Induction of Polyacetylene to a Chiral Smectic Liquid Crystal–Chiral Direct Conversion
Abstract
1. Introduction
2. Results
2.1. Synthesis of the Monosubstituted Polyacetylene
2.2. Characterization of Poly(Ac-Pyr)
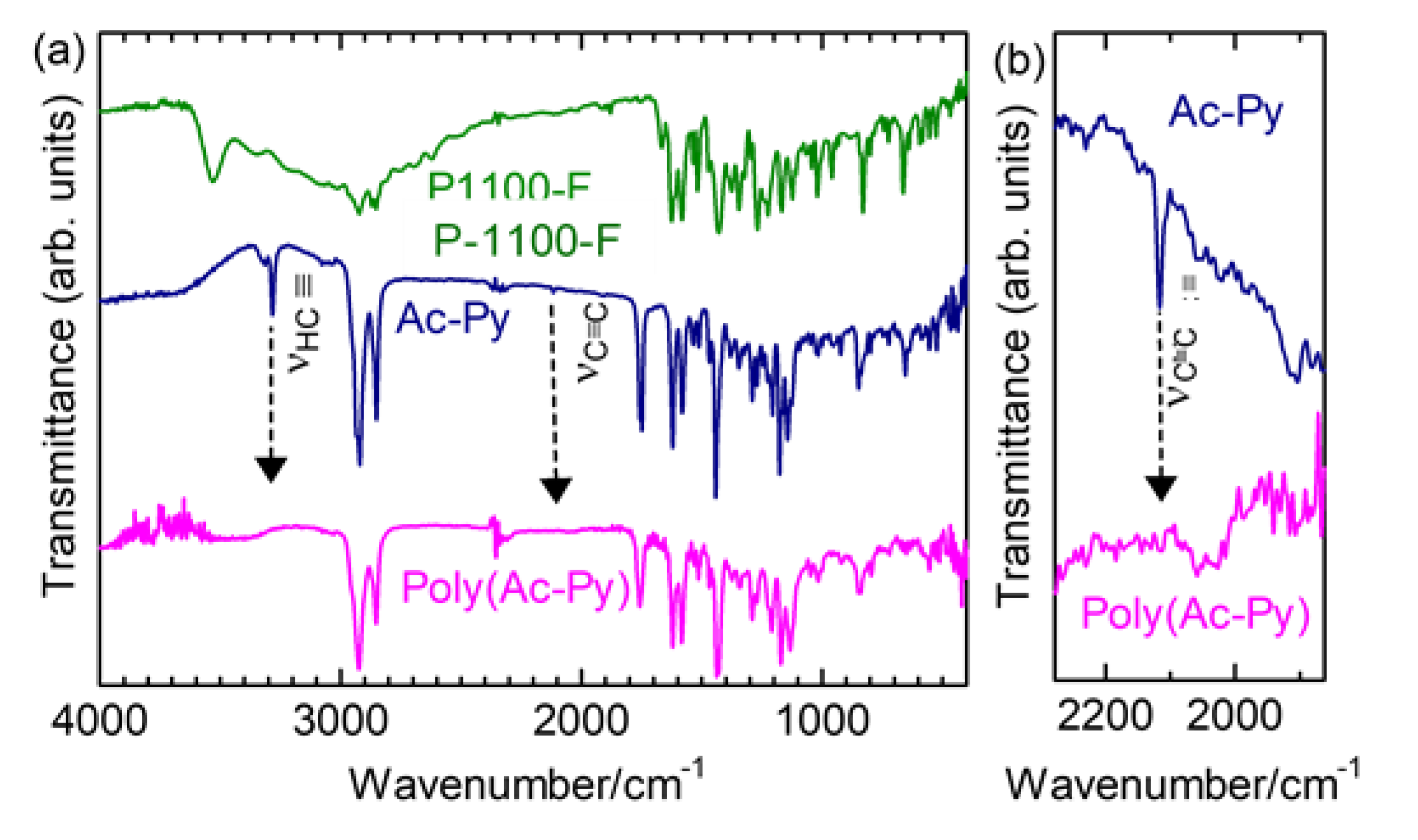
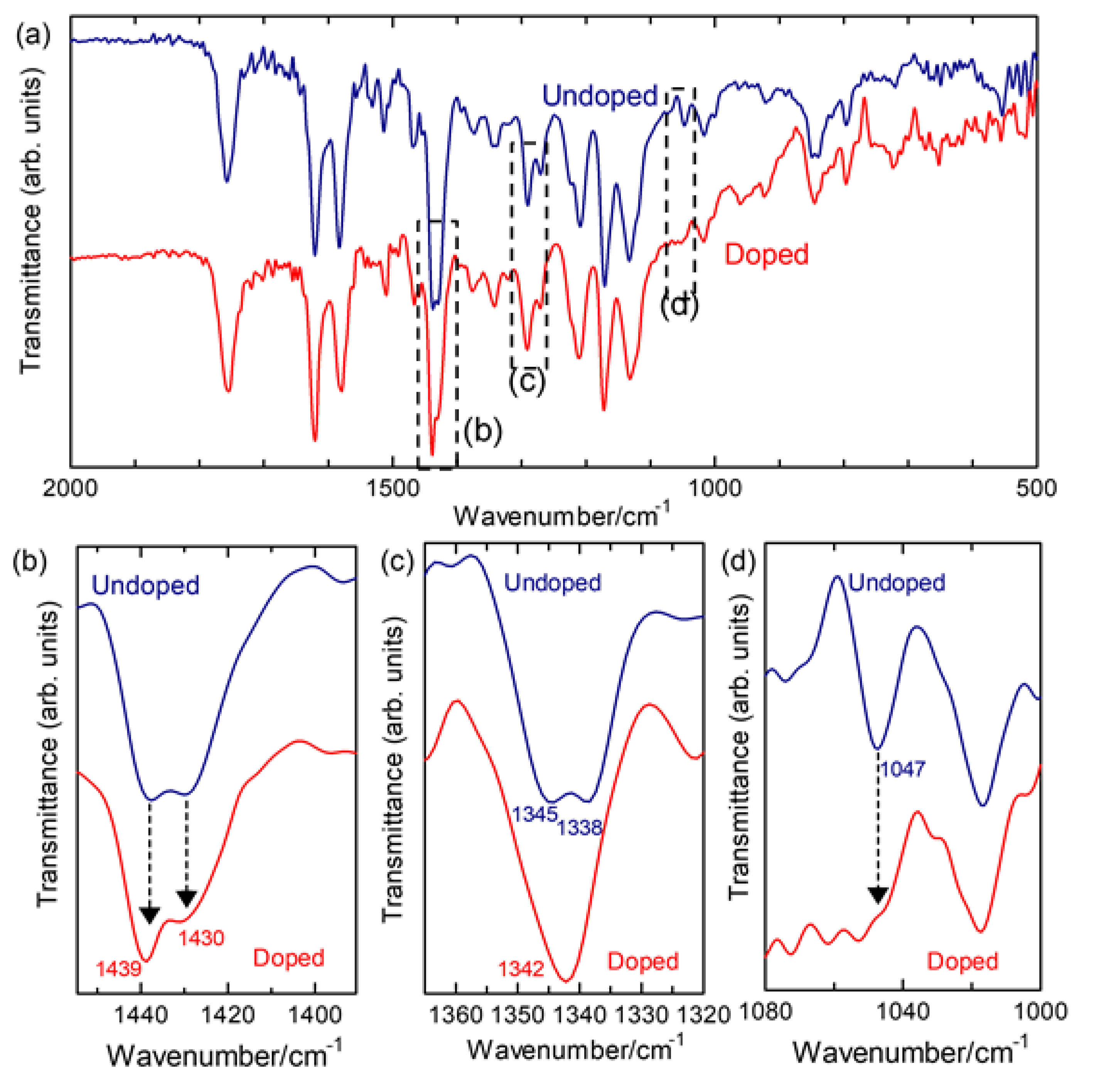
2.2.1. Infrared Absorption
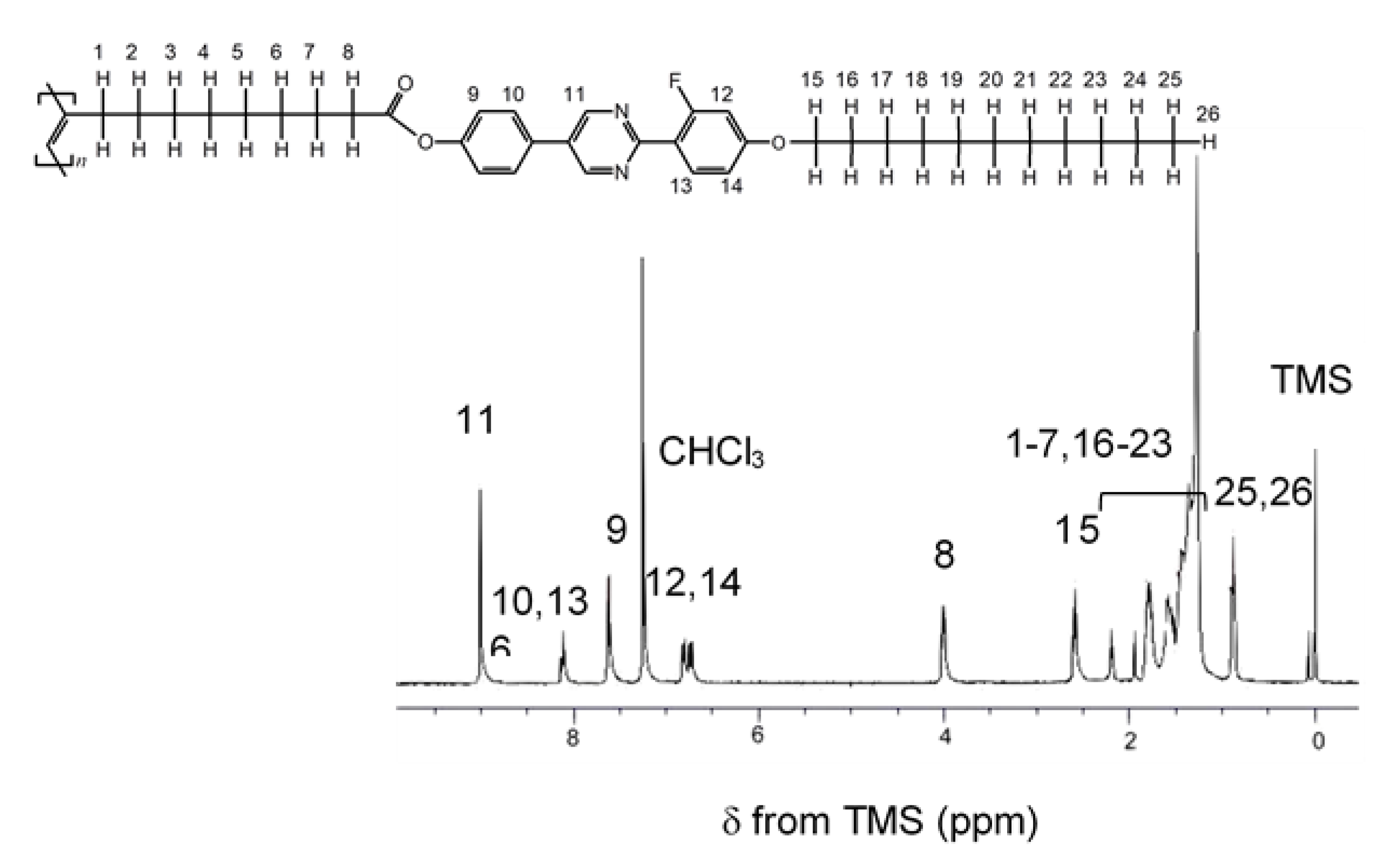
2.2.2. NMR
2.3. Physical Properties
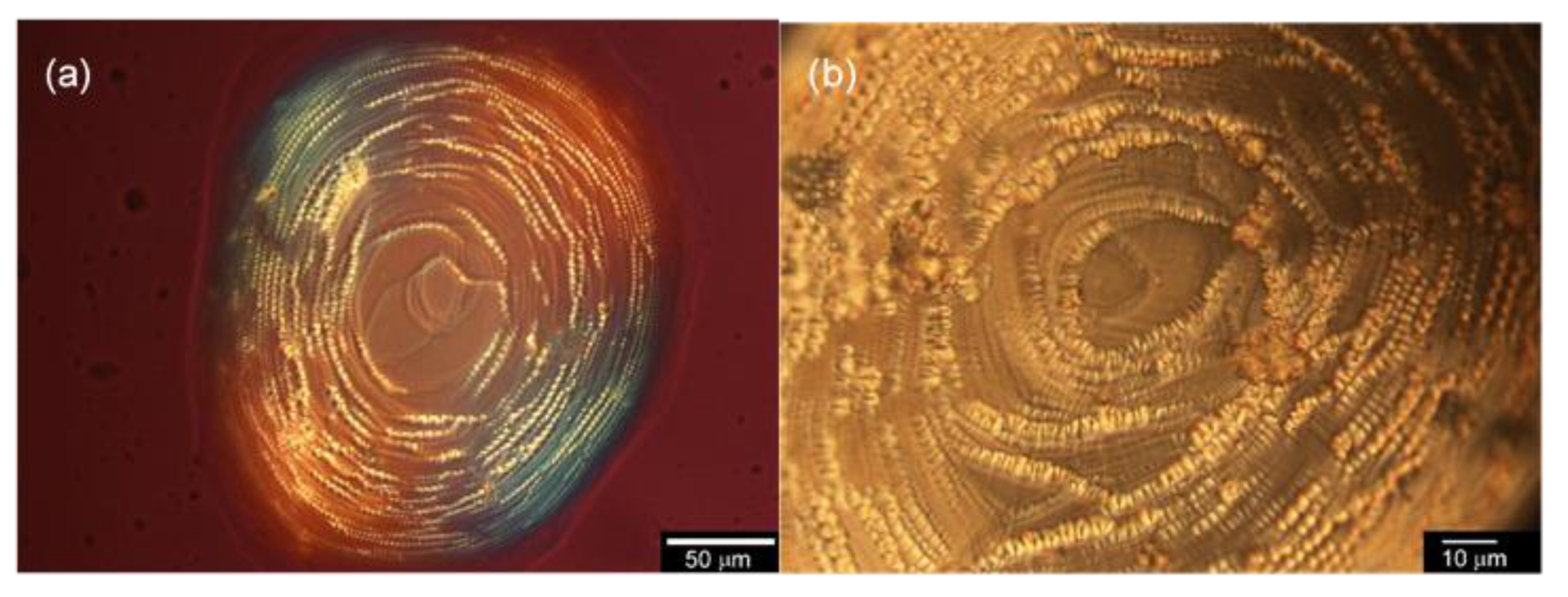
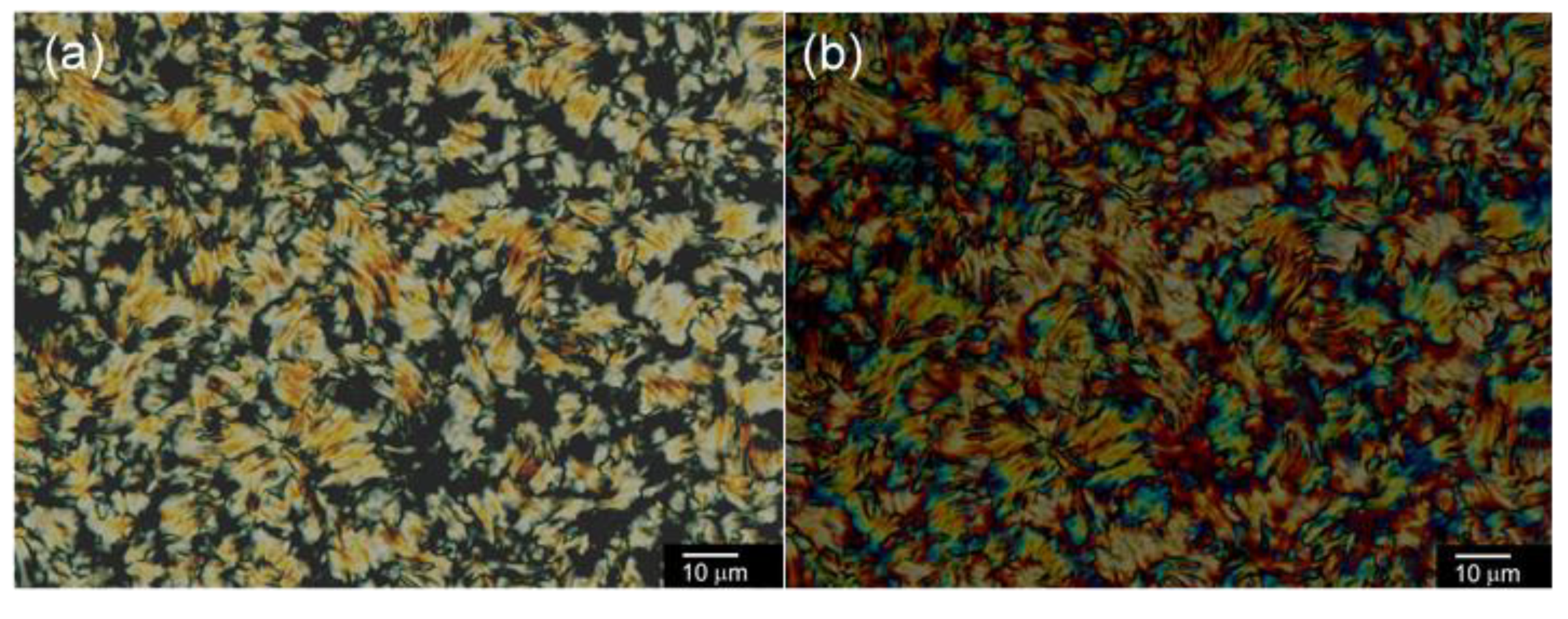
2.3.1. Optical Texture
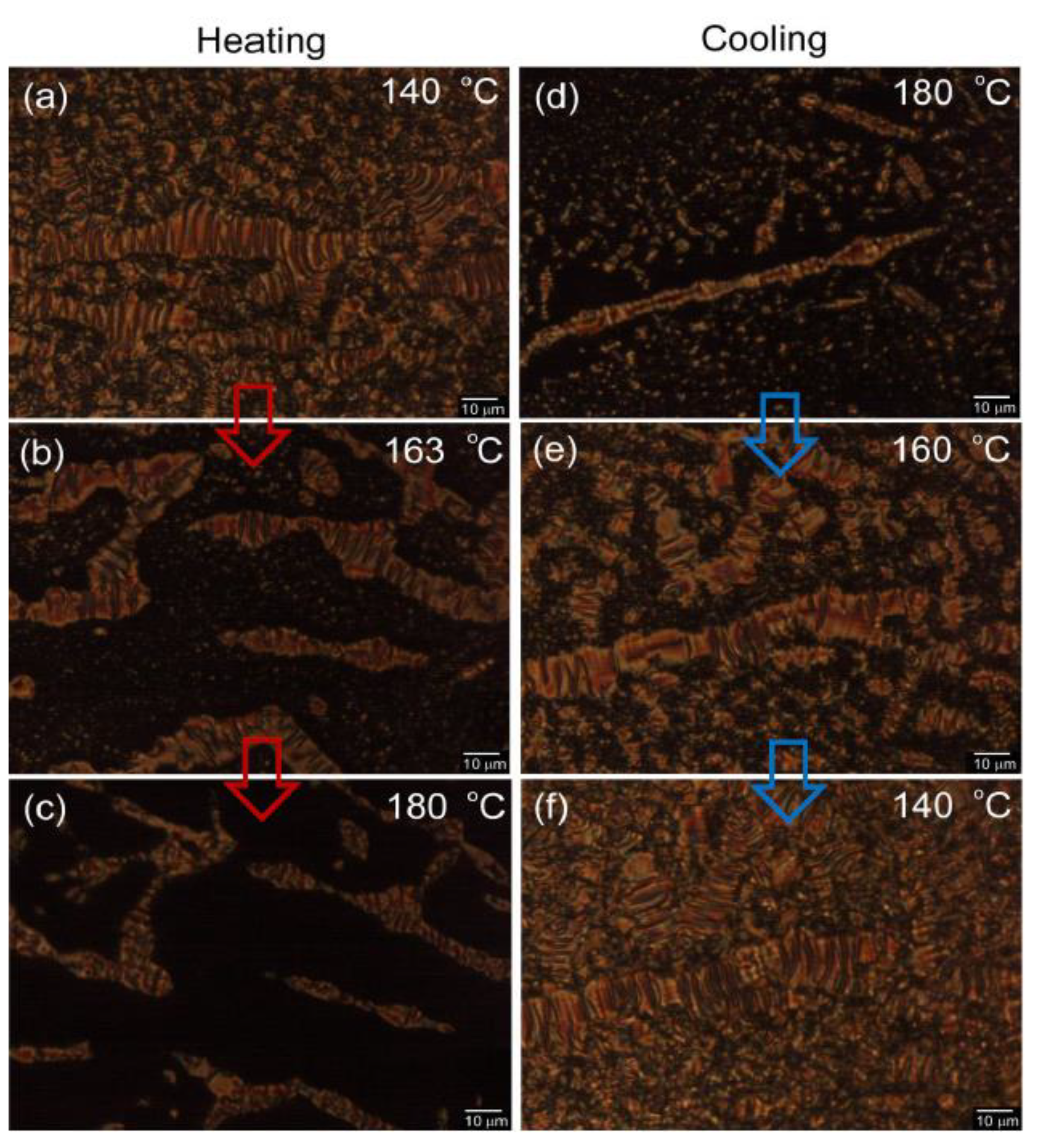
2.3.2. Modification of the Poly(Ac-Pyr) SmC Phase
2.3.3. Dynamic Scanning Calorimetry
2.4. Modification of the Poly(Ac–Pyr)
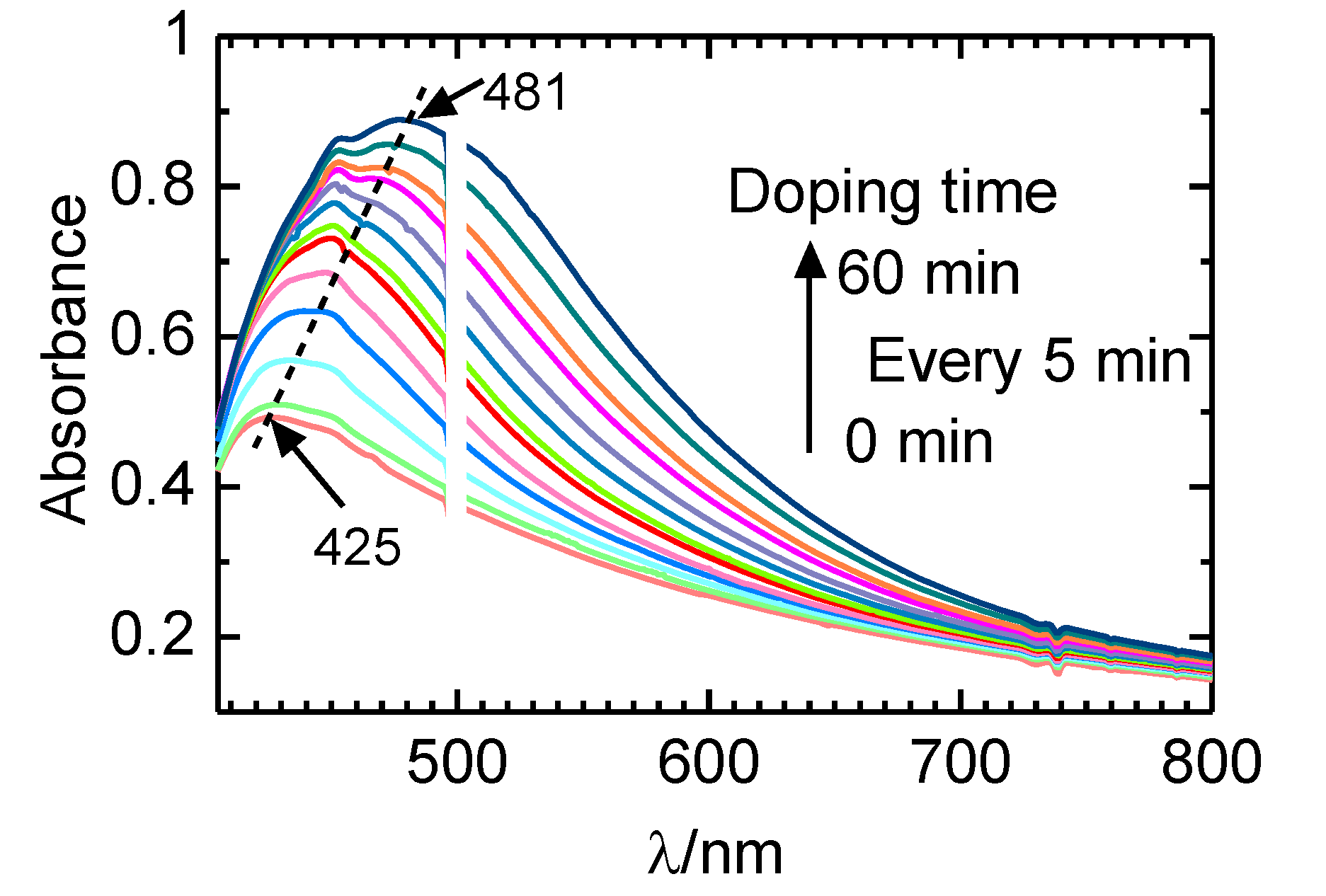
