Recent Advances in Polymeric Nanocomposites of Metal-Organic Frameworks (MOFs)
Abstract
1. Introduction
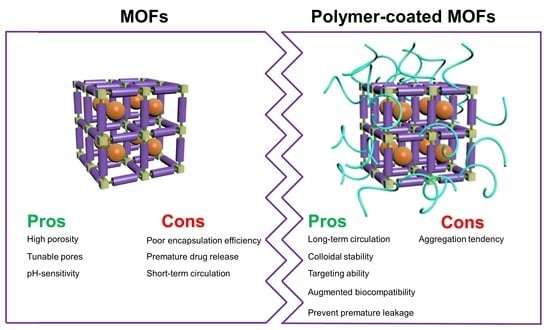
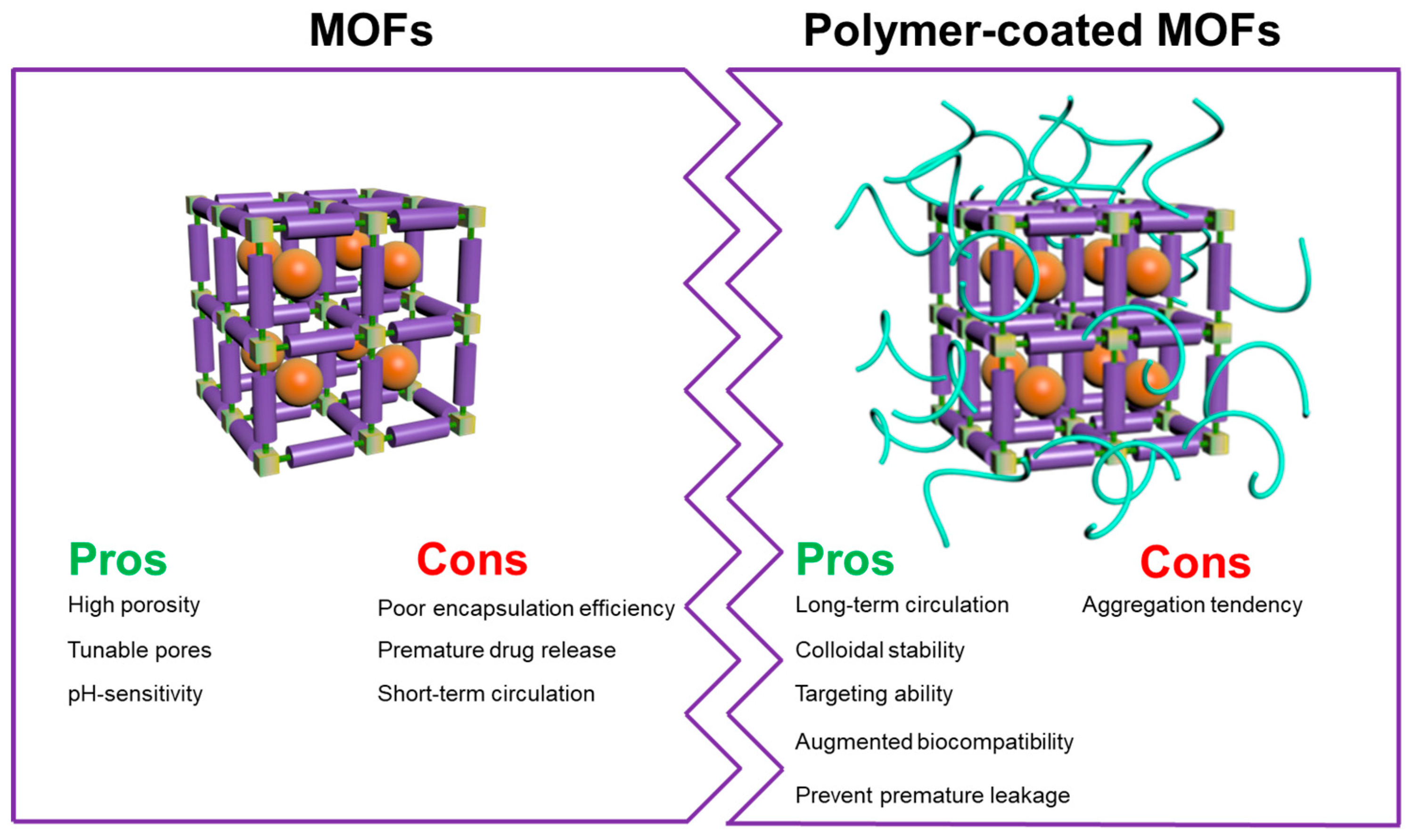
2. Surface-Coated MOFs
2.1. Chitosan
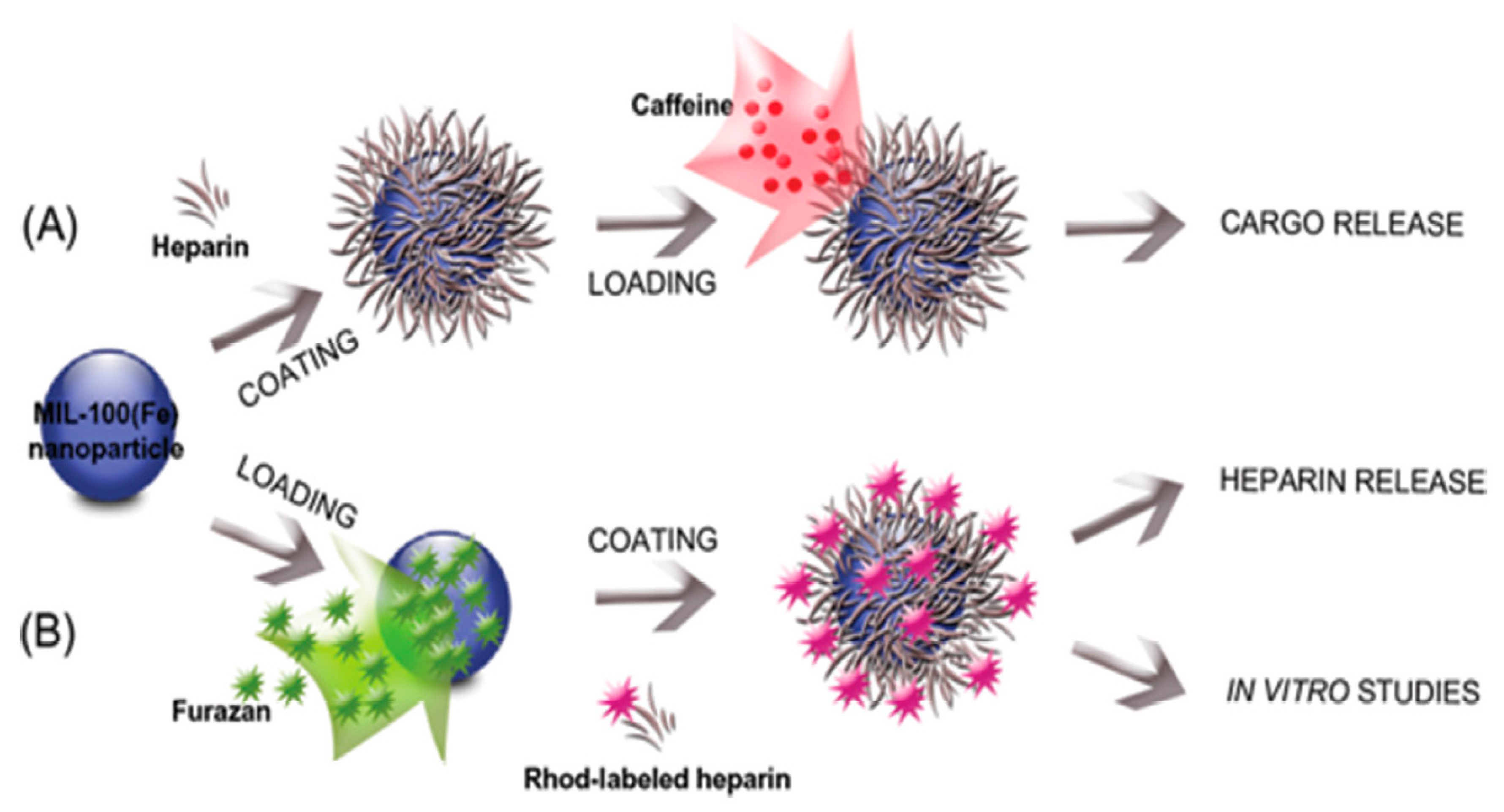
2.2. Heparin
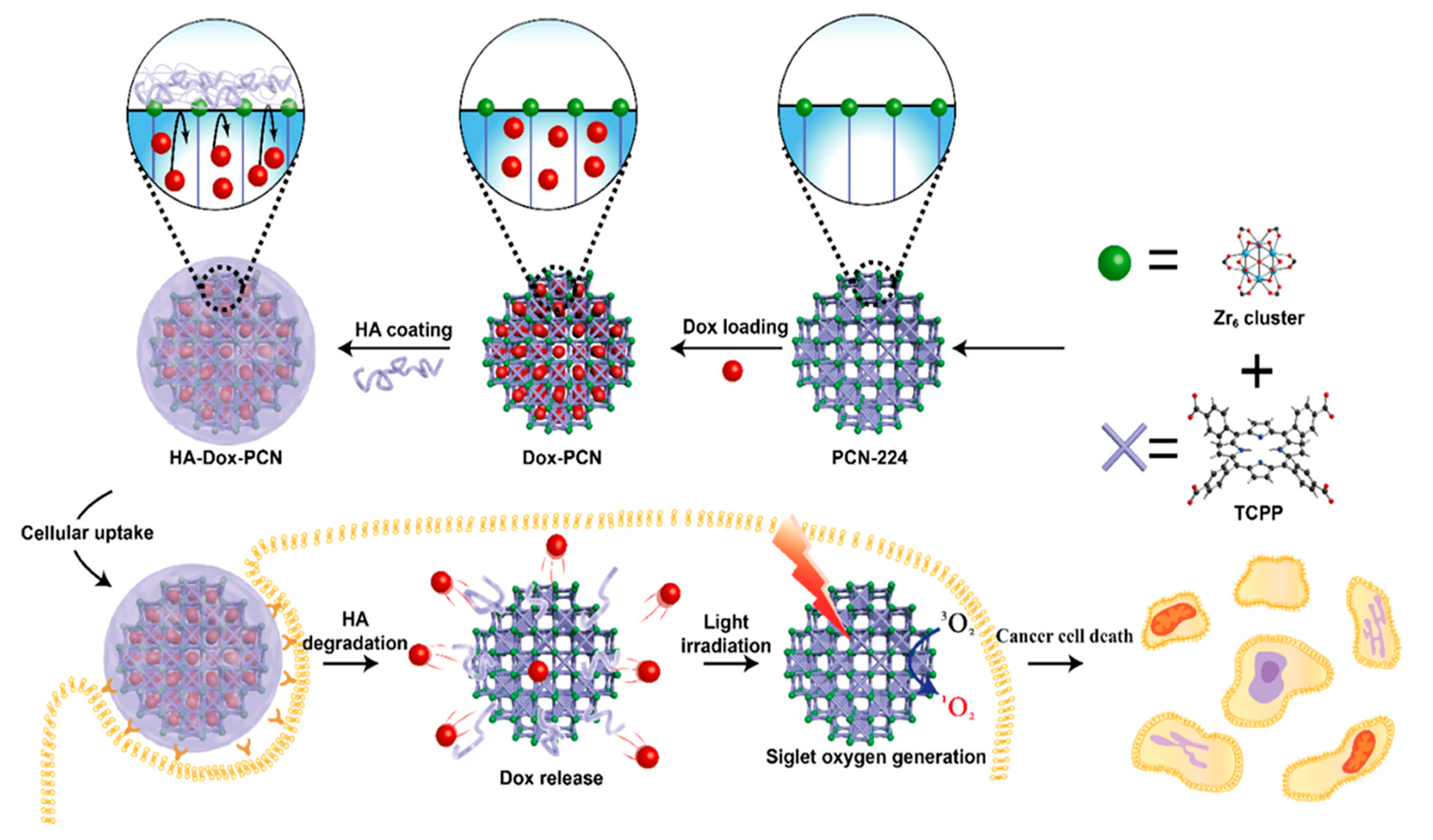
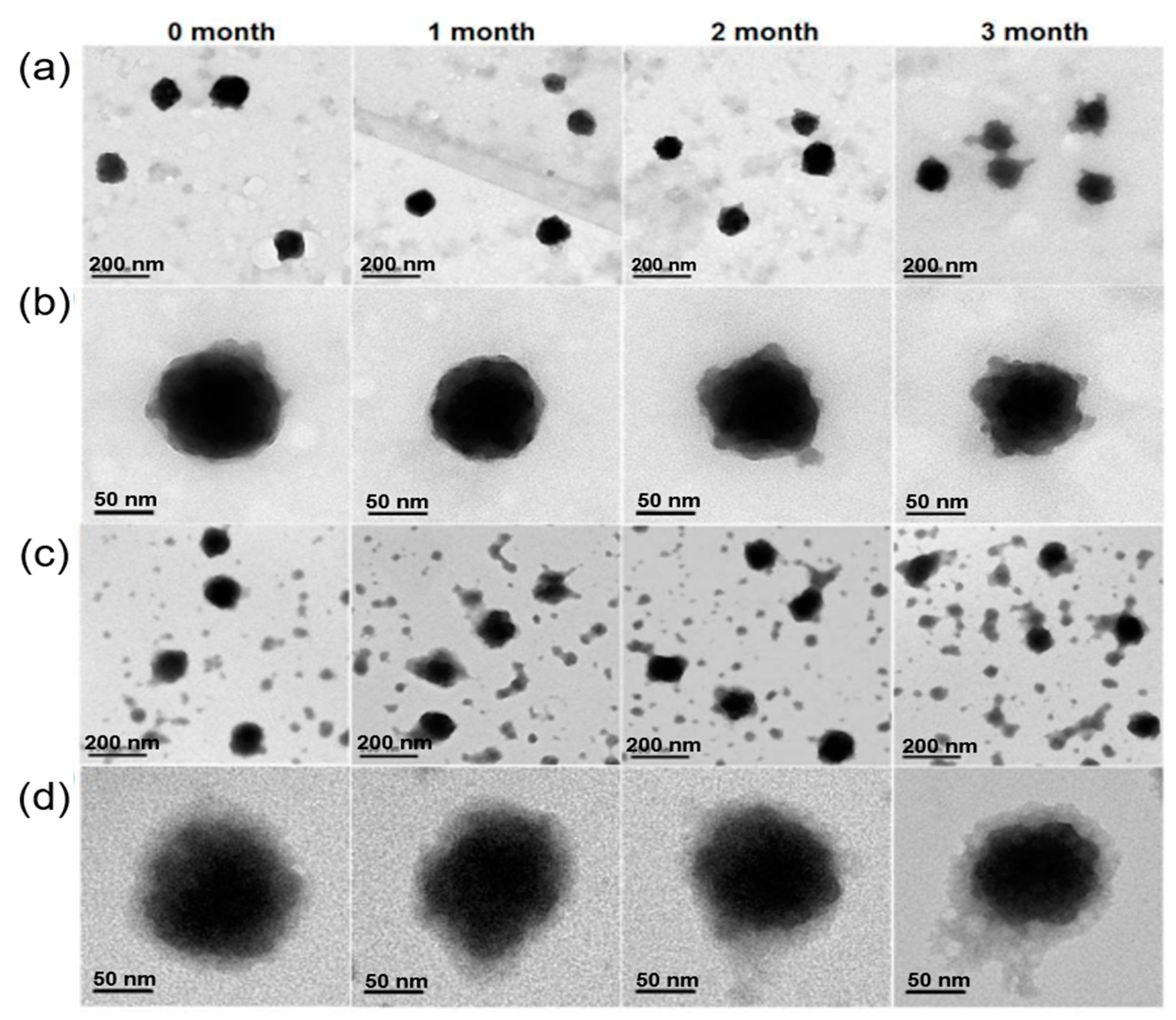
2.3. Hyaluronic acid
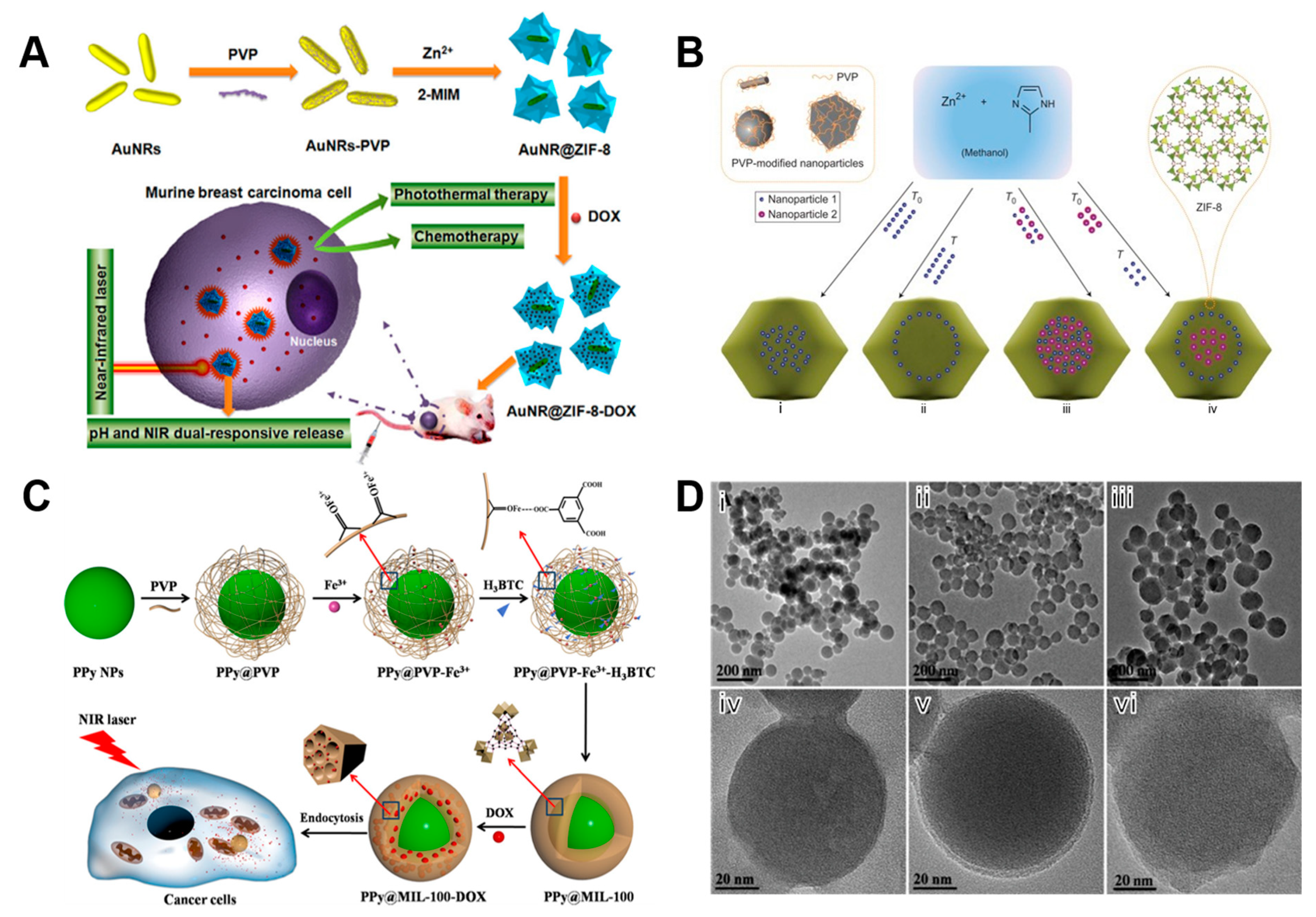
2.4. PVP
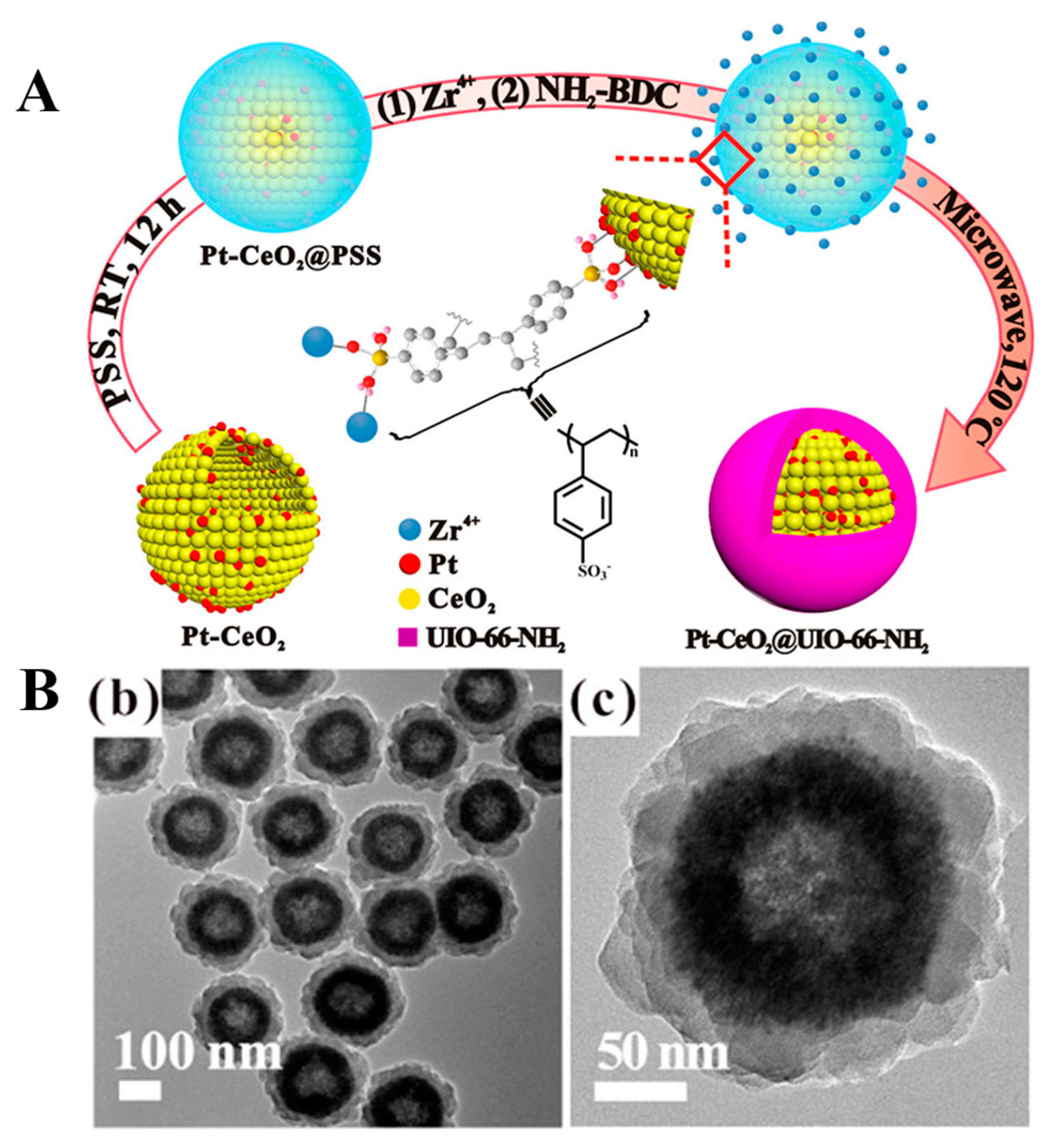
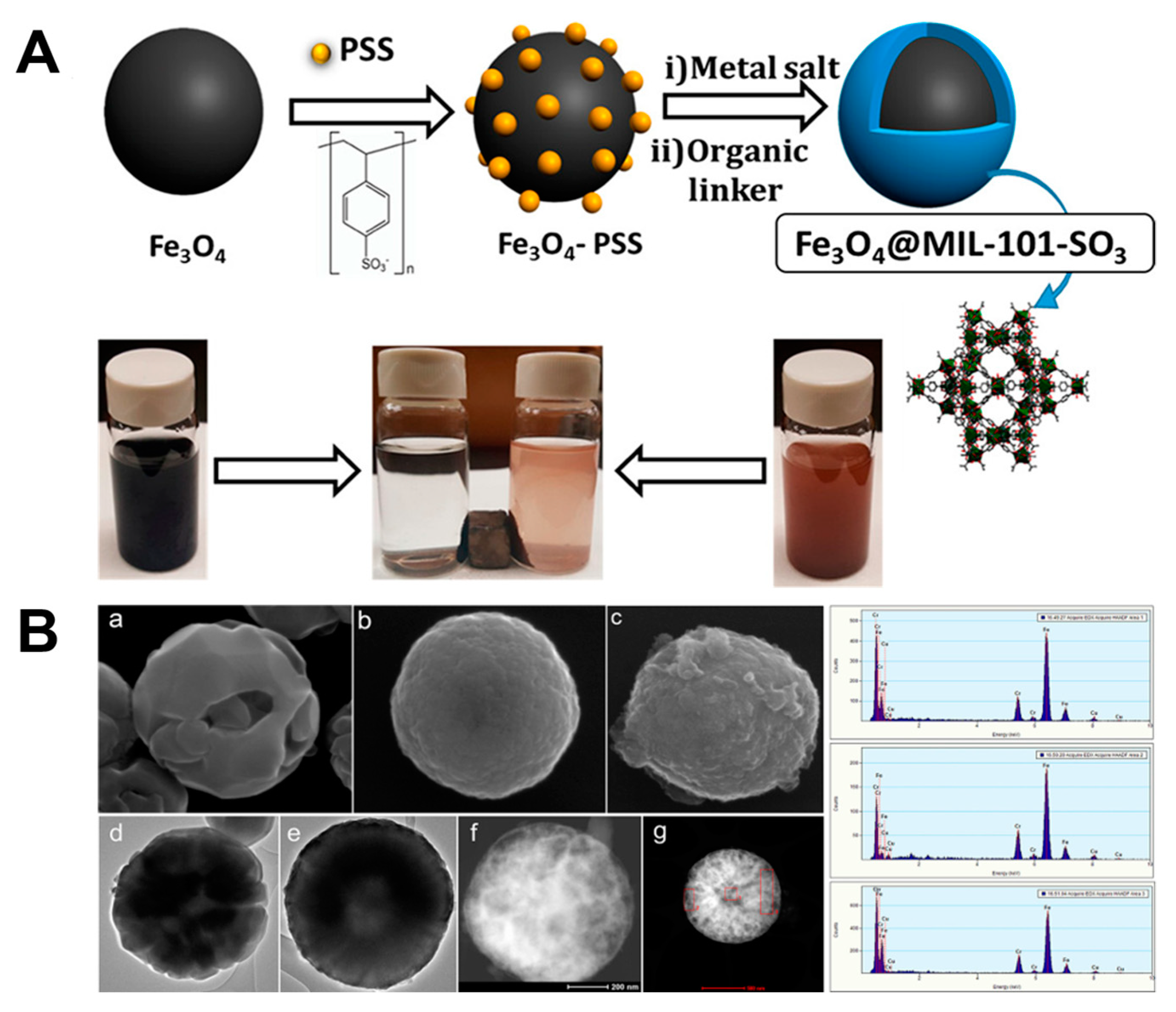
2.5. PSS
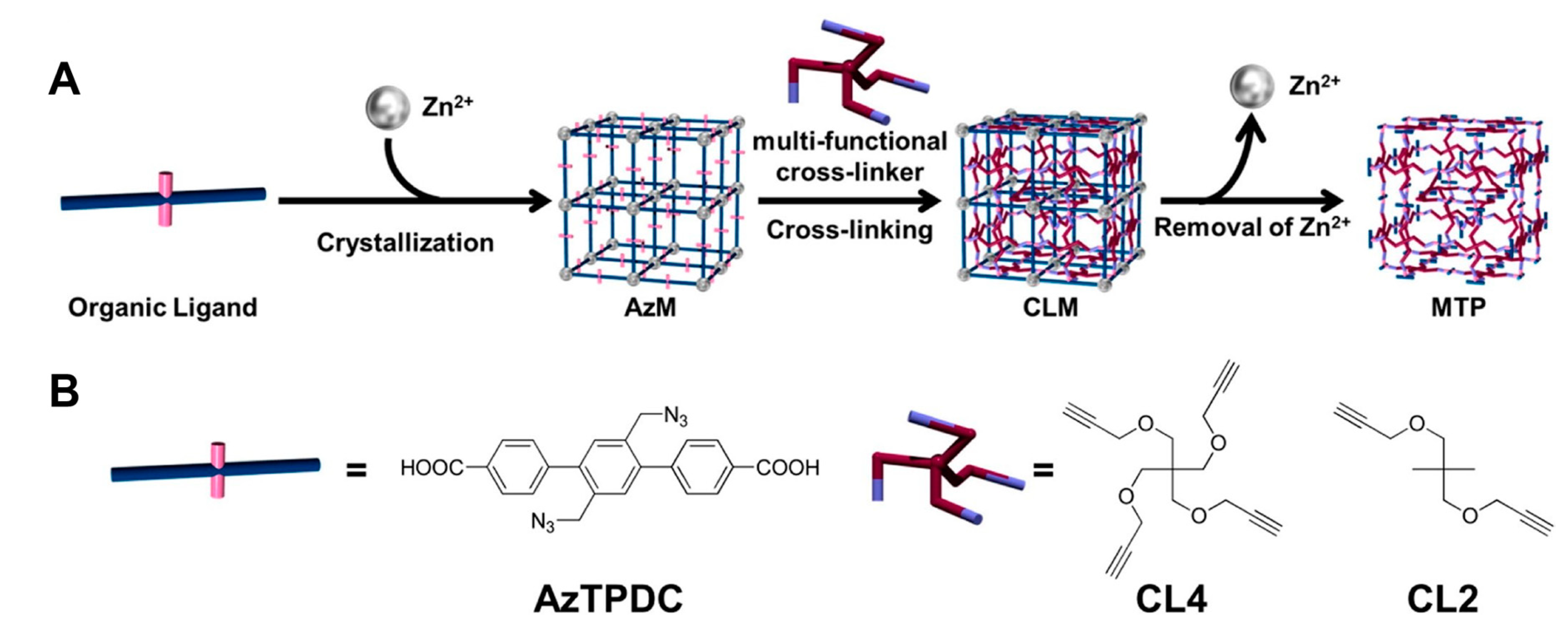
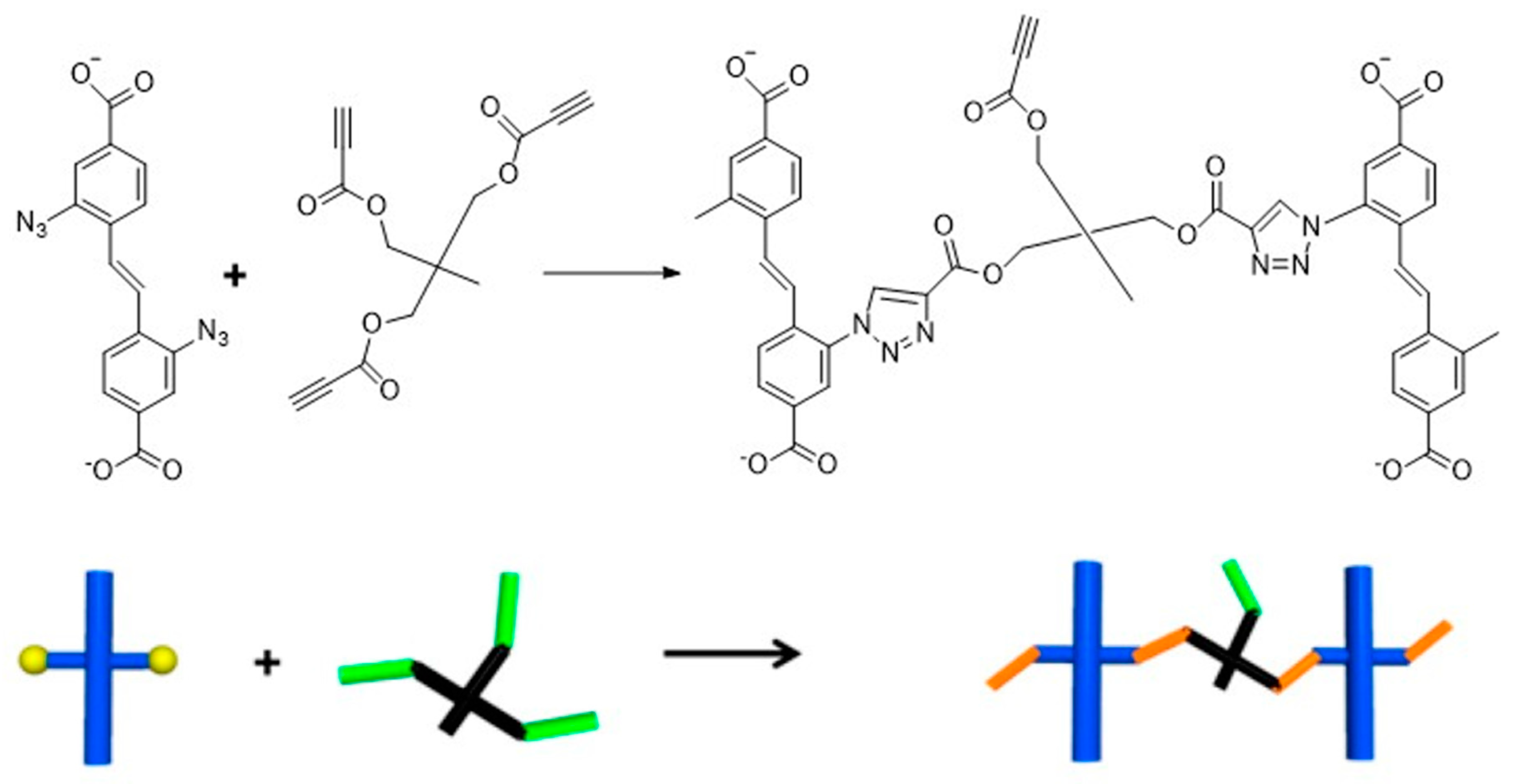
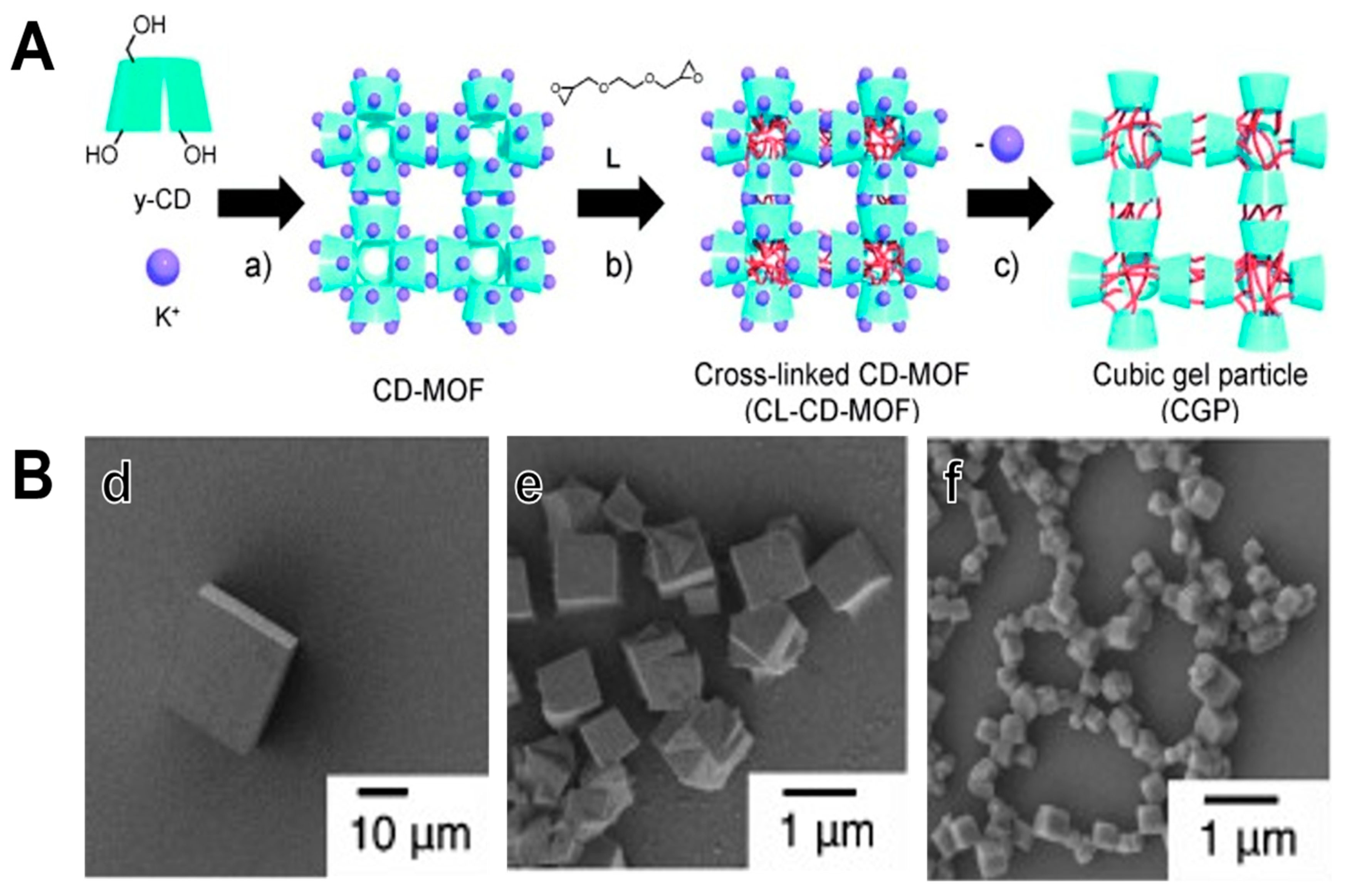
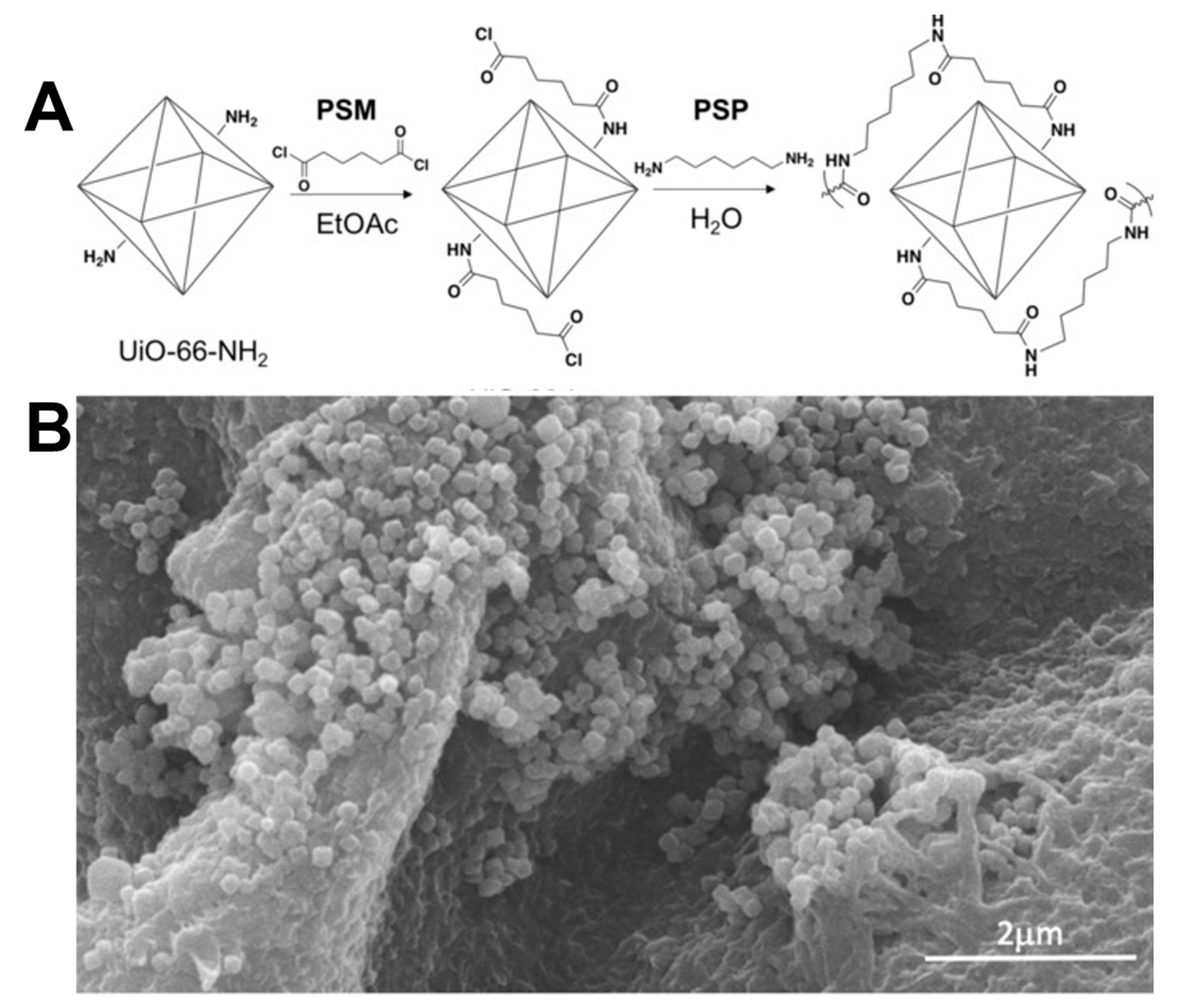
3. Cross Linking MOF to Polymer
4. Metal-Polymer Ligands
5. PolyMOF
6. Characterization of Polymer-Coated MOFs
7. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 1O2 | Singlet oxygen |
| 2-MIM | 2-methyl imidazole |
| AuNRs | Gold nanorods |
| AzM | Azide-tagged MOF |
| AzTPDC | diazide-triphenyl dicarboxylic acid |
| BDC | 1,4-benzene dicarboxylic acid |
| BSA | Bovine serum albumin |
| BTC | 1,3,5-benzene tricarboxylic acid |
| CD | Cyclodextrin |
| CGP | Cubic gel particles |
| CL4 | Acetylene cross-linker |
| CL2 | Diacetylene cross-linker |
| CLM | Cross-linked MOF |
| CTAB | Cetyltrimethylammonium bromide |
| DA-SBDC | Diazido-stilbenedicarboxylic acid |
| DH-Se | Di-(1-hydroxylundecyl) selenide |
| DOX | Doxorubicin |
| ECM | Extracellular matrix |
| EDX | Energy dispersive X-ray spectroscopy |
| FTIR | Fourier transform infrared |
| HA | Hyaluronic acid |
| PSS | Poly(sodium 4-styrenesulfonate) |
| PVP | Poly(N-vinylpyrrolidone) |
| PXRD | Powder X-ray diffraction |
| MAS | Microwave-assisted synthesis |
| MCS | Mechanochemical synthesis |
| ZIF | Zeolitic imidazolate framework |
| MOFs | Metal-organic frameworks |
| MSNs | Mesoporous silica nanoparticles |
| MTP | MOF-templated polymer |
| NMR | Nuclear magnetic resonance |
| PA-66 | Polyamide fiber |
| PAA | Poly(acrylic acid) |
| PCN | Porous coordination network |
| PDT | Photodynamic therapy |
| PEG | Poly(ethylene glycol) |
| PG | Polymer gel |
| PNIPAM | Poly-N-isopropylacrylamide |
| PolyLact | Poly-l-lactide |
| polyMOF | MOF-polymer composites |
| PPG | Poly(propylene glycol) |
| Ppy | Polypyrrole |
| PSM | Post-synthetic modification |
| PSP | Post-synthetic polymerization |
| Py–PGA-PEG-F3 | Pyrene-derived polyethylene glycol-F3 |
| ROS | Reactive oxygen species |
| SBU | Second building unit |
| SEM | Scanning electron microscope |
| STS | Solvothermal synthesis |
| TCPP | Tetrakis(4-carboxyphenyl) porphyrin |
| TEM | Transmission electron microscope |
| UCNPs | Upconverting nanoparticles |
| UiO | University of Oslo |
| ZIF | Zeolitic imidazolate framework |
References
- Zhou, H.-C.J.; Kitagawa, S. Metal-Organic Frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Kaskel, S.; Kitagawa, S. Special issue: New generations of porous metal-organic frameworks. Microporous Mesoporous Mater. 2015, 216. [Google Scholar] [CrossRef]
- Hunt, S.J.; Cliffe, M.J.; Hill, J.A.; Cairns, A.B.; Funnell, N.P.; Goodwin, A.L. Flexibility transition and guest-driven reconstruction in a ferroelastic metal-organic frameworkdagger. CrystEngComm 2015, 17, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Bennett, T.D.; Fuchs, A.H.; Cheetham, A.K.; Coudert, F.-X. Flexibility and disorder in metal-organic frameworks. Dalton Trans. 2016, 45, 4058–4059. [Google Scholar] [CrossRef] [PubMed]
- Long, J.R.; Yaghi, O.M. The pervasive chemistry of metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1213–1214. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to Metal-Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Astruc, D. State of the Art and Prospects in Metal-Organic Framework (MOF)-Based and MOF-Derived Nanocatalysis. Chem. Rev. 2019. [Google Scholar] [CrossRef]
- Xiao, J.-D.; Jiang, H.-L. Metal-Organic Frameworks for Photocatalysis and Photothermal Catalysis. Acc. Chem. Res. 2019, 52, 356–366. [Google Scholar] [CrossRef]
- Falcaro, P.; Ricco, R.; Doherty, C.M.; Liang, K.; Hill, A.J.; Styles, M.J. MOF positioning technology and device fabrication. Chem. Soc. Rev. 2014, 43, 5513–5560. [Google Scholar] [CrossRef]
- Zhan, Y.; Goncalves, M.; Yi, P.; Capelo, D.; Zhang, Y.; Rodrigues, J.; Liu, C.; Tomas, H.; Li, Y.; He, P. Thermo/redox/pH-triple sensitive poly (N-isopropylacrylamide-co-acrylic acid) nanogels for anticancer drug delivery. J. Mater. Chem. B 2015, 3, 4221–4230. [Google Scholar] [CrossRef]
- Rivera-Gil, P.; Jimenez de Aberasturi, D.; Wulf, V.; Pelaz, B.; del Pino, P.; Zhao, Y.; de la Fuente, J.M.; Ruiz de Larramendi, I.; Rojo, T.; Liang, X.J.; et al. The challenge to relate the physicochemical properties of colloidal nanoparticles to their cytotoxicity. Acc. Chem. Res. 2013, 46, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-G.; Han, Y.-H.; Zhang, J.-T.; Kankala, R.K.; Wang, S.-B.; Chen, A.-Z. Rerouting engineered metal-dependent shapes of mesoporous silica nanocontainers to biodegradable Janus-type (sphero-ellipsoid) nanoreactors for chemodynamic therapy. Chem. Eng. J. 2019, 370, 1188–1199. [Google Scholar] [CrossRef]
