Macroporous Oil-Sorbents with a High Absorption Capacity and High-Temperature Tolerance Prepared Through Cryo-Polymerization
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
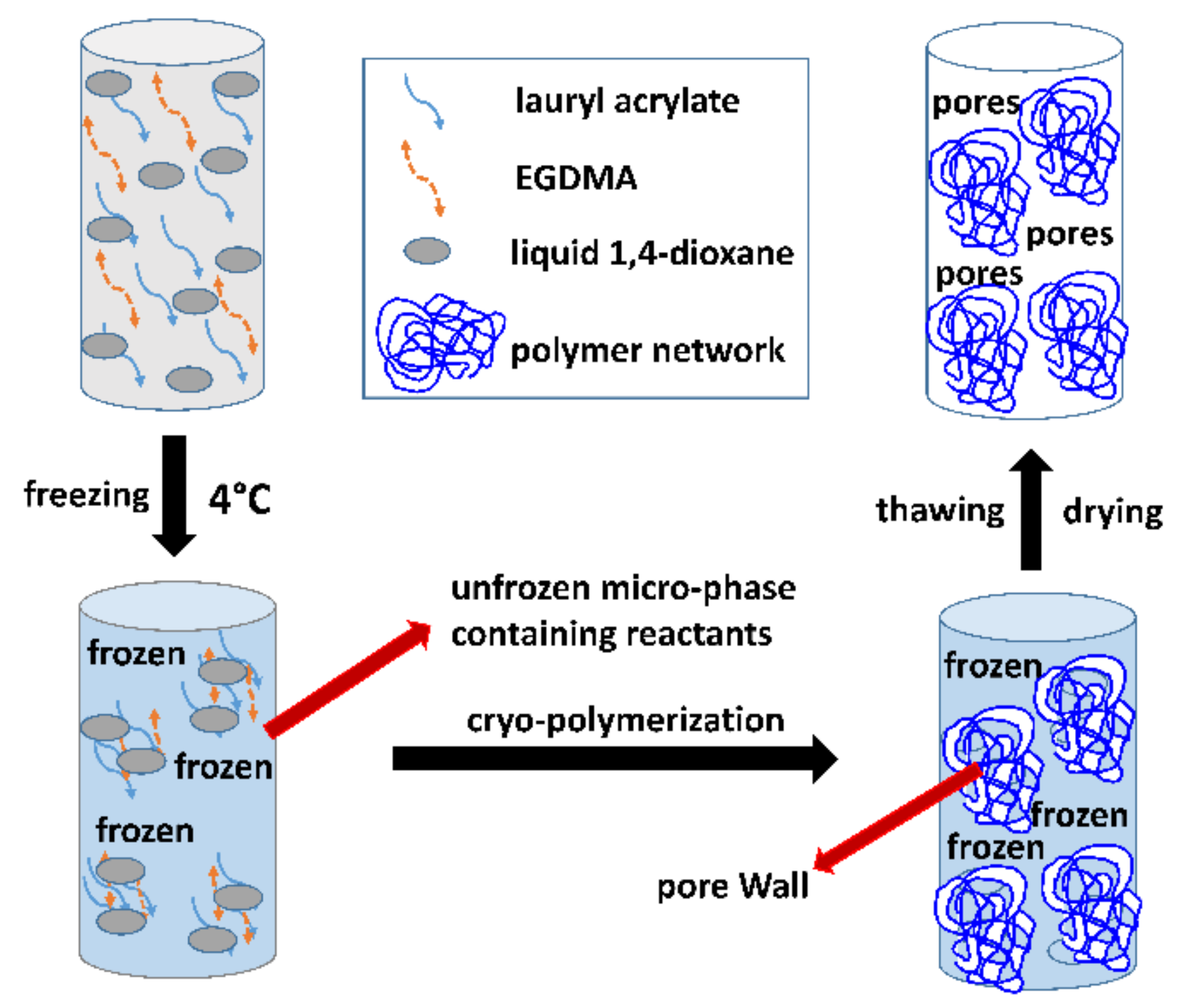
2.2. Preparation of Hydrophobic Cryogels through Cryo-Polymerization in Dioxane
2.3. Determination of Oil Absorption Capacity
2.4. Determination of Gel Fraction through Repeating Absorption
2.5. Instruments
3. Results and Discussion
3.1. Synthesis of Macro-Porous Hydrophobic Cryogels
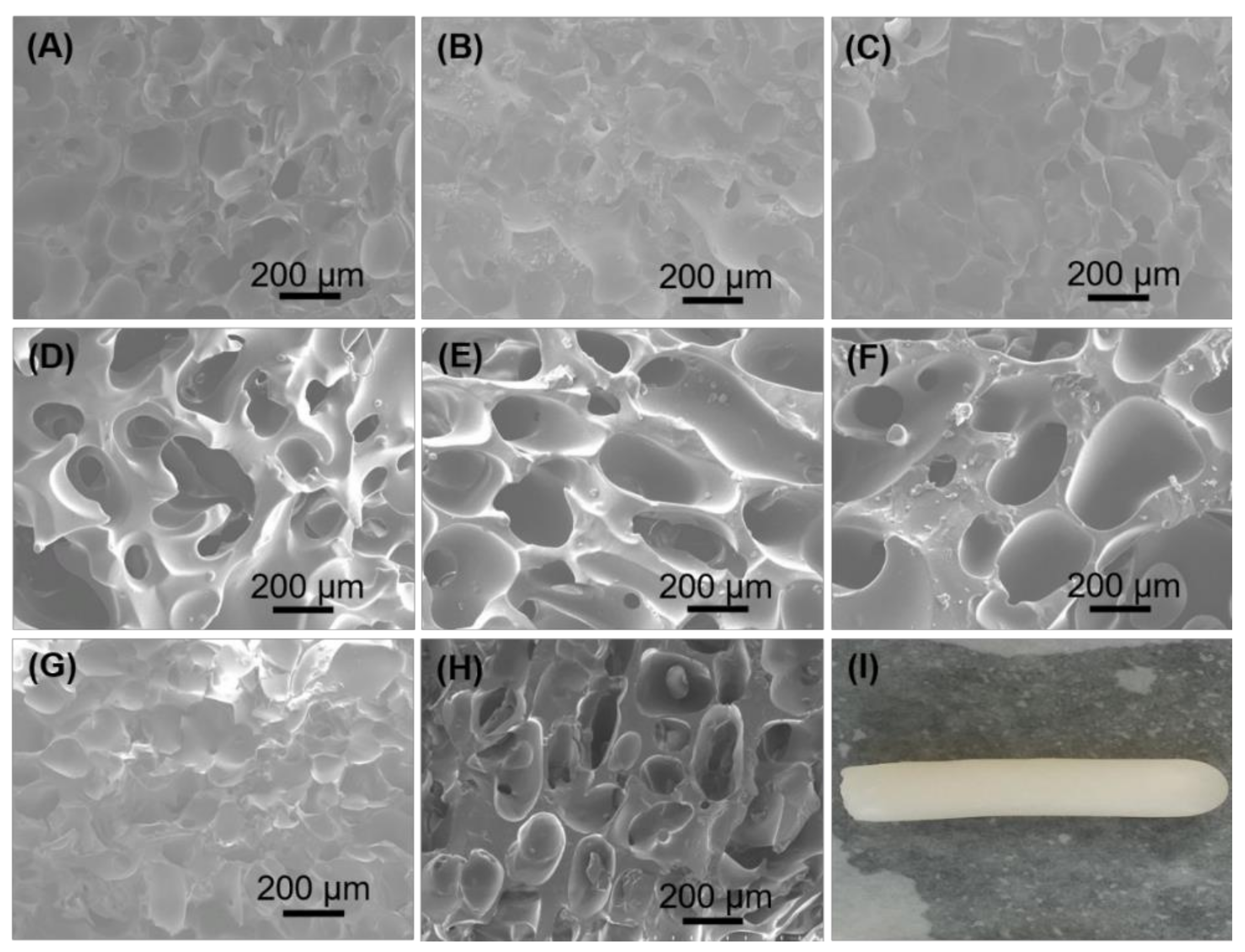
3.2. Morphology and Porosity of Cryogels
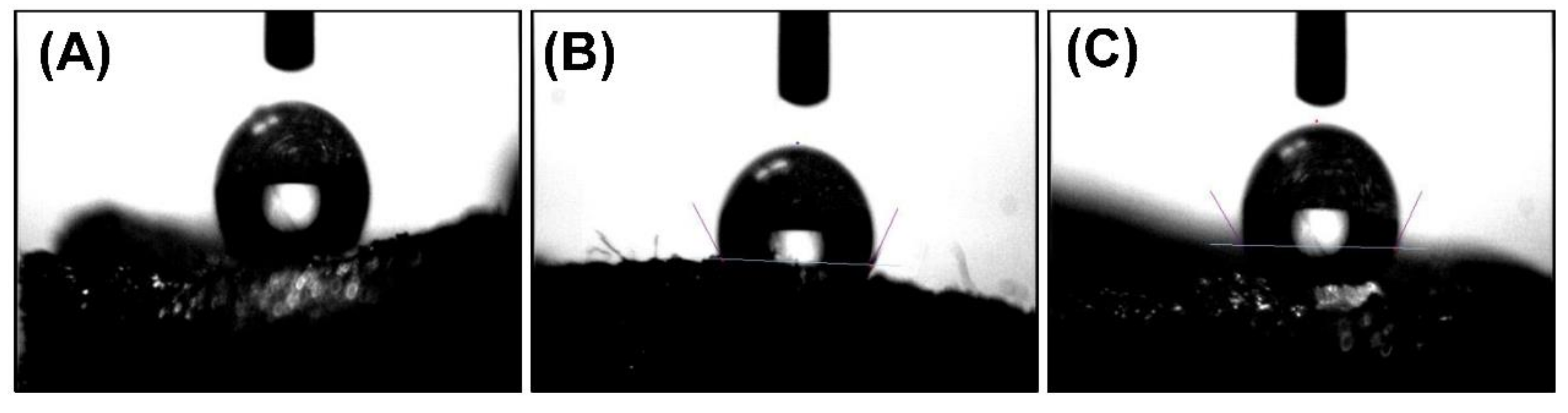
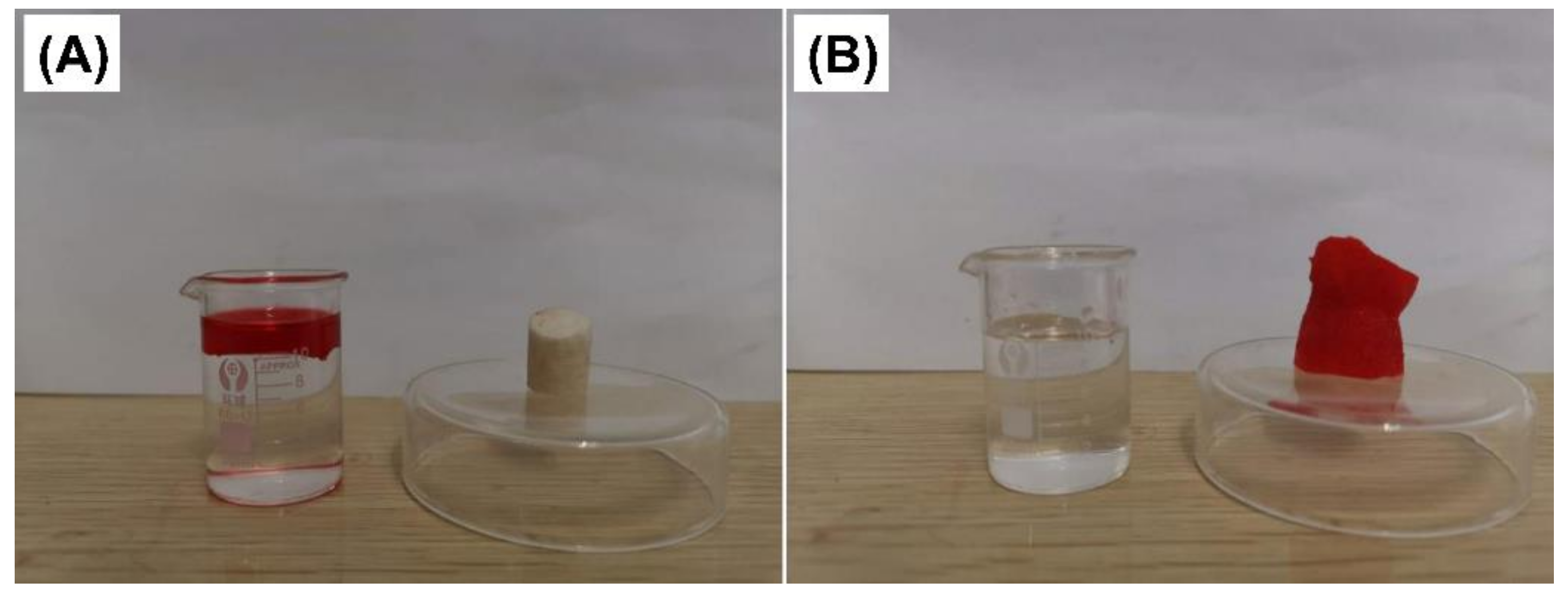
3.3. Hydrophobicity of PLA-EGDMA Cryogels
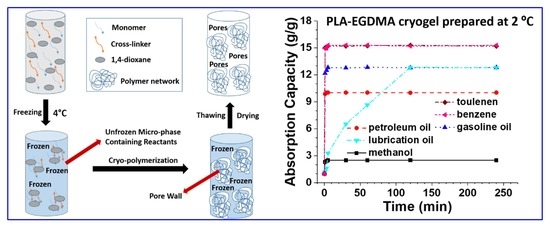
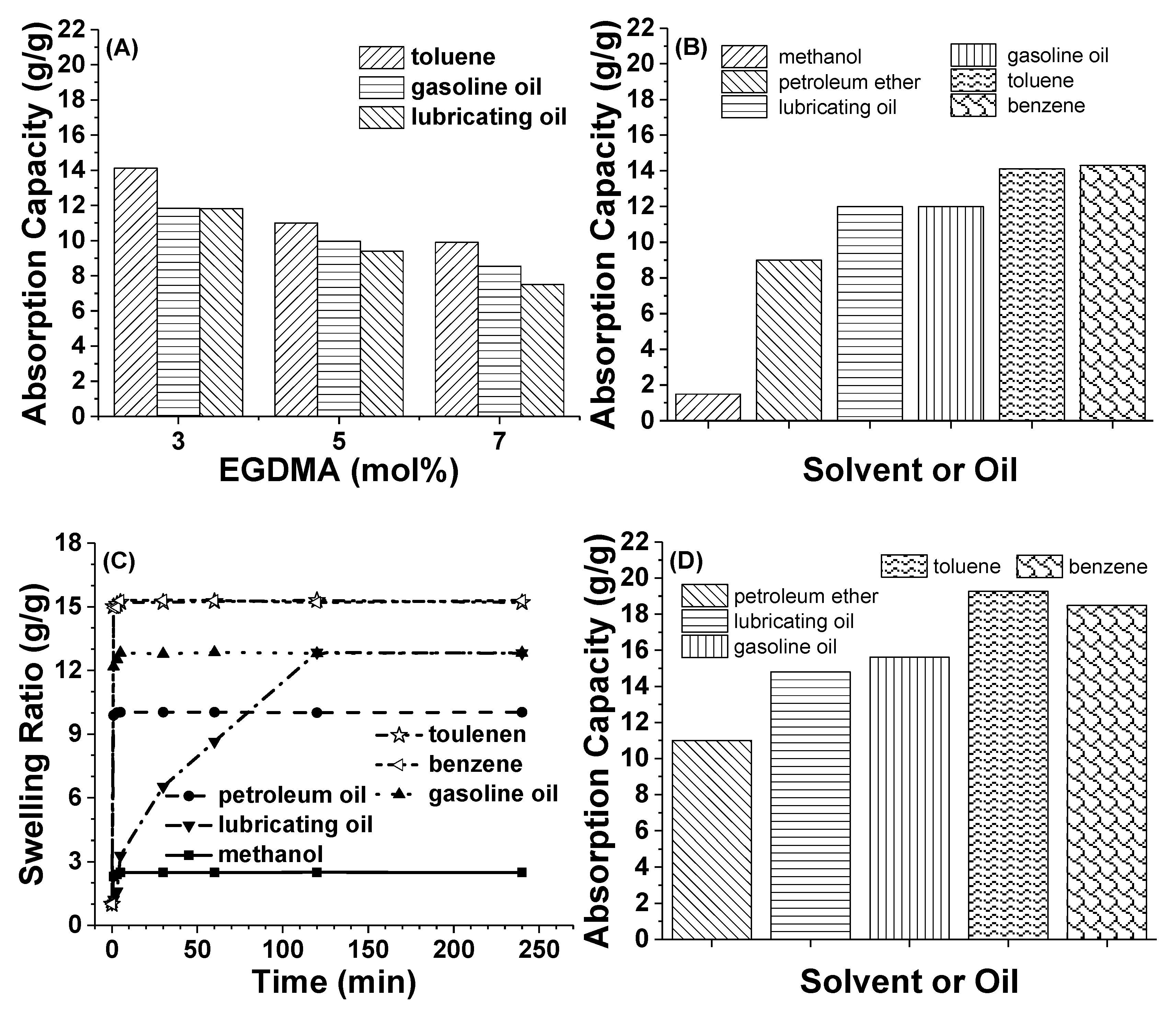
3.4. Influences of Different Parameters on the Absorption Capacity of Cryogels
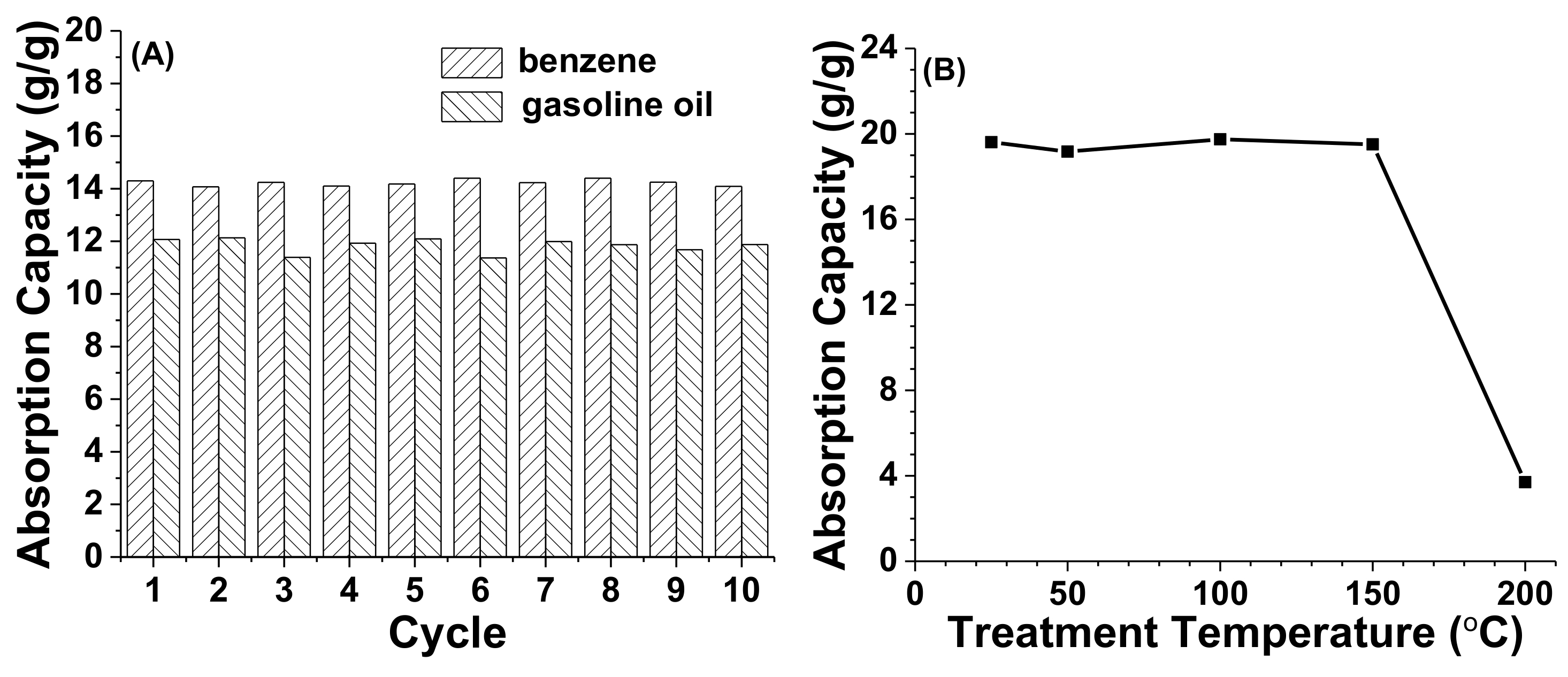
3.5. Repeating Absorption and High-Temperature Tolerance of Hydrophobic Cryogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aguilera, F.; Mendez, J.; Pasaro, E.; Laffon, B. Review on the effects of exposure to spilled oils on human health. J. Appl. Toxicol. 2010, 30, 291–301. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan-Gordo, C.; Orta-Martinez, M.; Kogevinas, M. Health effects of non-occupational exposure to oil extraction. Environ. Health 2016, 15, 56. [Google Scholar] [CrossRef]
- Li, P.; Cai, Q.H.; Lin, W.Y.; Chen, B.; Zhang, B.Y. Offshore oil spill response practices and emerging challenges. Mar. Pollut. Bull. 2016, 1101, 6–27. [Google Scholar] [CrossRef] [PubMed]
- Laffon, B.; Pasaro, E.; Valdiglesias, V. Effects of exposure to oil spills on human health: Updated review. J. Toxicol. Environ. Health Part B Crit. Rev. 2016, 19, 105–128. [Google Scholar] [CrossRef] [PubMed]