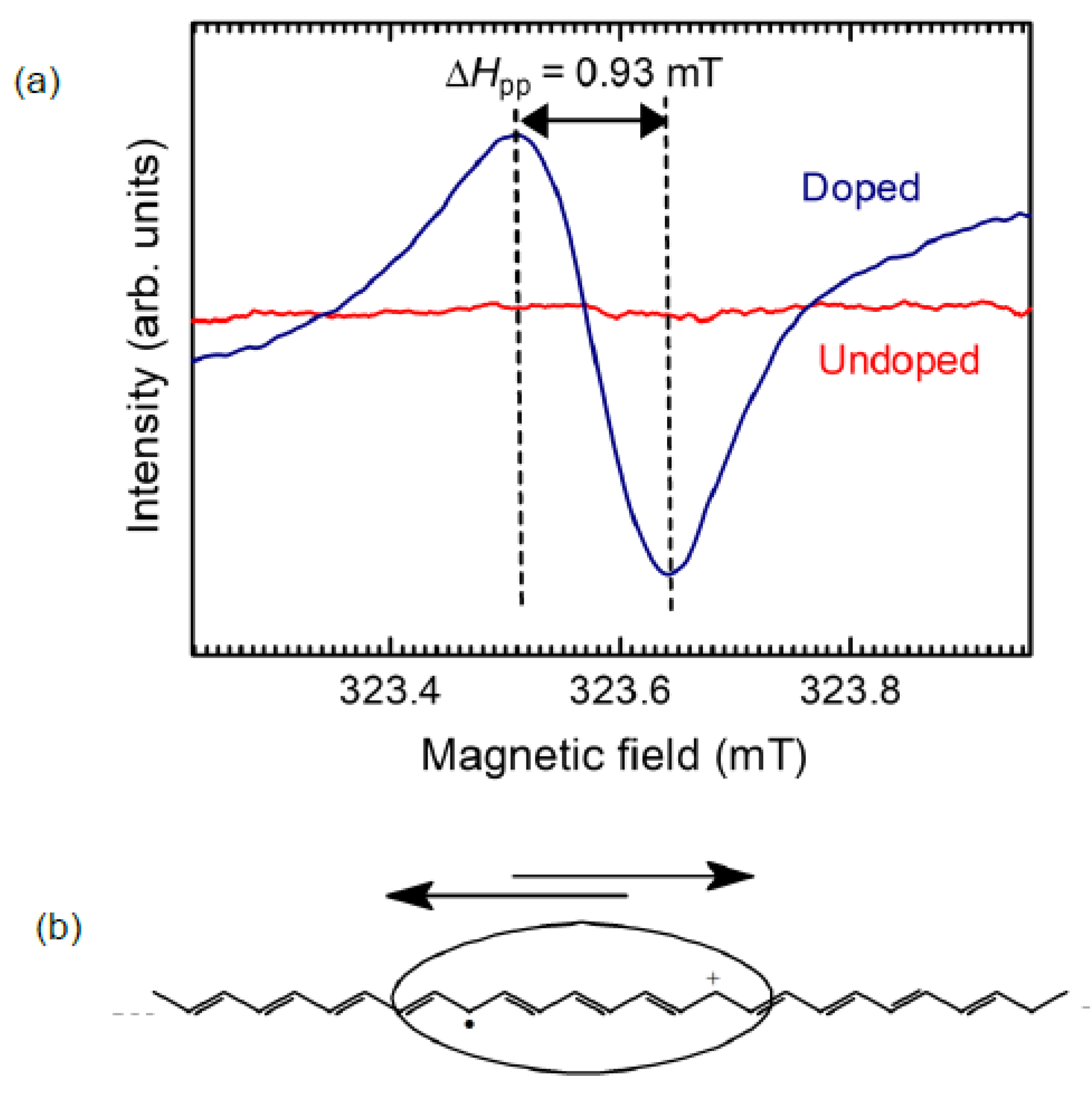
2.4.1. Influence on Polyacetylene Bandgap by Iodine Doping
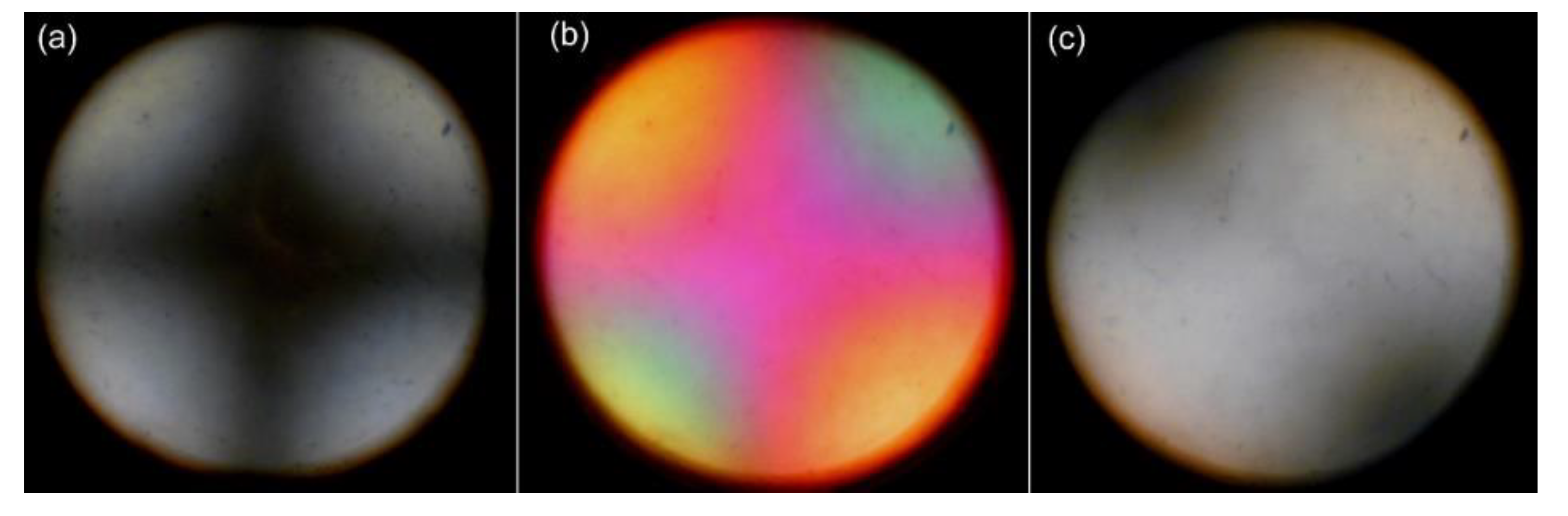
2.4.2. Inducting Uniaxial Alignment of Chiral Smectic LC
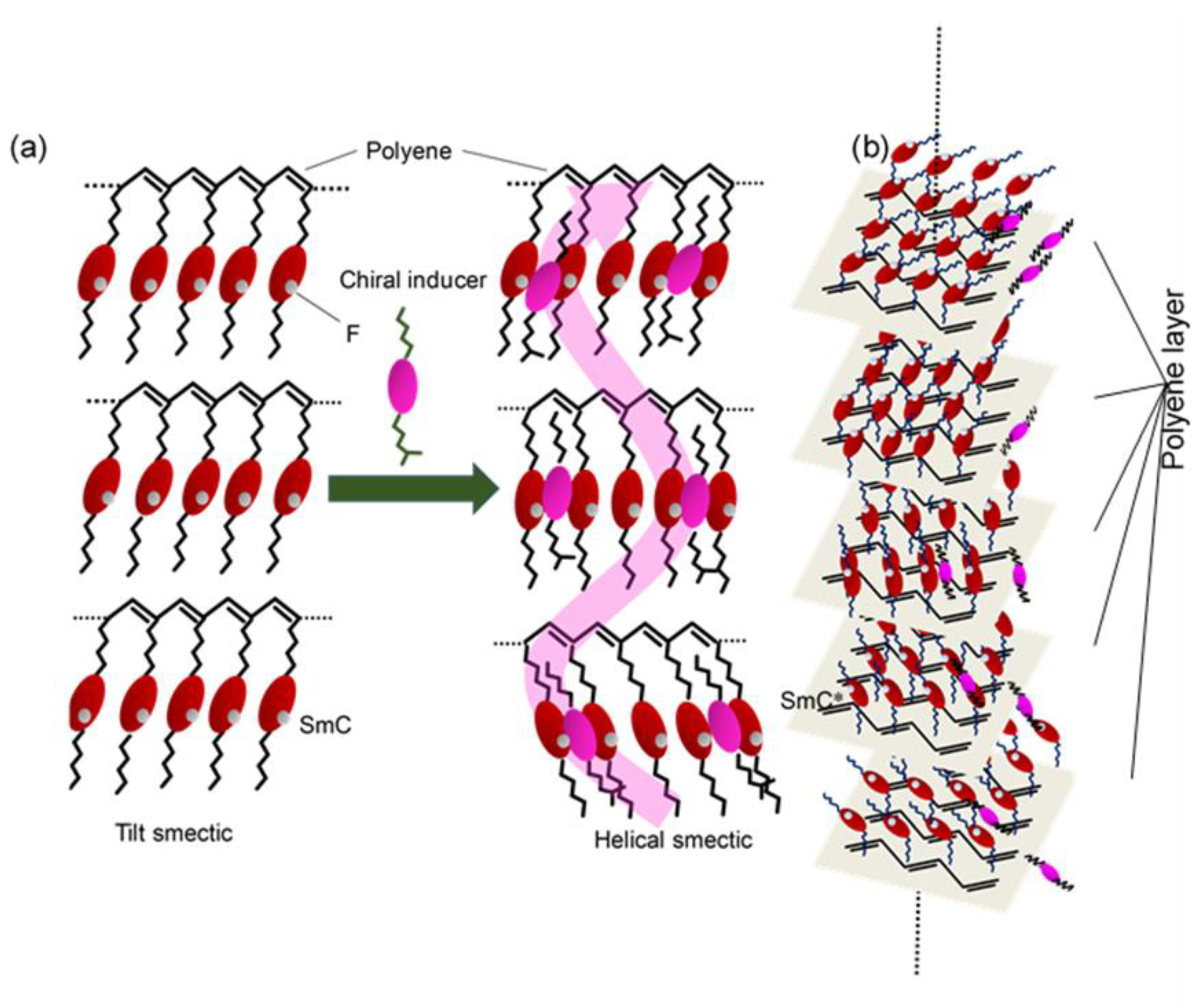
3. A Plausible Structure for the Chiral Phase
4. Conclusions
5. Instruments
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Araya, K.; Mukoh, A.; Narahara, T.; Shirakawa, H. Synthesis of highly-oriented polyacetylene film in a liquid crystal solvent. Synth. Met. 1986, 14, 199–206. [Google Scholar] [CrossRef]
- Brown, J.W.; Foot, P.J.S.; Gabaston, L.I.; Ibison, P.; Prevost, A. Synthesis of laser-alignable liquid crystalline conducting polymers. Macromol. Chem. Phys. 2004, 205, 1823–1828. [Google Scholar] [CrossRef]
