Preparation and Characterization of 3D Printed PLA-Based Conductive Composites Using Carbonaceous Fillers by Masterbatch Melting Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Characterization
2.3.1. Electrical Resistivity
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. Rheology Properties
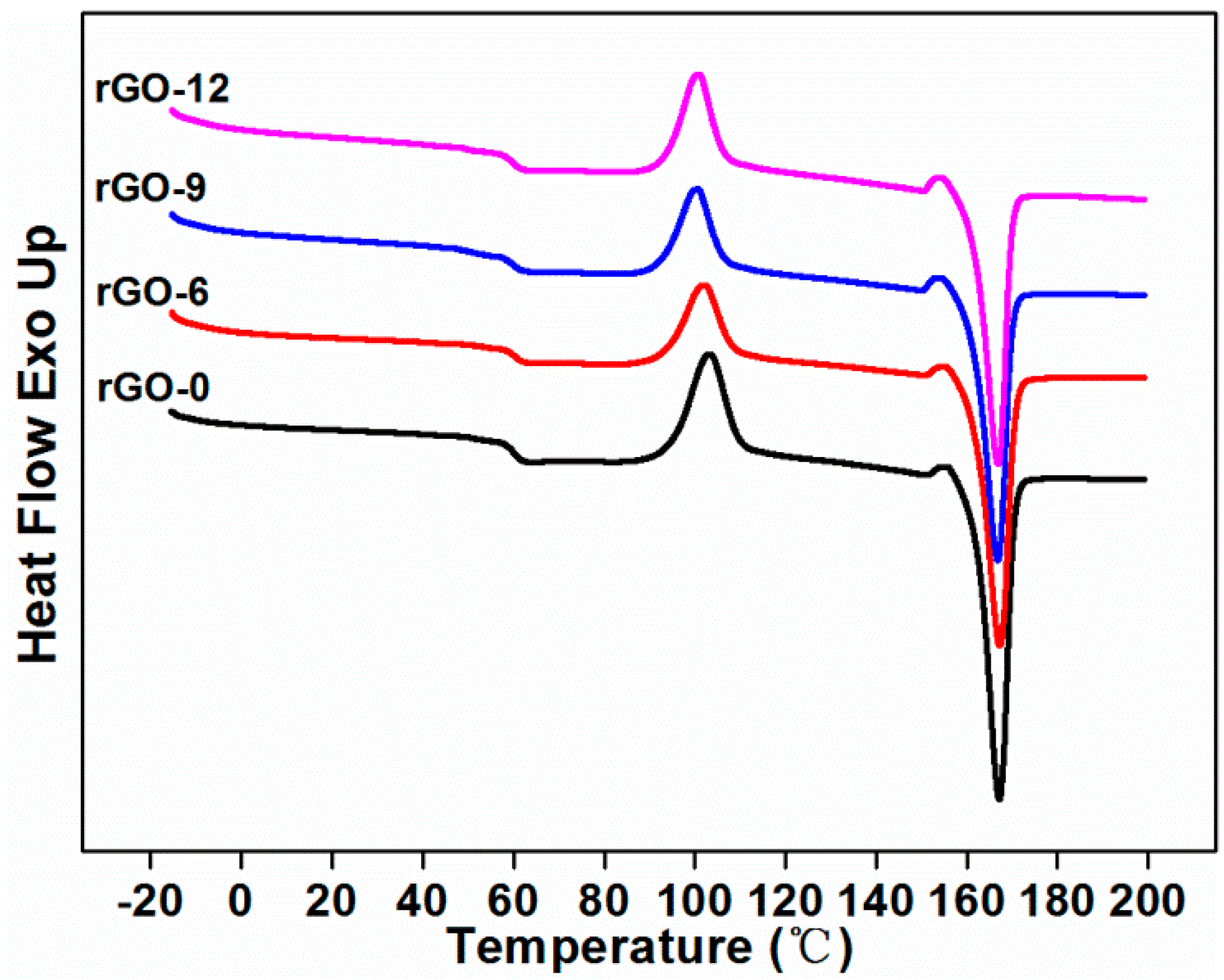
2.3.4. Differential Scanning Calorimetry (DSC)
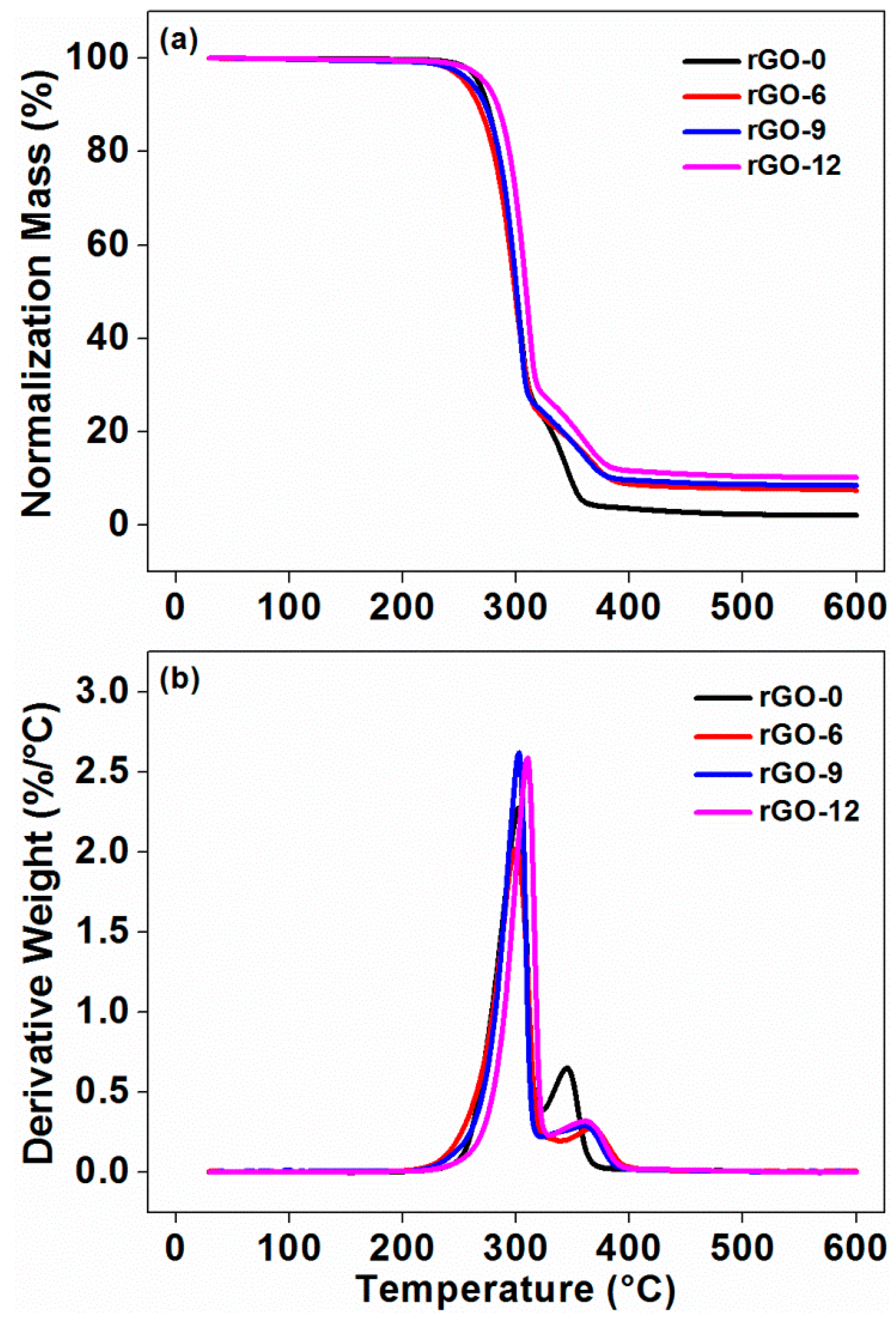
2.3.5. Thermalgravimetric Analysis (TGA)
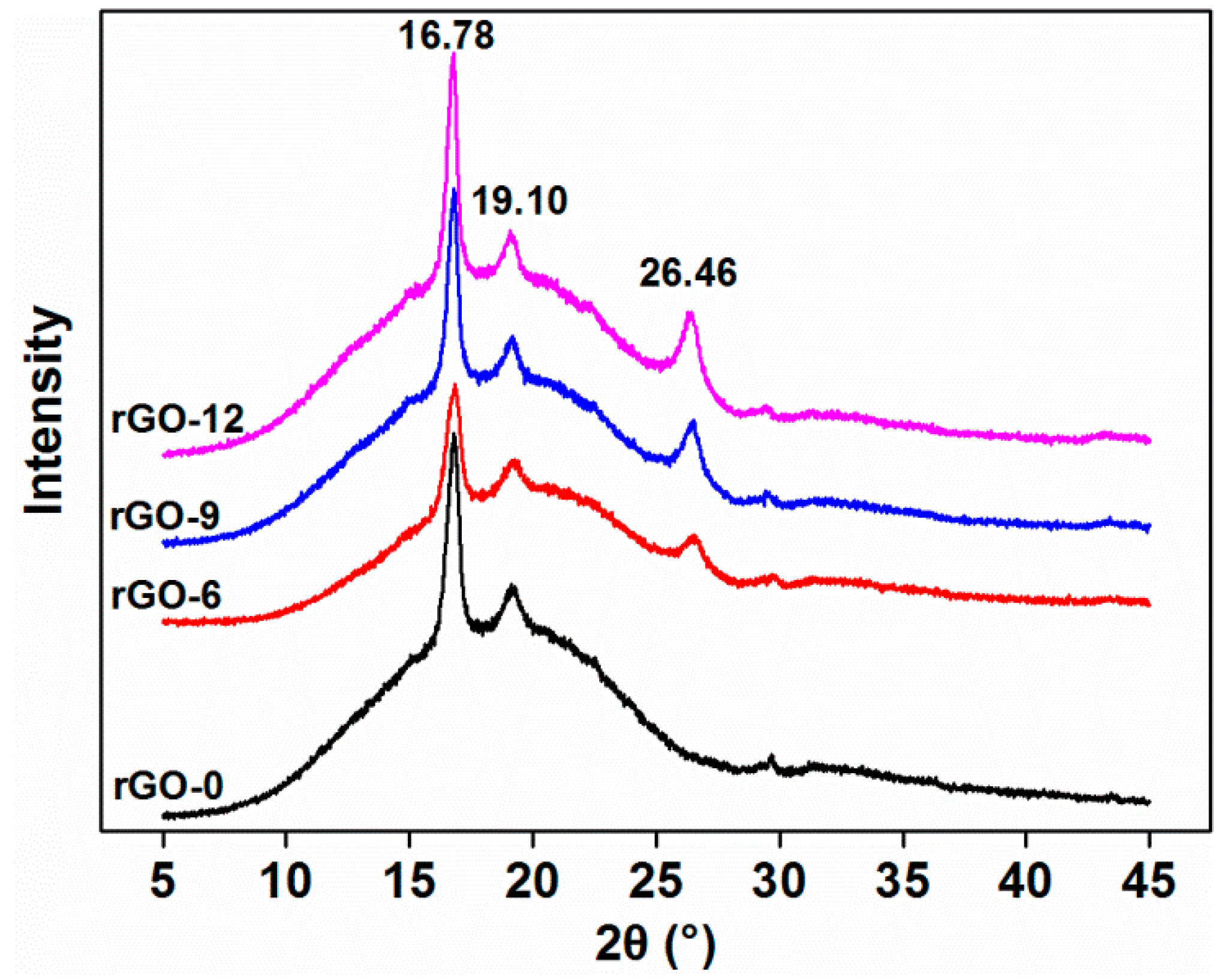
2.3.6. X-ray Diffraction (XRD)
2.3.7. Fourier-Transform Infrared Spectroscopy (FTIR)
3. Results and Discussion
3.1. Effect of the Carbonaceous Filler Types on PLA-Based Composites
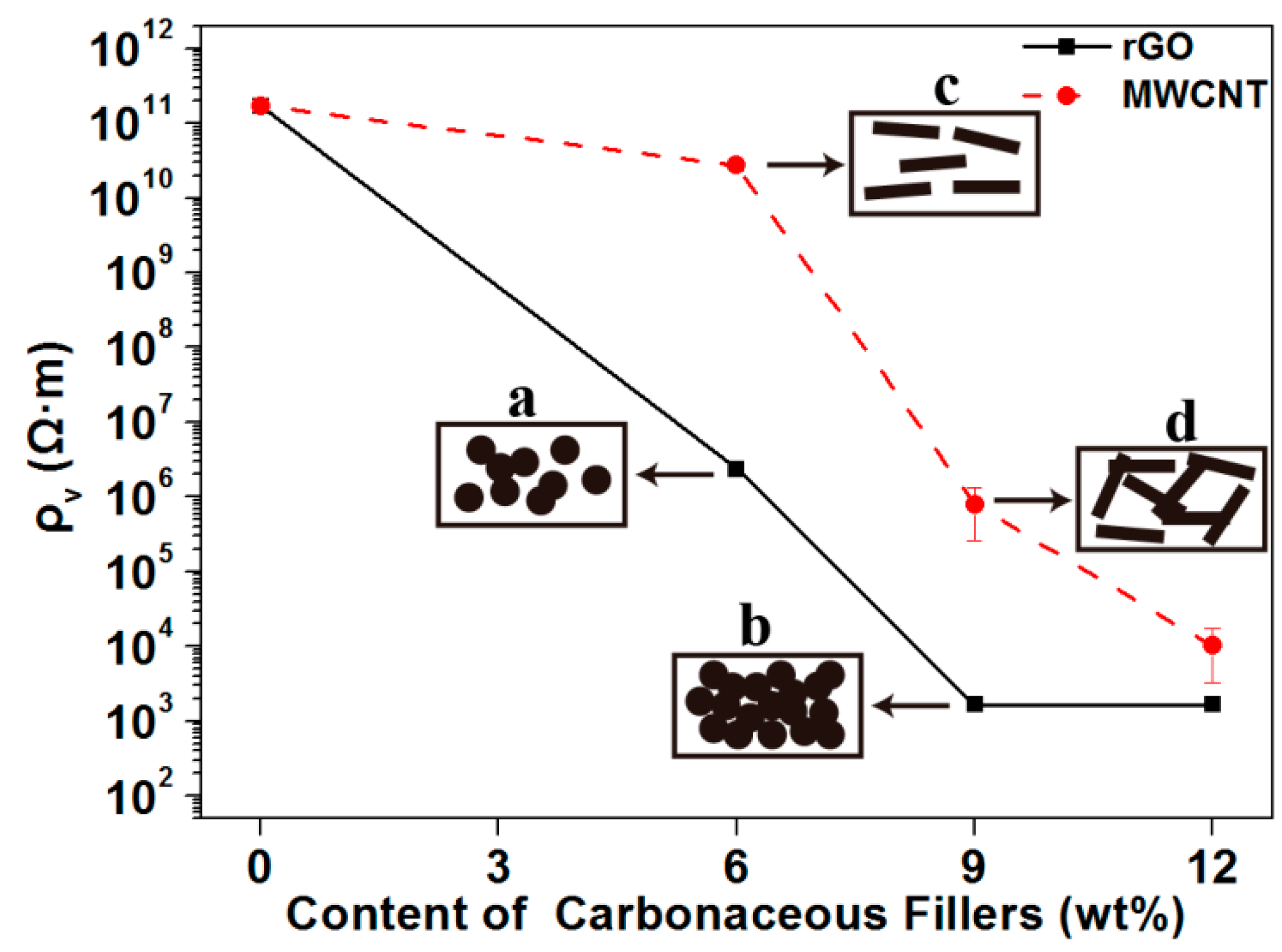
3.1.1. Electrical Resistivity of the PLA-Based Composites
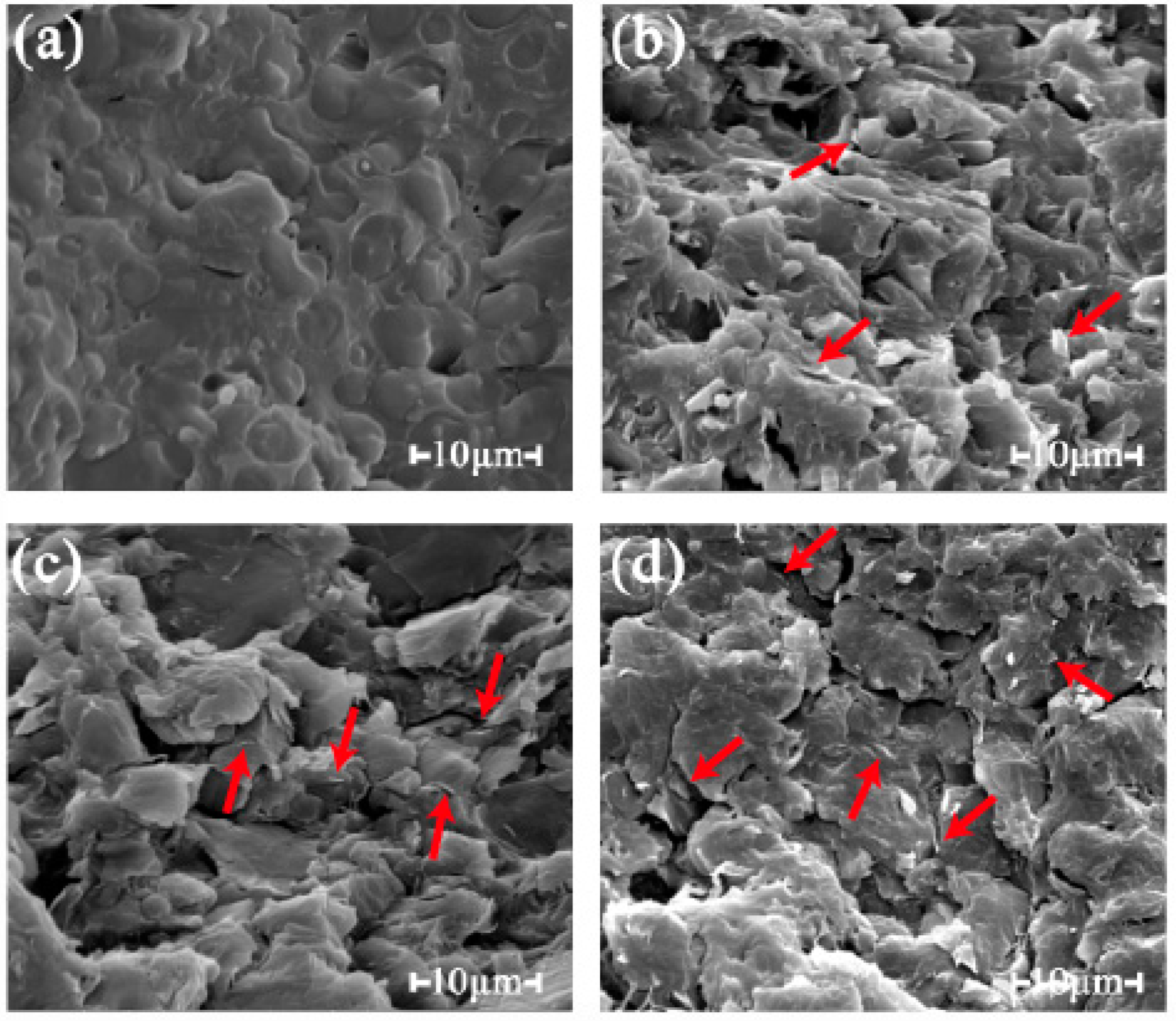
3.1.2. Morphology of the PLA-Based Composites
3.2. Effect of rGO Content on the PLA-Based Composites
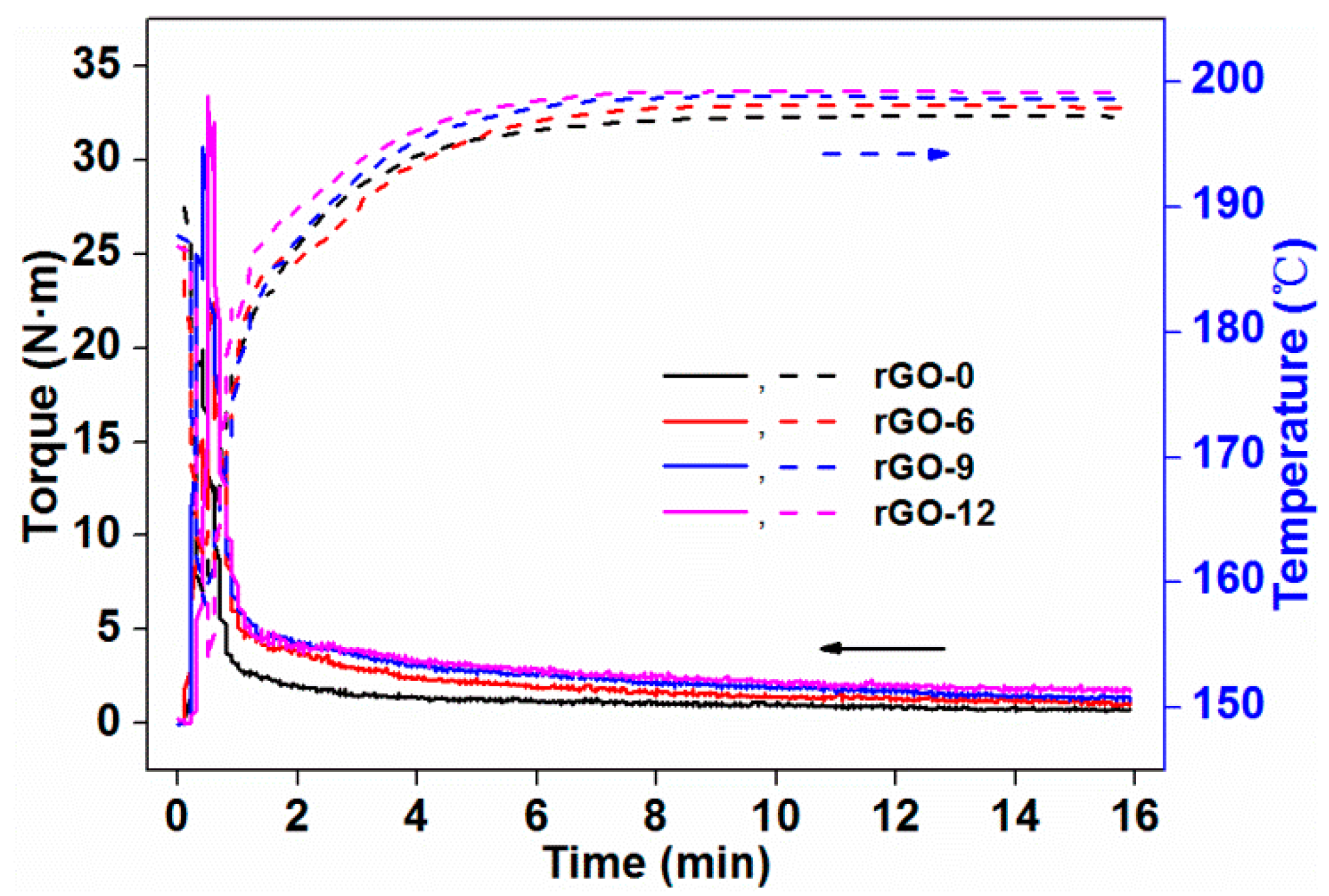
3.2.1. Rheological Properties
3.2.2. Thermal Stability
3.2.3. XRD Results
3.3. Properties of the Modified Composites
3.3.1. FTIR Results of the Masterbatch
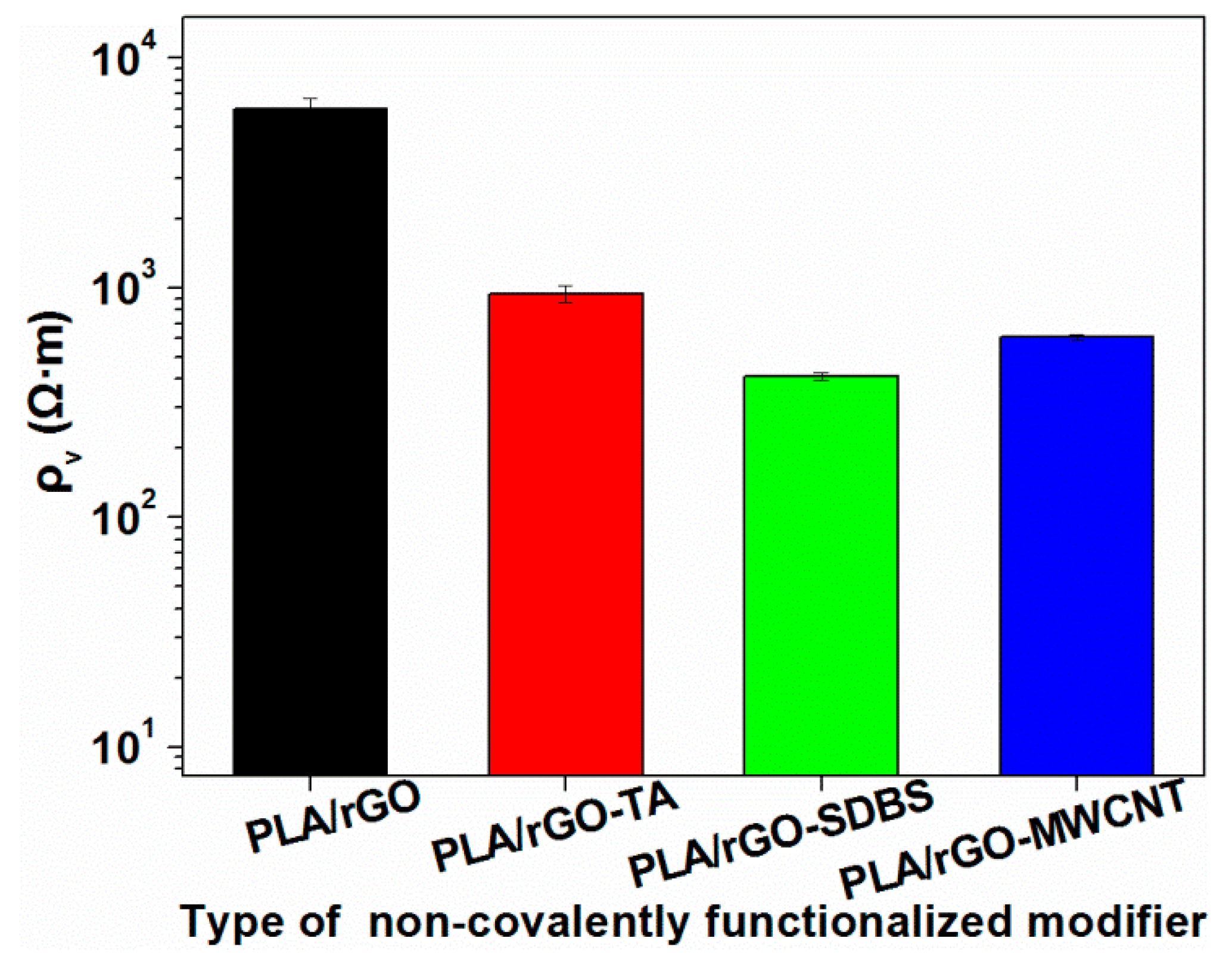
3.3.2. Electrical Resistivity of the rGO/PLA Composites
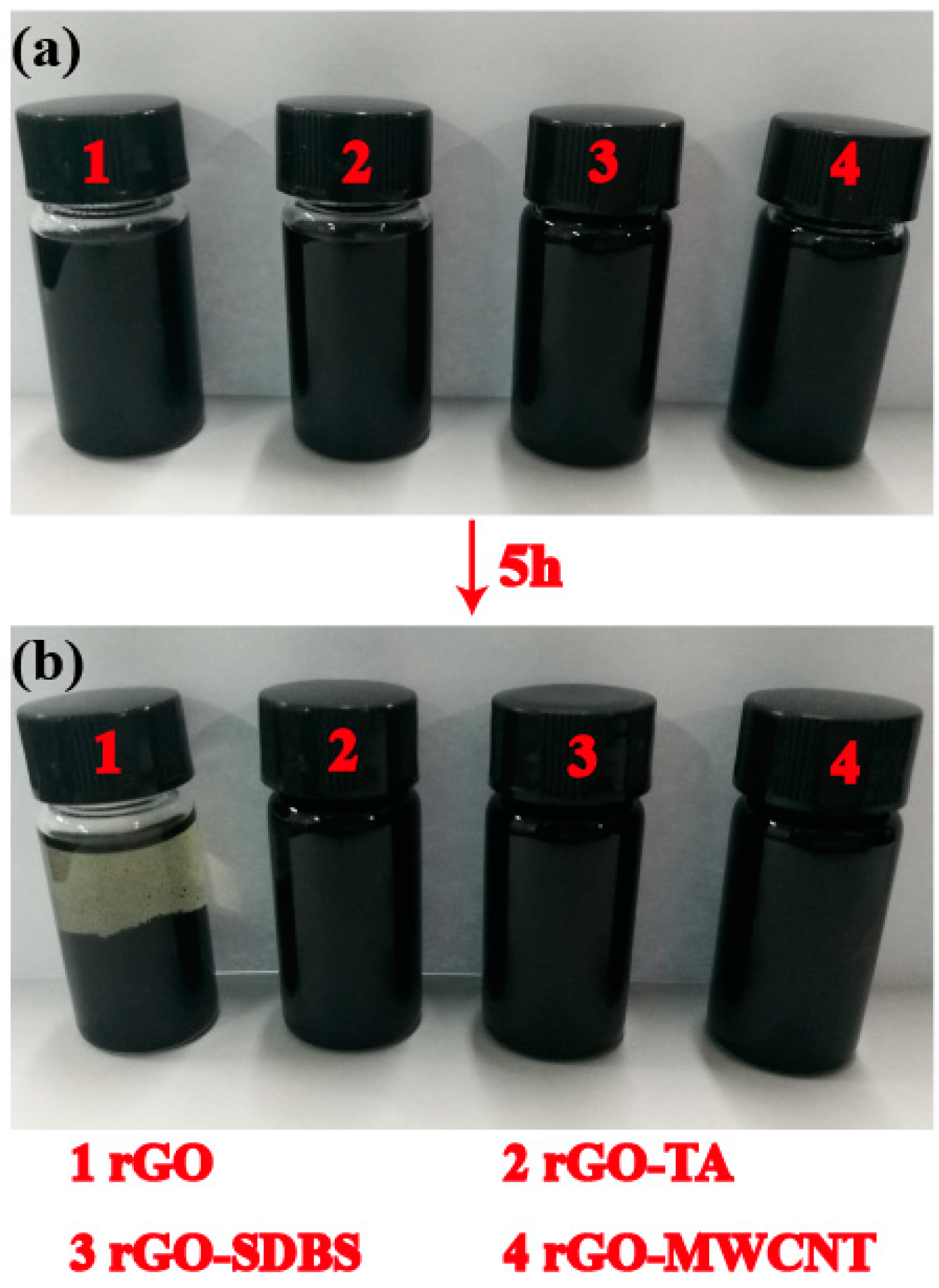
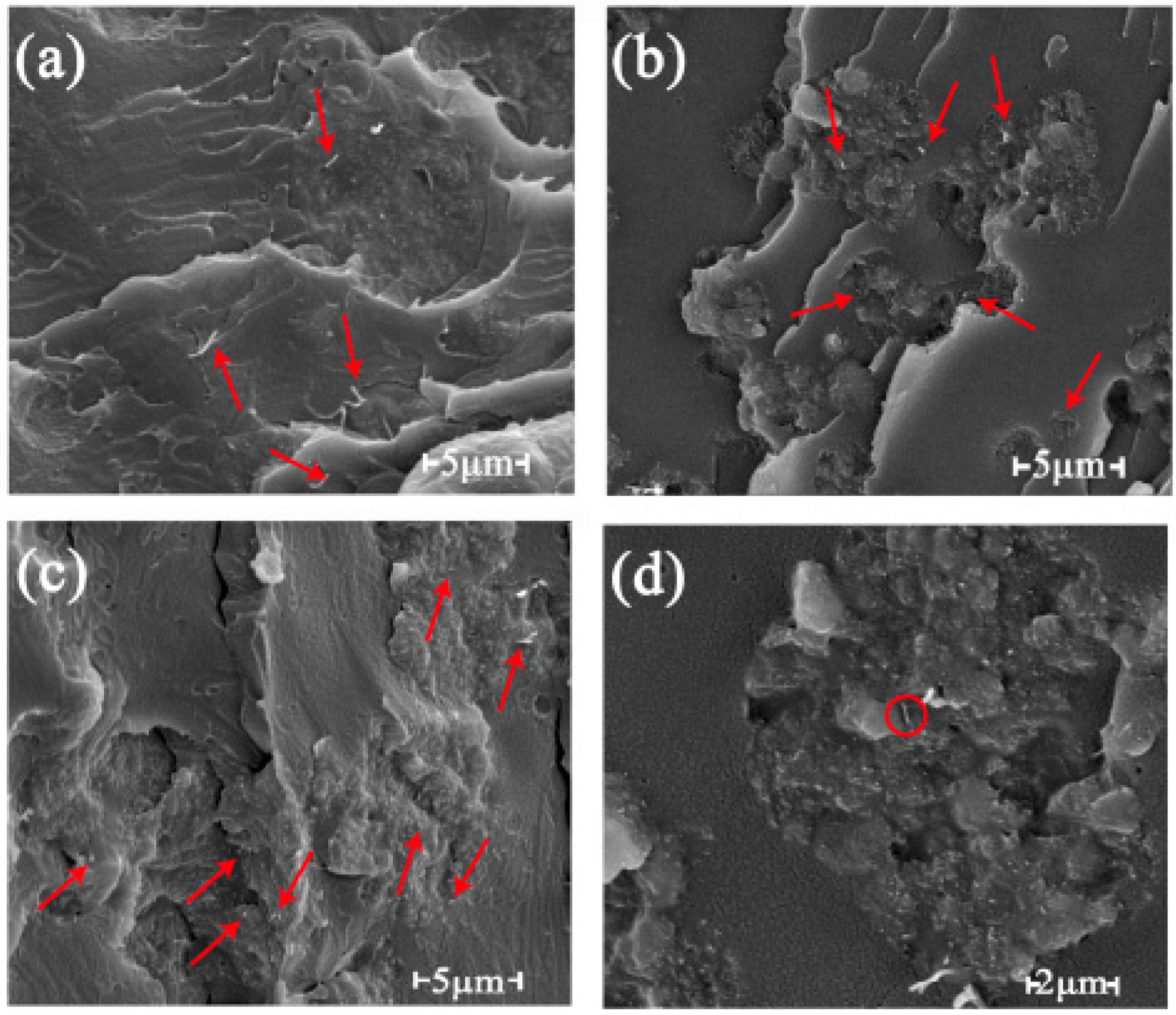
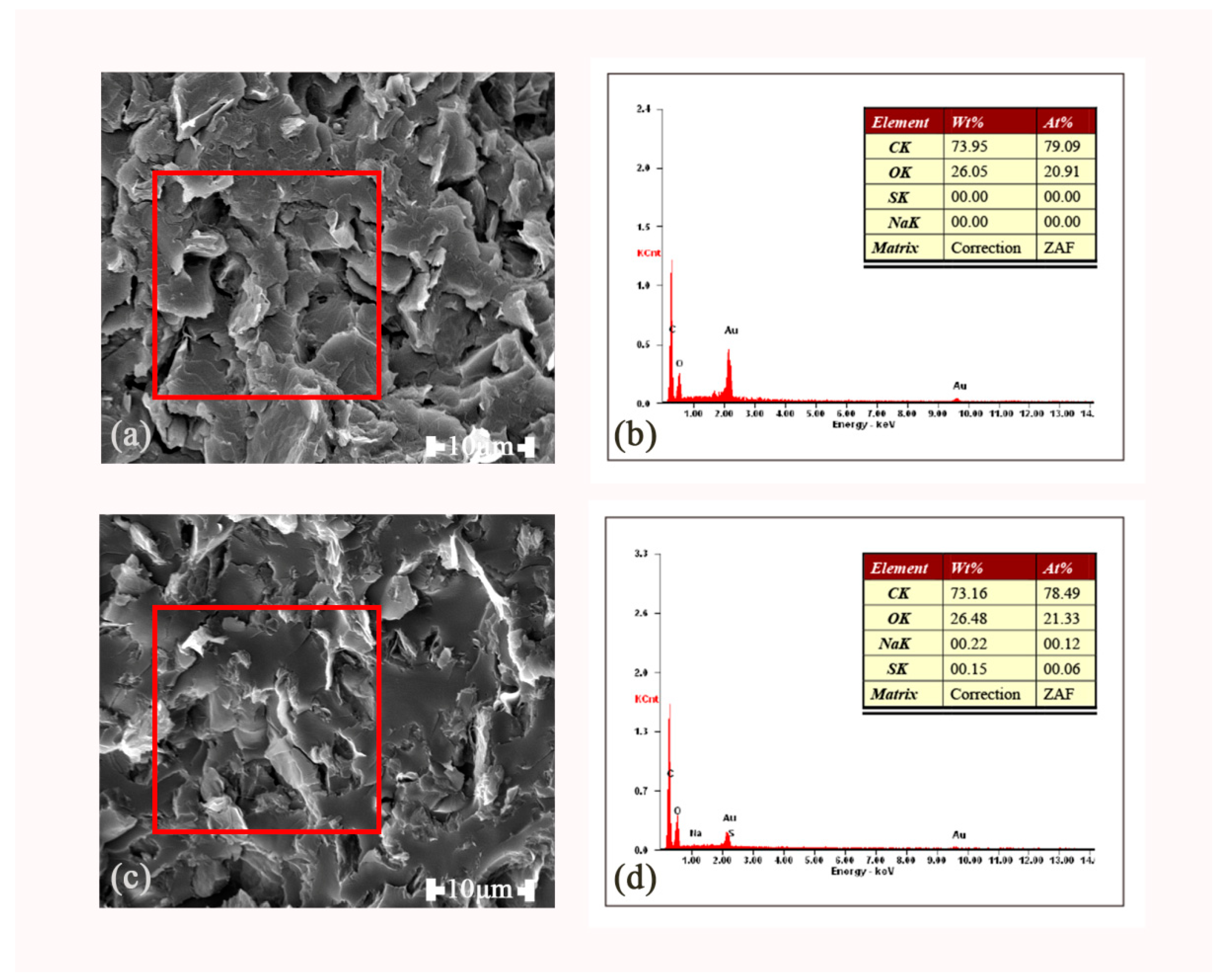
3.3.3. SEM Photographs and Energy-Dispersive Spectroscopy (EDX) Spectra of the rGO-SDBS/PLA Composites
