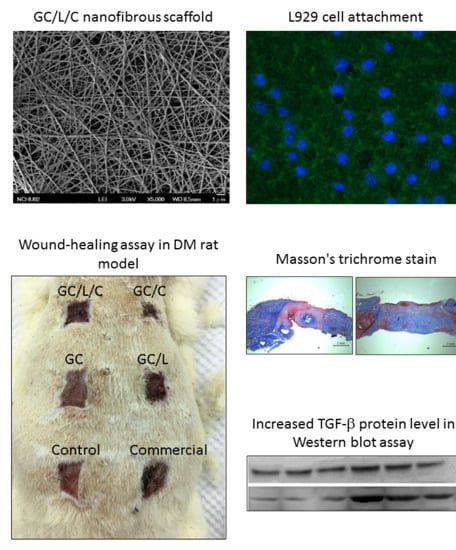
Effects of Bilayer Nanofibrous Scaffolds Containing Curcumin/Lithospermi Radix Extract on Wound Healing in Streptozotocin-Induced Diabetic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biochemical Analysis of Curcumin
2.1.1. Cell Viability Assay
2.1.2. Cell Migration Assay
2.1.3. Tyrosinase Inhibition Assay
2.2. Preparation of Gelatin/Chitosan Bilayer Nanofibrous Scaffolds (GC)
2.2.1. Preparation of Chitosan Scaffolds
2.2.2. Preparation of GC/L/C Bilayer Nanofibrous Scaffolds
2.3. Morphological and Physicochemical Characterization of Gelatin/PVA Nanofibers (GEL)
2.3.1. Microscopic Structure Evaluation
2.3.2. In Vitro Biocompatibility Assay
2.3.3. Immunofluorescence Staining
2.4. In Vivo Wound-Healing Assay
2.4.1. In Vivo Animal Evaluation
2.4.2. Streptozotocin (STZ)-Induced Diabetic Models in Rats
2.4.3. Wound-Healing Measurement in STZ Diabetic Rats
2.4.4. Histopathological Studies
2.4.5. Collagen Assay in the Wound Area
2.5. Western Blotting Assay
2.6. Inflammation Index Assay
2.7. Statistical Analysis
3. Results
3.1. Effects of Curcumin and LR Extract on Cell Viability and Biochemical Function
3.1.1. Cytotoxicity of Curcumin against L929 Cells
3.1.2. Effect of Curcumin on an in Vitro Cell Migration Assay
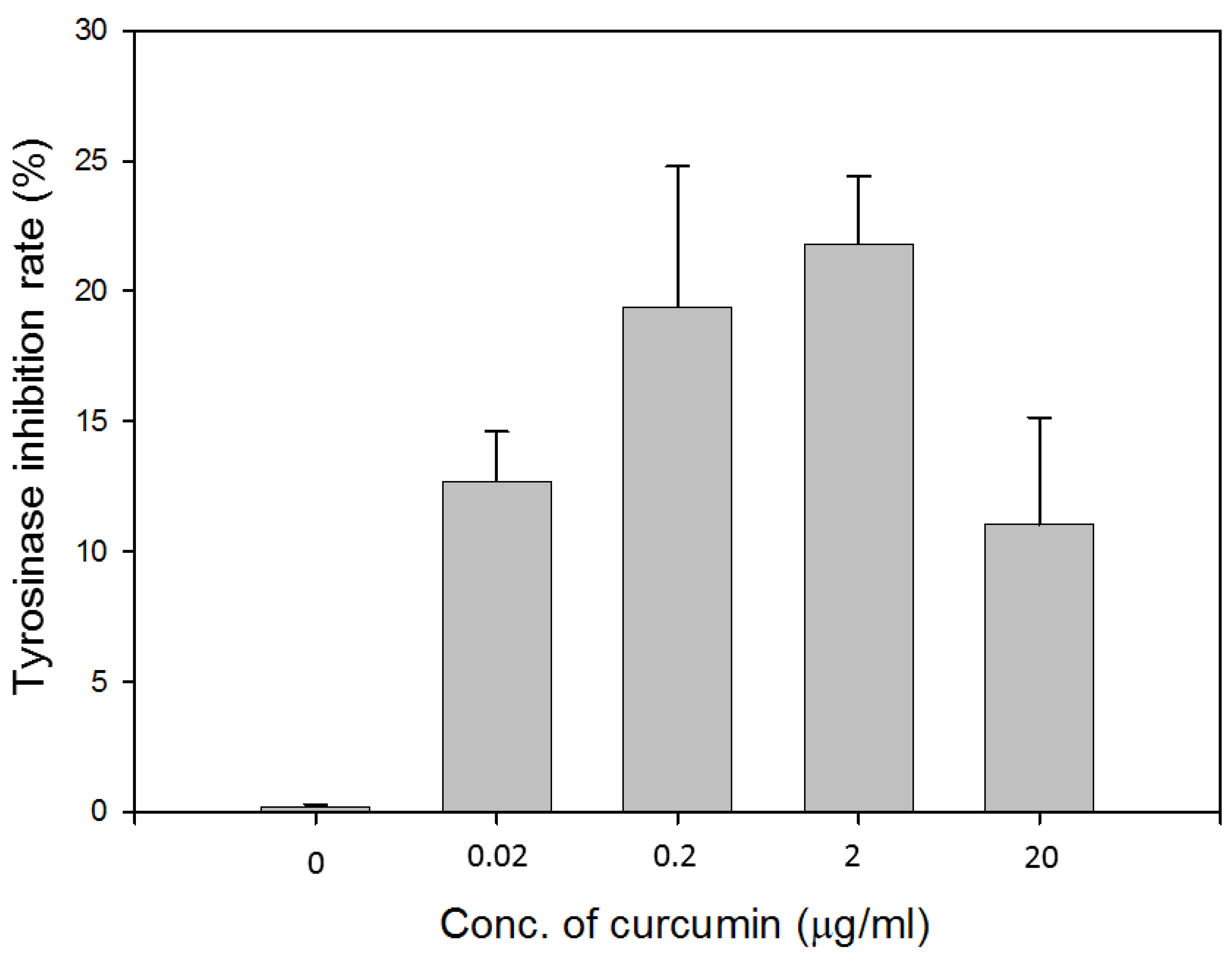
3.1.3. Inhibition Effect of Curcumin on Tyrosinase Activity
3.1.4. The Drug Loading Concentration of Curcumin and LR Extract
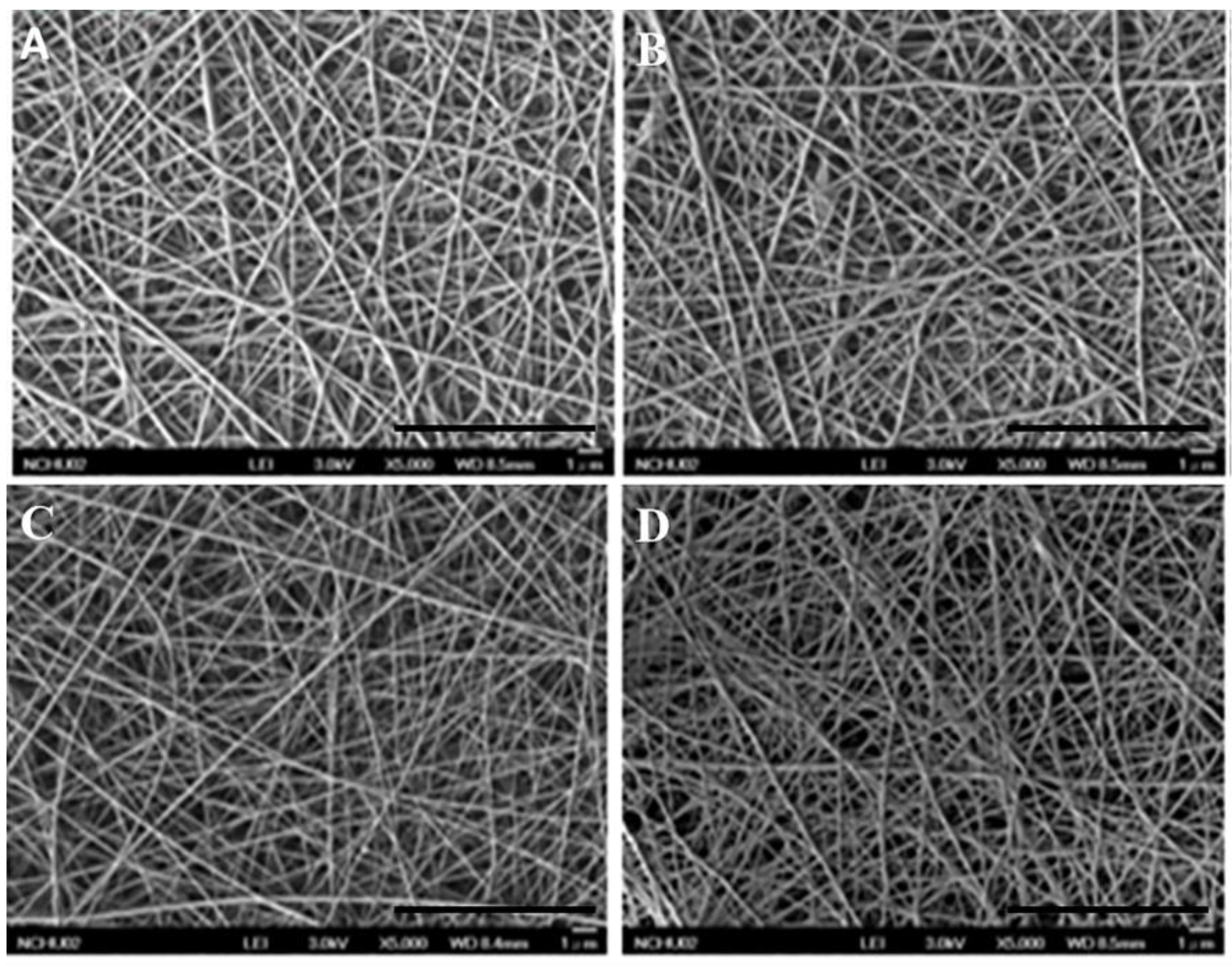