- Tariq, M.; Hameed, S.; Magnago, R.F.; Bechtold, I.H.; Merlo, A.A. Side-chain liquid-crystalline polymer tetrazoles: Synthesis and characterization. J. Braz. Chem. Soc. 2014, 25, 1275–1282. [Google Scholar] [CrossRef]
- Mizuta, K.; Katsushima, M.; Koga, Y.; Yamabuki, K.; Onimura, K.; Oishi, T. Synthesis of chiral side-chain liquid crystalline polyacetylenes bearing succinic acid spacer. Polym. Bull. 2012, 68, 623–634. [Google Scholar] [CrossRef]
- Roudini, D.; Foot, P.J.S.A. Side-chain liquid crystal conducting polymers. Sci. Prog. 2016, 99, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Lie, C.; Huanling, K.; Yiwang, C.; Fan, L.I. Synthesis of liquid crystalline polyacetylene containing chiral terphenyl and its “Jacket Effect”. Acta Agron. Sinica. 2012, 29, 1231–1239. [Google Scholar]
- Yu, Z.-Q.; Li, T.-T.; Zhang, Z.; Liu, J.-H.; Yuan, W.Z.; Lam, J.W.Y.; Yang, S.; Chen, E.-Q.; Tang, B.Z. Phase behaviors of side-chain liquid crystalline polyacetylenes with different length of spacer: Where will the decoupling effect appear? Macromolecules 2015, 48, 2886–2893. [Google Scholar] [CrossRef]
- Yu, Z.-Q.; Lam, J.W.Y.; Zhu, C.Z.; Chen, E.-Q.; Tang, B.Z. Side-chain liquid crystalline polyacetylenes with increasing length of alkyl tails: From highly ordered smectic to smectic c phase. Macromolecules 2013, 46, 588–596. [Google Scholar] [CrossRef]