3.3.4. Thermal Stability of the rGO-SDBS/PLA Composites
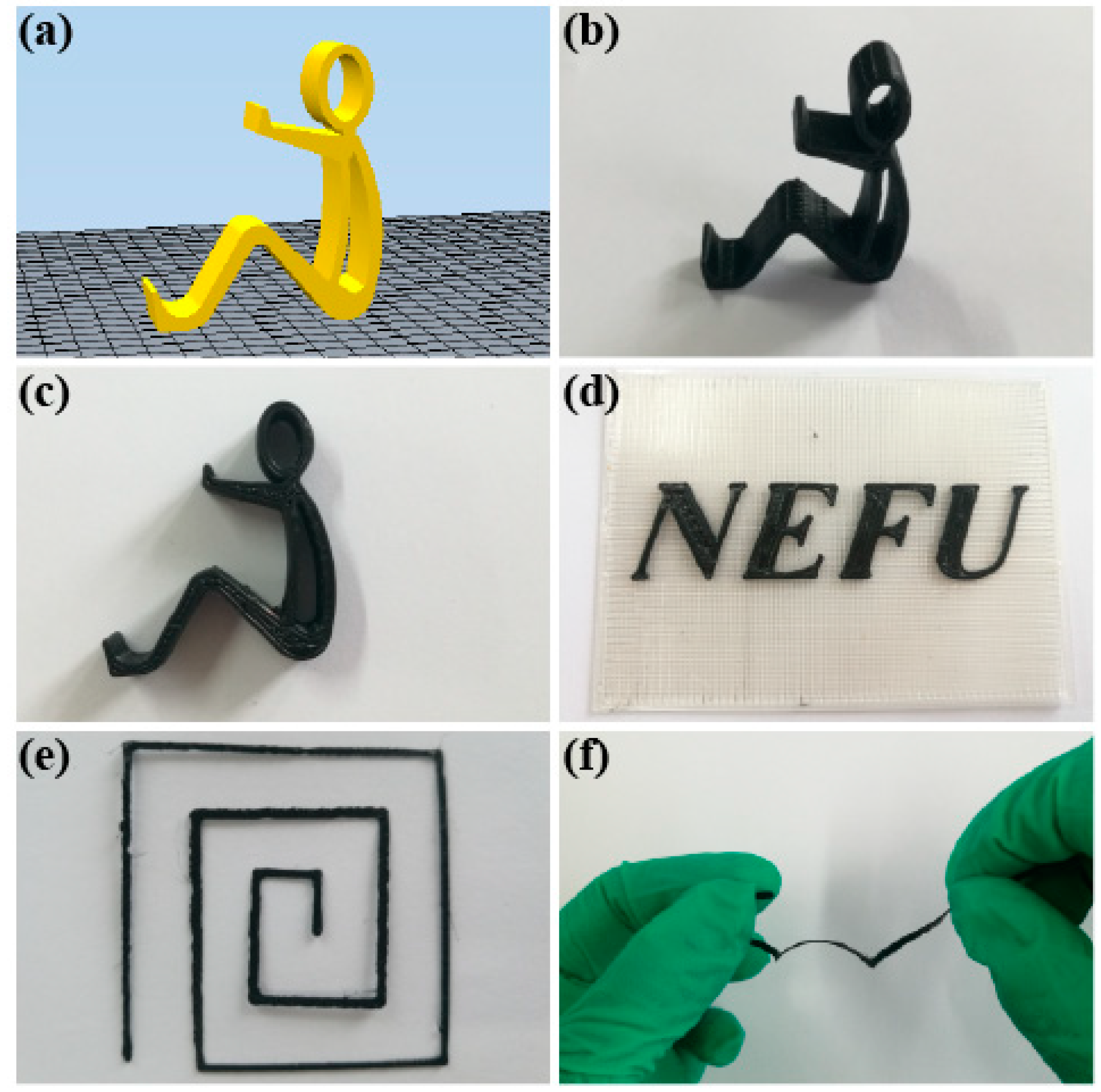
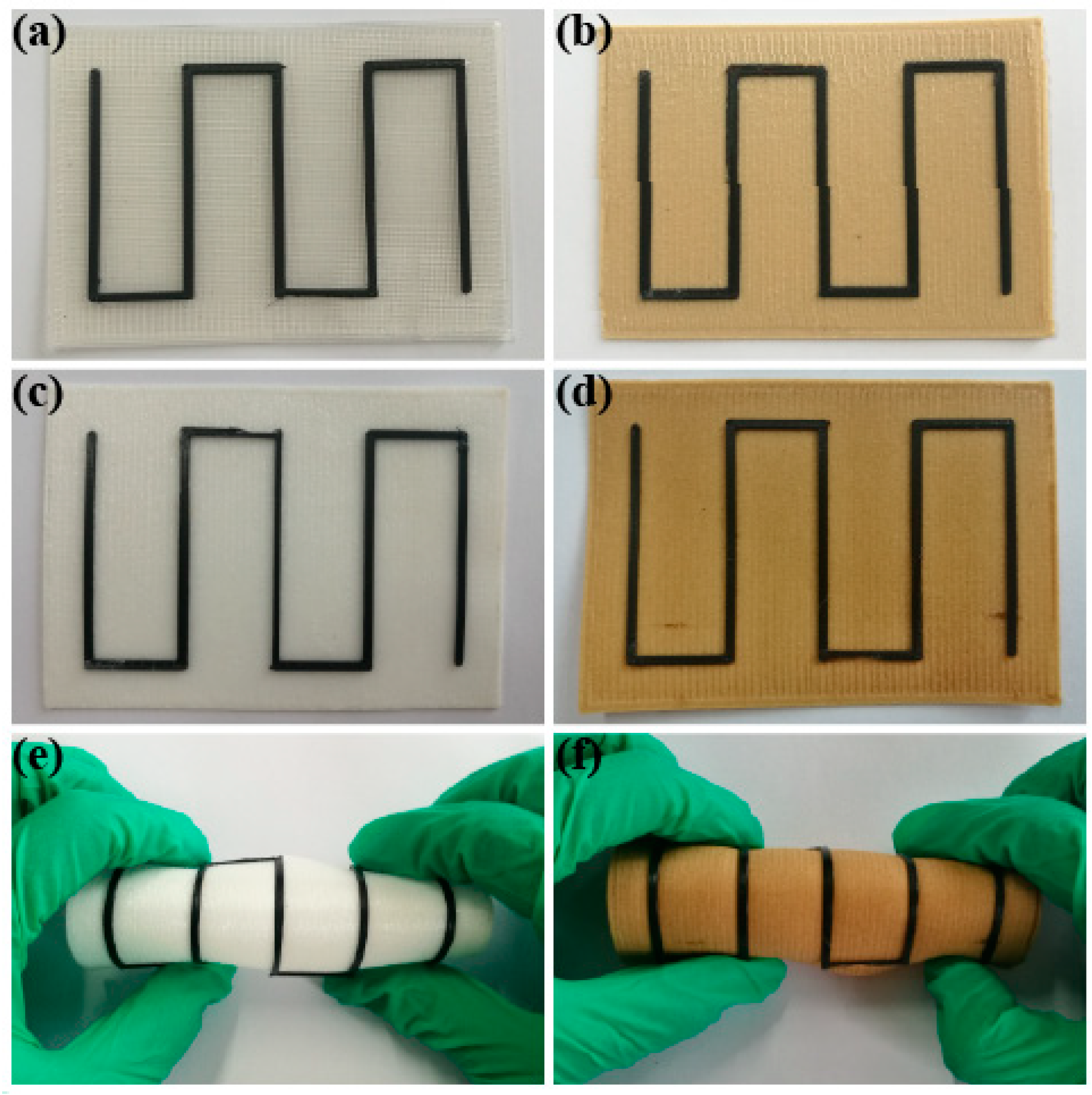
3.4. Models of PLA/rGO-SDBS Composites by FDM
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Thongruang, W.; Spontak, R.J.; Balik, C.M. Bridged double percolation in conductive polymer composites: An electrical conductivity, morphology and mechanical property study. Polymer 2002, 43, 3717–3725. [Google Scholar] [CrossRef]
- Tait, M.; Pegoretti, A.; Dorigato, A.; Kalaitzidou, K. The effect of filler type and content and the manufacturing process on the performance of multifunctional carbon/poly-lactide composites. Carbon 2011, 49, 4280–4290. [Google Scholar] [CrossRef]
- Teng, C.C.; Ma, C.C.M.; Lu, C.H.; Yang, S.Y.; Lee, S.H.; Hsiao, M.C.; Yen, M.Y.; Chiou, K.C.; Lee, T.M. Thermal conductivity and structure of non-covalent functionalized graphene/epoxy composites. Carbon 2011, 49, 5107–5116. [Google Scholar] [CrossRef]
- Coleman, J.N.; Khan, U.; Blau, W.J.; Gun’ko, Y.K. Small but strong: A review of the mechanical properties of carbon nanotube-polymer composites. Carbon 2006, 44, 1624–1652. [Google Scholar] [CrossRef]
- Lizundia, E.; Oleaga, A.; Salazar, A.; Sarasua, J.R. Nano-and microstructural effects on thermal properties of poly(l-lactide)/multi-wall carbon nanotube composites. Polymer 2012, 53, 2412–2421. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, L.S.; Zhang, J.W. Manipulating dispersion and distribution of graphene in PLA through novel interface engineering for improved conductive properties. ACS Appl. Mater. Interfaces 2014, 6, 14069–14075. [Google Scholar] [CrossRef]