- Bejarano, A.C.; Michel, J. Oil spills and their impacts on sand beach invertebrate communities: A literature review. Environ. Pollut. 2016, 218, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Pintor, A.M.A.; Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Oil and grease removal from wastewaters: Sorption treatment as an alternative to state-of-the-art technologies. A critical review. Chem. Eng. J. 2016, 297, 229–255. [Google Scholar] [CrossRef]
- Prendergast, D.P.; Gschwend, P.M. Assessing the performance and cost of oil spill remediation technologies. J. Cleaner Prod. 2014, 78, 233–242. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Bhaskarwar, A.N. A review on sorbent devices for oil-spill control. Environ. Poll. 2018, 243, 1758–1771. [Google Scholar] [CrossRef]
- Maxwell, K.; Kiessling, B.; Buckley, J. How clean is clean: A review of the social science of environmental cleanups. Environ. Res. Lett. 2018, 13, 083002. [Google Scholar] [CrossRef]
- Al-Majed, A.A.; Adebayo, A.R.; Hossain, M.E. A sustainable approach to controlling oil spills. J. Environ. Manag. 2012, 113, 213–227. [Google Scholar] [CrossRef]
- Guidi, G.; Sliskovic, M.; Violante, A.C.; Vukic, L. Best available techniques (BATs) for oil spill response in the Mediterranean Sea: Calm sea and presence of economic activities. Environ. Sci. Poll. Res. 2016, 23, 1944–1953. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, F.; Scoma, A.; Michoud, G.; Aulenta, F.; Boon, N.; Borin, S.; Kalogerakis, N.; Daffonchio, D. Biotechnologies for marine oil spill cleanup: Indissoluble ties with microorganisms. Trends Biotechnol. 2017, 35, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Varjani, S.J. Microbial degradation of petroleum hydrocarbons. Bioresour. Technol. 2017, 223, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Yavari, S.; Malakahmad, A.; Sapari, N.B. A review on phytoremediation of crude oil spills. Water Air Soil Poll. 2015, 226, 279. [Google Scholar] [CrossRef]
