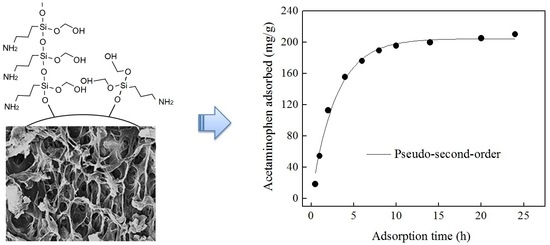
Ultrasound-Assisted Preparation of Chitosan/Nano-Activated Carbon Composite Beads Aminated with (3-Aminopropyl)Triethoxysilane for Adsorption of Acetaminophen from Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Adsorbent
2.3. Characterization of Adsorbents
2.4. Adsorption and Desorption Experiments
3. Results
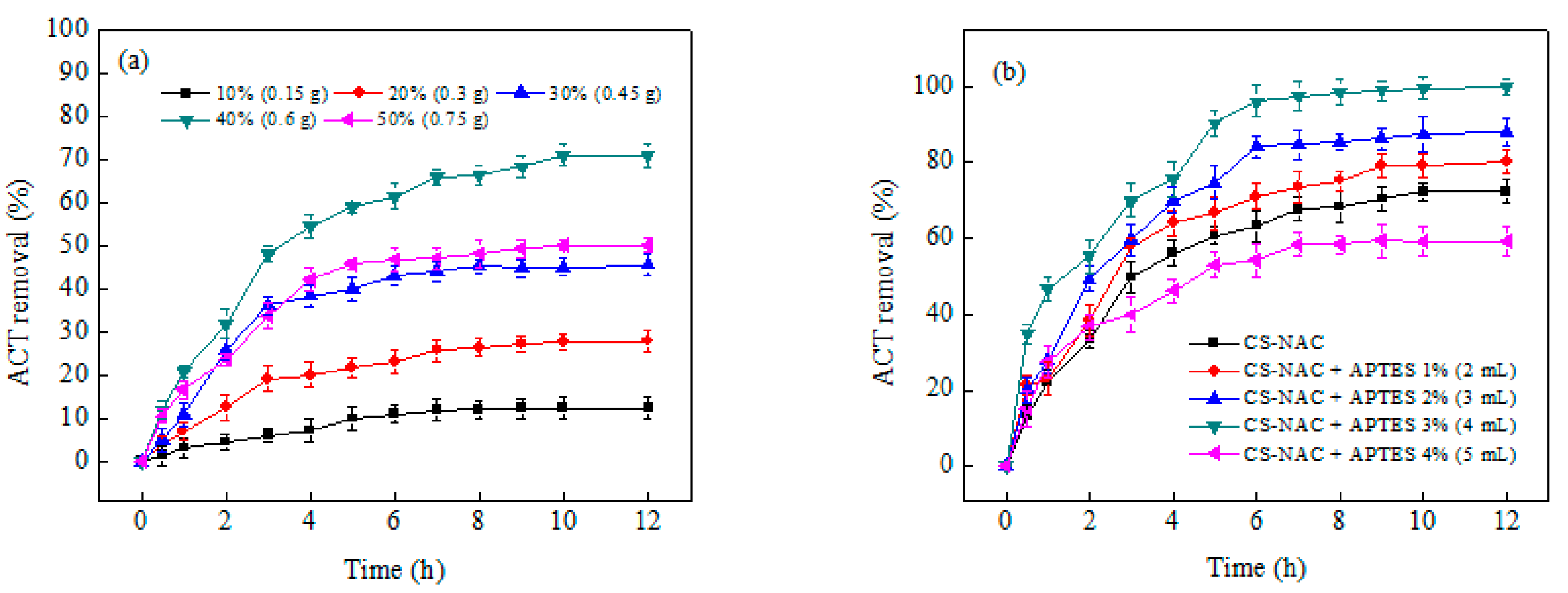
3.1. Effect of NAC Concentration
3.2. Effect of APTES Concentration
3.3. Characterization
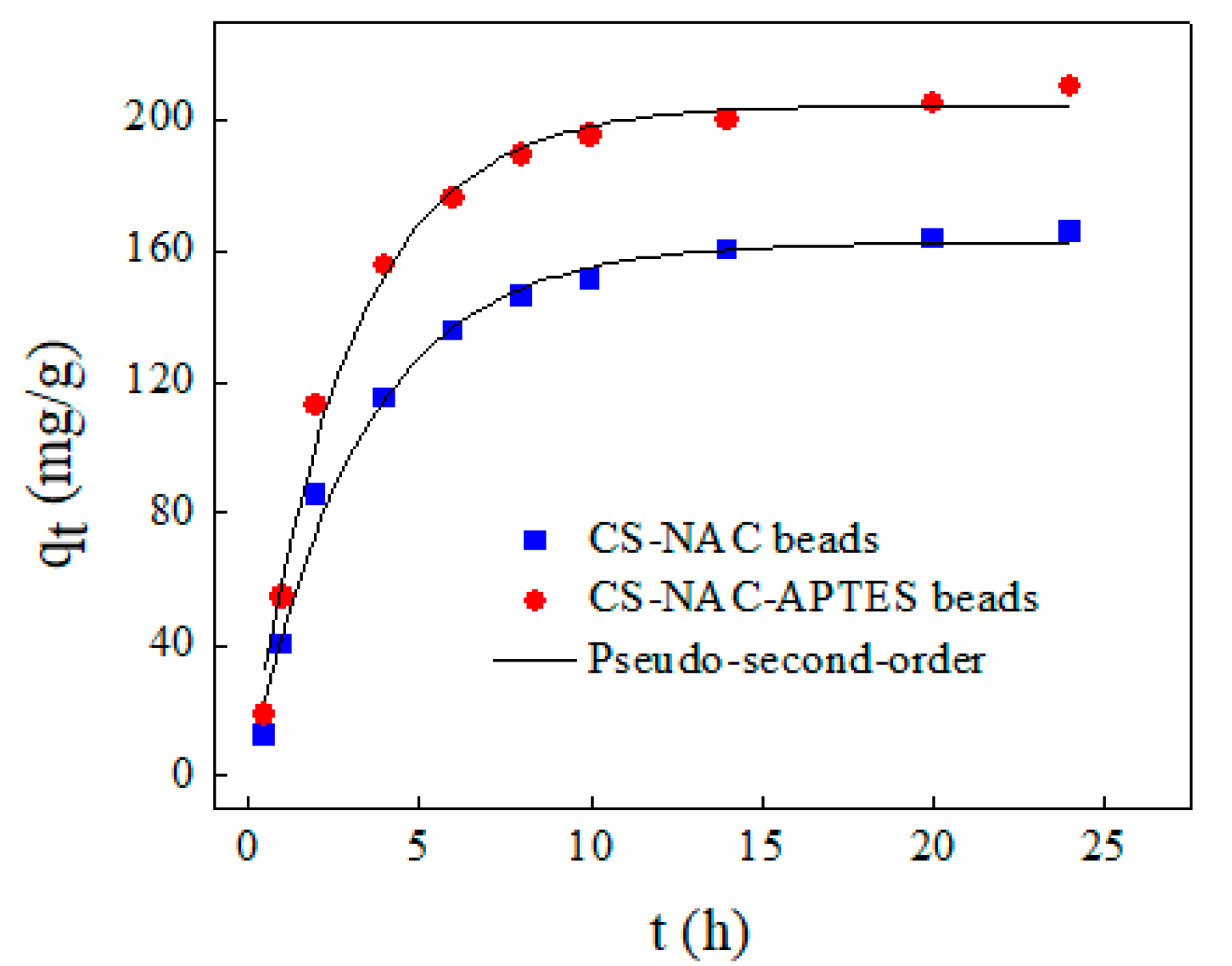
3.4. Adsorption Kinetics
3.5. Adsorption Isotherm
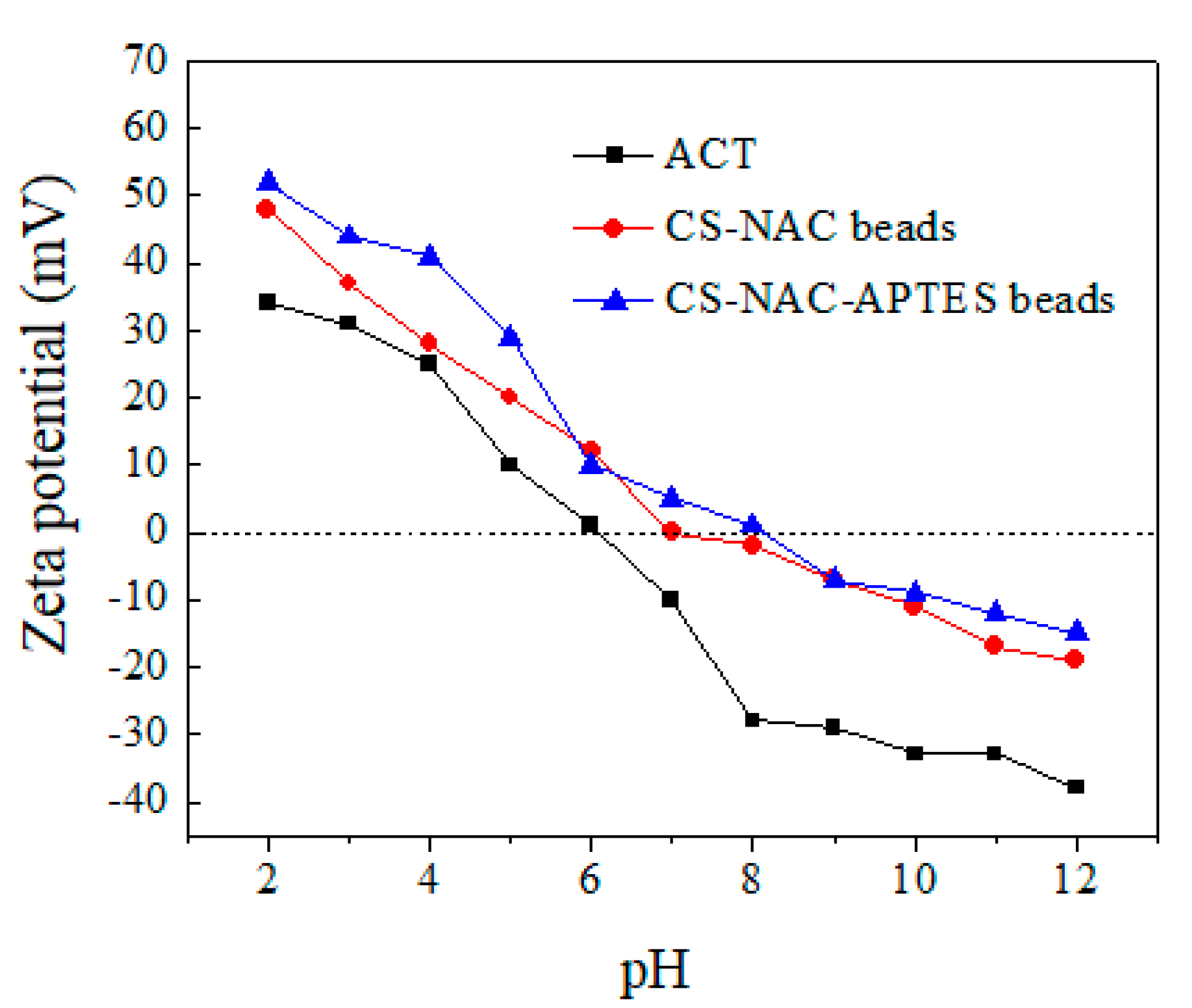
3.6. Adsorption Mechanism
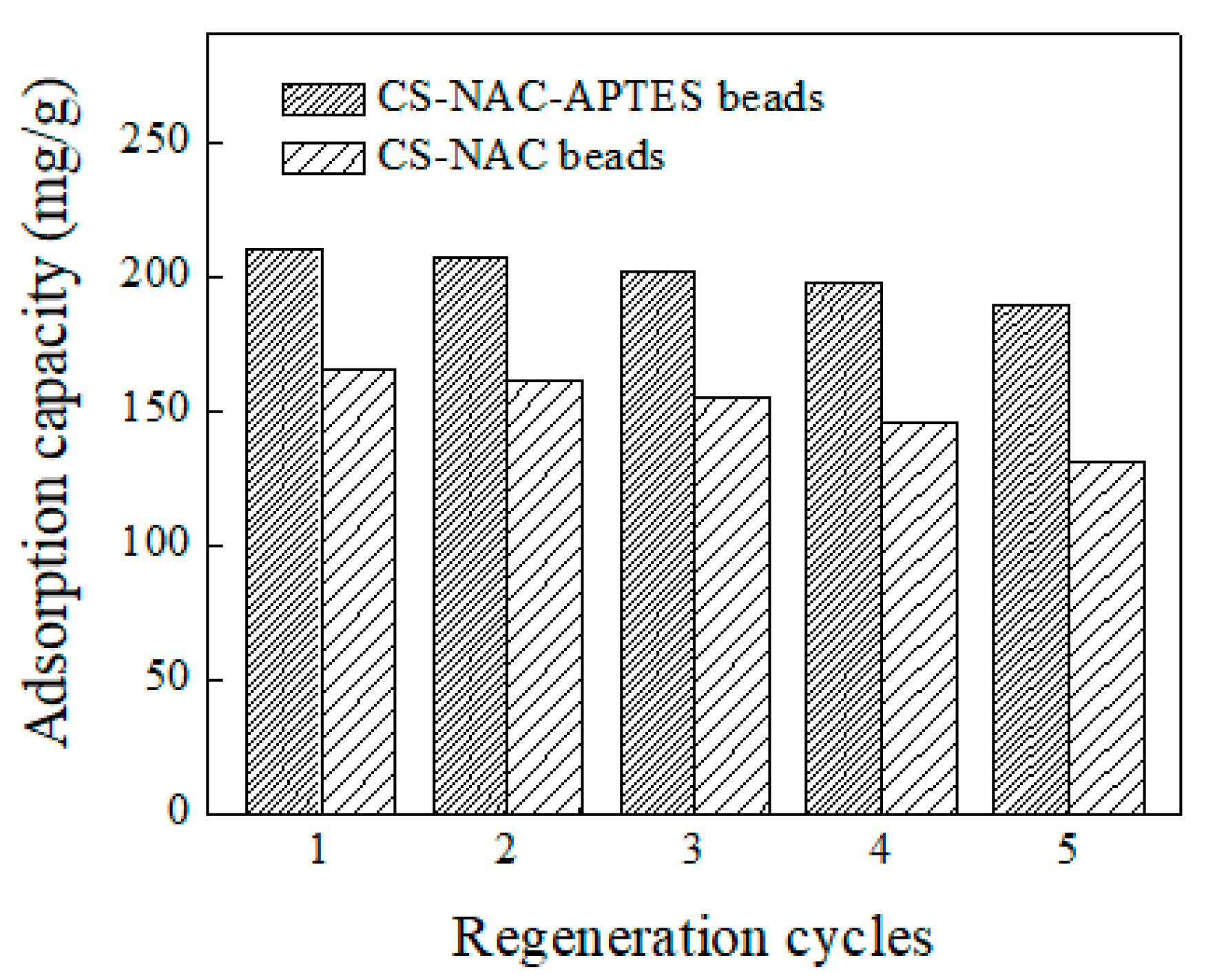
3.7. Reusability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.; Wang, B.; Cagnetta, G.; Duan, L.; Yang, J.; Deng, S.; Huang, J.; Wang, Y.; Yu, G. Typical pharmaceuticals in major WWTPs in Beijing, China: Occurrence, load pattern and calculation reliability. Water Res. 2018, 140, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Dudley, S.; McGinnis, M.; Trumble, J.; Gan, J. Acetaminophen detoxification in cucumber plants via induction of glutathione S-transferases. Sci. Total Environ. 2019, 649, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, J.; Lu, X.; Xu, W.; Gong, Q.; Ding, J.; Dan, B.; Xie, P. Removal of acetaminophen in the Fe2+/persulfate system: Kinetic model and degradation pathways. Chem. Eng. J. 2019, 358, 1091–1100. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, K.; Jung, J.; Park, S.; Kim, P.