3.2. Morphological and Physicochemical Characterization of GEL Nanofibers
3.2.1. Microscopic Structure Evaluation of GEL Nanofibers
3.2.2. Biocompatibility of Various GEL Nanofibers.
3.3. Wound Healing Effect of Bilayer Nanofibrous Scaffolds in STZ Diabetic Rats
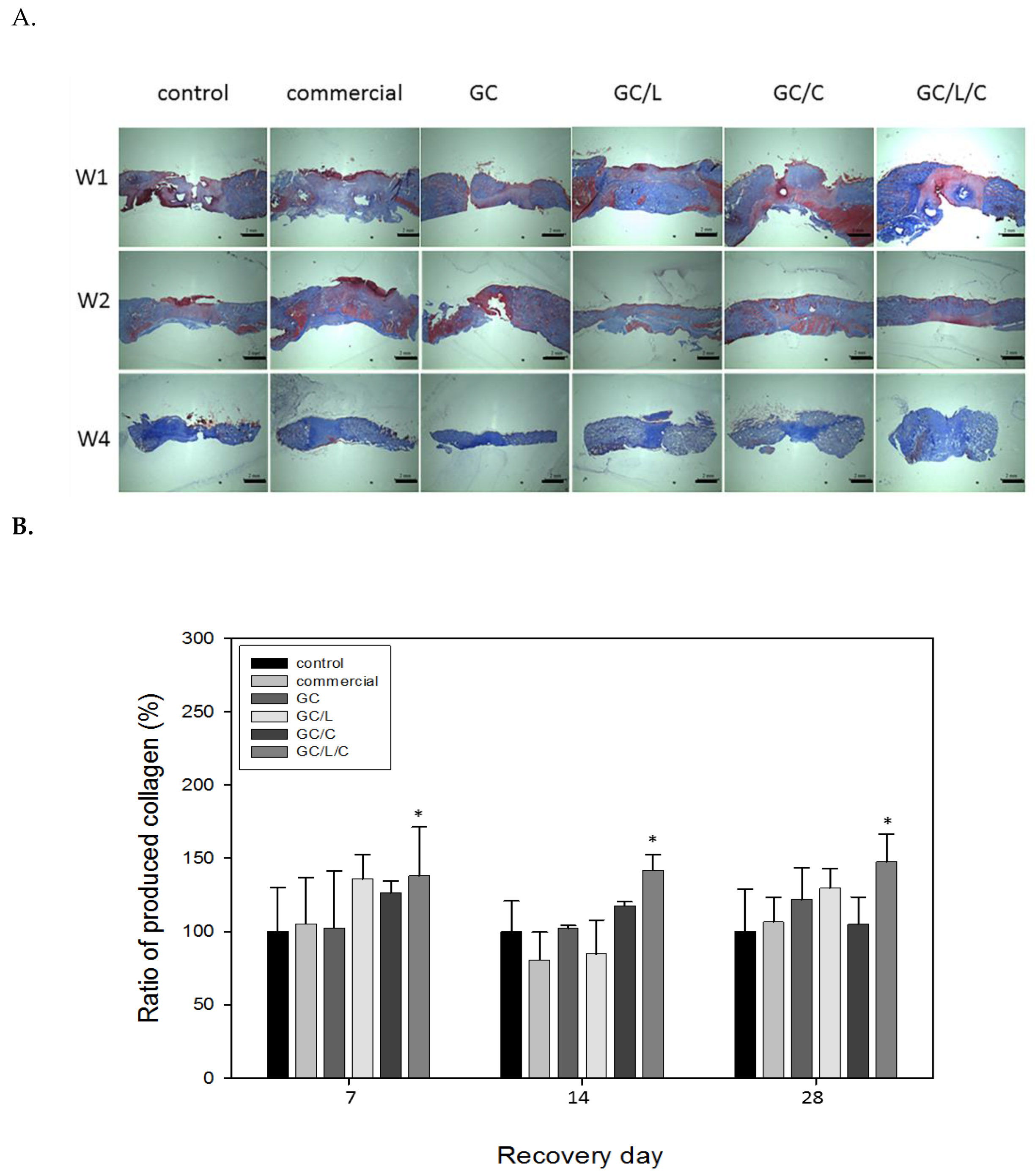
3.3.1. GC/L/C Bilayer Nanofibrous Scaffolds Accelerated Wound Recovery Rate
3.3.2. GC/L/C Bilayer Scaffold Increased Collagen Secretion in the Wound Area
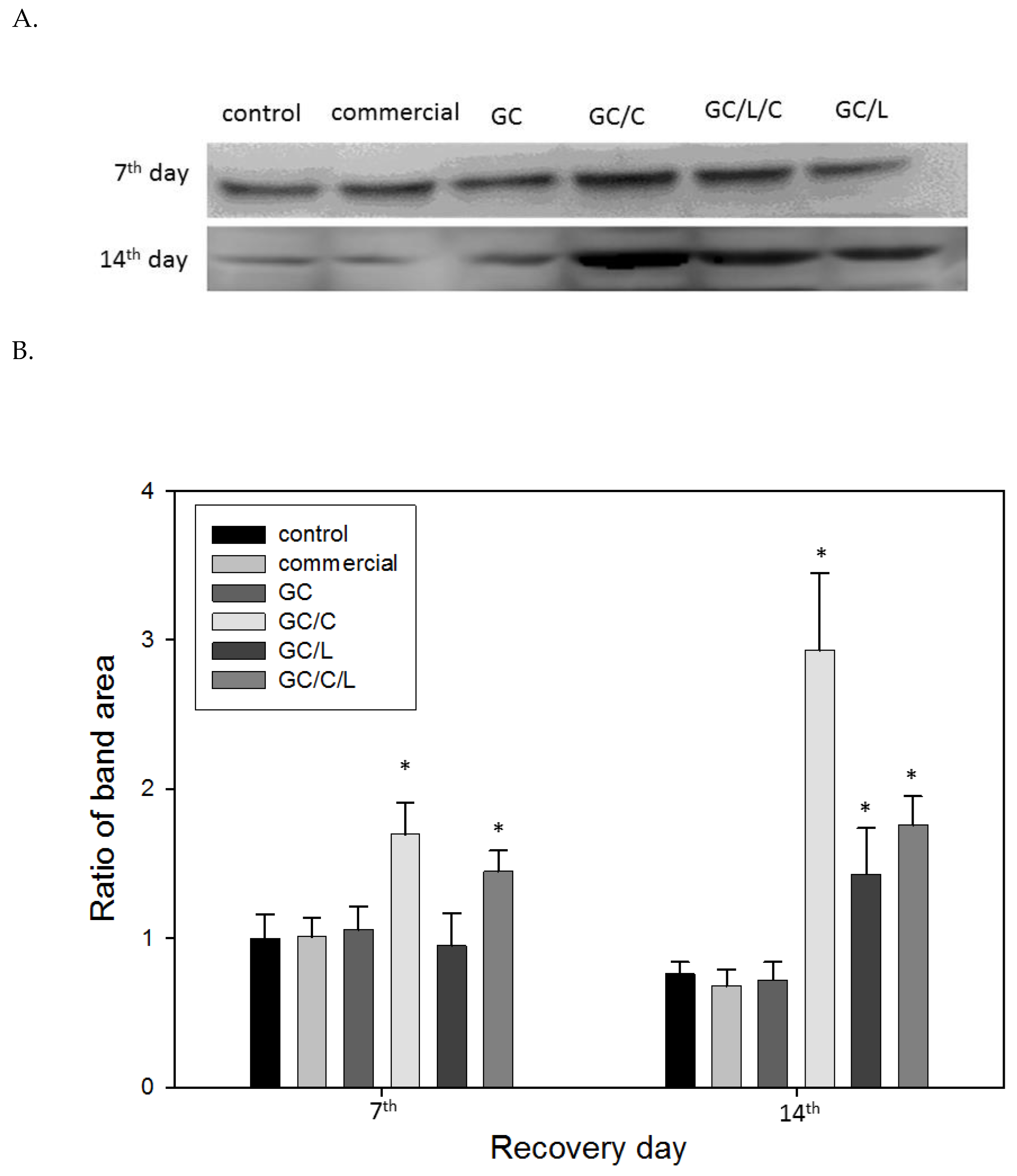
3.3.3. In Vivo Treatments with GC/C and GC/L/C Bilayer Scaffold Increased TGF-® Protein Level in Wounds
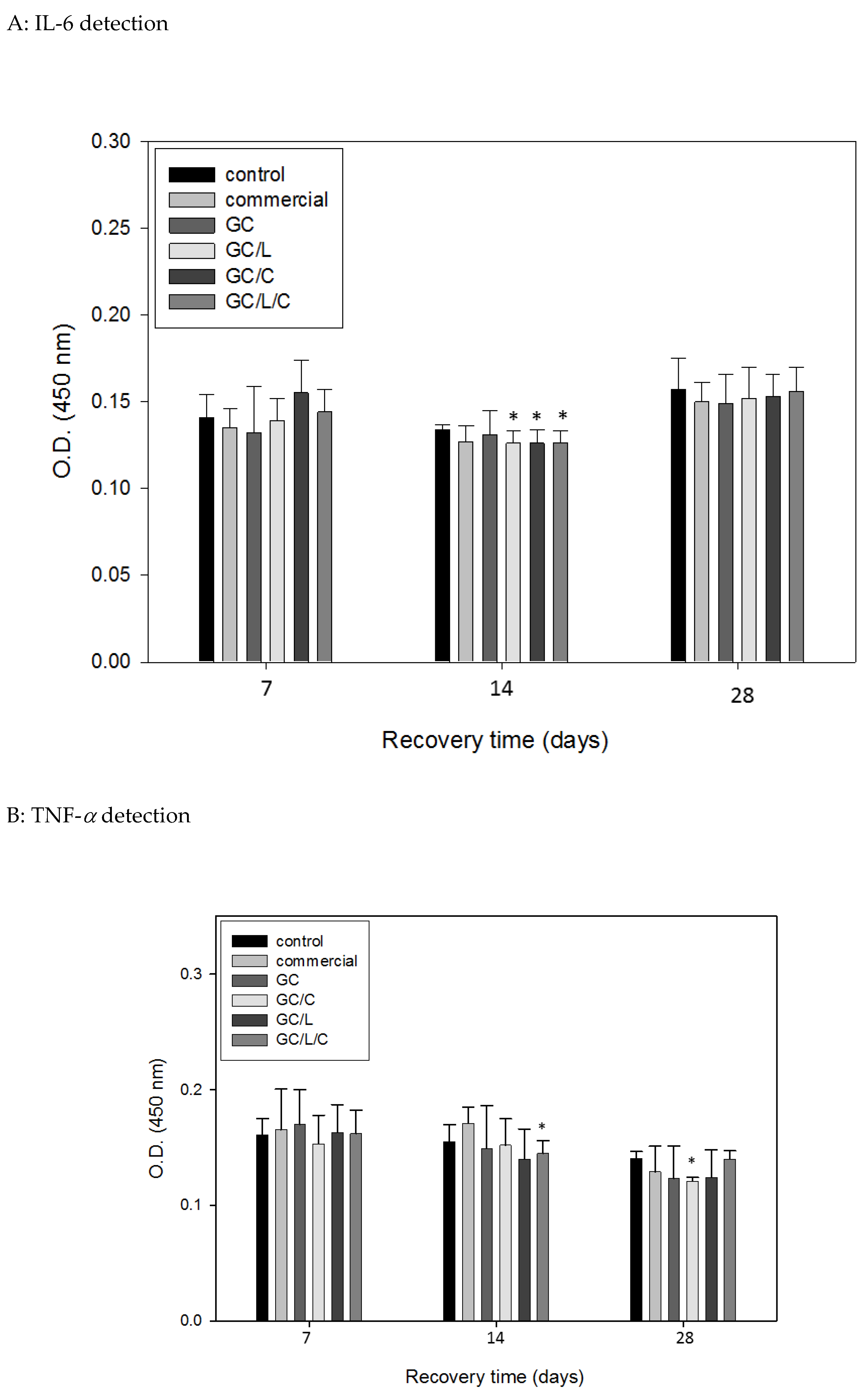
3.3.4. In Vivo Treatments with GC/L/C Nanofibers Reduced Pro-Inflammatory Cytokines IL-6 and TNF-α Protein Level in Wounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: an overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef]
- Wang, X.; Ding, B.; Li, B. Biomimetic electrospun nanofibrous structures for tissue engineering. Mater. Today 2013, 16, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, S.; Yar, M.; Siddiqi, S.A.; Mahmood, N.; Rauf, A.; Qureshi, Z.U.; Anwar, M.S.; Afzaal, S. Chitosan-based electrospun nanofibrous mats, hydrogels and cast films: Novel anti-bacterial wound dressing matrices. J. Mater. Sci. Mater. Med. 2015, 26, 136. [Google Scholar] [CrossRef] [PubMed]
