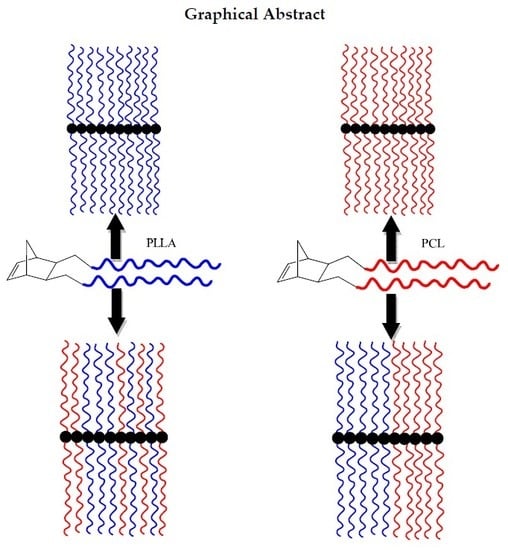
Macromolecular Brushes Based on Poly(L-Lactide) and Poly(ε-Caprolactone) Single and Double Macromonomers via ROMP. Synthesis, Characterization and Thermal Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Techniques
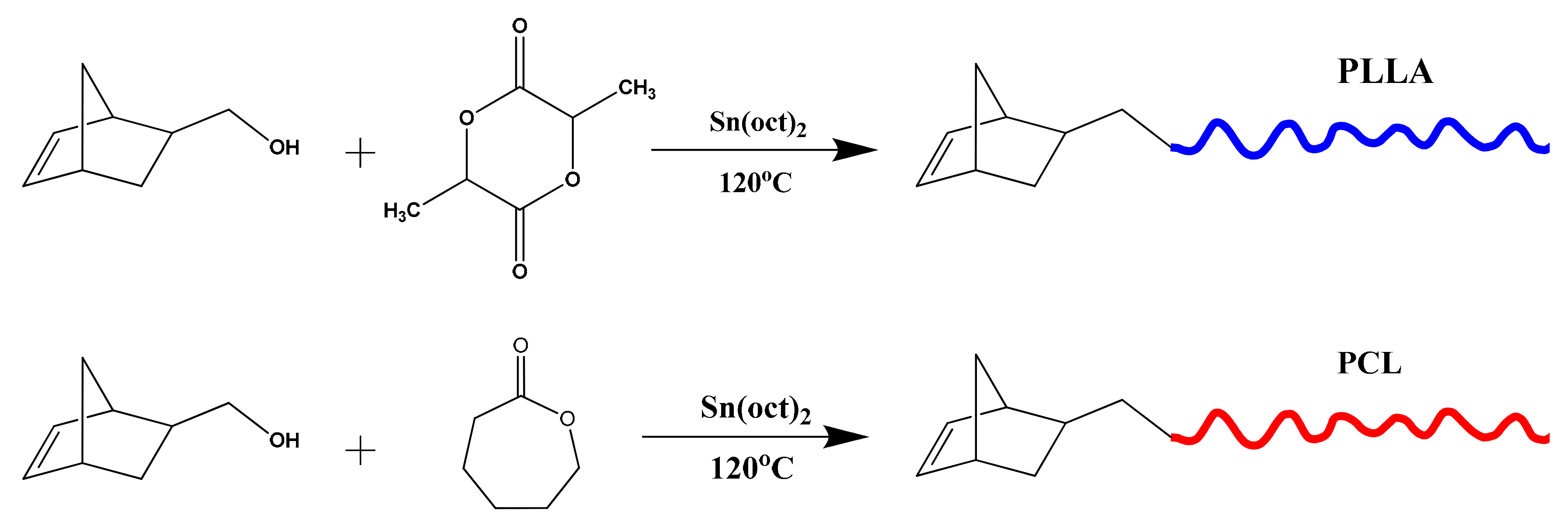
2.3. Synthesis of NBE-PCL Macromonomer
2.4. Synthesis of NBE-(PCL)2 Double Macromonomer
2.5. Synthesis of NBE-PLLA Macromonomer
2.6. Synthesis of NBE-(PLLA)2 Double Macromonomer
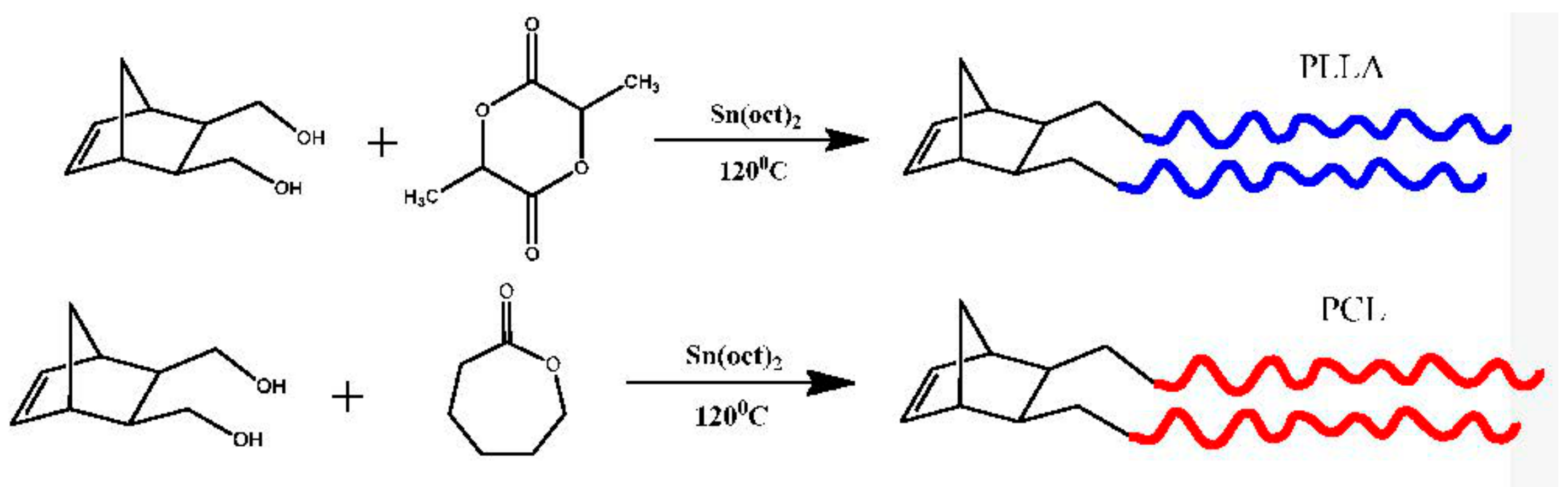
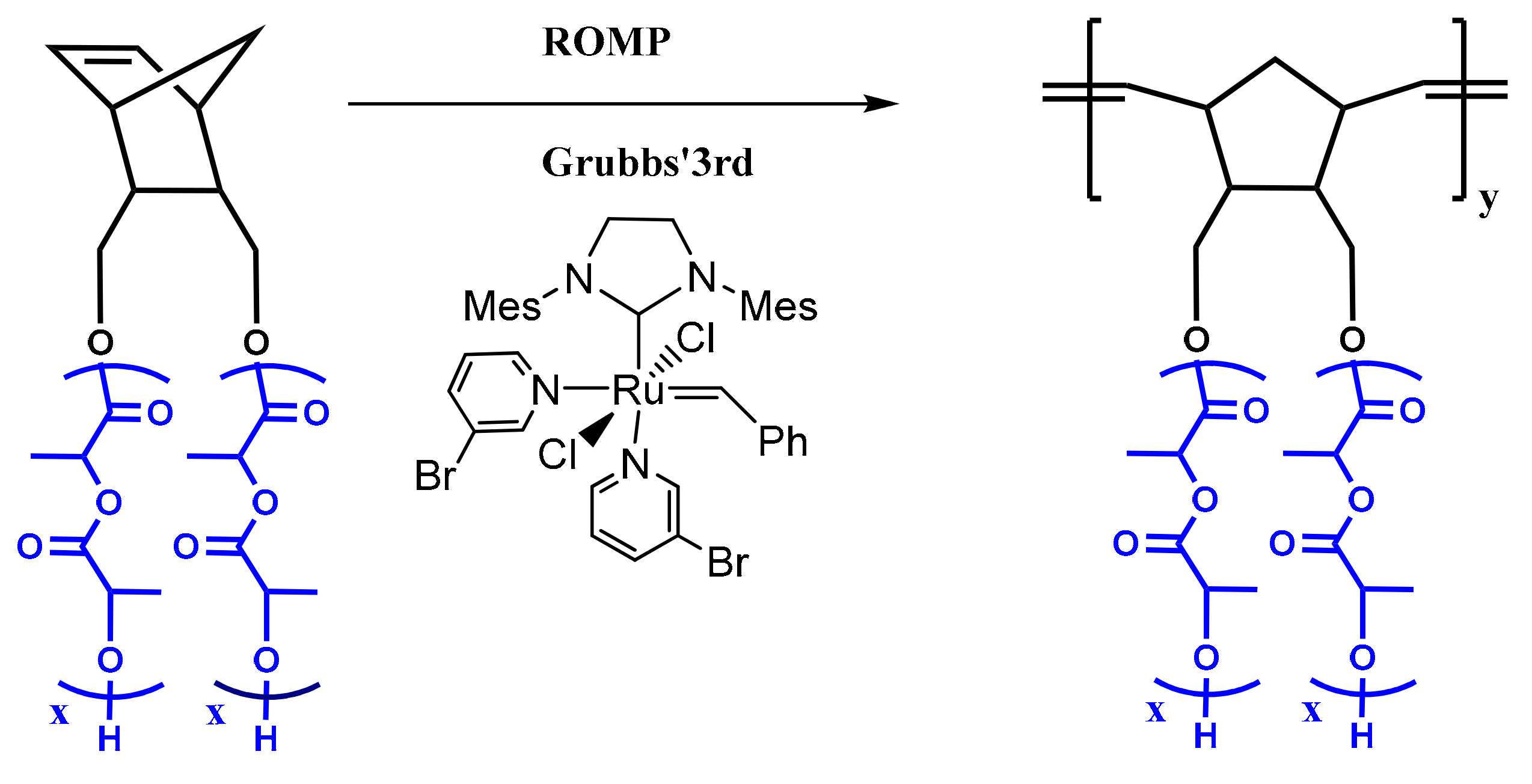
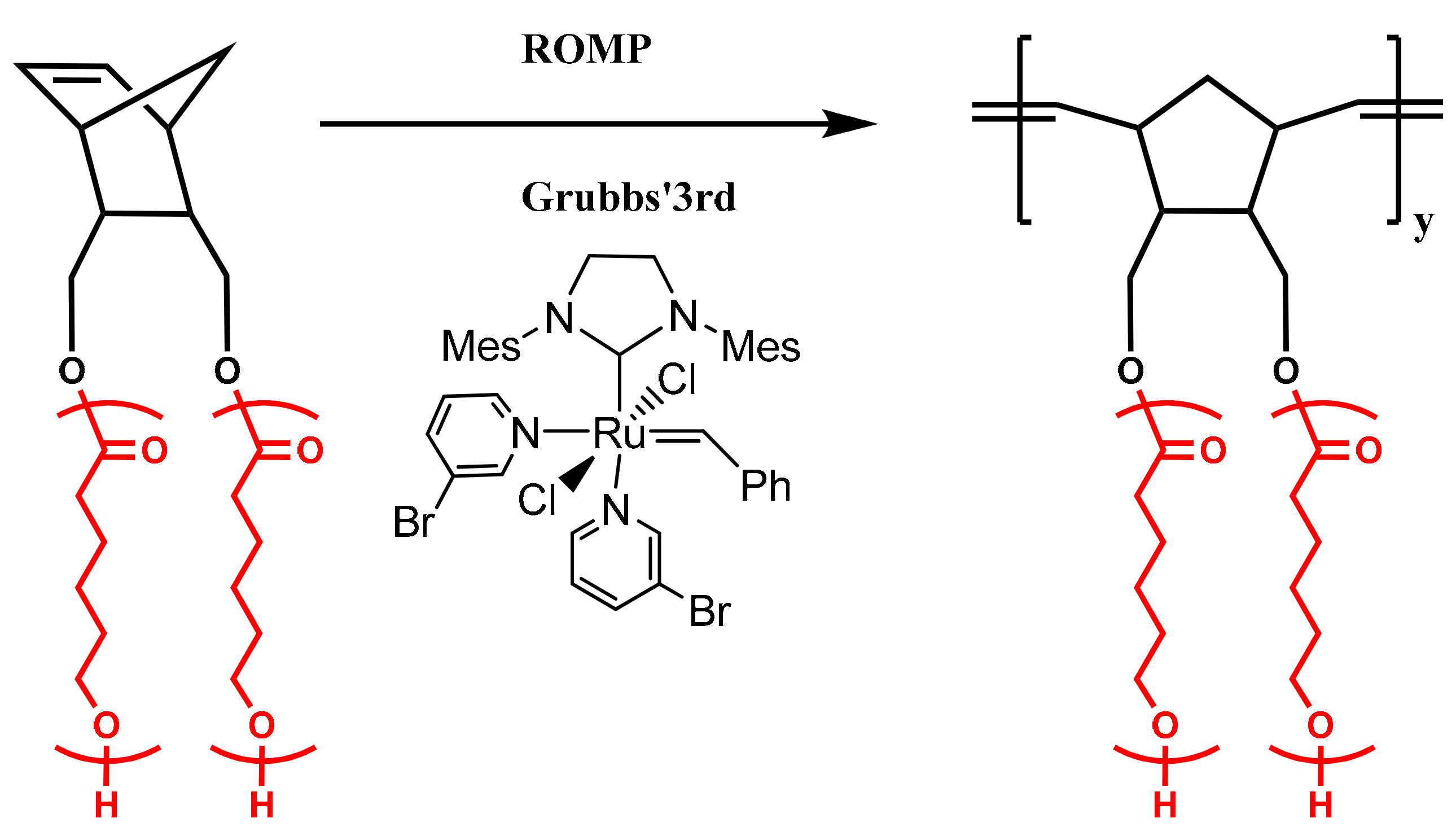
2.7. Synthesis of Double Brushes via Ring Opening Metathesis Polymerization of NBE-(PLLA)2 and NBE-(PCL)2 Macromonomers
2.8. Synthesis of Double Brushes Block Copolymer via Ring Opening Metathesis Polymerization of NBE-(PLLA)2 and NBE-(PCL)2 Macromonomers
2.9. Synthesis of Double Brushes Statistical Copolymer via Ring Opening Metathesis Polymerization of NBE-(PLLA)2 and NBE-(PCL)2 Macromonomers
2.10. Synthesis of Brush Triblock Copolymer via Ring Opening Metathesis Polymerization of NBE-PLLA and NBE-PCL Macromonomers
3. Results
3.1. Synthesis of Single and Double Macromonomers
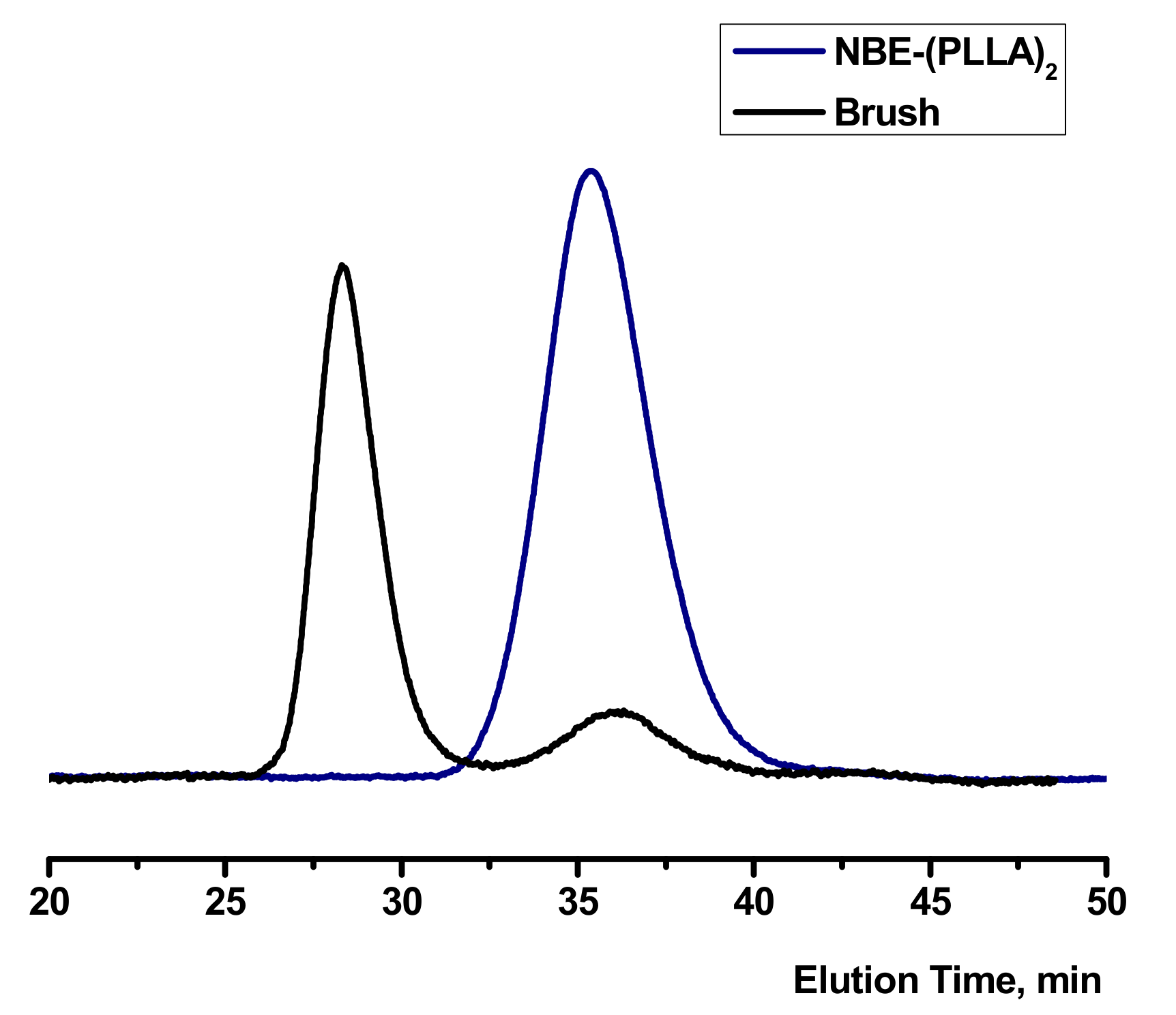
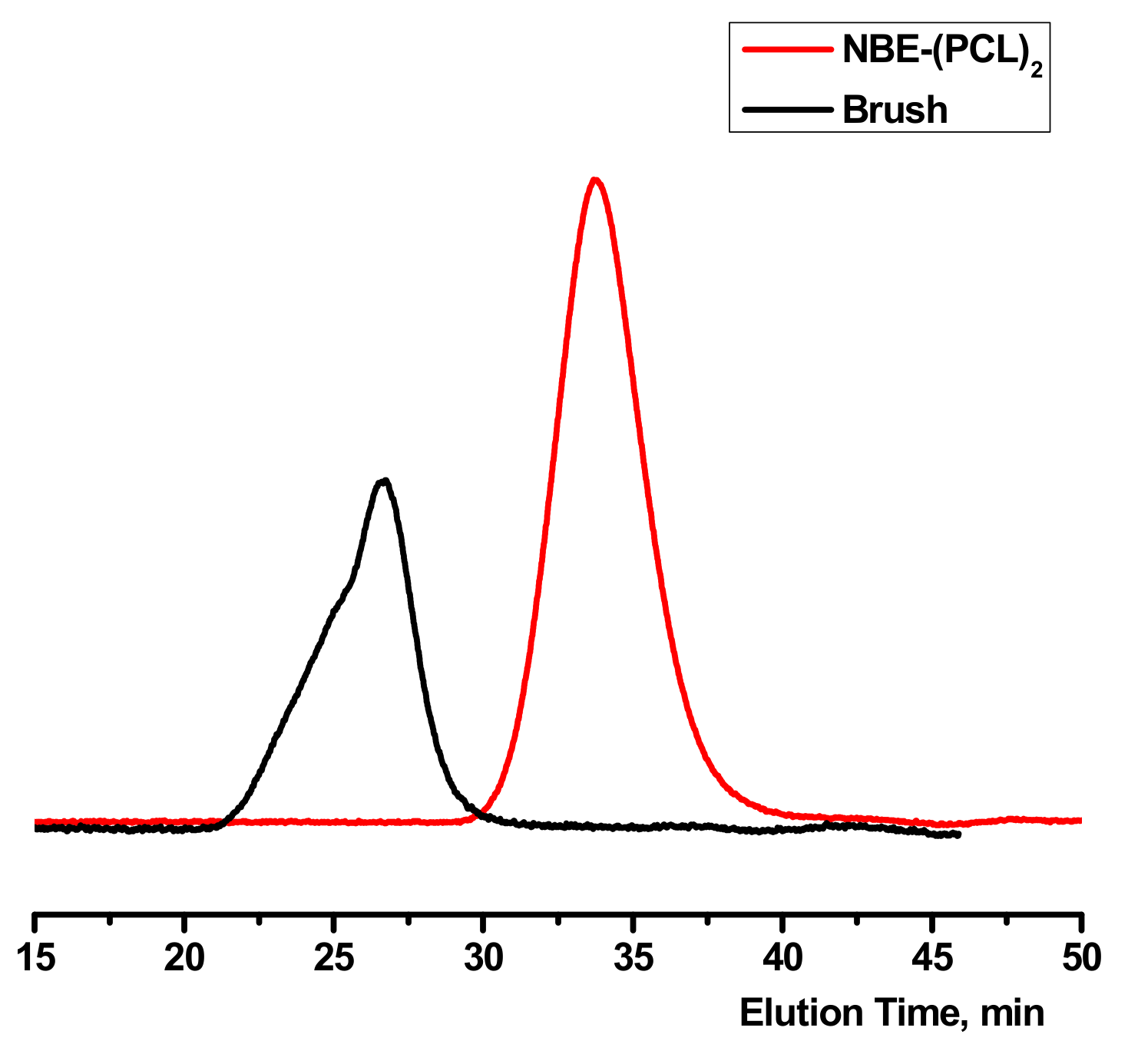
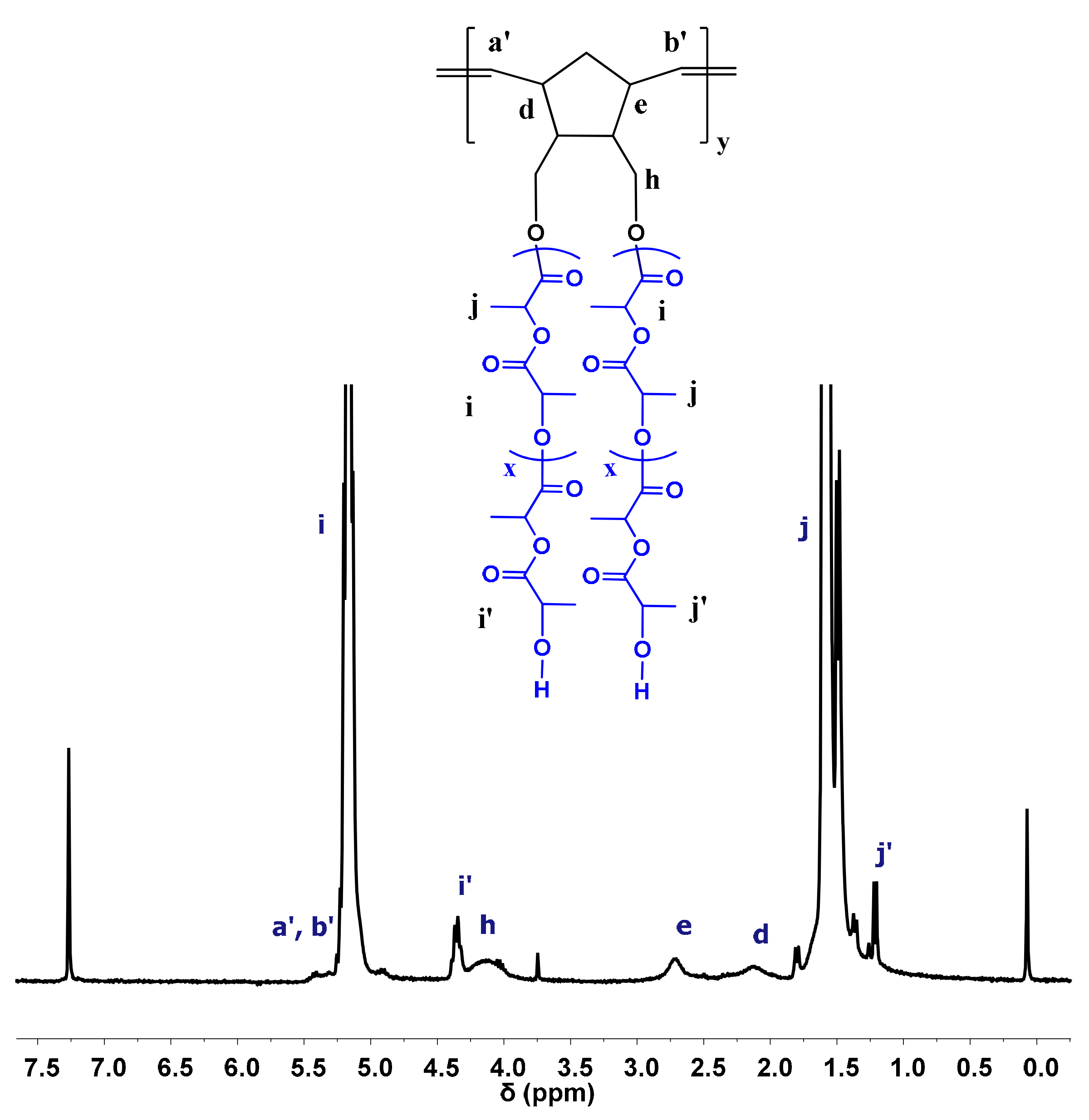
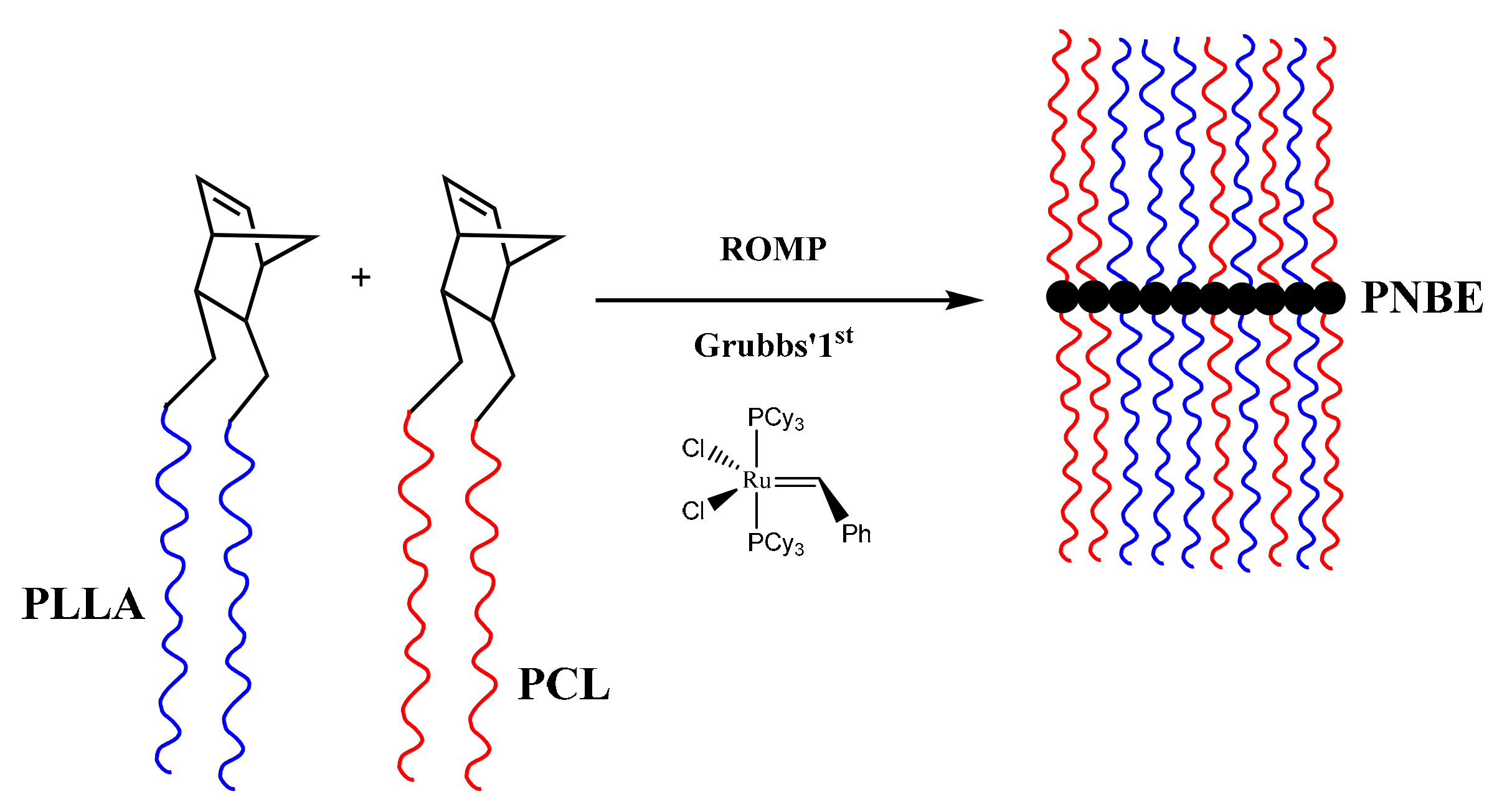
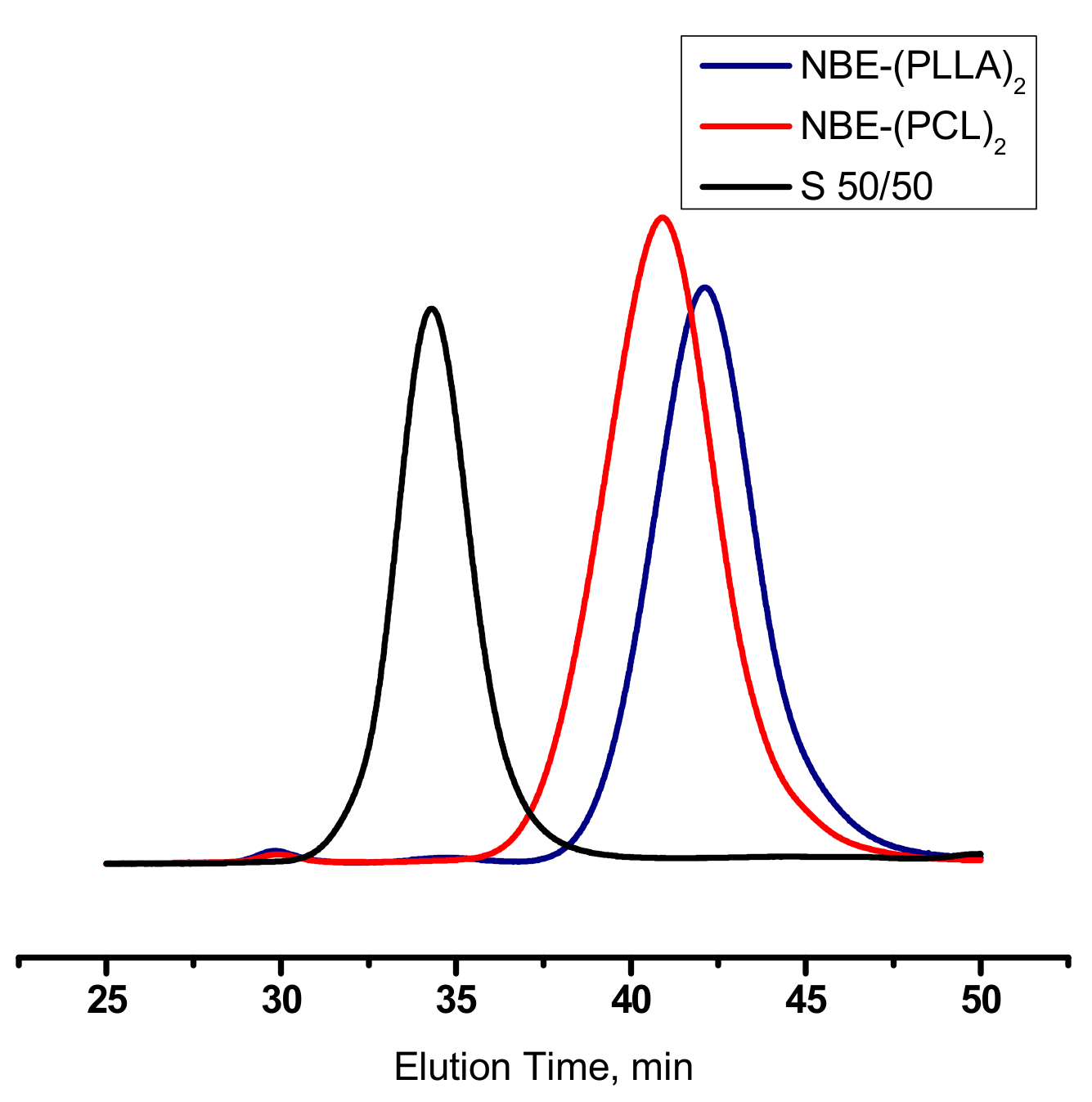
3.2. Synthesis of Homopolymer Double Brushes
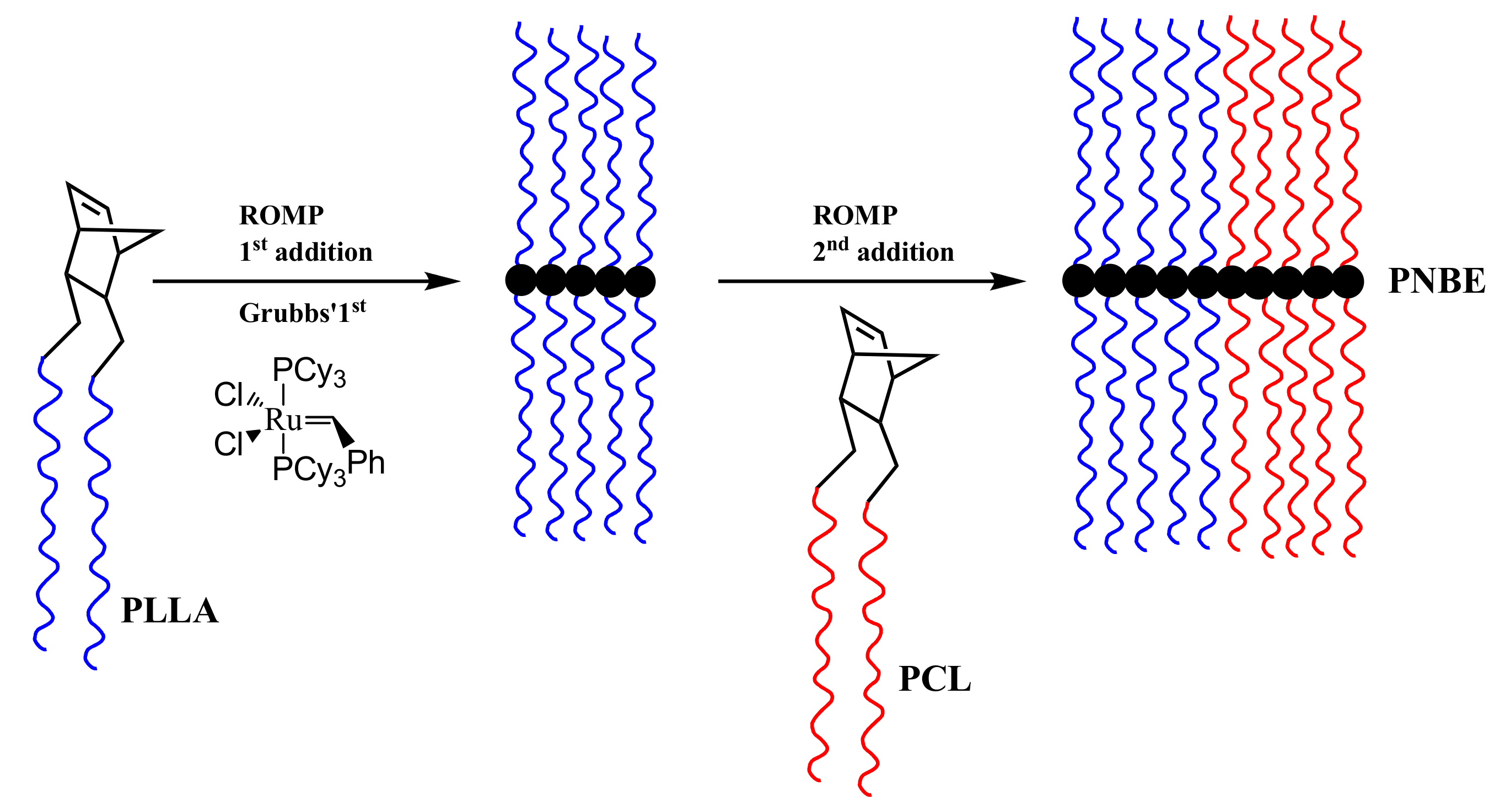
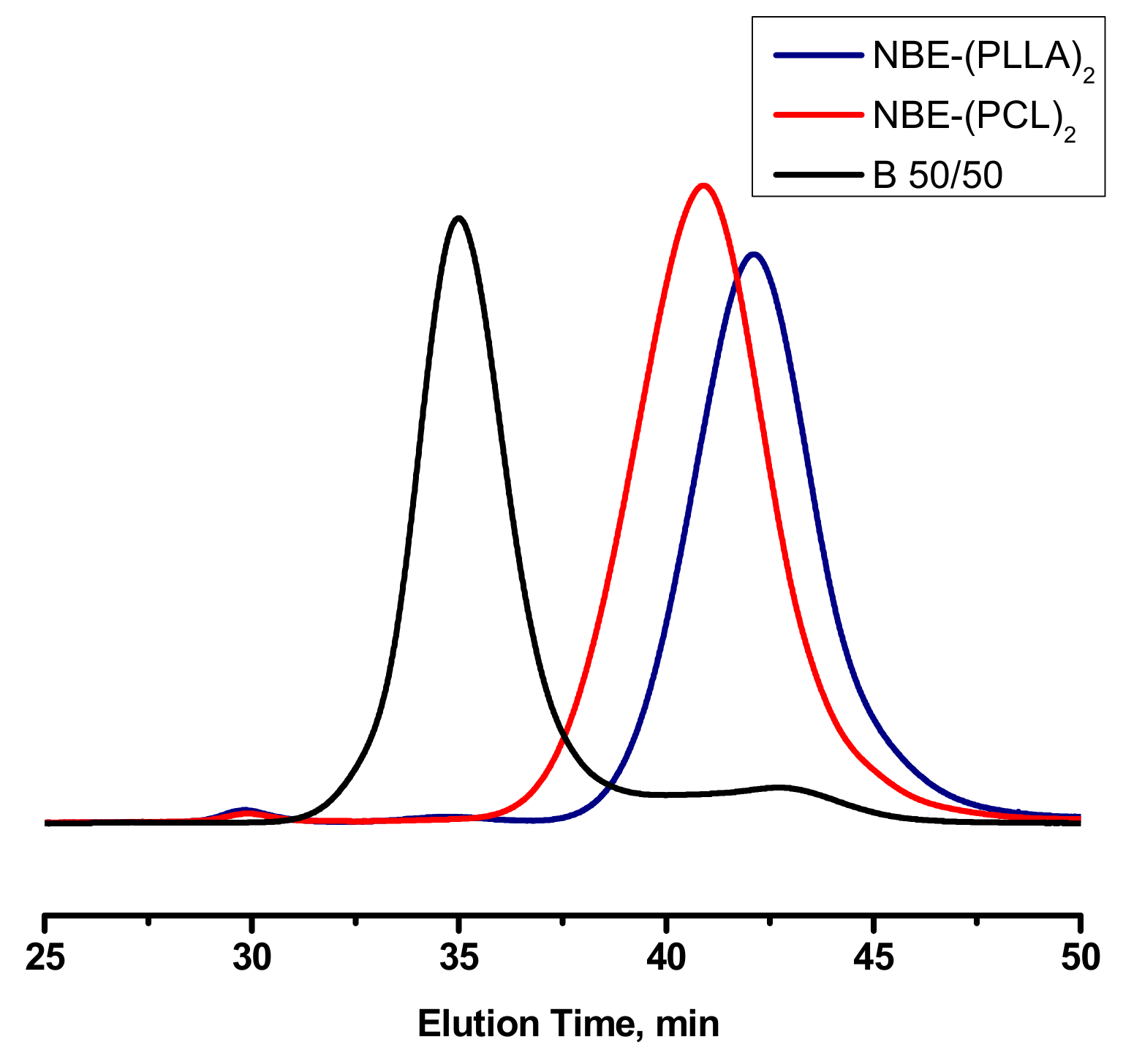
3.3. Synthesis of Statistical and Block Copolymeric Brushes via Ring Opening Metathesis Polymerization of the Double Macromonomers
3.4. Synthesis of Triblock Copolymer Brush via Ring Opening Metathesis Polymerization of Single Macromonomers
3.5. Thermal Decomposition of the Macromonomers and the Polymer Brushes
3.6. Thermal Transitions of the Macromonomers and the Polymer Brushes by Differential Scanning Calorimetry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pitsikalis, M.; Pispas, S.; Mays, J.W.; Hadjichristidis, N. Nonlinear block copolymer architectures. Adv. Polym. Sci. 1998, 135, 1–137. [Google Scholar]