- Hu, T.; Kong, H.; Chen, L.; Chen, Y.; Li, F.; Zha, D. Synthesis and properties of novel ferroelectric liquid crystalline polyacetylenes containing terphenyl mesogens with chiral groups. J. Therm. Anal. Calorim. 2011, 105, 995–1006. [Google Scholar] [CrossRef]
- Shirakawa, H.; Kadokura, K.; Goto, H.; Oh, S.-Y.; Akagi, K.; Araya, K. Synthesis of liquid crystalline polyacetylene derivatives. Mol. Cryst. Liq. Cryst. Sci. Tech. Sec. A Mol. Cryst. Liq. Cryst. 1994, 255, 213–219. [Google Scholar] [CrossRef]
- Kijima, M.; Setoh, K.; Shirakawa, H. Synthesis of novel ionic liquid crystalline pyrrole derivatives having a viologen moiety. Mol. Cryst. Liq. Cryst. 2001, 364, 911–918. [Google Scholar] [CrossRef]
- Ohkawa, S.; Ohta, R.; Kawabata, K.; Goto, H. Polymerization in liquid crystal medium: Preparation of polythiophene derivatives bearing a bulky pyrimidine substituent. Polymers 2010, 2, 393–406. [Google Scholar] [CrossRef]
- Kawabata, K.; Goto, H. Liquid crystalline π-conjugated copolymers bearing a pyrimidine type mesogenic group. Materials 2009, 2, 22–37. [Google Scholar] [CrossRef]
- Watanabe, M.; Tsuchiya, K.; Shinnai, T.; Kijima, M. Liquid crystalline polythiophene bearing phenylnaphthalene side-chain. Macromolecules 2012, 45, 1825–1832. [Google Scholar] [CrossRef]