- Movaffagh, J.; Fazly-Bazzaz, B.S.; Yazdi, A.T.; Sajadi-Tabassi, A.; Azizzadeh, M.; Najafi, E.; Amiri, N.; Taghanaki, H.B.; Ebrahimzadeh, M.H.; Moradi, A. Wound healing and antimicrobial effects of chitosan-hydrogel/honey compounds in a rat full-thickness wound model. Wounds A Compendium Clin. Res. Pract. 2019, 31, 228–235. [Google Scholar]
- Wang, M.; Roy, A.K.; Webster, T.J. Development of chitosan/poly(vinyl alcohol) electrospun nanofibers for infection related wound healing. Front. Physiol. 2016, 7, 683. [Google Scholar] [CrossRef]
- Staroszczyk, H.; Sztuka, K.; Wolska, J.; Wojtasz-Pajak, A.; Kolodziejska, I. Interactions of fish gelatin and chitosan in uncrosslinked and crosslinked with edc films: FT-IR study. Spectrochim. Acta Part A 2014, 117, 707–712. [Google Scholar] [CrossRef]
- Pillay, V.; Dott, C.; Choonara, Y.E.; Tyagi, C.; Tomar, L.; Kumar, P.; du Toit, L.C.; Ndesendo, V.M.K. A review of the effect of processing variables on the fabrication of electrospun nanofibers for drug delivery applications. J. Nanomater. 2013, 2013, 789289. [Google Scholar] [CrossRef]
- Yao, C.H.; Yeh, J.Y.; Chen, Y.S.; Li, M.H.; Huang, C.H. Wound-healing effect of electrospun gelatin nanofibres containing centella asiatica extract in a rat model. J. Tissue Eng. Regen. Med. 2017, 11, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Kim, M.Y.; Choi, C.H.; Kim, B.J.; Kim, K.Y.; Kim, G.Y.; Jeong, H.W.; Kim, H.W. Effect of lithospermi radix on contact dermatitis induced by dinitrofluorobenzene in mice. J. Pharmacopunct. 2012, 15, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Andujar, I.; Recio, M.C.; Giner, R.M.; Rios, J.L. Traditional chinese medicine remedy to jury: The pharmacological basis for the use of shikonin as an anticancer therapy. Curr. Med. Chem. 2013, 20, 2892–2898. [Google Scholar] [CrossRef]