- Hadjichristidis, N.; Iatrou, H.; Pitsikalis, M.; Pispas, S.; Avgeropoulos, A. Linear and non-linear triblock terpolymers. Synthesis, self-assembly in selective solvents and in bulk. Progr. Polym. Sci. 2005, 30, 725–782. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Pitsikalis, M.; Pispas, S.; Iatrou, H. Polymers with complex architecture by living anionic polymerization. Chem. Rev. 2001, 101, 3747–3792. [Google Scholar] [CrossRef] [PubMed]
- Hamley, I.W. The Physics of Block Copolymers; Oxford Science Publications: New York, NY, USA, 1998. [Google Scholar]
- Hamley, I.W. (Ed.) Developments in Block Copolymer Science and Technology; John Wiley & Sons, Ltd.: West Sussex, UK, 2004. [Google Scholar]
- Hadjichristidis, N.; Iatrou, H.; Pitsikalis, M.; Mays, J.W. Macromolecular architectures by living and controlled/living polymerizations. Progr. Polym. Sci. 2006, 31, 1068–1132. [Google Scholar] [CrossRef]
- Hsieh, H.L.; Quirk, R.P. Anionic Polymerization: Principles and Practical Applications; Marcel Dekker: New York, NY, USA, 1996. [Google Scholar]
- Kennedy, J.P. Living cationic polymerization of olefins. How did the discovery come about? J. Polym. Sci. Polym. Chem. 1999, 37, 2285–2293. [Google Scholar] [CrossRef]
- Webster, O.W. The discovery and commercialization of group transfer polymerization. J. Polym. Sci. Polym. Chem. 2000, 38, 2855–2860. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Xia, J. Atom transfer radical polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef]
- Kamigaito, M.; Ando, T.; Sawamoto, M. Metal-catalyzed living radical polymerization. Chem. Rev. 2001, 101, 3689–3746. [Google Scholar] [CrossRef]
- Hawker, C.J.; Bosman, A.W.; Harth, E. New polymer synthesis by nitroxide mediated living radical polymerizations. Chem. Rev. 2001, 101, 3661–3688. [Google Scholar] [CrossRef]
- Coates, G. Precise control of polyolefin stereochemistry using single-site metal catalysts. Chem. Rev. 2000, 100, 1223–1252. [Google Scholar] [CrossRef]
- Pitsikalis, M.; Maroudas, A.F. Synthesis of Complex Macromolecular Architectures by Metallocene and Half-Metallocene Complexes; Transworld Research Network: Kerala, India, 2013; ISBN 978-8-17-895597-1. [Google Scholar]