- Chien, J.C.W. Polyacetylene Chemistry, Physics, and material science; Academic Press: Orland, CA, USA, 1984. [Google Scholar]
- Ono, Y.; Terai, A. Motion of charged soliton in polyacetylene due to electric field. J. Phys. Soc. Jpn. 1990, 59, 2893–2904. [Google Scholar] [CrossRef]
- Meier, E.J.; An, F.A.; Gadway, B. Observation of the topological soliton state in the Su–Schrieffer–Heeger model. Nature Commun. 2016, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Su, W.P.; Schrieffer, J.R.; Heeger, A.J. Solitons in polyacetylene. Phys. Rev. Lett. 1979, 42, 1698. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yatsu, A.; Yonehara, T.; Goto, H. Induction of Polyacetylene to a Chiral Smectic Liquid Crystal–Chiral Direct Conversion. Polymers 2020, 12, 1547. https://doi.org/10.3390/polym12071547
Yatsu A, Yonehara T, Goto H. Induction of Polyacetylene to a Chiral Smectic Liquid Crystal–Chiral Direct Conversion. Polymers. 2020; 12(7):1547. https://doi.org/10.3390/polym12071547
Chicago/Turabian StyleYatsu, Akiko, Takuya Yonehara, and Hiromasa Goto. 2020. "Induction of Polyacetylene to a Chiral Smectic Liquid Crystal–Chiral Direct Conversion" Polymers 12, no. 7: 1547. https://doi.org/10.3390/polym12071547
APA StyleYatsu, A., Yonehara, T., & Goto, H. (2020). Induction of Polyacetylene to a Chiral Smectic Liquid Crystal–Chiral Direct Conversion. Polymers, 12(7), 1547. https://doi.org/10.3390/polym12071547