- Yin, S.Y.; Peng, A.P.; Huang, L.T.; Wang, Y.T.; Lan, C.W.; Yang, N.S. The phytochemical shikonin stimulates epithelial-mesenchymal transition (EMT) in skin wound healing. Evid. Based Complement. Altern. Med. 2013, 2013, 262796. [Google Scholar] [CrossRef] [PubMed]
- Fujita, N.; Sakaguchi, I.; Kobayashi, H.; Ikeda, N.; Kato, Y.; Minamino, M.; Ishii, M. An extract of the root of lithospermun erythrorhison accelerates wound healing in diabetic mice. Biol. Pharm. Bull. 2003, 26, 329–335. [Google Scholar] [CrossRef]
- Yao, C.H.; Chen, K.Y.; Chen, Y.S.; Li, S.J.; Huang, C.H. Lithospermi radix extract-containing bilayer nanofiber membrane for promoting wound healing in a rat model. Mater. Sci. Eng. C 2019, 96, 850–858. [Google Scholar] [CrossRef]
- Andujar, I.; Rios, J.L.; Giner, R.M.; Recio, M.C. Shikonin promotes intestinal wound healing in vitro via induction of tgf-beta release in iec-18 cells. Eur. J. Pharm. Sci 2013, 49, 637–641. [Google Scholar] [CrossRef]
- Guo, R.; Lan, Y.; Xue, W.; Cheng, B.; Zhang, Y.; Wang, C.; Ramakrishna, S. Collagen-cellulose nanocrystal scaffolds containing curcumin-loaded microspheres on infected full-thickness burns repair. J. Tissue Eng. Regen. Med. 2017, 11, 3544–3555. [Google Scholar] [CrossRef]