- Moses, J.E.; Moorhouse, A.D. The growing applications of click chemistry. Chem. Soc. Rev. 2007, 36, 1249–1262. [Google Scholar] [CrossRef] [PubMed]
- Buchspies, J.; Szostak, M. Recent advances in acyl-Suzuki cross coupling. Catalysts 2019, 9, 53. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Pitsikalis, M.; Iatrou, H.; Driva, P.; Sakellariou, G.; Chatzichristidi, M. Polymers with star-related structures: Synthesis, properties and applications. Polym. Sci. A Compr. Ref. 2012, 6, 29–111. [Google Scholar]
- Hadjichristidis, N.; Pispas, S.; Iatrou, H.; Pitsikalis, M. Linking chemistry and anionic polymerization. Curr. Org. Chem. 2002, 6, 155–176. [Google Scholar] [CrossRef]
- Grubbs, R.H.; Wenzel, A.G.; O’ Leary, D.J.; Khosravi, E. Handbook of Metathesis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015. [Google Scholar]
- Leitgeb, A.; Wappel, J.; Slugovc, C. The ROMP toolbox upgraded. Polymer 2010, 51, 2927–2946. [Google Scholar] [CrossRef]
- Hérogeuz, V.; Gnanou, Y.; Fontanille, M. Novel amphiphilic architectures by ring-opening metathesis polymerization of macromonomers. Macromolecules 1997, 30, 4791–4798. [Google Scholar] [CrossRef]
- Isono, T.; Kondo, Y.; Ozawa, S.; Chen, Y.; Sakai, R.; Sato, S.I.; Tajima, K.; Kakuchi, T.; Satoh, T. Stereoblock-like brush copolymers consisting of poly(l-lactide) and poly(d-lactide) side chains along poly(norbornene) backbone: Synthesis, stereocomplex formation and structure-property relationship. Macromolecules 2014, 47, 7118–7128. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Z.; Gnanou, Y.; Hadjichristidis, N. Well-defined polyethylene-based random, block and bilayered molecular cobrushes. Macromolecules 2015, 48, 3556–3562. [Google Scholar] [CrossRef]
- Gai, Y.; Song, D.P.; Yavitt, B.M.; Watkins, J.J. Polystyrene-block-poly (ethylene oxide) bottlebrush block copolymer morphology transitions: Influence of side chain length and volume fraction. Macromolecules 2017, 50, 1503–1511. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Pispas, S.; Pitsikalis, M.; Iatrou, H.; Lohse, D. Graft copolymers Encyclopedia of Polymer Science and Engineering; John Wiley & Sons: Hoboken, NJ, USA, 2002; Volume 6, p. 348. [Google Scholar]