- Doshi, B.; Sillanpaa, M.; Kalliola, S. A review of bio-based materials for oil spill treatment. Water Res. 2018, 135, 262–277. [Google Scholar] [CrossRef]
- Wahi, R.; Chuah, L.A.; Choong, T.S.Y.; Nagaini, Z.; Nourouzi, M. Oil removal from aqueous state by natural fibrous sorbent: An overview. Sep. Purif. Technol. 2013, 113, 51–63. [Google Scholar] [CrossRef]
- Saleem, J.; Riaz, M.A.; McKay, G. Oil sorbents from plastic wastes and polymers: A review. J. Hazard. Mater. 2018, 341, 424–437. [Google Scholar] [CrossRef]
- Pinto, J.; Athanassiou, A.; Fragouli, D. Surface modification of polymeric foams for oil spills remediation. J. Environ. Manag. 2018, 206, 872–889. [Google Scholar] [CrossRef]
- Gupta, S.; Tai, N.H. Carbon materials as oil sorbents: A review on the synthesis and performance. J. Mater. Chem. A 2016, 4, 1550–1565. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Li, C.; Liang, H.W.; Chen, J.F.; Yu, S.H. Ultralight, flexible, and fire-resistant carbon nanofiber aerogels from bacterial cellulose. Angew. Chem. Int. Ed. 2013, 52, 2925–2929. [Google Scholar] [CrossRef]
- Neuberger, N.; Adidharma, H.; Fan, M.H. Graphene: A review of applications in the petroleum industry. J. Pet. Sci. Eng. 2018, 167, 152–159. [Google Scholar] [CrossRef]
- Karakutuk, I.; Okay, O. Macroporous rubber gels as reusable sorbents for the removal of oil from surface waters. React. Funct. Polym. 2010, 70, 585–595. [Google Scholar] [CrossRef]
- Oh, Y.S.; Maeng, J.; Kim, S.J. Use of microorganism-immobilized polyurethane foams to absorb and degrade oil on water surface. Appl. Microbiol. Biotechnol. 