- Venkatasubbu, G.D.; Anusuya, T. Investigation on curcumin nanocomposite for wound dressing. Int. J. Biol. Macromol. 2017, 98, 366–378. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Sapudom, J.; Wu, X.; Chkolnikov, M.; Ansorge, M.; Anderegg, U.; Pompe, T. Fibroblast fate regulation by time dependent tgf-beta1 and il-10 stimulation in biomimetic 3d matrices. Biomater. Sci. 2017, 5, 1858–1867. [Google Scholar] [CrossRef] [PubMed]
- Carthy, J.M. Tgfbeta signaling and the control of myofibroblast differentiation: Implications for chronic inflammatory disorders. J. Cell. Physiol. 2018, 233, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Lenzen, S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 2008, 51, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, L.M.; Dos Santos, G.C.; Antonucci, G.A.; Dos Santos, A.C.; Bianchi Mde, L.; Antunes, L.M. Evaluation of the cytotoxicity and genotoxicity of curcumin in pc12 cells. Mutat. Res. 2009, 675, 29–34. [Google Scholar] [CrossRef]
- Cao, J.; Jiang, L.P.; Liu, Y.; Yang, G.; Yao, X.F.; Zhong, L.F. Curcumin-induced genotoxicity and antigenotoxicity in hepg2 cells. Toxicon 2007, 49, 1219–1222. [Google Scholar] [CrossRef]
- Mohanty, C.; Das, M.; Sahoo, S.K. Sustained wound healing activity of curcumin loaded oleic acid based polymeric bandage in a rat model. Mol. Pharm. 2012, 9, 2801–2811. [Google Scholar] [CrossRef]
- Sidhu, G.S.; Singh, A.K.; Thaloor, D.; Banaudha, K.K.; Patnaik, G.K.; Srimal, R.C.; Maheshwari, R.K. Enhancement of wound healing by curcumin in animals. Wound Repair Regen. 1998, 6, 167–177. [Google Scholar] [CrossRef]