-G.; Park, J. Aquatic toxicity of acetaminophen, carbamazepine, cimetidine, diltiazem and six major sulfonamides, and their potential ecological risks in Korea. Environ. Int. 2007, 33, 370–375. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Abdullah, A.Z.; Ibrahim, M.H.; Tan, K.B.; Gholami, Z.; Amouzgar, P. Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review. Carbohydr. Polym. 2014, 113, 115–130. [Google Scholar] [CrossRef]
- Gkika, D.A.; Liakos, E.V.; Vordos, N.; Kontogoulidou, C.; Magafas, L.; Bikiaris, D.N.; Bandekas, D.V.; Mitropoulos, A.C.; Kyzas, G.Z. Cost estimation of polymeric adsorbents. Polymers 2019, 11, 925. [Google Scholar] [CrossRef]
- Jun, B.-M.; Kim, Y.; Han, J.; Yoon, Y.; Kim, J.; Park, C.M. Preparation of activated biochar-supported magnetite composite for adsorption of polychlorinated phenols from aqueous solutions. Water 2019, 11, 1899. [Google Scholar] [CrossRef]
- Vakili, M.; Cagnetta, G.; Huang, J.; Yu, G.; Yuan, J. Synthesis and regeneration of a MXene-based pollutant adsorbent by mechanochemical methods. Molecules 2019, 24, 2478. [Google Scholar] [CrossRef]
- Kuroiwa, T.; Takada, H.; Shogen, A.; Saito, K.; Kobayashi, I.; Uemura, K.; Kanazawa, A. Cross-linkable chitosan-based hydrogel microbeads with pH-responsive adsorption properties for organic dyes prepared using size-tunable microchannel emulsification technique. Colloids Surface A 2017, 514, 69–78. [Google Scholar] [CrossRef]
- Li, G.; Rwo, K.H. Hydrophilic molecularly imprinted chitosan based on deep eutectic solvents for the enrichment of gallic acid in red ginseng tea. Polymers 2019, 11, 1434. [Google Scholar] [CrossRef]