- Ito, K. Polymeric design by macromonomer technique. Progr. Polym. Sci. 1998, 23, 581–620. [Google Scholar] [CrossRef]
- Zhang, M.; Müller, A.H. Cylindrical polymer brushes. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 3461–3481. [Google Scholar] [CrossRef]
- Xie, M.; Dang, J.; Han, H.; Wang, W.; Liu, J.; He, X.; Zhang, Y. Well-defined brush copolymers with high grafting density of amphiphilic side chains by combination of ROP, ROMP and ATRP. Macromolecules 2008, 41, 9004–9010. [Google Scholar] [CrossRef]
- Mecerreyes, A.; Dahan, D.; Lecomte, P.; Dubois, P.; Demonceau, A.; Noels, A.F.; Jerome, R. Ring opening metathesis polymerization of new α-norbornenyl poly(ε-caprolactone) macromonomers. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 2447–2455. [Google Scholar] [CrossRef]
- Yang, D.; Huang, W.; Yu, J.; Jiang, J.; Zhang, L.; Xie, M. A novel shape memory polynorbornene functionalized with poly(ε-caprolactone) side chain and cyano group through ring-opening metathesis polymerization. Polymer 2010, 51, 5100–5106. [Google Scholar] [CrossRef]
- Fu, Q.; Ren, J.M.; Qiao, G.G. Synthesis of novel cylindrical bottlebrush polypseudorotaxane via inclusion complexation of high density poly (ε-caprolactone) bottlebrush polymer and α-cyclodextrins. Polym. Chem. 2012, 3, 343–351. [Google Scholar] [CrossRef]
- N’Guyen, D.A.; Leroux, F.; Montembault, V.; Pascuala, S.; Fontaine, L. Synthesis and characterization of high grafting density bottle-brush poly (oxa)norbornene-g-poly (ε-caprolactone). Polym. Chem. 2016, 7, 1730–1738. [Google Scholar] [CrossRef]
- Jha, S.; Dutta, S.; Bowden, N.B. Synthesis of ultralarge molecular weight bottlebrush polymers using Grubbs’ catalysts. Macromolecules 2004, 37, 4365–4374. [Google Scholar] [CrossRef]
- Li, A.; Li, Z.; Zhang, S.; Sun, G.; Policarpio, D.M.; Wooley, K.L. Synthesis and direct visualization of dumbbell-shaped molecular brushes. ACS Macro Lett. 2012, 1, 241–245. [Google Scholar] [CrossRef]