- Han, Y.-H.; Kankala, R.K.; Wang, S.-B.; Chen, A.-Z. Leveraging Engineering of Indocyanine Green-Encapsulated Polymeric Nanocomposites for Biomedical Applications. Nanomaterials 2018, 8, 360. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Chen, B.-Q.; Liu, C.-G.; Tang, H.-X.; Wang, S.-B.; Chen, A.-Z. Solution-enhanced dispersion by supercritical fluids: An ecofriendly nanonization approach for processing biomaterials and pharmaceutical compounds. Int. J. Nanomed. 2018, 13, 4227–4245. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Liu, C.-G.; Chen, A.-Z.; Wang, S.-B.; Xu, P.-Y.; Mende, L.K.; Liu, C.-L.; Lee, C.-H.; Hu, Y.-F. Overcoming Multidrug Resistance through the Synergistic Effects of Hierarchical pH-Sensitive, ROS-Generating Nanoreactors. ACS Biomater. Sci. Eng. 2017, 3, 2431–2442. [Google Scholar] [CrossRef]
- Kankala, R.K.; Zhu, K.; Sun, X.-N.; Liu, C.-G.; Wang, S.-B.; Chen, A.-Z. Cardiac Tissue Engineering on the Nanoscale. ACS Biomater. Sci. Eng. 2018, 4, 800–818. [Google Scholar] [CrossRef]
- Kankala, R.K.; Zhang, Y.S.; Wang, S.-B.; Lee, C.-H.; Chen, A.-Z. Supercritical Fluid Technology: An Emphasis on Drug Delivery and Related Biomedical Applications. Adv. Healthcare Mater. 2017, 6, 1700433. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Zhang, H.; Liu, C.-G.; Kanubaddi, K.R.; Lee, C.-H.; Wang, S.-B.; Cui, W.; Santos, H.A.; Lin, K.; Chen, A.-Z. Metal Species—Encapsulated Mesoporous Silica Nanoparticles: Current Advancements and Latest Breakthroughs. Adv. Funct. Mater. 2019, 1902652. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, M.; Guan, X.; Xie, Z.; Huang, Y.; Jing, X. Unadulterated BODIPY-dimer nanoparticles with high stability and good biocompatibility for cellular imaging. Nanoscale 2014, 6, 5662–5665. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Yang, B.; Zhang, Y.; Wei, Y. A New Class of Red Fluorescent Organic Nanoparticles: Noncovalent Fabrication and Cell Imaging Applications. ACS Appl. Mater. Interfaces 2014, 6, 3600–3606. [Google Scholar] [CrossRef]
- Ben-Moshe, A.; Maoz, B.M.; Govorov, A.O.; Markovich, G. Chirality and chiroptical effects in inorganic nanocrystal systems with plasmon and exciton resonances. Chem. Soc. Rev. 2013, 42, 7028–7041. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Xu, L.; de Moura, A.F.; Wu, X.; Kuang, H.; Xu, C.; Kotov, N.A. Chiral Inorganic Nanostructures. Chem. Rev. 2017, 117, 8041–8093. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Gu, Y.; Zou, Y.; Song, J.; Xu, L.; Li, J.; Xue, J.; Li, X.; Zeng, H. Improving All-Inorganic Perovskite Photodetectors by Preferred Orientation and Plasmonic Effect. Small 2016, 12, 5622–5632. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Huang, P.; Duan, H.; Chen, X. Plasmonic Vesicles of Amphiphilic Nanocrystals: Optically Active Multifunctional Platform for Cancer Diagnosis and Therapy. Acc. Chem. Res. 2015, 48, 2506–2515. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. Doxil (R)—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, M.J.; Soon-Shiong, P.; Desai, N. Protein nanoparticles as drug carriers in clinical medicine. Adv. Drug Deliv. Rev. 2008, 60, 876–885. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.A.; Alrokayan, S.A.; Kumar, S. Targeted anticancer therapy: Overexpressed receptors and nanotechnology. Clin. Chim. Acta 2014, 436, 78–92. [Google Scholar] [CrossRef]
- Siefker, J.; Karande, P.; Coppens, M.-O. Packaging biological cargoes in mesoporous materials: Opportunities for drug delivery. Exp. Opin. Drug Deliv. 2014, 11, 1781–1793. [Google Scholar] [CrossRef]
- Li, R.; Jiang, J.; Liu, Q.; Xie, Z.; Zhai, J. Hybrid nanochannel membrane based on polymer/MOF for high-performance salinity gradient power generation. Nano Energy 2018, 53, 643–649. [Google Scholar] [CrossRef]
- Fu, Q.; Wen, L.; Zhang, L.; Chen, X.; Pun, D.; Ahmed, A.; Yang, Y.; Zhang, H. Preparation of Ice-Templated MOF–Polymer Composite Monoliths and Their Application for Wastewater Treatment with High Capacity and Easy Recycling. ACS Appl. Mater. Interfaces 2017, 9, 33979–33988. [Google Scholar] [CrossRef]
- Sabetghadam, A.; Seoane, B.; Keskin, D.; Duim, N.; Rodenas, T.; Shahid, S.; Sorribas, S.; Guillouzer, C.L.; Clet, G.; Tellez, C.; et al. Metal Organic Framework Crystals in Mixed-Matrix Membranes: Impact of the Filler Morphology on the Gas Separation Performance. Adv. Funct. Mater. 2016, 26, 3154–3163. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Chen, Y.; Lim, T.-T.; Wang, R.; Hu, X. A Novel Metal-Organic Framework (MOF)—Mediated Interfacial Polymerization for Direct Deposition of Polyamide Layer on Ceramic Substrates for Nanofiltration. Adv. Mater. Interfaces 2019, 6, 1900132. [Google Scholar] [CrossRef]