- Cheaburu-Yilmaz, C.N.; Yilmaz, O.; Aydin Kose, F.; Bibire, N. Chitosan-graft-poly(n-isopropylacrylamide)/pva cryogels as carriers for mucosal delivery of voriconazole. Polymers 2019, 11, 1432. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhao, H.; Chen, S.; Long, F.; Huang, B.; Yang, B.; Pan, X. A magnetically recyclable chitosan composite adsorbent functionalized with EDTA for simultaneous capture of anionic dye and heavy metals in complex wastewater. Chem. Eng. J. 2019, 356, 69–80. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Deliyanni, E.A.; Bikiaris, D.N.; Mitropoulos, A.C. Graphene composites as dye adsorbents: Review. Chem. Eng. Res. Des. 2018, 129, 75–88. [Google Scholar] [CrossRef]
- Rengga, W.D.P.; Chafidz, A.; Sudibandriyo, M.; Nasikin, M.; Abasaeed, A.E. Silver nano-particles deposited on bamboo-based activated carbon for removal of formaldehyde. J. Environ. Chem. Eng. 2017, 5, 1657–1665. [Google Scholar] [CrossRef]
- Zheng, Y.; Cheng, B.; You, W.; Yu, J.; Ho, W. 3D hierarchical graphene oxide-NiFe LDH composite with enhanced adsorption affinity to Congo red, methyl orange and Cr(VI) ions. J. Hazard. Mater. 2019, 369, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-R.; Xu, X.; Teng, J.; Zhou, N.; Zhou, Z.; Jiang, X.-Y.; Jiao, F.-P.; Yu, J.-G. Three-dimensional porous graphene oxide-maize amylopectin composites with controllable pore-sizes and good adsorption-desorption properties: Facile fabrication and reutilization, and the adsorption mechanism. Ecotoxicol. Environ. Saf. 2019, 176, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Li, Z.; Tan, W.