2000, 54, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Shang, Y.W.; Ding, B.; Yang, J.M.; Yu, J.Y.; Al-Deyab, S.S. Nanoporous polystyrene fibers for oil spill cleanup. Mar. Pollut. Bull. 2012, 64, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Hong, Y.J.; Hu, A.H. Using a composite material containing waste tire powder and polypropylene fiber cut end to recover spilled oil. Waste Manag. 2010, 30, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.F.; Mather, R.R.; Fotheringham, A.F.; Yang, R.D. Evaluation of nonwoven polypropylene oil sorbents in marine oil-spill recovery. Mar. Poll. Bull. 2003, 46, 780–783. [Google Scholar] [CrossRef]
- Guillen, G.R.; Pan, Y.J.; Li, M.H.; Hoek, E.M.V. Preparation and characterization of membranes formed by nonsolvent induced phase separation: A review. Ind. Eng. Chem. Res. 2011, 50, 3798–3817. [Google Scholar] [CrossRef]
- Zhang, T.; Guo, Q.P. Continuous preparation of polyHIPE monoliths from ionomer-stabilized high internal phase emulsions (HIPEs) for efficient recovery of spilled oils. Chem. Eng. J. 2017, 307, 812–819. [Google Scholar] [CrossRef]
- Guan, T.T.; Gao, Y.W.; Pan, M.M.; Wu, Y.W.; Zhang, S.H.; Xu, L.H.; Zhu, L.Y.; Yun, J.X. Slug flow hydrodynamics of immiscible fluids within a rectangular microchannel towards size-controllable fabrication of dextran-based cryogel beads. Chem. Eng. J. 2019, 369, 116–123. [Google Scholar] [CrossRef]
- Luo, G.X.; Teh, K.S.; Liu, Y.M.; Zang, X.N.; Wen, Z.Y.; Lin, L.W. Direct-write, self-aligned electrospinning on paper for controllable fabrication of three-dimensional structures. ACS Appl. Mater. Interfaces 2015, 7, 27765–27770. [Google Scholar] [CrossRef]
- Michalicova, P.; Mravec, F.; Pekar, M. Fluorescence study of freeze-drying as a method for support the interactions between hyaluronan and hydrophobic species. PLoS ONE 2017, 12, e0184558. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.Y.; Jing, X.; Liu, Y.J.; Li, L.W.; Li, H.; Peng, X.F.; Zhou, H.M. Highly durable superhydrophobic polymer foams fabricated by extrusion and supercritical CO2 foaming for selective oil absorption. ACS Appl. Mater. Interfaces 2019, 11, 7479–7487. [Google Scholar] [CrossRef] [PubMed]