- Ferey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Kitaura, R.; Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Zhu, C.; Liu, Y.; Cui, Y. Mesoporous metal-organic framework materials. Chem. Soc. Rev. 2012, 41, 1677–1695. [Google Scholar] [CrossRef] [PubMed]
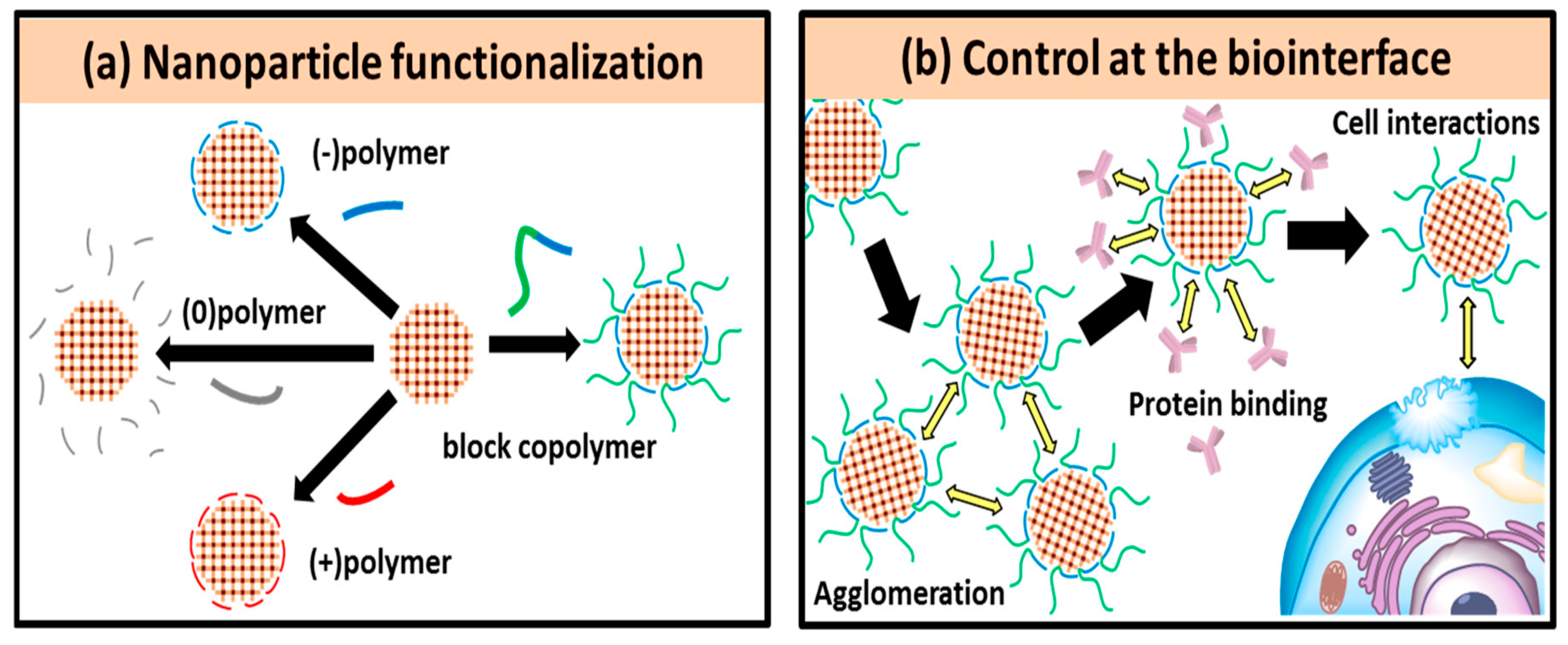
- Zimpel, A.; Al Danaf, N.; Steinborn, B.; Kuhn, J.; Höhn, M.; Bauer, T.; Hirschle, P.; Schrimpf, W.; Engelke, H.; Wagner, E.; et al. Coordinative Binding of Polymers to Metal-Organic Framework Nanoparticles for Control of Interactions at the Biointerface. ACS Nano 2019, 13, 3884–3895. [Google Scholar] [CrossRef] [PubMed]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef]
- Klinowski, J.; Almeida Paz, F.A.; Silva, P.; Rocha, J. Microwave-Assisted Synthesis of Metal-Organic Frameworks. Dalton Trans. 2011, 40, 321–330. [Google Scholar] [CrossRef]
- Qiu, L.-G.; Li, Z.-Q.; Wu, Y.; Wang, W.; Xu, T.; Jiang, X. Facile synthesis of nanocrystals of a microporous Metal-Organic framework by an ultrasonic method and selective sensing of organoamines. Chem. Commun. 2008, 3642–3644. [Google Scholar] [CrossRef]
- Khazalpour, S.; Safarifard, V.; Morsali, A.; Nematollahi, D. Electrochemical synthesis of pillared layer mixed ligand Metal-Organic framework: DMOF-1–Zn. RSC Adv. 2015, 5, 36547–36551. [Google Scholar] [CrossRef]
- Pichon, A.; Lazuen-Garay, A.; James, S.L. Solvent-free synthesis of a microporous Metal-Organic framework. CrystEngComm 2006, 8, 211–214. [Google Scholar] [CrossRef]
- Lu, W.; Wei, Z.; Gu, Z.-Y.; Liu, T.-F.; Park, J.; Park, J.; Tian, J.; Zhang, M.; Zhang, Q.; Gentle, T.; et al. Tuning the structure and function of Metal-Organic frameworks via linker design. Chem. Soc. Rev. 2014, 43, 5561–5593. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Chui, S.S.-Y.; Lo, S.M.-F.; Charmant, J.P.H.; Orpen, A.G.; Williams, I.D. A Chemically Functionalizable Nanoporous Material [Cu3(TMA)2(H2O)3]n. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef] [PubMed]
- Schaate, A.; Roy, P.; Godt, A.; Lippke, J.; Waltz, F.; Wiebcke, M.; Behrens, P. Modulated Synthesis of Zr-Based Metal-Organic Frameworks: From Nano to Single Crystals. Chem. Eur. J. 2011, 17, 6643–6651. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yuan, D.; Sun, D.; Zhou, H.-C. Stabilization of Metal-Organic Frameworks with High Surface Areas by the Incorporation of Mesocavities with Microwindows. J. Am. Chem. Soc. 2009, 131, 9186–9188. [Google Scholar] [CrossRef]
- He, Y.; Zhang, Z.; Xiang, S.; Fronczek, F.R.; Krishna, R.; Chen, B. A Microporous Metal-Organic Framework for Highly Selective Separation of Acetylene, Ethylene, and Ethane from Methane at Room Temperature. Chem. Eur. J. 2012, 18, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Schnobrich, J.K.; Lebel, O.; Cychosz, K.A.; Dailly, A.; Wong-Foy, A.G.; Matzger, A.J. Linker-Directed Vertex Desymmetrization for the Production of Coordination Polymers with High Porosity. J. Am. Chem. Soc. 2010, 132, 13941–13948. [Google Scholar] [CrossRef]
- Fan, J.; Yee, G.T.; Wang, G.; Hanson, B.E. Syntheses, Structures, and Magnetic Properties of Inorganic−Organic Hybrid Cobalt (II) Phosphites Containing Bifunctional Ligands. Inorg. Chem. 2006, 45, 599–608. [Google Scholar] [CrossRef]
- Wu, M.X.; Yang, Y.W. Metal-Organic Framework (MOF)-Based Drug/Cargo Delivery and Cancer Therapy. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Gao, P.F.; Zheng, L.L.; Liang, L.J.; Yang, X.X.; Li, Y.F.; Huang, C.Z. A new type of pH-responsive coordination polymer sphere as a vehicle for targeted anticancer drug delivery and sustained release. J. Mater. Chem. B 2013, 1. [Google Scholar] [CrossRef]
- Ke, F.; Yuan, Y.-P.; Qiu, L.-G.; Shen, Y.-H.; Xie, A.-J.; Zhu, J.-F.; Tian, X.-Y.; Zhang, L.-D. Facile fabrication of magnetic Metal-Organic framework nanocomposites for potential targeted drug delivery. J. Mater. Chem. 2011, 21. [Google Scholar] [CrossRef]
- An, J.; Geib, S.J.; Rosi, N.L. Cation-Triggered Drug Release from a Porous Zinc—Adeninate Metal-Organic Framework. J. Am. Chem. Soc. 2009, 131, 8376–8377. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S.; Kokado, K.; Sada, K. Metal-Organic framework tethering PNIPAM for ON–OFF controlled release in solution. Chem. Commun. 2015, 51, 8614–8617. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Zhang, L.; Hu, Q.; Zhao, D.; Xia, T.; Lin, W.; Yang, Y.; Cui, Y.; Yang, Y.; Qian, G. Pressure controlled drug release in a Zr-cluster-based MOF. J. Mater. Chem. B 2016, 4, 6398–6401. [Google Scholar] [CrossRef]
- Chen, Y.; Lykourinou, V.; Vetromile, C.; Hoang, T.; Ming, L.J.; Larsen, R.W.; Ma, S. How can proteins enter the interior of a MOF? Investigation of cytochrome c translocation into a MOF consisting of mesoporous cages with microporous windows. J. Am. Chem. Soc. 2012, 134, 13188–13191. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.; Senkovska, I.; Gedrich, K.; Stoeck, U.; Henschel, A.; Mueller, U.; Kaskel, S. A mesoporous metal-organic framework. Angew. Chem. Int. Ed. 2009, 48, 9954–9957. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.; Wong-Foy, A.G.; Matzge, A.J. A Porous Coordination Copolymer with over 5000 m2/g BET Surface Area. J. Am. Chem. Soc. 2008, 131, 4184–4185. [Google Scholar] [CrossRef]