-Z.; Liu, X.-H.; Sun, Z.-F.; Ren, P.-G.; Yan, D.-X. Facile preparation of 3D regenerated cellulose/graphene oxide composite aerogel with high-efficiency adsorption towards methylene blue. J. Colloid Interface Sci. 2018, 532, 58–67. [Google Scholar] [CrossRef]
- Shokry, H.; Elkady, M.; Hamad, H. Nano activated carbon from industrial mine coal as adsorbents for removal of dye from simulated textile wastewater: Operational parameters and mechanism study. J. Mater. Res. Technol. 2019, 8, 4477–4488. [Google Scholar] [CrossRef]
- Amouzgar, P.; Chan, E.-S.; Salamatinia, B. Effects of ultrasound on development of Cs/NAC nano composite beads through extrusion dripping for acetaminophen removal from aqueous solution. J. Clean. Prod. 2017, 165, 537–551. [Google Scholar] [CrossRef]
- Amouzgar, P.; Vakili, M.; Chan, E.-S.; Salamatinia, B. Effects of beading parameters for development of chitosan-nano-activated carbon biocomposite for acetaminophen elimination from aqueous sources. Environ. Eng. Sci. 2017, 34, 805–815. [Google Scholar] [CrossRef]
- Liang, X.X.; Ouyang, X.-K.; Wang, S.; Yang, L.-Y.; Huang, F.; Ji, C.; Chen, X. Efficient adsorption of Pb(II) from aqueous solutions using aminopropyltriethoxysilane-modified magnetic attapulgite@chitosan (APTS-Fe3O4/APT@CS) composite hydrogel beads. Int. J. Biol. Macromol. 2019, 137, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Lladó, J.; Solé-Sardans, M.; Lao-Luque, C.; Fuente, E.; Ruiz, B. Removal of pharmaceutical industry pollutants by coal-based activated carbons. Process Saf. Environ. 2016, 104, 294–303. [Google Scholar] [CrossRef]