- Mccullen, S.D.; Stano, K.L.; Stevens, D.R.; Roberts, W.A.; Monteiro-Riviere, N.A.; Clarke, L.I.; Gorga, R.E. Development, optimization, and characterization of electrospun poly(lactic acid) nanofibers containing multi-walled carbon nanotubes. J. Appl. Polym. Sci. 2010, 105, 1668–1678. [Google Scholar] [CrossRef]
- Villmow, T.; Pötschke, P.; Pegel, S.; Häussler, L.; Kretzschmar, B. Influence of twin-screw extrusion conditions on the dispersion of multi-walled carbon nanotubes in a poly(lactic acid) matrix. Polymer 2008, 49, 3500–3509. [Google Scholar] [CrossRef]
- Guo, R.; Ren, Z.; Bi, H.; Xu, M.; Cai, L. Electrical and thermal conductivity of polylactic acid (PLA)-based biocomposites by incorporation of nano-graphite fabricated with fused deposition modeling. Polymers 2019, 11, 549. [Google Scholar] [CrossRef]
- Wu, X.; Qi, S.; Jie, H.; Bo, C.; Duan, G. Synthesis of high conductivity polyaniline/Ag/graphite nanosheet composites via ultrasonic technique. J. Polym. Res. 2010, 17, 751–757. [Google Scholar] [CrossRef]
- Shi, S.L.; Zhang, L.Z.; Li, J.S. Electrical and dielectric properties of multiwall carbon nanotube/polyaniline composites. J. Polym. Res. 2009, 16, 395–399. [Google Scholar] [CrossRef]
- Xue, B.; Ye, J.; Zhang, J. Highly conductive Poly(L-lactic acid) composites obtained via in situ expansion of graphite. J. Polym. Res. 2015, 22, 112. [Google Scholar] [CrossRef]
