal of formaldehyde at room temperature over supported gold catalysts. Appl. Catal. B Environ. 2013, 132–133, 245–255. [Google Scholar] [CrossRef]
- Subbaraman, R.; Tripkovic, D.; Strmcnik, D.; Chang, K.-C.; Uchimura, M.; Paulikas, A.P.; Stamenkovic, V.; Markovic, N.M. Enhancing Hydrogen Evolution Activity in Water Splitting by Tailoring Li+-Ni(OH)2-Pt Interfaces. Science 2011, 334, 1256–1260. [Google Scholar] [CrossRef]
- Lee, H.; Rho, J.; Messersmith, P.B. Facile Conjugation of Biomolecules onto Surfaces via Mussel Adhesive Protein Inspired Coatings. Adv. Mater. 2009, 21, 431–434. [Google Scholar] [CrossRef]
- Arena, J.T.; McCloskey, B.; Freeman, B.D.; McCutcheon, J.R. Surface modification of thin film composite membrane support layers with polydopamine: Enabling use of reverse osmosis membranes in pressure retarded osmosis. J. Membr. Sci. 2011, 375, 55–62. [Google Scholar] [CrossRef]
- Wang, X.; Min, M.; Liu, Z.; Yang, Y.; Zhou, Z.; Zhu, M.; Chen, Y.; Hsiao, B.S. Poly(ethyleneimine) nanofibrous affinity membrane fabricated via one step wet-electrospinning from poly(vinyl alcohol)-doped poly(ethyleneimine) solution system and its application. J. Membr. Sci. 2011, 379, 191–199. [Google Scholar] [CrossRef]
- Hong, G.; Shen, L.; Wang, M.; Yang, Y.; Wang, X.; Zhu, M.; Hsiao, B.S. Nanofibrous polydopamine complex membranes for adsorption of Lanthanum (III) ions. Chem. Eng. J. 2014, 244, 307–316. [Google Scholar] [CrossRef]
- Fei, B.; Qian, B.; Yang, Z.; Wang, R.; Liu, W.C.; Mak, C.L.; Xin, J.H. Coating carbon nanotubes by spontaneous oxidative polymerization of dopamine. Carbon 2008, 46, 1795–1797. [Google Scholar] [CrossRef]
- Zhu, B.; Edmondson, S. Polydopamine-melanin initiators for Surface-initiated ATRP. Polymer 2011, 52, 2141–2149. [Google Scholar] [CrossRef]
- Casella, I.G.; Guascito, M.R.; Sannazzaro, M.G. Voltammetric and XPS investigations of nickel hydroxide electrochemically dispersed on gold surface electrodes. J. Electroanal. Chem. 1999, 462, 202–210. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Blume, R.; Zhang, A.; Schlögl, R.; Su, D.S. Surface-Modified Carbon Nanotubes Catalyze Oxidative Dehydrogenation of n-Butane. Science 2008, 322, 73–77. [Google Scholar] [CrossRef]
- Nathawat, R.; Kumar, A.; Acharya, N.K.; Vijay, Y.K. XPS and AFM surface study of PMMA irradiated by electron beam. Surf. Coat. Technol. 2009, 203, 2600–2604. [Google Scholar] [CrossRef]