- Rojas, S.; Wheatley, P.S.; Quartapelle-Procopio, E.; Gil, B.; Marszalek, B.; Morris, R.E.; Barea, E. Metal-Organic frameworks as potential multi-carriers of drugs. CrystEngComm 2013, 15. [Google Scholar] [CrossRef]
- Wang, X.; Ma, S.; Sun, D.; Parkin, S.; Zhou, H.-C. A Mesoporous Metal-Organic Framework with Permanent Porosity. J. Am. Chem. Soc. 2006, 128, 16474–16475. [Google Scholar] [CrossRef]
- Park, Y.K.; Choi, S.B.; Kim, H.; Kim, K.; Won, B.H.; Choi, K.; Choi, J.S.; Ahn, W.S.; Won, N.; Kim, S.; et al. Crystal structure and guest uptake of a mesoporous metal-organic framework containing cages of 3.9 and 4.7 nm in diameter. Angew. Chem. Int. Ed. 2007, 46, 8230–8233. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regi, M.; Colilla, M.; Gonzalez, B. Medical applications of organic-inorganic hybrid materials within the field of silica-based bioceramics. Chem. Soc. Rev. 2011, 40, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Johansson, E.M.; Ballem, M.A.; Cordoba, J.M.; Oden, M. Rapid synthesis of SBA-15 rods with variable lengths, widths, and tunable large pores. Langmuir 2011, 27, 4994–4999. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nor, Y.A.; Song, H.; Yang, Y.; Xu, C.; Yu, M.; Yu, C. Small-sized and large-pore dendritic mesoporous silica nanoparticles enhance antimicrobial enzyme delivery. J. Mater. Chem. B 2016, 4, 2646–2653. [Google Scholar] [CrossRef]
- Bruhwiler, D. Postsynthetic functionalization of mesoporous silica. Nanoscale 2010, 2, 887–892. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, Y.; Liu, L.; Wan, W.; Guo, P.; Nystrom, A.M.; Zou, X. One-pot Synthesis of Metal-Organic Frameworks with Encapsulated Target Molecules and Their Applications for Controlled Drug Delivery. J. Am. Chem. Soc. 2016, 138, 962–968. [Google Scholar] [CrossRef]
- Han, Y.R.; Park, J.W.; Kim, H.; Ji, H.; Lim, S.H.; Jun, C.H. A one-step co-condensation method for the synthesis of well-defined functionalized mesoporous SBA-15 using trimethallylsilanes as organosilane sources. Chem. Commun. 2015, 51, 17084–17087. [Google Scholar] [CrossRef]
- Motakef-Kazemi, N.; Shojaosadati, S.A.; Morsali, A. In situ synthesis of a drug-loaded MOF at room temperature. Microporous Mesoporous Mater. 2014, 186, 73–79. [Google Scholar] [CrossRef]
- Hintz, H.; Wuttke, S. Postsynthetic modification of an amino-tagged MOF using peptide coupling reagents: A comparative study. Chem. Commun. 2014, 50, 11472–11475. [Google Scholar] [CrossRef]
- Hintz, H.; Wuttke, S. Solvent-Free and Time Efficient Postsynthetic Modification of Amino-Tagged Metal-Organic Frameworks with Carboxylic Acid Derivatives. Chem. Mater. 2014, 26, 6722–6728. [Google Scholar] [CrossRef]
- Wuttke, S.; Dietl, C.; Hinterholzinger, F.M.; Hintz, H.; Langhals, H.; Bein, T. Turn-on fluorescence triggered by selective internal dye replacement in MOFs. Chem. Commun. 2014, 50, 3599–3601. [Google Scholar] [CrossRef] [PubMed]
- Deria, P.; Mondloch, J.E.; Karagiaridi, O.; Bury, W.; Hupp, J.T.; Farha, O.K. Beyond post-synthesis modification: Evolution of metal-organic frameworks via building block replacement. Chem. Soc. Rev. 2014, 43, 5896–5912. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, T.; Rosi, N.L. Strain-Promoted “Click” Modification of a Mesoporous Metal-Organic Framework. J. Am. Chem. Soc. 2012, 134, 18886–18888. [Google Scholar] [CrossRef] [PubMed]
- Flanigen, E.M.; Bennett, J.M.; Grose, R.W.; Cohen, J.P.; Patton, R.L.; Kirchner, R.M.; Smith, J.V. Silicalite, a new hydrophobic crystalline silica molecular sieve. Nature 1978, 271, 512–516. [Google Scholar] [CrossRef]
- Liang, K.; Ricco, R.; Doherty, C.M.; Styles, M.J.; Bell, S.; Kirby, N.; Mudie, S.; Haylock, D.; Hill, A.J.; Doonan, C.J.; et al. Biomimetic mineralization of metal-organic frameworks as protective coatings for biomacromolecules. Nat. Commun. 2015, 6, 7240. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Namgung, U.; Hong, K.-E. Regenerative Effects of Moxibustion on Skeletal Muscle in Collagen-Induced Arthritic Mice. J. Acupunct. Meridian Stud. 2012, 5, 126–135. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qin, Y.-T.; Peng, H.; He, X.-W.; Li, W.-Y.; Zhang, Y.-K. pH-Responsive Polymer-Stabilized ZIF-8 Nanocomposites for Fluorescence and Magnetic Resonance Dual-Modal Imaging-Guided Chemo-/Photodynamic Combinational Cancer Therapy. ACS. Appl. Mater. Interfaces 2019, 11, 34268–34281. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Qin, C.; Wang, C.-G.; Su, Z.-M.; Wang, S.; Wang, X.-L.; Yang, G.-S.; Shao, K.-Z.; Lan, Y.-Q.; Wang, E.-B. Chiral Nanoporous Metal-Organic Frameworks with High Porosity as Materials for Drug Delivery. Adv. Mater. 2011, 23, 5629–5632. [Google Scholar] [CrossRef]