- Vakili, M.; Mojiri, A.; Zwain, H.M.; Yuan, J.; Giwa, A.S.; Wang, W.; Gholami, F.; Guo, X.; Cagnetta, G.; Yu, G. Effect of beading parameters on cross-linked chitosan adsorptive properties. React. Funct. Polym. 2019, 144, 104354. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Ibrahim, M.H.; Abdullah, A.Z. Elimination of reactive blue 4 from aqueous solutions using 3-aminopropyl triethoxysilane modified chitosan beads. Carbohydr. Polym. 2015, 132, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Mahaninia, M.H.; Wilson, L.D. Phosphate uptake studies of cross-linked chitosan bead materials. J. Colloid Interface Sci. 2017, 485, 201–212. [Google Scholar] [CrossRef]
- Rahman, I.A.; Padavettan, V. Synthesis of silica nanoparticles by sol-gel: Size-dependent properties, surface modification, and applications in silica-polymer nanocomposites—a review. J. Nanomater. 2012, 2012, 1–15. [Google Scholar] [CrossRef]
- Ren, L.; Xu, J.; Zhang, Y.; Zhou, J.; Chen, D.; Chang, Z. Preparation and characterization of porous chitosan microspheres and adsorption performance for hexavalent chromium. Int. J. Biol. Macromol. 2019, 135, 898–906. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Ibrahim, M.H.; Abdullah, A.Z.; Gholami, Z.; Salamatinia, B. Enhancing reactive blue 4 adsorption through chemical modification of chitosan with hexadecylamine and 3-aminopropyl triethoxysilane. J. Water Process. Eng. 2017, 15, 49–54. [Google Scholar] [CrossRef]