- Nikonorov, V.V.; Ivanov, R.V.; Kildeeva, N.R.; Bulatnikova, L.N.; Lozinsky, V.I. Synthesis and characteristics of cryogels of chitosan crosslinked by glutaric aldehyde. Polym. Sci. Series A 2010, 52, 828–834. [Google Scholar] [CrossRef]
- Sun, X.L.; He, W.D.; Pan, T.T.; Ding, Z.L.; Zhang, Y.J. RAFT cryopolymerizations of acrylamides and acrylates in dioxane at −5 °C. Polymer 2010, 51, 110–114. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Vainerman, E.S.; Rogozhin, S.V. Study of cryostructurization of polymer systems 2. The influence of freezing of a reacting mass on the properties of products in the preparation of covalently cross-linked gels. Colloid Polym. Sci. 1982, 268, 776–780. [Google Scholar] [CrossRef]
- Chen, X.L.; Sui, W.W.; Ren, D.Y.; Ding, Y.Y.; Zhu, X.L.; Chen, Z.Y. Synthesis of hydrophobic polymeric cryogels with supermacroporous structure. Macromol. Mater. Eng. 2016, 301, 659–664. [Google Scholar] [CrossRef]
- Chen, K.L.; Zhao, Y.H.; Yuan, X.Y. Grafting of poly(lauryl acrylate) onto nano-silica by ‘click chemistry’. Chem. Res. Chin. Univ. 2014, 30, 339–342. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Ivanov, R.V.; Kalinina, E.V.; Timofeeva, G.I.; Khokhlov, A.R. Redox-initiated radical polymerisation of acrylamide in moderately frozen water solutions. Macromol. Rapid Commun. 2001, 22, 1441–1446. [Google Scholar] [CrossRef]
- Anovitz, L.M.; Cole, D.R. Characterization and Analysis of Porosity and Pore Structures. Pore Scale Geochem. Process. 2015, 80, 61–164. [Google Scholar]