- Floudas, G.; Reiter, G.; Lambert, O.; Dumas, P. Structure and dynamics of structure formation in model triarm star block copolymers of polystyrene, poly (ethylene oxide) and poly(ε-caprolactone). Macromolecules 1998, 31, 7279–7290. [Google Scholar] [CrossRef]
- Palacios, J.K.; Tercjak, A.; Liu, G.; Wang, D.; Zhao, J.; Hadjichristidis, N.; Müller, A.J. Trilayered morphology of an ABC triple crystalline triblock terpolymer. Macromolecules 2018, 50, 7268–7281. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Pitsikalis, M.; Iatrou, H.; Pispas, S. The strength of the macromonomer strategy for complex macromolecular architecture: Molecular characterization, properties and applications of polymacromonomers. Macromol. Rapid Commun. 2003, 24, 979–1013. [Google Scholar] [CrossRef]
- Zamurovic, M.; Christodoulou, S.; Vazaios, A.; Iatrou, E.; Pitsikalis, M.; Hadjichristidis, N. Micallization behaviour of complex comblike block copolymer architectures. Macromolecules 2007, 40, 5835–5849. [Google Scholar] [CrossRef]
- Nikovia, C.; Theodoridis, L.; Alexandris, S.; Bilalis, P.; Hadjichristidis, N.; Floudas, G.; Pitsikalis, M. Macromolecular brushes by combination of ring-opening metathesis polymerization. Synthesis, self-assembly, thermodynamics and dynamics. Macromolecules 2018, 51, 8940–8955. [Google Scholar] [CrossRef]
- Theodosopoulos, G.V.; Zisis, C.; Charalambidis, G.; Nikolaou, V.; Coutsolelos, A.G.; Pitsikalis, M. Synthesis, characterization and thermal properties of poly (ethylene oxide), PEO, polymacromonomers via anionic and ring opening metathesis polymerization. Polymers 2017, 9, 145. [Google Scholar] [CrossRef] [PubMed]
- Choinopoulos, I.; Patias, G.; Koinis, S.; Pitsikalis, M. Synthesis and characterization of brush diblock and triblock copolymers bearing polynorbornene backbone and poly (l-lactide) and/or poly (hexyl isocyanate) side chains by a combination of coordination and ring opening metathesis polymerization. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3455–3465. [Google Scholar] [CrossRef]
- Choinopoulos, I. Grubbs’ and Schrock’s Catalysts, Ring Opening Metathesis Polymerization and Molecular Brushes—Synthesis, Characterization, Properties and Applications. Polymers 2019, 11, 298. [Google Scholar] [CrossRef] [PubMed]