- Huxford, R.C.; Della Rocca, J.; Lin, W. Metal-Organic frameworks as potential drug carriers. Curr. Opin. Chem. Biol. 2010, 14, 262–268. [Google Scholar] [CrossRef]
- Collet, G.; Lathion, T.; Besnard, C.; Piguet, C.; Petoud, S. On-Demand Degradation of Metal-Organic Framework Based on Photocleavable Dianthracene-Based Ligand. J. Am. Chem. Soc. 2018, 140, 10820–10828. [Google Scholar] [CrossRef]
- Wan, X.; Zhong, H.; Pan, W.; Li, Y.; Chen, Y.; Li, N.; Tang, B. Programmed Release of Dihydroartemisinin for Synergistic Cancer Therapy using CaCO3 Mineralized Metal-Organic Framework. Angew. Chem. Int. Ed. 2019. [Google Scholar] [CrossRef] [PubMed]
- Wuttke, S.; Braig, S.; Preiß, T.; Zimpel, A.; Sicklinger, J.; Bellomo, C.; Rädler, J.O.; Vollmar, A.M.; Bein, T. MOF nanoparticles coated by lipid bilayers and their uptake by cancer cells. Chem. Commun. 2015, 51, 15752–15755. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.-L.; Li, H.; Zhou, Y.; Zhang, Y.; Feng, X.; Wang, B.; Yang, Y.-W. Zn2+-Triggered Drug Release from Biocompatible Zirconium MOFs Equipped with Supramolecular Gates. Small 2015, 11, 3807–3813. [Google Scholar] [CrossRef] [PubMed]
- McGuire, C.V.; Forgan, R.S. The surface chemistry of metal-organic frameworks. Chem. Commun. 2015, 51, 5199–5217. [Google Scholar] [CrossRef] [PubMed]
- Nanjwade, B.K.; Bechra, H.M.; Derkar, G.K.; Manvi, F.V.; Nanjwade, V.K. Dendrimers: Emerging polymers for drug-delivery systems. Eur. J. Pharm. Sci. 2009, 38, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Svenson, S. Dendrimers as versatile platform in drug delivery applications. Eur. J. Pharm. Biopharm. 2009, 71, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regi, M.; Balas, F.; Arcos, D. Mesoporous materials for drug delivery. Angew. Chem. Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef]
- Zhu, J.; Shi, X. Dendrimer-based nanodevices for targeted drug delivery applications. J. Mater. Chem. B 2013, 1. [Google Scholar] [CrossRef]
- He, C.; Lu, K.; Liu, D.; Lin, W. Nanoscale metal-organic frameworks for the co-delivery of cisplatin and pooled siRNAs to enhance therapeutic efficacy in drug-resistant ovarian cancer cells. J. Am. Chem. Soc. 2014, 136, 5181–5184. [Google Scholar] [CrossRef]
- Wang, S. Ordered mesoporous materials for drug delivery. Microporous Mesoporous Mater. 2009, 117, 1–9. [Google Scholar] [CrossRef]
- Mintzer, M.A.; Grinstaff, M.W. Biomedical applications of dendrimers: A tutorial. Chem. Soc. Rev. 2011, 40, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Ganta, S.; Devalapally, H.; Shahiwala, A.; Amiji, M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. Control. Release 2008, 126, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Mamaeva, V.; Sahlgren, C.; Lindén, M. Mesoporous silica nanoparticles in medicine—Recent advances. Adv. Drug Deliv. Rev. 2013, 65, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Hu, Q.; Jiang, K.; Yang, Y.; Yang, Y.; Cui, Y.; Qian, G. A porphyrin-based Metal-Organic framework as a pH-responsive drug carrier. J. Solid State Chem. 2016, 237, 307–312. [Google Scholar] [CrossRef]
- Romberg, B.; Hennink, W.E.; Storm, G. Sheddable coatings for long-circulating nanoparticles. Pharm. Res. 2008, 25, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Sethi, M.; Sukumar, R.; Karve, S.; Werner, M.E.; Wang, E.C.; Moore, D.T.; Kowalczyk, S.R.; Zhang, L.; Wang, A.Z. Effect of drug release kinetics on nanoparticle therapeutic efficacy and toxicity. Nanoscale 2014, 6, 2321–2327. [Google Scholar] [CrossRef] [PubMed]
- Müller-Buschbaum, K.; Beuerle, F.; Feldmann, C. MOF based luminescence tuning and chemical/physical sensing. Microporous Mesoporous Mater. 2015, 216, 171–199. [Google Scholar] [CrossRef]
- Carne-Sanchez, A.; Bonnet, C.S.; Imaz, I.; Lorenzo, J.; Toth, E.; Maspoch, D. Relaxometry studies of a highly stable nanoscale metal-organic framework made of Cu (II), Gd (III), and the macrocyclic DOTP. J. Am. Chem. Soc. 2013, 135, 17711–17714. [Google Scholar] [CrossRef]
- Liberman, A.; Mendez, N.; Trogler, W.C.; Kummel, A.C. Synthesis and surface functionalization of silica nanoparticles for nanomedicine. Surf. Sci. Rep. 2014, 69, 132–158. [Google Scholar] [CrossRef]
- Kleist, W.; Jutz, F.; Maciejewski, M.; Baiker, A. Mixed-Linker Metal-Organic Frameworks as Catalysts for the Synthesis of Propylene Carbonate from Propylene Oxide and CO2. Eur. J. Inorg. Chem. 2009, 2009, 3552–3561. [Google Scholar] [CrossRef]
- Smaldone, R.A.; Forgan, R.S.; Furukawa, H.; Gassensmith, J.J.; Slawin, A.M.; Yaghi, O.M.; Stoddart, J.F. Metal-organic frameworks from edible natural products. Angew. Chem. Int. Ed. 2010, 49, 8630–8634. [Google Scholar] [CrossRef] [PubMed]
- Krug, H.F. Nanosafety research—Are we on the right track? Angew. Chem. Int. Ed. 2014, 53, 12304–12319. [Google Scholar] [CrossRef] [PubMed]