- Soboleva, T.; Zhao, X.S.; Malek, K.; Xie, Z.; Nevessin, T.; Holdcroft, S. On the micro-, meso- and macroporous structures of polymer electrolyte membrane fuel cell catalyst layers. ACS Appl. Mater. Interfaces 2010, 2, 375–384. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems. Pure Appl. Chem. 1982, 54, 2201–2218. [Google Scholar] [CrossRef]
- Wang, J.T.; Wang, Q.; Zheng, Y.; Wang, A.Q. Synthesis and oil absorption of poly(butylmethacrylate)/organo-attapulgite nanocomposite by suspended emulsion polymerization. Polym. Compos. 2013, 34, 274–281. [Google Scholar] [CrossRef]
- Jin, C.D.; Han, S.J.; Li, J.P.; Sun, Q.F. Fabrication of cellulose-based aerogels from waste newspaper without any pretreatment and their use for absorbents. Carbohydr. Polym. 2015, 123, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Zou, F.X.; Peng, L.; Fu, W.X.; Zhang, J.L.; Li, Z.B. Flexible superhydrophobic polysiloxane aerogels for oil-water separation via one-pot synthesis in supercritical CO2. RSC Adv. 2015, 5, 76346–76351. [Google Scholar] [CrossRef]
- Fu, Q.L.; Ansari, F.; Zhou, Q.; Berglund, L.A. Wood nanotechnology for strong, mesoporous, and hydrophobic biocomposites for selective separation of oil/water mixtures. ACS Nano. 2018, 12, 2222–2230. [Google Scholar] [CrossRef] [PubMed]
- Rotaru, A.; Cojocaru, C.; Cretescu, I.; Pinteala, M.; Timpu, D.; Sacarescu, L.; Harabagiu, V. Performances of clay aerogel polymer composites for oil spill sorption: Experimental design and modeling. Sep. Purif. Technol. 2014, 133, 260–275. [Google Scholar] [CrossRef]
- Cojocaru, C.; Pricop, L.; Samoila, P.; Rotaru, R.; Harabagiu, V. Surface hydrophobization of polyester fibers with poly(methylhydro-dimethyl)siloxane copolymers: Experimental design for testing of modified nonwoven materials as oil spill sorbents. Polym. Test. 2017, 59, 377–389. [Google Scholar] [CrossRef]
- Cojocaru, C.; Dorneanu, P.P.; Airinei, A.; Olaru, N.; Samoila, P.; Rotaru, A. Design and evaluation of electrospun polysulfone fibers and polysulfone/NiFe2O4 nanostructured composite as sorbents for oil spill cleanup. J. Taiwan Ins. Chem. Eng. 2017, 70, 267–281. [Google Scholar] [CrossRef]

