Application of DNA Quadruplex Hydrogels Prepared from Polyethylene Glycol-Oligodeoxynucleotide Conjugates to Cell Culture Media
Abstract
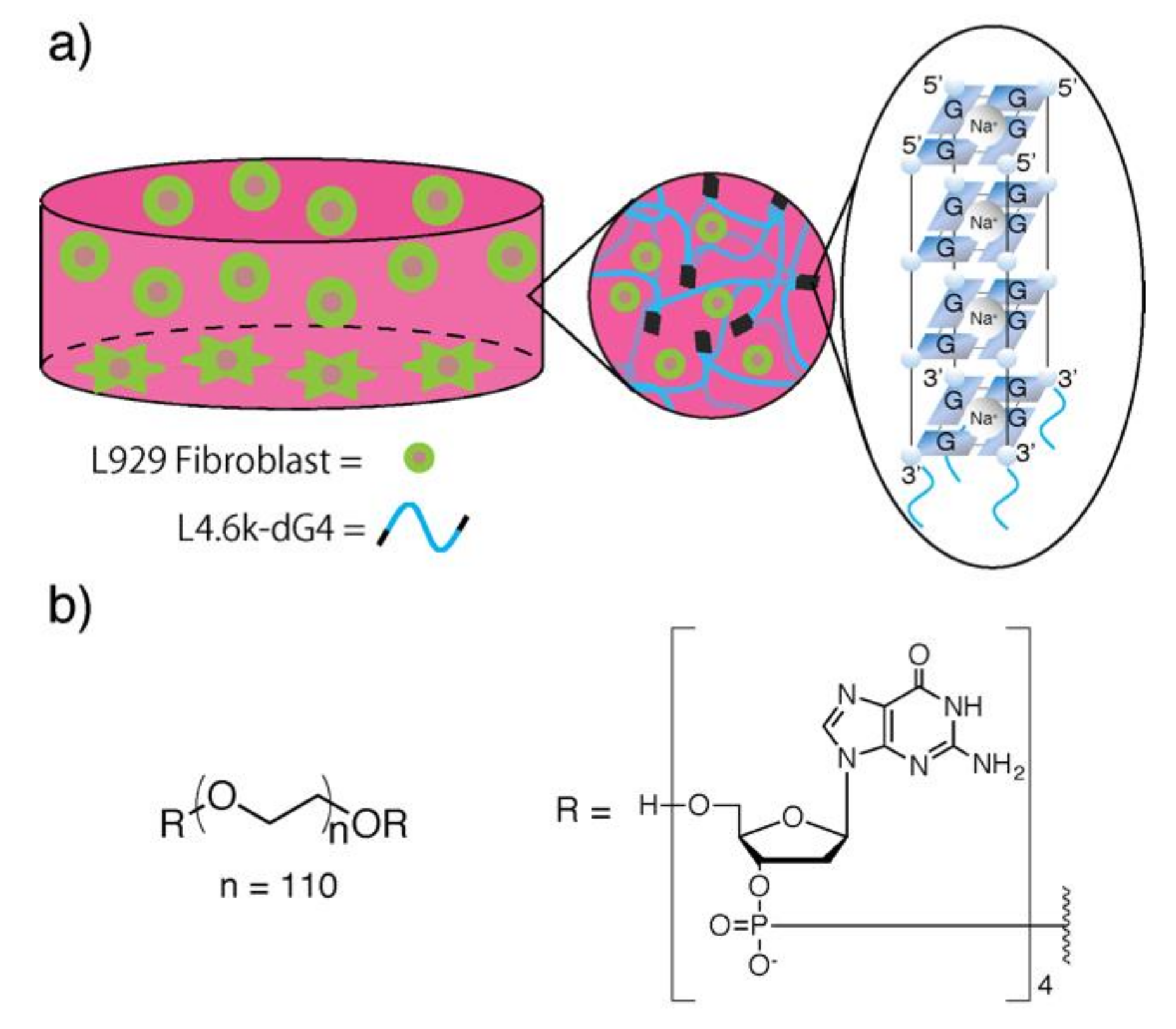
1. Introduction
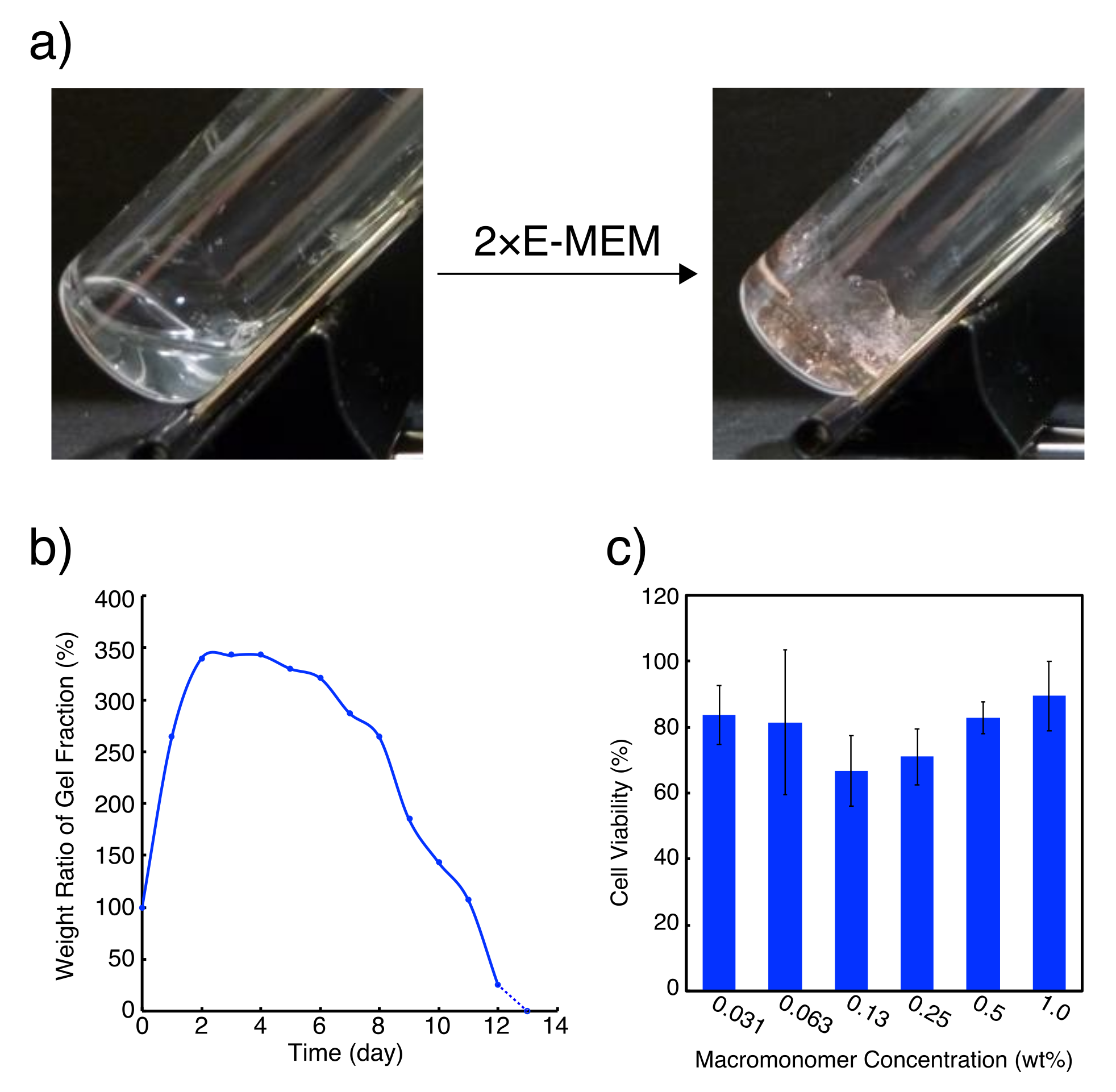
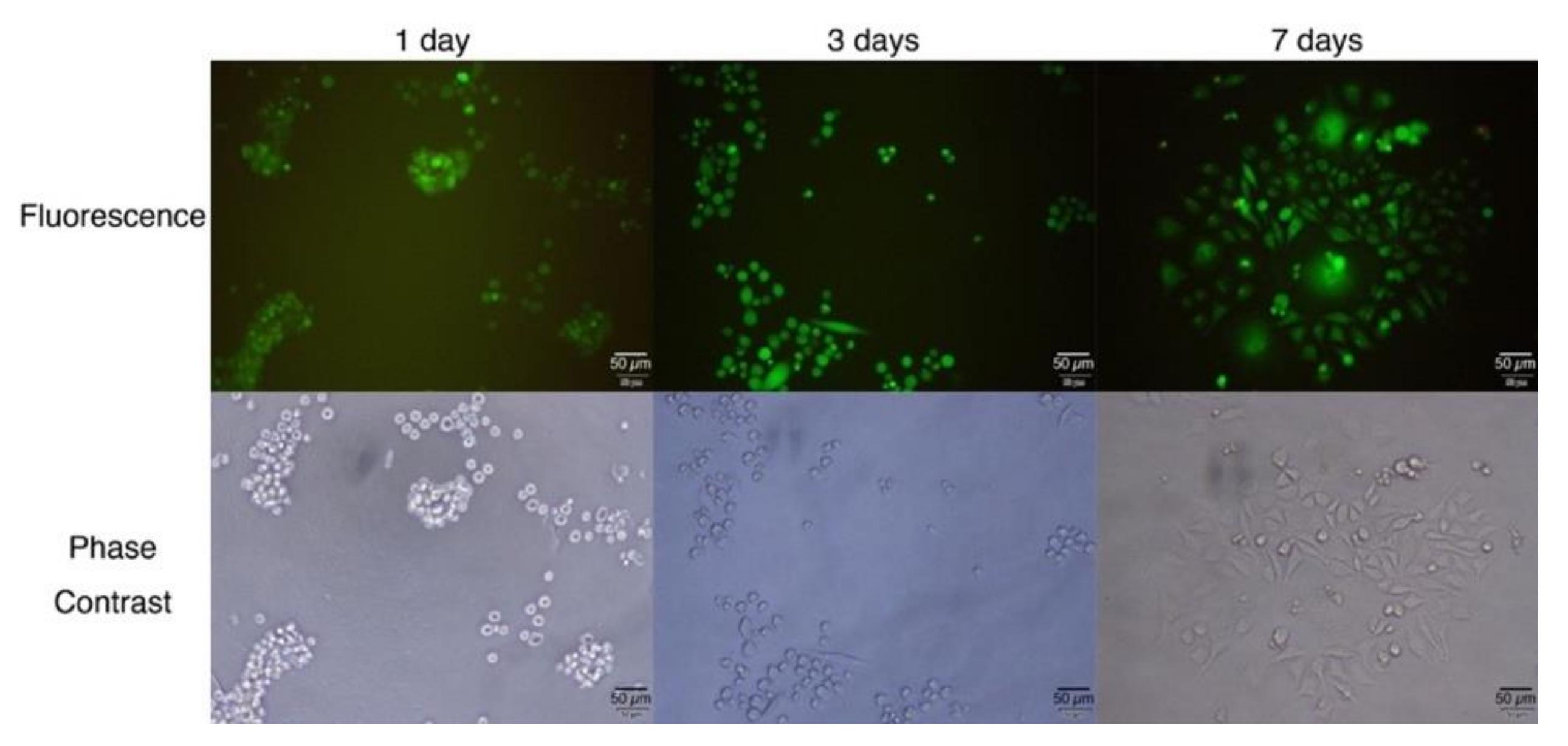
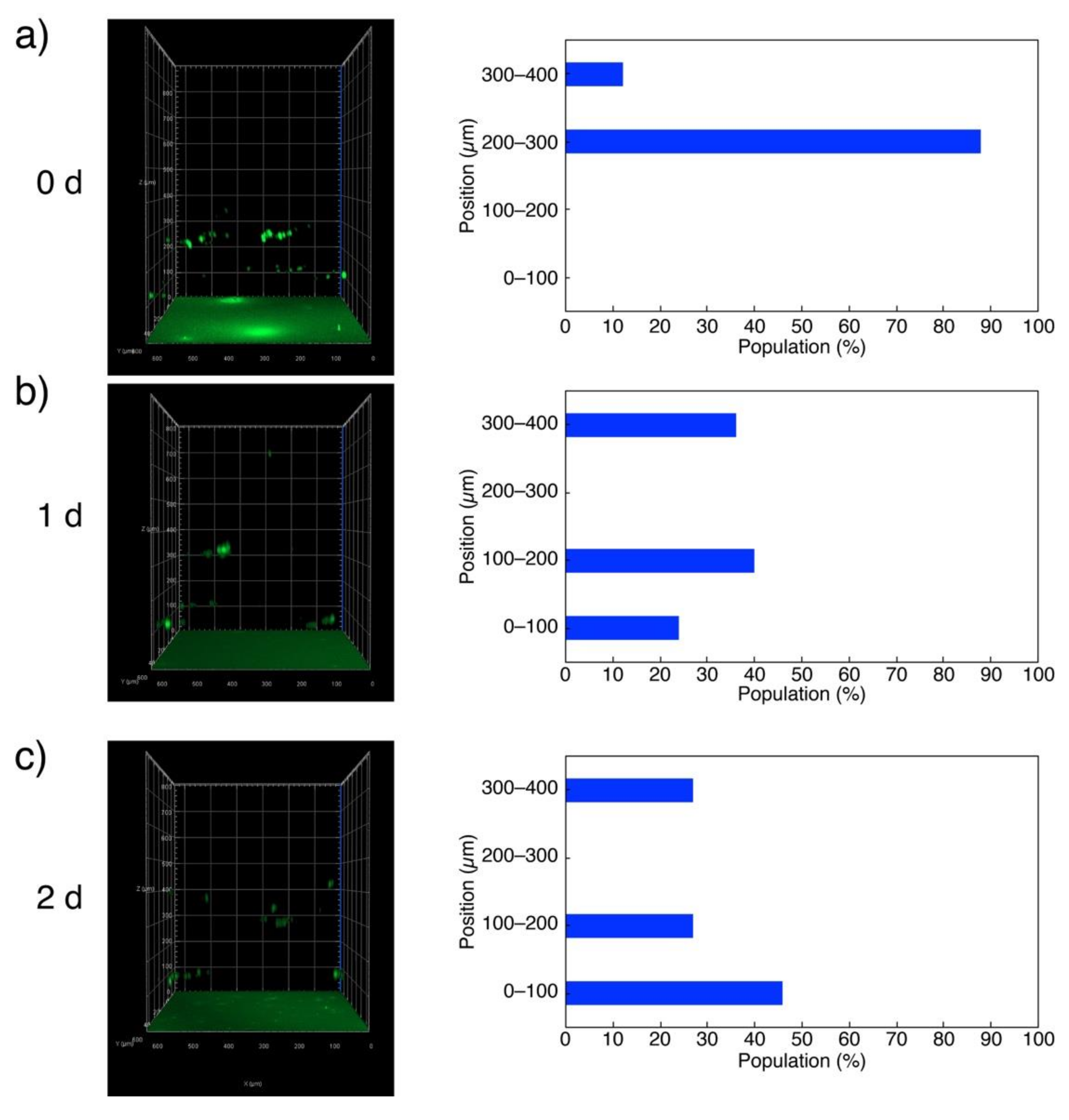
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of G-Quadruplex Hydrogel Involving 1×E-MEM
4.3. Exposure of G-Quadruplex Hydrogel to Cell Culture Media
4.4. Cytotoxicity Estimation of PEG-ODN Conjugate
4.5. Culture of Cells Deposited on G-Quadruplex Hydrogel Surface
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Xiong, X.; Wu, C.; Zhou, C.; Zhu, G.; Chen, Z.; Tan, W. Responsive DNA-based hydrogels and their applications. Macromol. Rapid Commun. 2013, 34, 1271–1283. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Jia, H.; Cao, T.; Liu, D. Supramolecular hydrogels based on DNA self-assembly. Acc. Chem. Res. 2017, 50, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Kahn, J.S.; Hu, Y.; Willner, I. Stimuli-responsive DNA-based hydrogels: From basic principles to applications. Acc. Chem. Res. 2017, 50, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Um, S.H.; Lee, J.B.; Park, N.; Kwon, S.Y.; Umbach, C.C.; Luo, D. Enzyme-catalysed assembly of DNA hydrogel. Nat. Mater. 2006, 5, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Umeno, D.; Kano, T.; Maeda, M. Affinity adsorption separation of mutagenic molecules by polyacrylamide hydrogels comprising double-stranded DNA. Anal. Chim. Acta 1998, 365, 101–108. [Google Scholar] [CrossRef]