- Sidhu, G.S.; Mani, H.; Gaddipati, J.P.; Singh, A.K.; Seth, P.; Banaudha, K.K.; Patnaik, G.K.; Maheshwari, R.K. Curcumin enhances wound healing in streptozotocin induced diabetic rats and genetically diabetic mice. Wound Repair Regen. 1999, 7, 362–374. [Google Scholar] [CrossRef]
- Chung, C.C.; Kao, Y.H.; Liou, J.P.; Chen, Y.J. Curcumin suppress cardiac fibroblasts activities by regulating proliferation, migration, and the extracellular matrix. Acta Cardiol. Sin. 2014, 30, 474–482. [Google Scholar]
- de Campos, P.S.; Matte, B.F.; Diel, L.F.; Jesus, L.H.; Bernardi, L.; Alves, A.M.; Rados, P.V.; Lamers, M.L. Low doses of curcuma longa modulates cell migration and cell-cell adhesion. Phytother. Res. 2017, 31, 1433–1440. [Google Scholar] [CrossRef]
- Krausz, A.E.; Adler, B.L.; Cabral, V.; Navati, M.; Doerner, J.; Charafeddine, R.A.; Chandra, D.; Liang, H.; Gunther, L.; Clendaniel, A.; et al. Curcumin-encapsulated nanoparticles as innovative antimicrobial and wound healing agent. Nanomedicine 2015, 11, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Thomopoulos, S.; Xia, Y. Electrospun nanofibers for regenerative medicine. Adv. Healthc. Mater. 2012, 1, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Mogosanu, G.D.; Grumezescu, A.M. Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014, 463, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Garg, T.; Singh, O.; Arora, S.; Murthy, R. Scaffold: A novel carrier for cell and drug delivery. Crit. Rev. Ther. Drug Carr. Syst. 2012, 29, 1–63. [Google Scholar] [CrossRef]