| Cryogels Code a | EGDMA (mol%) b | Temperature (°C) | Swelling Ratio c | Gel Fraction (%) |
|---|---|---|---|---|
| C-63p4 | 3 | +4 | 21–22 | 79 |
| C-65p4 | 5 | +4 | 14–15 | 80 |
| C-67p4 | 7 | +4 | 12–13 | 81 |
| C-63p2 | 3 | +2 | 15–16 | 95 |
| C-65p2 | 5 | +2 | 12–13 | 98 |
| C-67p2 | 7 | +2 | 10–11 | 97 |
| C-63z0 | 3 | 0 | not measured | 31 |
| C-65z0 | 5 | 0 | not measured | 51 |
| C-67z0 | 7 | 0 | not measured | 54 |
| Material and Fabrication | Absorption Capacity (g/g) | Reference |
|---|---|---|
| Polypropylene/polytetrafluoroethylene foams through twin-screw extrusion and supercritical CO2 foaming | Gasoline oil: ~6.5; benzene and toluene: ~8.0; olive oil: 6.8; dichloromethane: 9.7 | [32] |
| Poly(butyl methacrylate)/organo-attapulgite nano-composite through solution polymerization with emulsified water phase | Dependent on composition; toluene: 15~21; gasoline oil: 8~13; chloroform: 13~36 | [42] |
| Poly(lauryl methacrylate-co-divinylbenzene) cryogels through cryo-polymerization in acetic acid | Toluene: 12; crude oil: 13.5 30 min to reach saturation for crude oil | [36] |
| Chitin sponge through freezing dryness and vapor deposition of methyltrichlorosilane | Gasoline oil: about 33; toluene: about 36; hexane: about 30 | [10] |
| Polyethylene ordinary shock absorption foams doped with titanium dioxide NPs | Lubricating oil: 35~43; tetrachloromethane: 51~63 | [12] |
| Macroporous poly(butyl acrylate) monoliths from photo-initiated high internal phase emulsion (HIPE) polymerization | Benzene: 8.2; toluene: 7.8; gasoline oil: 6.4; hexane: 5.6 | [28] |
| Cellulose-based aerogels through freeze-drying and trimethylchlorosilane vapor deposition | Benzene: 14; toluene: 13.5; waste engineering oil: 16.8 | [43] |