- Vernardou, D.; Vasilopoulos, K.C.; Kenanakis, G. 3D printed graphene-based electrodes with high electrochemical performance. Appl. Phys. A 2017, 123, 623. [Google Scholar] [CrossRef]
- Lahiri, D.; Hec, F.; Thiesse, M.; Durygin, A.; Cheng, Z.; Agarwal, A. Nanotribological behavior of graphene nanoplatelet reinforced ultra high molecular weight polyethylene composites. Tribol. Int. 2014, 70, 165–169. [Google Scholar] [CrossRef]
- Tehrani, M.; Safdari, M.; Al-Haik, M.S. Nanocharacterization of creep behavior of multiwall carbon nanotubes/epoxy nanocomposite. Int. J. Plast. 2011, 27, 887–901. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, D.W.; Makotchenko, V.G.; Nazarov, A.S.; Fedorov, V.E.; Yoo, J.H.; Yu, S.M.; Choi, J.Y.; Kim, J.M.; Yoo, J.B. The superior dispersion of easily soluble graphite. Small 2010, 6, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Zirnstein, B.; Tabaka, W.; Frasca, D.; Schulze, D.; Schartel, B. Graphene/hydrogenated acrylonitrile-butadiene rubber nanocomposites: Dispersion, curing, mechanical reinforcement, multifunctional filler. Polym. Test. 2018, 66, 268–279. [Google Scholar] [CrossRef]
- Bustillos, J.; Montero, D.; Nautiyal, P.; Loganathan, A.; Boesl, B.; Agarwal, A. Integration of graphene in poly(lactic) acid by 3D printing to develop creep and wear-resistant hierarchical nanocomposites. Polym. Compos. 2018, 39, 3877–3888. [Google Scholar] [CrossRef]