- Hadjichristidis, N.; Iatrou, H.; Pispas, S.; Pitsikalis, M. Anionic polymerization: High vacuum techniques. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 3211–3234. [Google Scholar] [CrossRef]
- Uhrig, D.; Mays, J.W. Experimental techniques in high-vacuum anionic polymerization. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 6179–6222. [Google Scholar] [CrossRef]
- Hirao, A.; Hadjichristidis, N. (Eds.) Anionic Polymerization: Principles, Practice, Strength, Consequences and Applications; Springer: Tokyo, Japan, 2015; Chapter 1; p. 3. [Google Scholar]
- Hirao, A.; Hadjichristidis, N. (Eds.) Anionic Polymerization: Principles, Practice, Strength, Consequences and Applications; Springer: Tokyo, Japan, 2015; Chapter 2; p. 19. [Google Scholar]
- Love, J.A.; Morgan, J.P.; Trnka, T.M.; Grubbs, R.H. A practical and highly active Ruthenium-based catalyst that effects the cross metathesis of acrylonitrile. Angew. Chem. Int. Ed. 2002, 41, 4035–4037. [Google Scholar] [CrossRef]
- Choi, T.-L.; Grubbs, R.H. Controlled Living Ring-Opening-Metathesis Polymerization by a Fast-Initiating Ruthenium Catalyst. Angew. Chem. Int. Ed. 2003, 42, 1743–1746. [Google Scholar] [CrossRef]
- Slugovc, C.; Riegler, S.; Hayn, G.; Saf, R.; Stelzer, F. Highly Defined ABC Triblock Cooligomers and Copolymers Prepared by ROMP Usingan N-Heterocyclic-Carbene-Substituted Ruthenium Benzylidene Initiator. Macromol. Rapid Commun. 2003, 24, 435–439. [Google Scholar] [CrossRef]
- Slugovc, C.; Demel, S.; Riegler, S.; Hobisch, J.; Stelzer, F. The Resting State Makes the Difference: The Influence of the Anchor Group in the ROMP of Norbornene Derivatives. Macromol. Rapid Commun. 2004, 25, 475–480. [Google Scholar] [CrossRef]
- Feast, W.J.; Gibson, V.C.; Johnson, A.F.; Khosravi, E.; Moshin, M.A. Tailored copolymers via coupled anionic and ring opening metathesis polymerization. Synthesis and polymerization of bicycle [2.2.1] hept-5-ene-2,3-trans-bis (polystyrylcarboxylate) s. Polymer 1994, 35, 3542–3548. [Google Scholar] [CrossRef]
- Czelusniak, I.; Khosravi, E.; Kenwright, A.M.; Ansell, C.W.G. Synthesis, characterization, and hydrolytic degradation of polylactide-functionalized polyoxanorbornenes. Macromolecules 2007, 40, 1444–1452. [Google Scholar] [CrossRef]