- Ikezoe, Y.; Fang, J.; Wasik, T.L.; Shi, M.; Uemura, T.; Kitagawa, S.; Matsui, H. Peptide-Metal Organic Framework Swimmers that Direct the Motion toward Chemical Targets. Nano Lett. 2015, 15, 4019–4023. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.G.; Dong, Z.Y.; Cheng, H.; Wan, S.S.; Chen, W.H.; Zou, M.Z.; Huo, J.W.; Deng, H.X.; Zhang, X.Z. A multifunctional metal-organic framework based tumor targeting drug delivery system for cancer therapy. Nanoscale 2015, 7, 16061–16070. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Morris, W.; Liu, Y.; McGuirk, C.M.; Zhou, Y.; Hupp, J.T.; Farha, O.K.; Mirkin, C.A. Surface-Specific Functionalization of Nanoscale Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2015, 54, 14738–14742. [Google Scholar] [CrossRef] [PubMed]
- Feliu, N.; Docter, D.; Heine, M.; Del Pino, P.; Ashraf, S.; Kolosnjaj-Tabi, J.; Macchiarini, P.; Nielsen, P.; Alloyeau, D.; Gazeau, F.; et al. In vivo degeneration and the fate of inorganic nanoparticles. Chem. Soc. Rev. 2016, 45, 2440–2457. [Google Scholar] [CrossRef]
- Wang, H.; Huang, Q.; Chang, H.; Xiao, J.; Cheng, Y. Stimuli-responsive dendrimers in drug delivery. Biomater. Sci. 2016, 4, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Gil, P.; De Koker, S.; De Geest, B.G.; Parak, W.J. Intracellular Processing of Proteins Mediated by Biodegradable Polyelectrolyte Capsules. Nano Lett. 2009, 9, 4398–4402. [Google Scholar] [CrossRef]
- Brown, J.W.; Henderson, B.L.; Kiesz, M.D.; Whalley, A.C.; Morris, W.; Grunder, S.; Deng, H.; Furukawa, H.; Zink, J.I.; Stoddart, J.F.; et al. Photophysical pore control in an azobenzene-containing Metal-Organic framework. Chem. Sci. 2013, 4, 2858–2864. [Google Scholar] [CrossRef]
- Hatakeyama, W.; Sanchez, T.J.; Rowe, M.D.; Serkova, N.J.; Liberatore, M.W.; Boyes, S.G. Synthesis of Gadolinium Nanoscale Metal-Organic Framework with Hydrotropes: Manipulation of Particle Size and Magnetic Resonance Imaging Capability. ACS Appl. Mater. Interfaces 2011, 3, 1502–1510. [Google Scholar] [CrossRef]
- Rieter, W.J.; Taylor, K.M.L.; An, H.; Lin, W.; Lin, W. Nanoscale Metal−Organic Frameworks as Potential Multimodal Contrast Enhancing Agents. J. Am. Chem. Soc. 2006, 128, 9024–9025. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, T.; Giménez-Marqués, M.; Bellido, E.; Avila, J.; Asensio, M.C.; Salles, F.; Lozano, M.V.; Guillevic, M.; Simón-Vázquez, R.; González-Fernández, A.; et al. Chitosan-coated mesoporous MIL-100 (Fe) nanoparticles as improved bio-compatible oral nanocarriers. Sci. Rep. 2017, 7, 43099. [Google Scholar] [CrossRef]
- Bellido, E.; Hidalgo, T.; Lozano, M.V.; Guillevic, M.; Simón-Vázquez, R.; Santander-Ortega, M.J.; González-Fernández, Á.; Serre, C.; Alonso, M.J.; Horcajada, P. Heparin-Engineered Mesoporous Iron Metal-Organic Framework Nanoparticles: Toward Stealth Drug Nanocarriers. Adv. Healthc. Mater. 2015, 4, 1246–1257. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.E.; Rimmer, S.; MacNeil, S. Examination of the effects of poly (N-vinylpyrrolidinone) hydrogels in direct and indirect contact with cells. Biomaterials 2006, 27, 2806–2812. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Song, S.; Li, J.; Wu, L.; Wang, Q.; Liu, Y.; Jin, R.; Zhang, H. Pt/CeO2@MOF Core@Shell Nanoreactor for Selective Hydrogenation of Furfural via the Channel Screening Effect. ACS Catal. 2018, 8, 8506–8512. [Google Scholar] [CrossRef]
- Filippousi, M.; Turner, S.; Leus, K.; Siafaka, P.I.; Tseligka, E.D.; Vandichel, M.; Nanaki, S.G.; Vizirianakis, I.S.; Bikiaris, D.N.; Van Der Voort, P.; et al. Biocompatible Zr-based nanoscale MOFs coated with modified poly(ε-caprolactone) as anticancer drug carriers. Int. J. Pharm. 2016, 509, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zhu, Y.; Huddleston, S.; Li, P.; Xiao, B.; Farha, O.K.; Ameer, G.A. Copper Metal-Organic Framework Nanoparticles Stabilized with Folic Acid Improve Wound Healing in Diabetes. ACS Nano 2018, 12, 1023–1032. [Google Scholar] [CrossRef]
- Onishi, H.; Machida, Y. Biodegradation and distribution of water-soluble chitosan in mice. Biomaterials 1999, 20, 175–182. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Human enzymatic activities related to the therapeutic administration of chitin derivatives. Cell. Mol. Life Sci. 1997, 53, 131–140. [Google Scholar] [CrossRef]
- Khor, E.; Lim, L.Y. Implantable applications of chitin and chitosan. Biomaterials 2003, 24, 2339–2349. [Google Scholar] [CrossRef]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan—A review. J. Control. Release 2006, 114. [Google Scholar] [CrossRef] [PubMed]