- Daniel, F.; Patoary, N.H.; Moore, A.L.; Weiss, L.; Radadia, A.D. Temperature-dependent electrical resistance of conductive polylactic acid filament for fused deposition modeling. Int. J. Adv. Manuf. Technol. 2018, 99, 1215–1224. [Google Scholar] [CrossRef]
- Wei, X.; Li, D.; Jiang, W.; Gu, Z.; Wang, X.; Zhang, Z.; Sun, Z. 3D printable graphene composite. Sci. Rep. 2015, 5, 11181. [Google Scholar] [CrossRef]
- Kwok, S.W.; Goh, K.H.H.; Tan, Z.D.; Tan, S.T.M.; Tjiu, W.W.; Soh, J.Y.; Ng, Z.J.G.; Chan, Y.Z.; Hui, H.K.; Goh, K.E.J. Electrically conductive filament for 3D-printed circuits and sensors. Appl. Mater. Today 2017, 9, 167–175. [Google Scholar] [CrossRef]
- Shakouri, Z.; Nazockdast, H. Microstructural development and mechanical performance of PLA/TPU blends containing geometrically different cellulose nanocrystals. Cellulose 2018, 25, 7167–7188. [Google Scholar] [CrossRef]
- Manafi, P.; Ghasemi, I.; Karrabi, M.; Azizi, H.; Ehsaninamin, P. Effect of graphene nanoplatelets on crystallization kinetics of poly(lactic acid). Soft Mater. 2014, 12, 433–444. [Google Scholar] [CrossRef]
- Guo, R.; Ren, Z.C.; Bi, H.J.; Song, Y.M.; Xu, M. Effect of toughening agents on the properties of poplar wood flour/poly(lactic acid) composites fabricated with Fused Deposition Modeling. Eur. Polym. J. 2018, 107, 34–45. [Google Scholar] [CrossRef]