- He, X.; Tao, R.; Zhou, T.; Wang, C.; Xie, K. Structure and properties of cotton fabrics treated with functionalized dialdehyde chitosan. Carbohydr. Polym. 2014, 103, 558–565. [Google Scholar] [CrossRef]
- Nayak, N.; Huertas, R.; Crespo, J.G.; Portugal, C.A.M. Surface modification of alumina monolithic columns with 3-aminopropyltetraethoxysilane (APTES) for protein attachment. Sep. Purif. Technol. 2019, 229, 115674. [Google Scholar] [CrossRef]
- Badnore, A.U.; Chaudhari, A.P.; Patel, J.K.; Pandit, A.B. Effect of solvents on properties of the ultrasound assisted synthesized ceria nanoparticles and its performance as an adsorbent. Adv. Powder Technol. 2019, 30, 1058–1066. [Google Scholar] [CrossRef]
- Singh, I.; Birajdar, B. Effective La-Na Co-doped TiO2 nano-particles for dye adsorption: Synthesis, characterization and study on adsorption kinetics. Nanomaterials 2019, 9, 400. [Google Scholar] [CrossRef] [PubMed]
- Vakili, M.; Rafatullah, M.; Ibrahim, M.H.; Abdullah, A.Z.; Salamatinia, B.; Gholami, Z. Chitosan hydrogel beads impregnated with hexadecylamine for improved reactive blue 4 adsorption. Carbohydr. Polym. 2016, 137, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Nie, T.; Hao, P.; Zhao, Z.; Zhou, W.; Zhu, L. Effect of oxidation-induced aging on the adsorption and co-adsorption of tetracycline and Cu2+ onto biochar. Sci. Total Environ. 2019, 637, 522–532. [Google Scholar] [CrossRef]
- Vakili, M.; Deng, S.; Li, T.; Wang, W.; Wang, W.; Yu, G. Novel crosslinked chitosan for enhanced adsorption of hexavalent chromium in acidic solution. Chem. Eng. J. 2018, 347, 782–790. [Google Scholar] [CrossRef]