- Liedl, T.; Dietz, H.; Yurke, B.; Simmel, F. Controlled trapping and release of quantum dots in a DNA-swichable hydrogel. Small 2007, 3, 1688–1693. [Google Scholar] [CrossRef]
- Qi, H.; Ghodousi, M.; Du, Y.; Grun, C.; Bae, H.; Yin, P.; Khademhosseini, A. DNA-directed self-assembly of shape-controlled hydrogels. Nat. Commun. 2013, 4, 2275. [Google Scholar] [CrossRef]
- Song, J.; Im, K.; Hwang, S.; Hur, J.; Nam, J.; Ahn, G.-O.; Hwang, S.; Kim, S.; Park, N. DNA hydrogel delivery vehicle for light-triggered and synergistic cancer therapy. Nanoscale 2015, 7, 9433–9437. [Google Scholar] [CrossRef]
- Bomboi, F.; Romano, F.; Leo, M.; Fernandez-Castanon, J.; Cerbino, R.; Bellini, T.; Bordi, F.; Filetici, P.; Sciortino, F. Re-entrant DNA gels. Nat. Commun. 2016, 7, 13191. [Google Scholar] [CrossRef] [PubMed]
- Adleman, L.M. Molecular computation of solutions to combinatorial problems. Science 1994, 266, 1021–1024. [Google Scholar] [CrossRef] [PubMed]
- Seeman, N.C. Nucleic acid junctions and lattices. J. Theor. Biol. 1982, 99, 237–247. [Google Scholar] [CrossRef]
- Tanaka, S.; Kuzuya, A. Hydrogels utilizing G-quadruplexes. MOJ Poly. Sci. 2017, 1, 00033. [Google Scholar] [CrossRef][Green Version]