- Maurel, A.; Courty, M.; Fleutot, B.; Tortajada, H.; Prashantha, K.; Armand, M.; Grugeon, S.; Panier, S.; Dupont, L. Highly loaded graphite–polylactic acid composite-based filaments for lithium-ion battery three-dimensional printing. Chem. Mater. 2018, 30, 7484–7493. [Google Scholar] [CrossRef]
- Huang, H.D.; Ren, P.G.; Xu, J.Z.; Xu, L.; Zhong, G.J.; Hsiao, B.S.; Li, Z.M. Improved barrier properties of poly(lactic acid) with randomly dispersed graphene oxide nanosheets. J. Membr. Sci. 2014, 464, 110–118. [Google Scholar] [CrossRef]
- Pluta, M.; Murariu, M.; Alexandre, M.; Galeski, A.; Dubois, P. Polylactide compositions. The influence of ageing on the structure, thermal and viscoelastic properties of PLA/calcium sulfate composites. Polym. Degrad. Stab. 2008, 93, 925–931. [Google Scholar] [CrossRef]
- Chen, Q.; Mangadlao, J.D.; Wallat, J.; de Leon, A.; Pokorski, J.K.; Advincula, R.C. 3D printing biocompatible polyurethane/poly(lactic acid)/graphene oxide nanocomposites: Anisotropic properties. ACS Appl. Mater. Interfaces 2017, 9, 4015–4023. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wang, H.; Zuo, D.; Ji, A.; Liu, Y. Research on the application of MWCNTs/PLA composite material in the manufacturing of conductive composite products in 3D printing. Micromachines 2018, 9, 635. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, J.; Xu, G.; Wei, J.; Zhang, Z.; Li, X. Tuning the conductivity and inner structure of electrospun fibers to promote cardiomyocyte elongation and synchronous beating. Mater. Sci. Eng. C 2016, 69, 865–874. [Google Scholar] [CrossRef]