- Vougioukalakis, G.C.; Grubbs, R.H. Ruthenium-Based Heterocyclic Carbene-Coordinated Olefin Metathesis Catalysts. Chem. Rev. 2010, 110, 1746–1787. [Google Scholar] [CrossRef]
- Pretula, J.; Slomkowski, S.; Penczek, S. Polylactides-Methods of synthesis and characterization. Adv. Drug Deliv. Rev. 2016, 107, 3–16. [Google Scholar] [CrossRef]
- Gupta, B.; Ray, A.R. Preparation of Poly (ε-caprolactone)/Poly (ε-caprolactone-colactide) (PCL/PLCL) Blend Filament by Melt Spinning. J. Appl. Polym. Sci. 2012, 123, 1944–1950. [Google Scholar] [CrossRef]
- Wang., Y.; Mano, J.F. Multiple melting behaviour of poly (L-lactide-co-glycolide) investigated by DSC. Polym. Test. 2009, 28, 452–455. [Google Scholar] [CrossRef]
- Zhang, J.; Tashiro, K.; Tsuji, H.; Domb, A. Disorder-to-order phase transition and multiple melting behavior of poly (L-lactide) investigated by simultaneous measurements of WAXD and DSC. Macromolecules 2008, 41, 1352–1357. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Duan, B.; Wang, M.; Cheung, W.L. Isothermal and non-isothermal crystallization kinetics of poly (L-Lactide)/carbonated hydroxyapatite nanocomposite microspheres. Advances in diverse industrial applications of nanocomposites. J. Appl. Polym. Sci. 2009, 113, 4100–4115. [Google Scholar] [CrossRef]


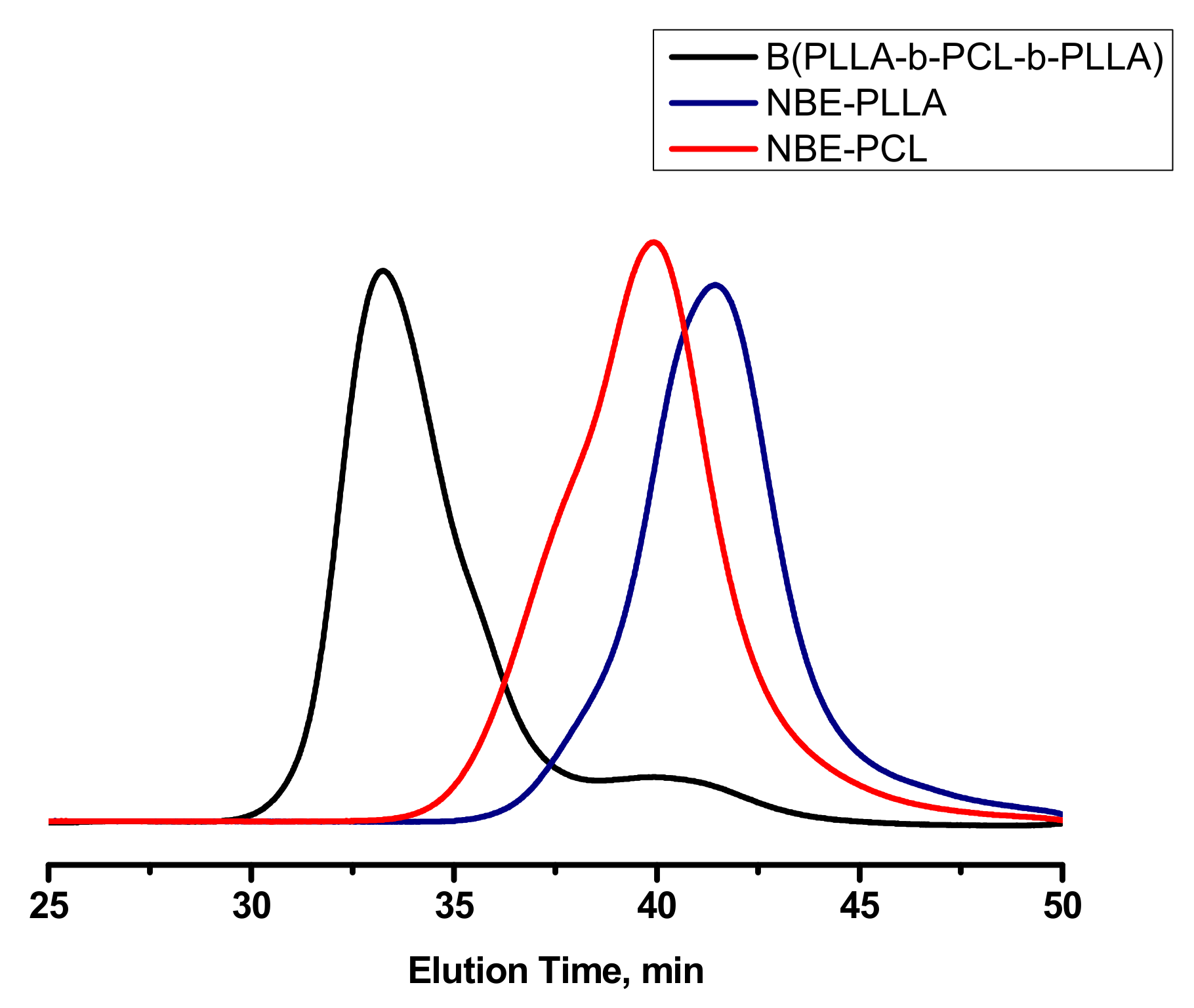

| Macromonomers | Mw, SEC b (g mol −1) | Mw/Mn b | Mn, NMR c (g mol −1) |
|---|---|---|---|
| NBE-PLLA a | 5800 | 1.18 | 4200 |
| NBE-PCL a | 11,400 | 1.32 | 12,400 |
| NBE-(PLLA)2 #1 a | 4200 | 1.30 | 2900 |
| NBE-(PLLA)2 #2 a | 9000 | 1.17 | 4270 |
| NBE-(PCL)2 #1 a | 6480 | 1.30 | 4550 |
| NBE-(PCL)2 #2 a | 11,300 | 1.13 | 8500 |
| Sample | Mw a | Mw/Mn a | f b |
|---|---|---|---|
| DB-PLLA c | 36,900 | 1.10 | 9 |
| DB-PCL d | 116,350 | 1.40 | 18 |
| Sample | Mw a | Mw/Mn a | % PLLA (w/w) b | f c |
|---|---|---|---|---|
| SDBC d | 47,000 | 1.13 | 61 | 7/3 |
| BDBC e | 37,900 | 1.13 | 62 | 6/3 |
| B-(PLLA-b-PCL-b-PLLA) | 60,300 | 1.17 | 76 | 8/2 |
| Sample | Peak 1, °C | Peak 2, °C | Peak 3, °C |
|---|---|---|---|