- Whitelock, J.M.; Iozzo, R.V. Heparan Sulfate: A Complex Polymer Charged with Biological Activity. Chem. Rev. 2005, 105, 2745–2764. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chang, Y.-T. Discovery of heparin chemosensors through diversity oriented fluorescence library approach. Chem. Commun. 2008, 1173–1175. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.R.E.; Laurent, T.C.; Laurent, U.B.G. Hyaluronan: Its nature, distribution, functions and turnover. J. Int. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, S.M.; Lee, S.A.; Powell, J.W.; Weidlich, T.; Demarco, C.; Lewen, G.D.; Tao, N.J.; Rupprecht, A. The origin of the A to B transition in DNA fibers and films. Biopolymers 1988, 27, 1015–1043. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, S.; Jin, E.; Palanikumar, L.; Lee, J.H.; Kim, J.C.; Nam, J.S.; Jana, B.; Kwon, T.-H.; Kwak, S.K.; et al. MOF × Biopolymer: Collaborative Combination of Metal-Organic Framework and Biopolymer for Advanced Anticancer Therapy. ACS Appl. Mater. Interfaces 2019, 11, 27512–27520. [Google Scholar] [CrossRef]
- Yu, M.; Jambhrunkar, S.; Thorn, P.; Chen, J.; Gu, W.; Yu, C. Hyaluronic acid modified mesoporous silica nanoparticles for targeted drug delivery to CD44-overexpressing cancer cells. Nanoscale 2013, 5, 178–183. [Google Scholar] [CrossRef]
- Min, H.; Wang, J.; Qi, Y.; Zhang, Y.; Han, X.; Xu, Y.; Xu, J.; Li, Y.; Chen, L.; Cheng, K.; et al. Biomimetic Metal-Organic Framework Nanoparticles for Cooperative Combination of Antiangiogenesis and Photodynamic Therapy for Enhanced Efficacy. Adv. Mater. 2019, 31, 1808200. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Wu, Z.; Chen, H. Poly (N-vinylpyrrolidone)-Modified Surfaces for Biomedical Applications. Macromol. Biosci. 2013, 13, 147–154. [Google Scholar] [CrossRef]
- Chen, T.-T.; Yi, J.-T.; Zhao, Y.-Y.; Chu, X. Biomineralized Metal-Organic Framework Nanoparticles Enable Intracellular Delivery and Endo-Lysosomal Release of Native Active Proteins. J. Am. Chem. Soc. 2018, 140, 9912–9920. [Google Scholar] [CrossRef]
- Li, Y.; Jin, J.; Wang, D.; Lv, J.; Hou, K.; Liu, Y.; Chen, C.; Tang, Z. Coordination-responsive drug release inside gold nanorod@metal-organic framework core-shell nanostructures for near-infrared-induced synergistic chemo-photothermal therapy. Nano Res. 2018, 11, 3294–3305. [Google Scholar] [CrossRef]
- Lu, G.; Li, S.; Guo, Z.; Farha, O.K.; Hauser, B.G.; Qi, X.; Wang, Y.; Wang, X.; Han, S.; Liu, X.; et al. Imparting functionality to a Metal-Organic framework material by controlled nanoparticle encapsulation. Nat. Chem. 2012, 4, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-D.; Chen, S.-P.; Zhao, H.; Yang, Y.; Chen, X.-Q.; Sun, J.; Fan, H.-S.; Zhang, X.-D. PPy@MIL-100 Nanoparticles as a pH- and Near-IR-Irradiation-Responsive Drug Carrier for Simultaneous Photothermal Therapy and Chemotherapy of Cancer Cells. ACS Appl. Mater. Interfaces 2016, 8, 34209–34217. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xia, Y. Shape-Controlled Synthesis of Gold and Silver Nanoparticles. Science 2002, 298, 2176. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Hou, Z.; Li, X.; Li, C.; Zhang, Y.; Deng, X.; Cheng, Z.; Lin, J. Aptamer-Mediated Up-conversion Core/MOF Shell Nanocomposites for Targeted Drug Delivery and Cell Imaging. Sci. Rep. 2015, 5, 7851. [Google Scholar] [CrossRef]
- Elsaidi, S.K.; Sinnwell, M.A.; Banerjee, D.; Devaraj, A.; Kukkadapu, R.K.; Droubay, T.C.; Nie, Z.; Kovarik, L.; Vijayakumar, M.; Manandhar, S.; et al. Reduced Magnetism in Core–Shell Magnetite@MOF Composites. Nano Lett. 2017, 17, 6968–6973. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, G.; Matsuda, R.; Sato, H.; Takata, M.; Kitagawa, S. Cellulose hydrolysis by a new porous coordination polymer decorated with sulfonic acid functional groups. Adv. Mater. 2011, 23, 3294–3297. [Google Scholar] [CrossRef]
- Zhao, X.; Han, X.; Li, Z.; Huang, H.; Liu, D.; Zhong, C. Enhanced removal of iodide from water induced by a metal-incorporated porous Metal-Organic framework. Appl. Surf. Sci. 2015, 351, 760–764. [Google Scholar] [CrossRef]
- Elsaidi, S.K.; Sinnwell, M.A.; Devaraj, A.; Droubay, T.C.; Nie, Z.; Murugesan, V.; McGrail, B.P.; Thallapally, P.K. Extraction of rare earth elements using magnetite@MOF composites. J. Mater. Chem. A 2018, 6, 18438–18443. [Google Scholar] [CrossRef]
- Chen, D.; Yang, D.; Dougherty, C.A.; Lu, W.; Wu, H.; He, X.; Cai, T.; Van Dort, M.E.; Ross, B.D.; Hong, H. In Vivo Targeting and Positron Emission Tomography Imaging of Tumor with Intrinsically Radioactive Metal-Organic Frameworks Nanomaterials. ACS Nano 2017, 11, 4315–4327. [Google Scholar] [CrossRef]
- Tian, C.; Zhu, L.; Lin, F.; Boyes, S.G. Poly (acrylic acid) Bridged Gadolinium Metal-Organic Framework—Gold Nanoparticle Composites as Contrast Agents for Computed Tomography and Magnetic Resonance Bimodal Imaging. ACS Appl. Mater. Interfaces 2015, 7, 17765–17775. [Google Scholar] [CrossRef] [PubMed]