- Du, J.; Wang, Y.; Xie, X.; Xu, M.; Song, Y. Styrene-assisted maleic anhydride grafted poly(lactic acid) as an effective compatibilizer for wood flour/poly(lactic acid) bio-composites. Polymers 2017, 9, 623. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liu, Z.-Y.; Shan, G.-F.; Li, Z.-M.; Xie, B.-H.; Yang, M.-B. Study on the melt flow behavior of glass bead filled polypropylene. Polym. Test. 2005, 24, 490–497. [Google Scholar] [CrossRef]
- Pan, P.; Bo, Z.; Kai, W.; Dong, T.; Inoue, Y. Polymorphic transition in disordered poly(l-lactide) crystals induced by annealing at elevated temperatures. Macromolecules 2008, 41, 4296–4304. [Google Scholar] [CrossRef]
- Cheng, C.-C.; Liao, Z.-S.; Huang, J.-J.; Huang, S.-Y.; Fan, W.-L. Incorporation of supramolecular polymer-functionalized graphene: Towards the development of bio-based high electrically conductive polymeric nanocomposites. Compos. Sci. Technol. 2017, 148, 89–96. [Google Scholar] [CrossRef]
- Cao, Y.; Feng, J.; Wu, P. Preparation of organically dispersible graphene nanosheet powders through a lyophilization method and their poly(lactic acid) composites. Carbon 2010, 48, 3834–3839. [Google Scholar] [CrossRef]
- Wang, W.Y.; Jie, Z.; Jin, R.W.; Xin, Z.W.; Yu, H.D. Incorporation of graphitic nano-filler and poly(lactic acid) in fused deposition modeling. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Ramanathan, T.; Abdala, A.A.; Stankovich, S.; Dikin, D.A.; Herrera-Alonso, M.; Piner, R.D.; Adamson, D.H.; Schniepp, H.C.; Chen, X.; Ruoff, R.S. Functionalized graphene sheets for polymer nanocomposites. Nat. Nanotechnol. 2008, 3, 327–331. [Google Scholar] [CrossRef]