| NBE-PLLA | 267.51 | ||
| NBE-PCL | 319.66 | ||
| NBE-(PLLA)2 #1 | 261.44 | ||
| NBE-(PLLA)2 #2 | 254.77 | ||
| NBE-(PCL)2 #1 | 326.94 | ||
| NBE-(PCL)2 #2 | 319.06 | ||
| DB-PLLA | 266.90 | 440.95 | |
| DB-PCL | 319.66 | 442.77 | |
| SDBC | 277.82 | 331.18 | 442.77 |
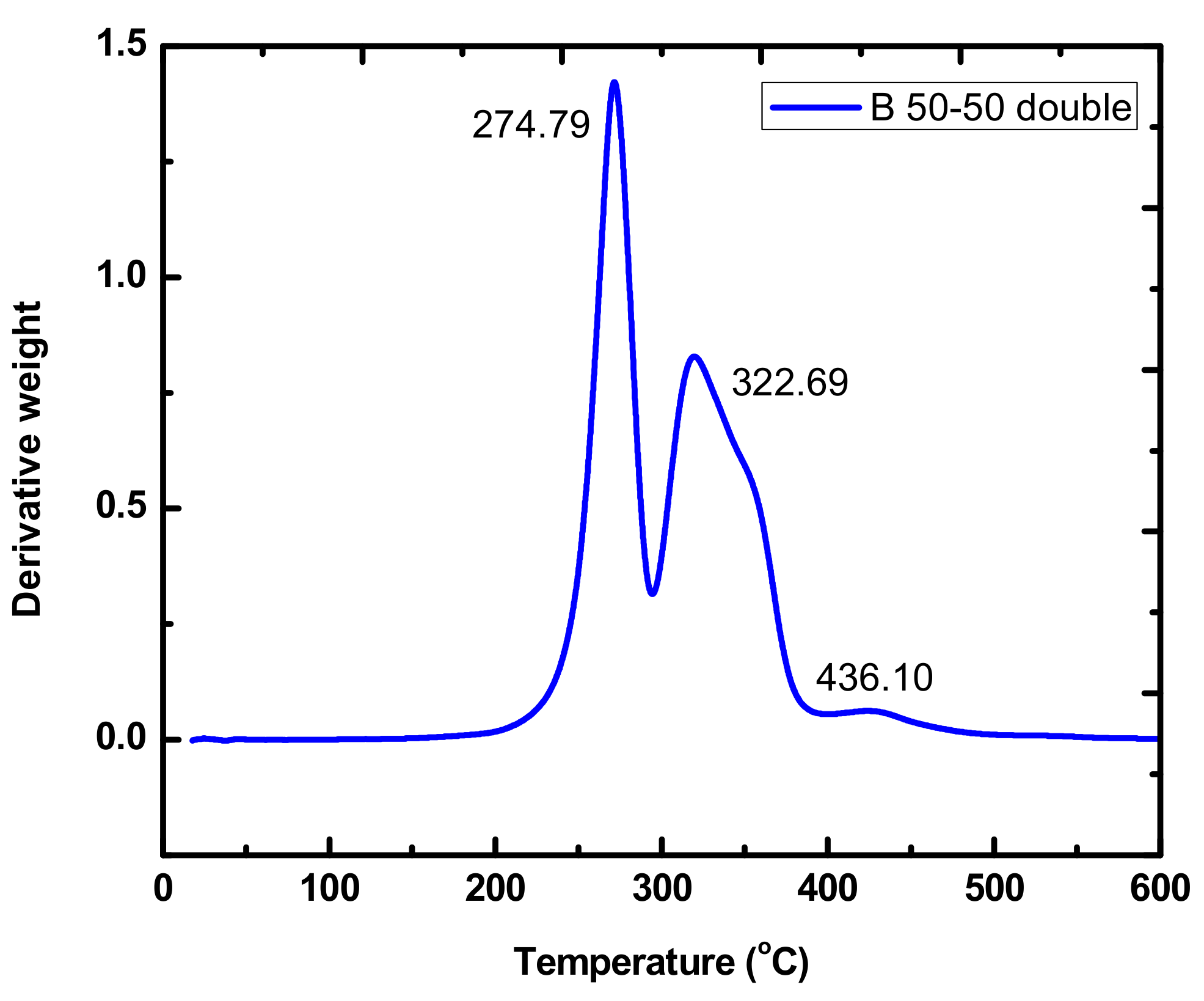
| BDBC | 274.79 | 322.69 | 436.10 |
| B-(PLLA-b-PCL-b-PLLA) | 291.77 | 335.43 | 435.49 |
| Sample | (Tm1)PCL, °C | (ΔHm1)PCL, J/g a | (Tm2)PLLA, °C | (Tm3)PLLA, °C | (ΔHm)PLLA, J/g a | (Tcc)PLLA, °C |
|---|---|---|---|---|---|---|
| NBE-PCL | 53 | 85 (60.5%) | ||||
| NBE-(PCL)2 #1 | 51 | 80 (57.3%) | ||||
| NBE-PLLA | 139 | 52 (38.4%) | 82 | |||
| NBE-(PLLA)2 #1 | 106 | 131 | 7 (5.0%) | |||
| DB-PCL | 46 | 62 (44.3%) | ||||
| DB-PLLA | 138 | 12 (9%) | ||||
| BDBC | 43 | 69 (49.4%) | 104 | 5 (3.8%) | ||
| B-(PLLA-b-PCL-b-PLLA) | 48 | 59 (42.3%) | 122 | 132 | 30 (22.0%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikovia, C.; Sougioltzoupoulou, E.; Rigas, V.; Pitsikalis, M. Macromolecular Brushes Based on Poly(L-Lactide) and Poly(ε-Caprolactone) Single and Double Macromonomers via ROMP. Synthesis, Characterization and Thermal Properties. Polymers 2019, 11, 1606. https://doi.org/10.3390/polym11101606
Nikovia C, Sougioltzoupoulou E, Rigas V, Pitsikalis M. Macromolecular Brushes Based on Poly(L-Lactide) and Poly(ε-Caprolactone) Single and Double Macromonomers via ROMP. Synthesis, Characterization and Thermal Properties. Polymers. 2019; 11(10):1606. https://doi.org/10.3390/polym11101606
Chicago/Turabian StyleNikovia, Christiana, Eleftheria Sougioltzoupoulou, Vyron Rigas, and Marinos Pitsikalis. 2019. "Macromolecular Brushes Based on Poly(L-Lactide) and Poly(ε-Caprolactone) Single and Double Macromonomers via ROMP. Synthesis, Characterization and Thermal Properties" Polymers 11, no. 10: 1606. https://doi.org/10.3390/polym11101606
APA StyleNikovia, C., Sougioltzoupoulou, E., Rigas, V., & Pitsikalis, M. (2019). Macromolecular Brushes Based on Poly(L-Lactide) and Poly(ε-Caprolactone) Single and Double Macromonomers via ROMP. Synthesis, Characterization and Thermal Properties. Polymers, 11(10), 1606. https://doi.org/10.3390/polym11101606