- Nagahama, H.; Rani, V.V.; Shalumon, K.T.; Jayakumar, R.; Nair, S.V.; Koiwa, S.; Furuike, T.; Tamura, H. Preparation, characterization, bioactive and cell attachment studies of alpha-chitin/gelatin composite membranes. Int. J. Biol. Macromol. 2009, 44, 333–337. [Google Scholar] [CrossRef]
- Panchatcharam, M.; Miriyala, S.; Gayathri, V.S.; Suguna, L. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol. Cell. Biochem. 2006, 290, 87–96. [Google Scholar] [CrossRef]
- Kirsner, R.S.; Eaglstein, W.H. The wound healing process. Dermatol. Clin. 1993, 11, 629–640. [Google Scholar] [CrossRef]
- Diegelmann, R.F.; Evans, M.C. Wound healing: an overview of acute, fibrotic and delayed healing. Front. Biosci. 2004, 9, 283–289. [Google Scholar] [CrossRef]
- Ghosh, P.K.; Gaba, A. Phyto-extracts in wound healing. J. Pharm. Pharm. Sci. 2013, 16, 760–820. [Google Scholar] [CrossRef]
- Choudhary, V.; Shivakumar, H.G. A review on curcumin: Wound healing properties and biomarkers of wound healing. Int. Res. J. Pharm 2008, 9, 1–5. [Google Scholar] [CrossRef]
- Prasad, R.; Kumar, D.; Kant, V.; Tandan, S.K.; Kumar, D. Curcumin enhanced cutaneous wound healing by modulating cytokines and transforming growth factor in excision wound model in rats. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2263–2273. [Google Scholar] [CrossRef]