- Wei, B.; Cheng, I.; Luo, K.Q.; Mi, Y. Capture and release of protein by a reversible DNA-induced sol-gel transition system. Angew. Chem. Int. Ed. 2008, 47, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Li, C.; Zhou, X.; Chen, P.; Yang, Z.; Li, Z.; Liu, D. Responsive polypeptide-DNA hydrogel crosslinked by G-quadruplex. Acta Chim. Sin. 2015, 73, 815–818. [Google Scholar] [CrossRef]
- Xiang, B.; He, K.; Zhu, R.; Liu, Z.; Zeng, S.; Huang, Y.; Nie, Z.; Yao, S. Self-assembled DNA hydrogel based on enzymatically polymerized DNA for protein encapsulation and enzyme/DNAzyme hybrid cascade reaction. ACS Appl. Mater. Interfaces 2016, 8, 22801–22807. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, B.; Liu, J. Molecular imprinting for substrate selectivity and enhanced activity of enzyme mimics. Small 2017, 13, 1602730. [Google Scholar] [CrossRef]
- Zhao, H.; Jiang, G.; Weng, J.; Ma, Q.; Zhang, H.; Ito, Y.; Liu, M. A signal-accumulating DNAzyme- crosslinked hydrogel for colorimetric sensing of hydrogen peroxide. J. Mater. Chem. B 2016, 4, 4648–4651. [Google Scholar] [CrossRef]
- Hasuike, E.; Akimoto, A.M.; Kuroda, R.; Matsukawa, K.; Hiruta, Y.; Kanazawa, H.; Yoshida, R. Reversible conformational changes in the parallel type G quadruplex structure inside a thermoresponsive hydrogel. Chem. Commun. 2017, 53, 3142–3144. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, W.; Liu, G.; Tian, L. A Pure DNA Hydrogel with Stable Catalytic Ability Produced by One-Step Rolling Circle Amplification. Chem. Commun. 2017, 53, 3038–3041. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-H.; Qi, X.-J.; Orbach, R.; Yang, H.-H.; Mironi-Harpaz, I.; Seliktar, D.; Willner, I. Switchable Catalytic Acrylamide Hydrogels Cross-Linked by Hemin/G-Quadruplexes. Nano Lett. 2013, 13, 1298–1302. [Google Scholar] [CrossRef] [PubMed]
- Kahn, J.S.; Trifonov, A.; Cecconello, A.; Guo, W.; Fan, C.; Willner, I. Integration of Switchable DNA-Based Hydrogels with Surfaces by the Hybridization Chain Reaction. Nano Lett. 2015, 15, 7773–7778. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-H.; Guo, W.; Qi, X.-J.; Neubauer, A.; Paltiel, Y.; Willner, I. Hemin−G-Quadruplex-Crosslinked Poly-N-Isopropylacrylamide Hydrogel: A Catalytic Matrix for the Deposition of Conductive Polyaniline. Chem. Sci. 2015, 6, 6659–6664. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Wakabayashi, K.; Fukushima, K.; Yukami, S.; Maezawa, R.; Takeda, Y.; Tatsumi, K.; Ohya, Y.; Kuzuya, A. Intelligent, Biodegradable, and Self-Healing Hydrogels Utilizing DNA Quadruplexes. Chem. Asian J. 2017, 12, 2388–2392. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Chan, S.K.; Lim, T.S.; Ohya, Y.; Kuzuya, A. DNA Quadruplex Hydrogel Beads Showing Peroxidase Activity. J. Electrochem. Soc. 2019, 166, B3271–B3273. [Google Scholar] [CrossRef]