| Polysiloxane aerogels through silyl-hydrogen addition in supercritical CO2 | Petroleum ether: 6.7~9.2; kerosene: 6~8; toluene: 6.5~11.7 | [44] |
| Wood/epoxy bio-composites through template-doping technique | Hexane: 8.5; diesel oil: 9; biodiesel: 14 | [45] |
| Clay aerogel/polymer composites | Dodecane: 23.63: motor oil: 25.84 | [46] |
| Surface hydrophobization of polyester fibers with poly(methylhydro-dimethyl)siloxane copolymers | Dodecane: 5.52; motor oil: 10.03 | [47] |
| Electrospun polysulfone fibers and polysulfone/NiFe2O4 nanostructured composite | Dodecane: 9.20; motor oil: 15.11 | [48] |
| P(LA-co-EGDMA) cryogels through cryo-polymerization in 1,4-dioxane | 20–21 | Our work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haleem, A.; Wang, J.-Y.; Li, H.-J.; Hu, C.-S.; Li, X.-C.; He, W.-D. Macroporous Oil-Sorbents with a High Absorption Capacity and High-Temperature Tolerance Prepared Through Cryo-Polymerization. Polymers 2019, 11, 1620. https://doi.org/10.3390/polym11101620
Haleem A, Wang J-Y, Li H-J, Hu C-S, Li X-C, He W-D. Macroporous Oil-Sorbents with a High Absorption Capacity and High-Temperature Tolerance Prepared Through Cryo-Polymerization. Polymers. 2019; 11(10):1620. https://doi.org/10.3390/polym11101620
Chicago/Turabian StyleHaleem, Abdul, Jia-Yun Wang, Hui-Juan Li, Chuan-Shan Hu, Xi-Chuan Li, and Wei-Dong He. 2019. "Macroporous Oil-Sorbents with a High Absorption Capacity and High-Temperature Tolerance Prepared Through Cryo-Polymerization" Polymers 11, no. 10: 1620. https://doi.org/10.3390/polym11101620
APA StyleHaleem, A., Wang, J.-Y., Li, H.-J., Hu, C.-S., Li, X.-C., & He, W.-D. (2019). Macroporous Oil-Sorbents with a High Absorption Capacity and High-Temperature Tolerance Prepared Through Cryo-Polymerization. Polymers, 11(10), 1620. https://doi.org/10.3390/polym11101620