- Vakili, M.; Deng, S.; Cagnetta, G.; Wang, W.; Meng, P.; Liu, D.; Yu, G. Regeneration of chitosan-based adsorbents used in heavy metal adsorption: A review. Sep. Purif. Technol. 2019, 224, 373–387. [Google Scholar] [CrossRef]
- Yanyan, L.; Kurniawan, T.A.; Albadarin, A.B.; Walker, G. Enhanced removal of acetaminophen from synthetic wastewater using multi-walled carbon nanotubes (MWCNTs) chemically modified with NaOH, HNO3/H2SO4, ozone, and/or chitosan. J. Mol. Liq. 2018, 251, 369–377. [Google Scholar] [CrossRef]
- Vakili, M.; Deng, S.; Shen, L.; Shan, D.; Liu, D.; Yu, G. Regeneration of chitosan-based adsorbents for eliminating dyes from aqueous solutions. Sep. Purif. Rev. 2019, 48, 1–13. [Google Scholar] [CrossRef]
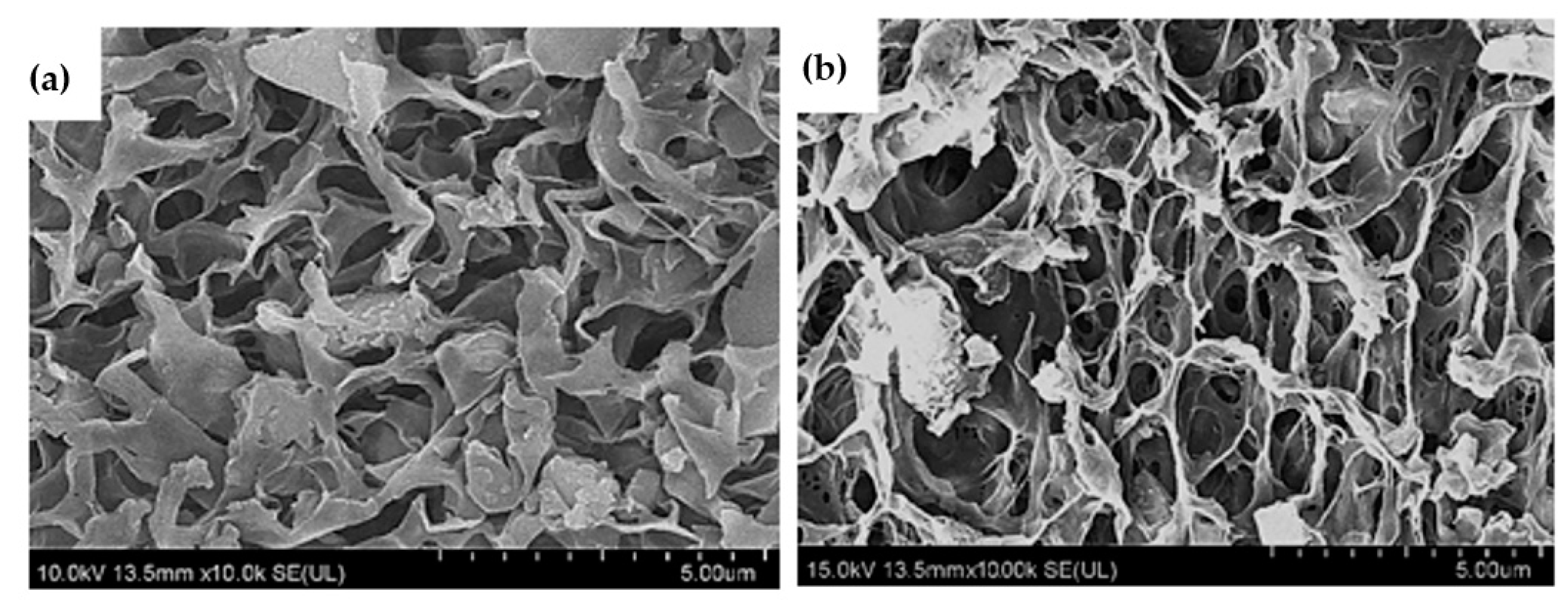
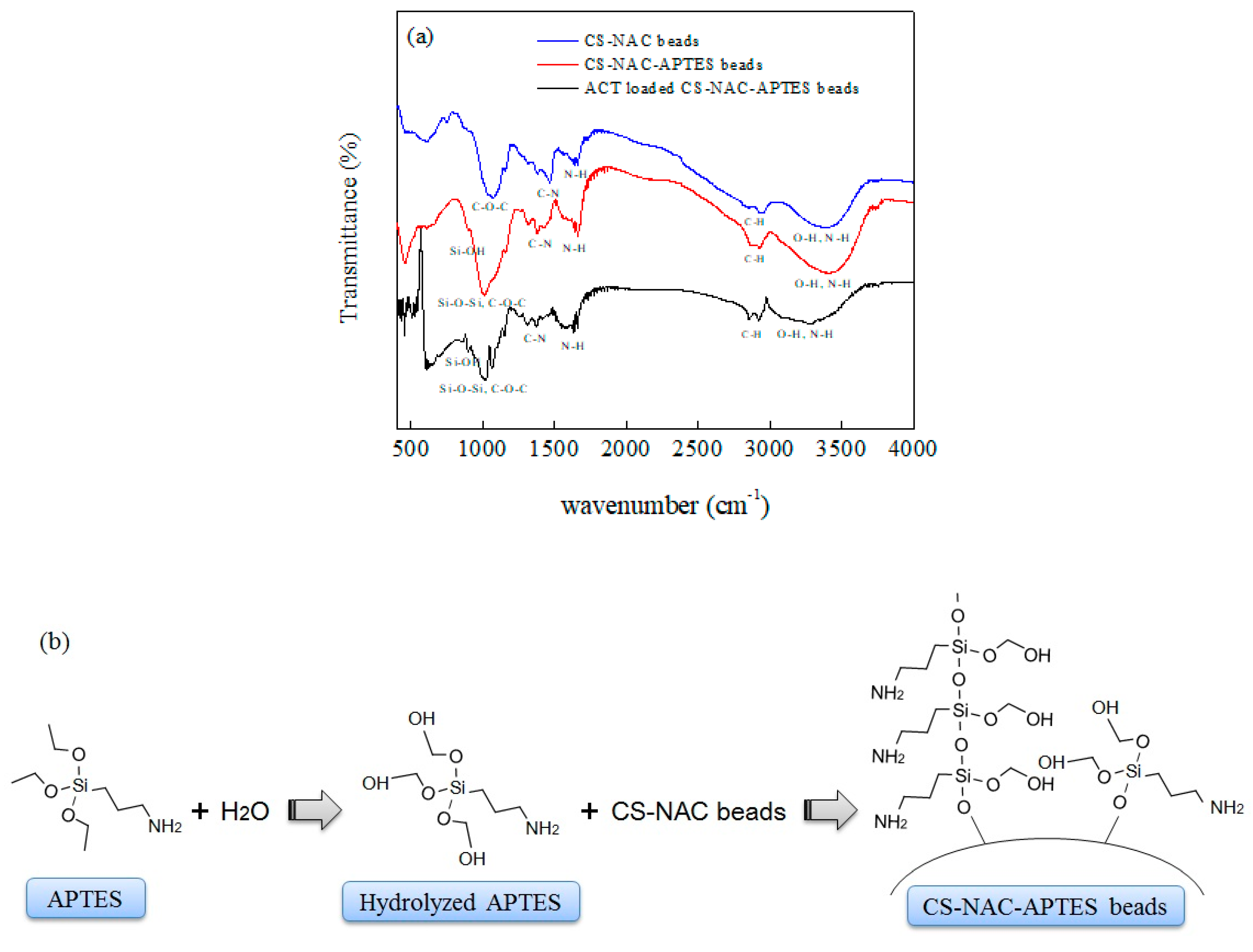

| Sample | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|
| CS-NAC beads | 70.91 | 0.070 | 6.64 |
| CS-NAC-APTES beads | 82.32 | 0.072 | 6.96 |
| Kinetic Model | Adsorbent | |
|---|---|---|
| CS-NAC Beads | CS-NAC-APTES Beads | |
| Pseudo-First-Order Model | ||
| C0 (mg/L) | 200 | 200 |
| qexp (mg/g) | 165.78 | 210.19 |
| qcal (mg/g) | 192.09 | 239.5 |
| (1/min) | 0.307 | 0.344 |
| 0.977 | 0.973 | |
| 65.44 | 123.3 | |
| Pseudo-Second-Order Model | ||
| C0 (mg/L) | 200 | 200 |
| qexp (mg/g) | 165.78 | 210.19 |
| qcal (mg/g) | 162.5 | 204.5 |
| (mg/mg/h) | 66.89 | 94.78 |
| 0.988 | 0.988 | |
| 35.9 | 51.175 | |
| Adsorbent | Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|---|
| b (L/mg) | KF (mg/g) | n | ||||||
| CS-NAC beads | 278.4 | 142.5 | 0.976 | 62.88 | 7.36 | 1.69 | 0.996 | 10.62 |