- Tanaka, S.; Yukami, S.; Fukushima, K.; Wakabayashi, K.; Ohya, Y.; Kuzuya, A. Bulk pH-Responsive DNA Quadruplex Hydrogels Prepared by Liquid-Phase, Large-Scale DNA Synthesis. ACS Macro Lett. 2018, 7, 295–299. [Google Scholar] [CrossRef]
- Bonora, G.M.; Zaramella, S.; Veronese, F.M. Synthesis by High-Efficiency Liquid-Phase (HELP) Method of Oligonucleotides Conjugated with High-Molecular Weight Poly (ethylene glycols) (PEGs). Biol. Proced. Online 1998, 1, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Ellington, E.; Pollard, J.D. Synthesis and Purification of Oligonucleotides. Curr. Protoc. Mol. Biol. 2001, 42, 2–11. [Google Scholar] [CrossRef]
- Burdick, J.A.; Anseth, K.S. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials 2002, 32, 4315–4323. [Google Scholar] [CrossRef]
- Nuttelman, C.R.; Tripodi, M.C.; Anseth, K.S. Synthetic hydrogel niches that promote hMSC viability. Matrix Biol. 2005, 24, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Kloxin, A.M.; Kasko, A.M.; Salinas, C.N.; Anseth, K.S. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science 2009, 324, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Huebsch, N.; Arany, P.R.; Mao, A.S.; Shvartsman, D.; Ali, O.A.; Bencherif, S.A.; Rivera-Feliciano, J.; Mooney, D.J. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 2010, 9, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Phelps, E.A.; Enemuchukwu, N.O.; Fiore, V.F.; Sy, J.C.; Murthy, N.; Sulchek, T.A.; Barker, T.H.; García, A.J. Maleimide Cross-Linked Bioactive PEG Hydrogel Exhibits Improved Reaction Kinetics and Cross-Linking for Cell Encapsulation and in Situ Delivery. Adv. Mater. 2012, 24, 64–70. [Google Scholar] [CrossRef]
- Kim, H.D.; Heo, J.; Hwang, Y.; Kwak, S.Y.; Park, O.K.; Kim, H.; Varghese, S.; Hwang, N.S. Extracellular-Matrix-Based and Arg-Gly-Asp–Modified Photopolymerizing Hydrogels for Cartilage Tissue Engineering. Tissue Eng. Part A 2015, 21, 757–766. [Google Scholar] [CrossRef]
- Sargeant, T.D.; Desai, A.P.; Banerjee, S.; Agawu, A.; Stopek, J.B. An in situ forming collagen-PEG hydrogel for tissue regeneration. Acta Biomater. 2012, 8, 124–132. [Google Scholar] [CrossRef]
- Noh, M.; Kim, S.H.; Kim, J.; Lee, J.R.; Jeong, G.J.; Yoon, J.K.; Kang, S.; Bhang, S.H.; Yoon, H.H.; Lee, J.C.; et al. Graphene oxide reinforced hydrogels for osteogenic differentiation of human adipose-derived stem cells. RSC Adv. 2017, 7, 20779–20788. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, Y.; Zhang, L.; Wu, L.; Chen, Y.; Xie, D.; Chen, W. Hydrogel Cryopreservation System: An Effective Method for Cell Storage. Int. J. Mol. Sci. 2018, 19, 3330. [Google Scholar] [CrossRef]
- Choi, J.; Konno, T.; Ishihara, K. Phospholipid polymer multilayered hydrogels for releasing cell growth factor protein. Biomater. Biomed. Eng. 2014, 1, 1–12. [Google Scholar] [CrossRef]