- Luo, R.; Tang, L.; Wang, J.; Zhao, Y.; Tu, Q.; Weng, Y.; Shen, R.; Huang, N. Improved immobilization of biomolecules to quinone-rich polydopamine for efficient surface functionalization. Colloids Surf. B Biointerfaces 2013, 106, 66–73. [Google Scholar] [CrossRef]
- Zangmeister, R.A.; Morris, T.A.; Tarlov, M.J. Characterization of Polydopamine Thin Films Deposited at Short Times by Autoxidation of Dopamine. Langmuir 2013, 29, 8619–8628. [Google Scholar] [CrossRef]
- Wang, G.-J.; Gao, Y.-Z.; Wang, Z.-B.; Du, C.-Y.; Wang, J.-J.; Yin, G.-P. Investigation of PtNi/C anode electrocatalysts for direct borohydride fuel cell. J. Power Sources 2010, 195, 185–189. [Google Scholar] [CrossRef]
- Huang, H.; Hu, X.; Zhang, J.; Su, N.; Cheng, J. Facile Fabrication of Platinum-Cobalt Alloy Nanoparticles with Enhanced Electrocatalytic Activity for a Methanol Oxidation Reaction. Sci. Rep. 2017, 7, 45555. [Google Scholar] [CrossRef]
- Shen, C.-T.; Wang, K.-W.; Tseng, C.-J.; Lee, K.-R.; Hsueh, Y.-J. The oxygen reduction reaction of ordered porous carbon-supported PtSn catalysts. RSC Adv. 2016, 6, 44205–44211. [Google Scholar] [CrossRef]
- Deivaraj, T.C.; Chen, W.; Lee, J.Y. Preparation of PtNi nanoparticles for the electrocatalytic oxidation of methanol. J. Mater. Chem. 2003, 13, 2555–2560. [Google Scholar] [CrossRef]
- Nie, L.; Yu, J.; Fu, J. Complete Decomposition of Formaldehyde at Room Temperature over a Platinum-Decorated Hierarchically Porous Electrospun Titania Nanofiber Mat. ChemCatChem 2014, 6, 1983–1989. [Google Scholar] [CrossRef]
- Chen, B.-B.; Zhu, X.-B.; Crocker, M.; Wang, Y.; Shi, C. Complete oxidation of formaldehyde at ambient temperature over γ-Al2O3 supported Au catalyst. Catal. Commun. 2013, 42, 93–97. [Google Scholar] [CrossRef]
- Huo, Y.; Wang, X.; Rui, Z.; Yang, X.; Ji, H. Identification of the Nearby Hydroxyls’ Role in Promoting HCHO Oxidation over a Pt Catalyst. Ind. Eng. Chem. Res. 2018, 57, 8183–8189. [Google Scholar] [CrossRef]
- Araña, J.; Garriga i Cabo, C.; Doña-Rodríguez, J.M.; González-Díaz, O.; Herrera-Melián, J.A.; Pérez-Peña, J. FTIR study of formic acid interaction with TiO2 and TiO2 doped with Pd and Cu in photocatalytic processes. Appl. Surf. Sci. 2004, 239, 60–71. [Google Scholar] [CrossRef]
- Zhu, X.; Shen, M.; Lobban, L.L.; Mallinson, R.G. Structural effects of Na promotion for high water gas shift activity on Pt–Na/TiO2. J. Catal. 2011, 278, 123–132. [Google Scholar] [CrossRef]

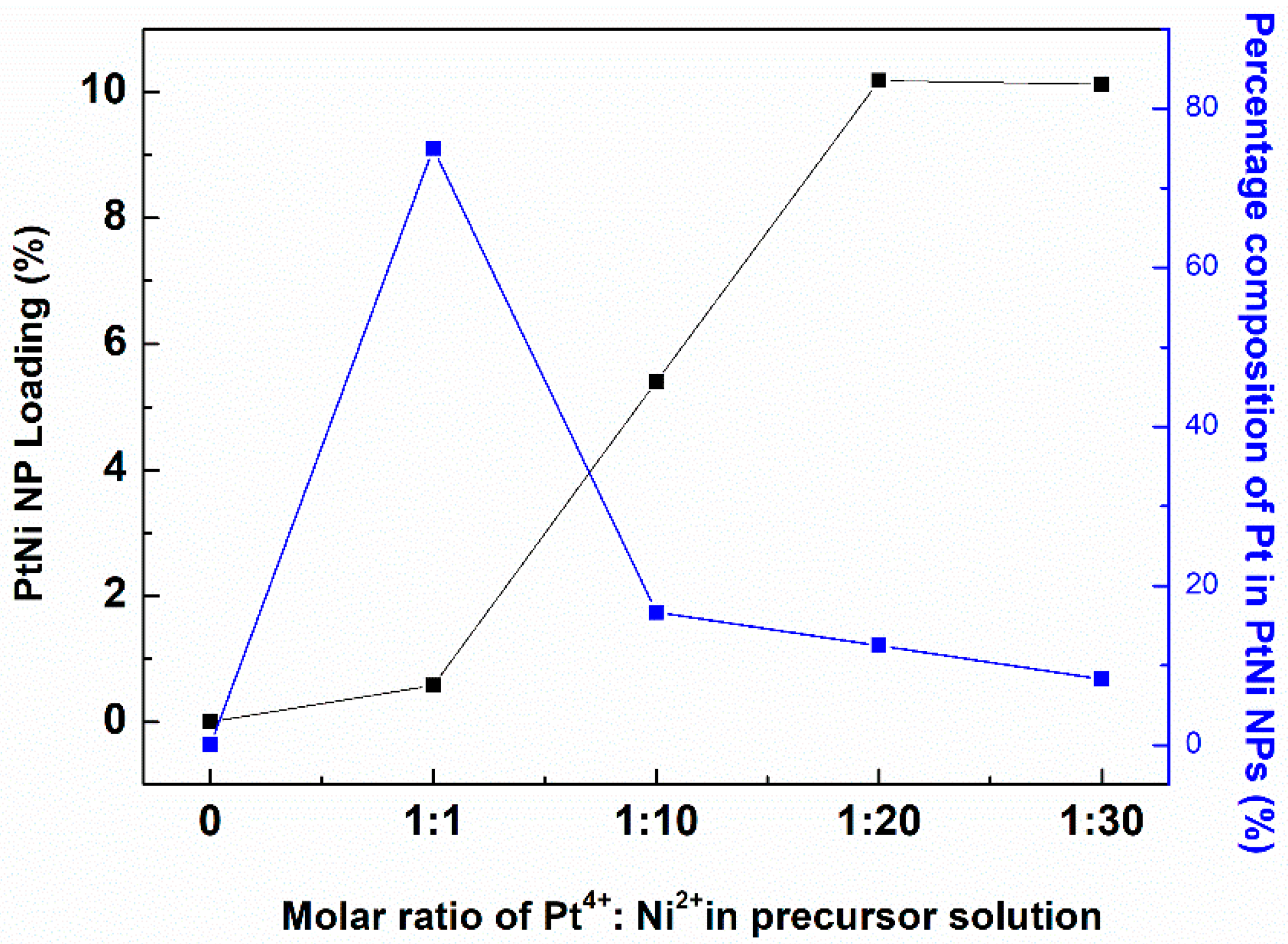
| Sample ID | Precursor Solution a | Deposition of PtNi NPs b | |||||
|---|---|---|---|---|---|---|---|