| CS-NAC-APTES beads | 407.83 | 177.99 | 0.894 | 448.83 | 9.6 | 1.7 | 0.956 | 186.28 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vakili, M.; Amouzgar, P.; Cagnetta, G.; Wang, B.; Guo, X.; Mojiri, A.; Zeimaran, E.; Salamatinia, B. Ultrasound-Assisted Preparation of Chitosan/Nano-Activated Carbon Composite Beads Aminated with (3-Aminopropyl)Triethoxysilane for Adsorption of Acetaminophen from Aqueous Solutions. Polymers 2019, 11, 1701. https://doi.org/10.3390/polym11101701
Vakili M, Amouzgar P, Cagnetta G, Wang B, Guo X, Mojiri A, Zeimaran E, Salamatinia B. Ultrasound-Assisted Preparation of Chitosan/Nano-Activated Carbon Composite Beads Aminated with (3-Aminopropyl)Triethoxysilane for Adsorption of Acetaminophen from Aqueous Solutions. Polymers. 2019; 11(10):1701. https://doi.org/10.3390/polym11101701
Chicago/Turabian StyleVakili, Mohammadtaghi, Parisa Amouzgar, Giovanni Cagnetta, Baozhen Wang, Xiaogang Guo, Amin Mojiri, Ehsan Zeimaran, and Babak Salamatinia. 2019. "Ultrasound-Assisted Preparation of Chitosan/Nano-Activated Carbon Composite Beads Aminated with (3-Aminopropyl)Triethoxysilane for Adsorption of Acetaminophen from Aqueous Solutions" Polymers 11, no. 10: 1701. https://doi.org/10.3390/polym11101701
APA StyleVakili, M., Amouzgar, P., Cagnetta, G., Wang, B., Guo, X., Mojiri, A., Zeimaran, E., & Salamatinia, B. (2019). Ultrasound-Assisted Preparation of Chitosan/Nano-Activated Carbon Composite Beads Aminated with (3-Aminopropyl)Triethoxysilane for Adsorption of Acetaminophen from Aqueous Solutions. Polymers, 11(10), 1701. https://doi.org/10.3390/polym11101701