- Yan, C.; Mackay, M.E.; Czymmek, K.; Nagarkar, R.O.; Schneider, J.P.; Pochan, D.J. Injectable solid peptide hydrogel as a cell carrier: Effects of shear flow on hydrogels and cell payload. Langmuir 2012, 28, 6076–6087. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, S.; Yukami, S.; Hachiro, Y.; Ohya, Y.; Kuzuya, A. Application of DNA Quadruplex Hydrogels Prepared from Polyethylene Glycol-Oligodeoxynucleotide Conjugates to Cell Culture Media. Polymers 2019, 11, 1607. https://doi.org/10.3390/polym11101607
Tanaka S, Yukami S, Hachiro Y, Ohya Y, Kuzuya A. Application of DNA Quadruplex Hydrogels Prepared from Polyethylene Glycol-Oligodeoxynucleotide Conjugates to Cell Culture Media. Polymers. 2019; 11(10):1607. https://doi.org/10.3390/polym11101607
Chicago/Turabian StyleTanaka, Shizuma, Shinsuke Yukami, Yuhei Hachiro, Yuichi Ohya, and Akinori Kuzuya. 2019. "Application of DNA Quadruplex Hydrogels Prepared from Polyethylene Glycol-Oligodeoxynucleotide Conjugates to Cell Culture Media" Polymers 11, no. 10: 1607. https://doi.org/10.3390/polym11101607
APA StyleTanaka, S., Yukami, S., Hachiro, Y., Ohya, Y., & Kuzuya, A. (2019). Application of DNA Quadruplex Hydrogels Prepared from Polyethylene Glycol-Oligodeoxynucleotide Conjugates to Cell Culture Media. Polymers, 11(10), 1607. https://doi.org/10.3390/polym11101607