- Abánades Lázaro, I.; Haddad, S.; Rodrigo-Muñoz, J.M.; Orellana-Tavra, C.; del Pozo, V.; Fairen-Jimenez, D.; Forgan, R.S. Mechanistic Investigation into the Selective Anticancer Cytotoxicity and Immune System Response of Surface-Functionalized, Dichloroacetate-Loaded, UiO-66 Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 5255–5268. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lv, X.-L.; Feng, D.; Xie, L.-H.; Zhang, J.; Li, M.; Xie, Y.; Li, J.-R.; Zhou, H.-C. Highly Stable Zr (IV)-Based Metal-Organic Frameworks for the Detection and Removal of Antibiotics and Organic Explosives in Water. J. Am. Chem. Soc. 2016, 138, 6204–6216. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, T.; Du, P.; Zhang, L.; Lei, J. Metal-Organic Framework (MOF) Hybrid as a Tandem Catalyst for Enhanced Therapy against Hypoxic Tumor Cells. Angew. Chem. Int. Ed. 2019, 58, 7808–7812. [Google Scholar] [CrossRef] [PubMed]
- Ishiwata, T.; Furukawa, Y.; Sugikawa, K.; Kokado, K.; Sada, K. Transformation of Metal-Organic Framework to Polymer Gel by Cross-Linking the Organic Ligands Preorganized in Metal-Organic Framework. J. Am. Chem. Soc. 2013, 135, 5427–5432. [Google Scholar] [CrossRef] [PubMed]
- Howarth, A.J.; Liu, Y.; Li, P.; Li, Z.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Chemical, thermal and mechanical stabilities of Metal-Organic frameworks. Nat. Rev. Mater. 2016, 1, 15018. [Google Scholar] [CrossRef]
- Hanke, M.; Arslan, H.K.; Bauer, S.; Zybaylo, O.; Christophis, C.; Gliemann, H.; Rosenhahn, A.; Wöll, C. The Biocompatibility of Metal-Organic Framework Coatings: An Investigation on the Stability of SURMOFs with Regard to Water and Selected Cell Culture Media. Langmuir 2012, 28, 6877–6884. [Google Scholar] [CrossRef]
- Tsotsalas, M.; Liu, J.; Tettmann, B.; Grosjean, S.; Shahnas, A.; Wang, Z.; Azucena, C.; Addicoat, M.; Heine, T.; Lahann, J.; et al. Fabrication of Highly Uniform Gel Coatings by the Conversion of Surface-Anchored Metal-Organic Frameworks. J. Am. Chem. Soc. 2014, 136, 8–11. [Google Scholar] [CrossRef]
- Furukawa, Y.; Ishiwata, T.; Sugikawa, K.; Kokado, K.; Sada, K. Nano- and Microsized Cubic Gel Particles from Cyclodextrin Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2012, 51, 10566–10569. [Google Scholar] [CrossRef]
- DeCoste, J.B.; Denny, M.S., Jr.; Peterson, G.W.; Mahle, J.J.; Cohen, S.M. Enhanced aging properties of HKUST-1 in hydrophobic mixed-matrix membranes for ammonia adsorption. Chem. Sci. 2016, 7, 2711–2716. [Google Scholar] [CrossRef]
- Lu, A.X.; McEntee, M.; Browe, M.A.; Hall, M.G.; DeCoste, J.B.; Peterson, G.W. MOFabric: Electrospun Nanofiber Mats from PVDF/UiO-66-NH2 for Chemical Protection and Decontamination. ACS Appl. Mater. Interfaces 2017, 9, 13632–13636. [Google Scholar] [CrossRef] [PubMed]
- Kalaj, M.; Denny, M.S., Jr.; Bentz, K.C.; Palomba, J.M.; Cohen, S.M. Nylon-MOF Composites through Postsynthetic Polymerization. Angew. Chem. Int. Ed. 2019, 58, 2336–2340. [Google Scholar] [CrossRef] [PubMed]
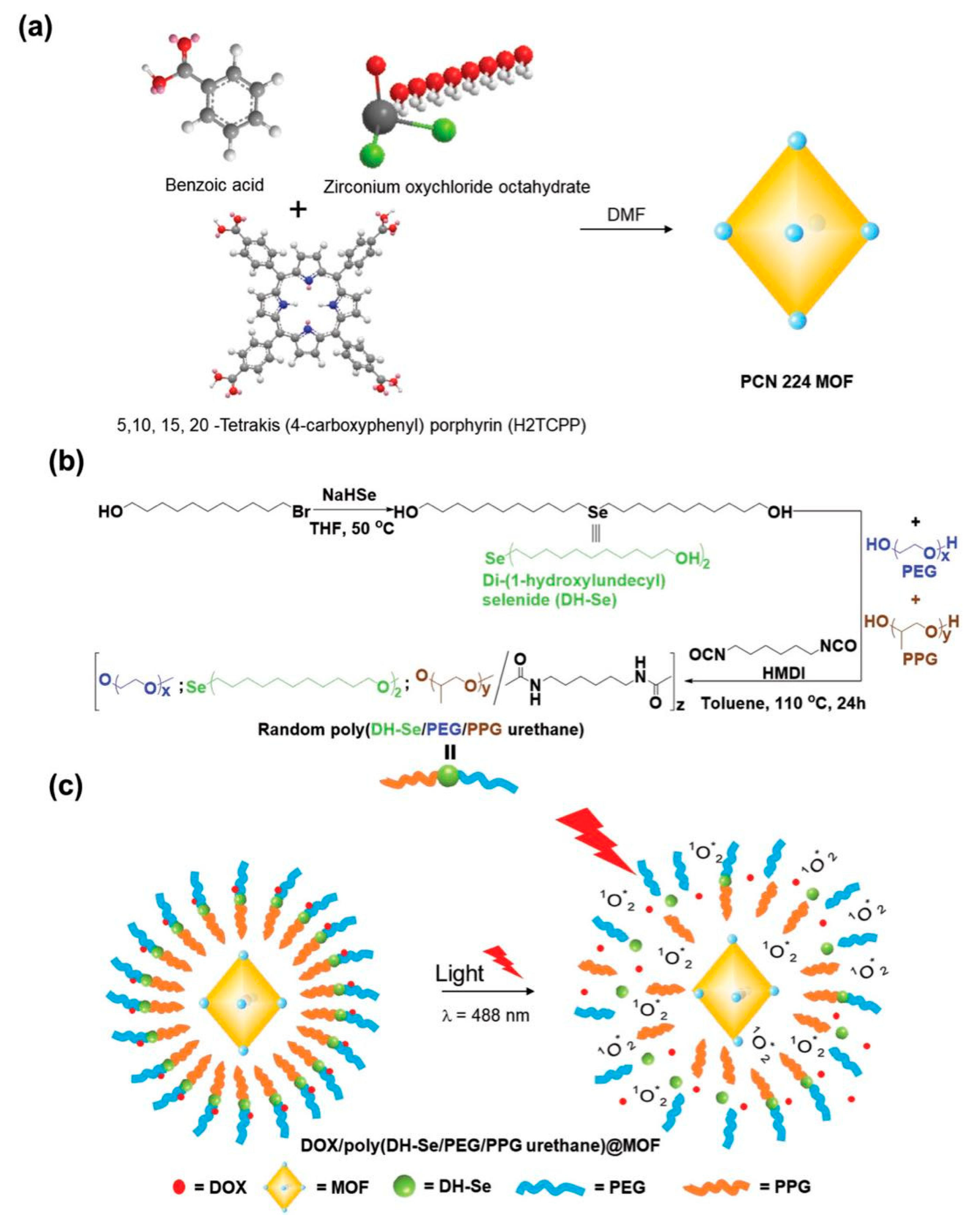
- Luo, Z.; Jiang, L.; Yang, S.; Li, Z.; Soh, W.M.W.; Zheng, L.; Loh, X.J.; Wu, Y.-L. Light-Induced Redox-Responsive Smart Drug Delivery System by Using Selenium-Containing Polymer@MOF Shell/Core Nanocomposite. Adv. Healthc. Mater. 2019, 8, 1900406. [Google Scholar] [CrossRef] [PubMed]
| MOF@Polymer | Organic Linkers | Synthetic Process | Particle Size (nm) | Outcome | Reference | |
|---|---|---|---|---|---|---|
| MOFs | MOF@Polymer | |||||
| MIL-100(Fe)@ chitosan | BTC | MAS | 135 ± 20 | 204 ± 32 | Improved biocompatibility of oral nanocarriers | [112] |
| MIL-100(Fe)@ heparin | BTC | MAS | 155 ± 61 | 178 ± 44 | Toward stealth drug nanocarriers | [113] |
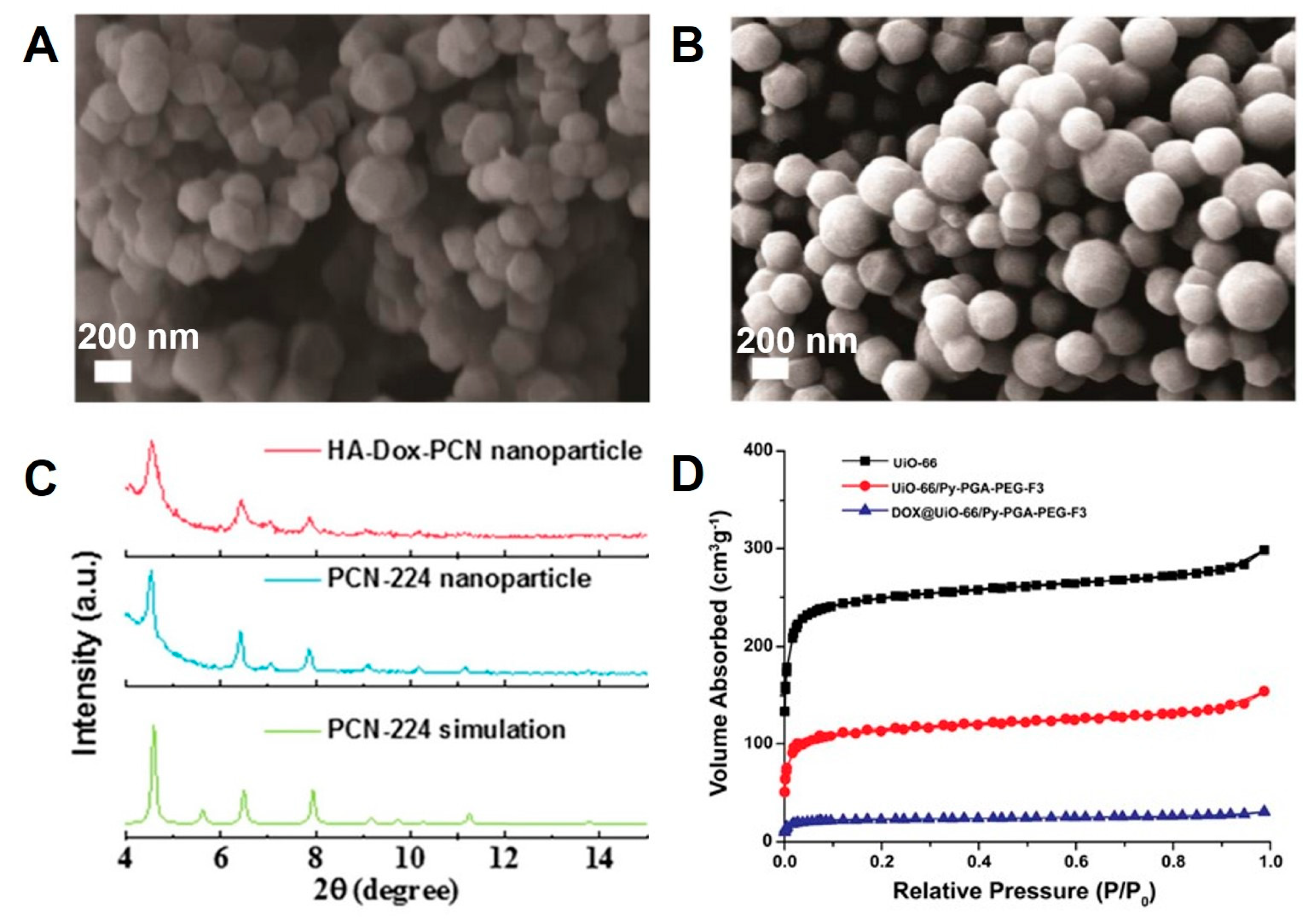
| PCN-224@HA | TCPP | STS | 164 ±20 | 250 ± 20 | Advanced anticancer therapy | [126] |
| BSA@ZIF-8@PVP | 2-MIM | MCS | 53±3.1 | 10 ± 1.6 | Intracellular delivery and endo-lysosomal release of native active proteins | [130] |
| Zr-UiO-66/Py-PGA-PEG-F3 | BDC | STS | 220 | 250 | In vivo targeting and positron emission tomography imaging of tumor | [140] |
| GdMOF@PAA | BDC | STS | 155 ± 30 (l) and 30 ± 11 (w) | 158 ± 30 (l) and 33 ± 11 (w) | Contrast agents for computed tomography and magnetic resonance bimodal imaging | [141] |
| UiO-66-L1-PolyLact | BDC | STS | 143 ± 31 | 177 ± 25 | Selective anticancer cytotoxicity and immune system response | [142] |
| UiO-66-L2-PNIPAM | BDC | STS | 142 ± 14 | 177 ± 24 | Selective anticancer cytotoxicity and immune system response | [142] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, J.; Kankala, R.K.; Wang, S.-B.; Chen, A.-Z. Recent Advances in Polymeric Nanocomposites of Metal-Organic Frameworks (MOFs). Polymers 2019, 11, 1627. https://doi.org/10.3390/polym11101627
Zhong J, Kankala RK, Wang S-B, Chen A-Z. Recent Advances in Polymeric Nanocomposites of Metal-Organic Frameworks (MOFs). Polymers. 2019; 11(10):1627. https://doi.org/10.3390/polym11101627
Chicago/Turabian StyleZhong, Jun, Ranjith Kumar Kankala, Shi-Bin Wang, and Ai-Zheng Chen. 2019. "Recent Advances in Polymeric Nanocomposites of Metal-Organic Frameworks (MOFs)" Polymers 11, no. 10: 1627. https://doi.org/10.3390/polym11101627
APA StyleZhong, J., Kankala, R. K., Wang, S.-B., & Chen, A.-Z. (2019). Recent Advances in Polymeric Nanocomposites of Metal-Organic Frameworks (MOFs). Polymers, 11(10), 1627. https://doi.org/10.3390/polym11101627