| Content (mM) | Pt4+:Ni2+ | Loading Capacity (wt %)- | Pt:Ni | ||||
| Pt4+ | Ni2+ | Pt | Ni | PtNi NPs | |||
| PtNi/PMMA@PDA-1/1 | 0.494 | 0.494 | 1:1 | 0.53 | 0.05 | 0.58 | 3:1 |
| PtNi/PMMA@PDA-1/10 | 0.494 | 4.940 | 1:10 | 2.16 | 3.24 | 5.40 | 1:5 |
| PtNi/PMMA@PDA-1/20 | 0.494 | 9.880 | 1:20 | 3.19 | 6.99 | 10.18 | 1:7 |
| PtNi/PMMA@PDA-1/30 | 0.494 | 14.82 | 1:30 | 2.4 | 7.72 | 10.12 | 1:11 |
| Pt/PMMA@PDA-1/0 | 0.494 | 0.000 | 1:0 | 0.23 | --- | 0.23 | --- |
| Ni/PMMA@PDA-0/10 | 0.000 | 4.940 | 0:10 | --- | 5.95 | 5.95 | --- |
| Sample ID | Molar Ratio of Pt:Ni in PtNi NPs | HCHO Conversion(%) |
|---|---|---|
| PtNi/PMMA@PDA-1/1 | 3:1 | 26% |
| PtNi/PMMA@PDA-1/10 | 1:5 | 90% |
| PtNi/PMMA@PDA-1/20 | 1:7 | 66% |
| PtNi/PMMA@PDA-1/30 | 1:11 | 25% |
| Pt/PMMA@PDA-1/0 | 1:0 | 17% |
| Ni/PMMA@PDA-0/10 | 0:10 | <5% |
| Sample ID | Type of Reactor | HCHO Conc. (ppm) | Flow Rate (mL/min) | Volume of Catalyst (mL) | Weight of Catalyst (g) | GHSV (h−1) | HCHO Oxidation Efficiency (mgHCHO g−1 h−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| PtNi/PMMA@PDA | Fixed bed reactor | 10 | 100 | 0.25 | 0.02 | 24 000 | 3.75 | This work |
| Au/ZrO2/PET | Fixed bed reactor | 0.5 | 200 | 1.5 | 0.3 | 8 000 | 0.025 | Ref. [12] |
| MnOx/PET | Fixed bed reactor | 0.48 | 1000 | 3.5 | 0.5 | 17 000 | 0.072 | Ref. [13] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, F.-G.; Du, B.; Sharma, G.; Stadler, F.J. Highly Efficient Polydopamine-coated Poly(methyl methacrylate) Nanofiber Supported Platinum–Nickel Bimetallic Catalyst for Formaldehyde Oxidation at Room Temperature. Polymers 2019, 11, 674. https://doi.org/10.3390/polym11040674
He F-G, Du B, Sharma G, Stadler FJ. Highly Efficient Polydopamine-coated Poly(methyl methacrylate) Nanofiber Supported Platinum–Nickel Bimetallic Catalyst for Formaldehyde Oxidation at Room Temperature. Polymers. 2019; 11(4):674. https://doi.org/10.3390/polym11040674
Chicago/Turabian StyleHe, Fa-Gui, Bing Du, Gaurav Sharma, and Florian J. Stadler. 2019. "Highly Efficient Polydopamine-coated Poly(methyl methacrylate) Nanofiber Supported Platinum–Nickel Bimetallic Catalyst for Formaldehyde Oxidation at Room Temperature" Polymers 11, no. 4: 674. https://doi.org/10.3390/polym11040674
APA StyleHe, F.-G., Du, B., Sharma, G., & Stadler, F. J. (2019). Highly Efficient Polydopamine-coated Poly(methyl methacrylate) Nanofiber Supported Platinum–Nickel Bimetallic Catalyst for Formaldehyde Oxidation at Room Temperature. Polymers, 11(4), 674. https://doi.org/10.3390/polym11040674