- Yang, S.; Xia, C.; Li, S.; Du, L.; Zhang, L.; Hu, Y. Mitochondrial dysfunction driven by the lrrk2-mediated pathway is associated with loss of purkinje cells and motor coordination deficits in diabetic rat model. Cell Death Dis. 2014, 5, e1217. [Google Scholar] [CrossRef] [PubMed]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.-Y.; Hu, C.-H.; Huang, W.-C.; Ho, C.-Y.; Yao, C.-H.; Huang, C.-H. Effects of Bilayer Nanofibrous Scaffolds Containing Curcumin/Lithospermi Radix Extract on Wound Healing in Streptozotocin-Induced Diabetic Rats. Polymers 2019, 11, 1745. https://doi.org/10.3390/polym11111745
Yang B-Y, Hu C-H, Huang W-C, Ho C-Y, Yao C-H, Huang C-H. Effects of Bilayer Nanofibrous Scaffolds Containing Curcumin/Lithospermi Radix Extract on Wound Healing in Streptozotocin-Induced Diabetic Rats. Polymers. 2019; 11(11):1745. https://doi.org/10.3390/polym11111745
Chicago/Turabian StyleYang, Bo-Yin, Chung-Hsuan Hu, Wei-Chien Huang, Chien-Yi Ho, Chun-Hsu Yao, and Chiung-Hua Huang. 2019. "Effects of Bilayer Nanofibrous Scaffolds Containing Curcumin/Lithospermi Radix Extract on Wound Healing in Streptozotocin-Induced Diabetic Rats" Polymers 11, no. 11: 1745. https://doi.org/10.3390/polym11111745
APA StyleYang, B.-Y., Hu, C.-H., Huang, W.-C., Ho, C.-Y., Yao, C.-H., & Huang, C.-H. (2019). Effects of Bilayer Nanofibrous Scaffolds Containing Curcumin/Lithospermi Radix Extract on Wound Healing in Streptozotocin-Induced Diabetic Rats. Polymers, 11(11), 1745. https://doi.org/10.3390/polym11111745