- Villar-Rodil, S.; Paredes, J.I.; Martínez-Alonso, A.; Tascón, J.M.D. Preparation of graphene dispersion and graphene–polymer composites in organic media. J. Mater. Chem. 2009, 19, 3591–3593. [Google Scholar] [CrossRef]
- Bao, C.; Lei, S.; Wilkie, C.A.; Yuan, B.; Gong, X. Graphite oxide, graphene, and metal-loaded graphene for fire safety applications of polystyrene. J. Mater. Chem. 2012, 22, 16399–16406. [Google Scholar] [CrossRef]
- Song, P.A.; Yu, Y.; Zhang, T.; Fu, S.; Fang, Z.; Wu, Q. Permeability, viscoelasticity, and flammability performances and their relationship to polymer nanocomposites. Ind. Eng. Chem. Res. 2012, 51, 7255–7263. [Google Scholar] [CrossRef]
- Xu, Y.; Hong, W.; Hua, B.; Li, C.; Shi, G. Strong and ductile poly(vinyl alcohol)/graphene oxide composite films with a layered structure. Carbon 2009, 47, 3538–3543. [Google Scholar] [CrossRef]
- Mano, J.F.; Wang, Y.; Viana, J.C.; Denchev, Z.; Oliveira, M.J. Cold crystallization of PLLA studied by simultaneous SAXS and WAXS. Macromol. Mater. Eng. 2010, 289, 910–915. [Google Scholar] [CrossRef]
- Fernández-Merino, M.J.; Paredes, J.I.; Villar-Rodil, S.; Guardia, L.; Solís-Fernández, P.; Salinas-Torres, D.; Cazorla-Amorós, D.; Morallón, E.; Martínez-Alonso, A.; Tascón, J.M.D. Investigating the influence of surfactants on the stabilization of aqueous reduced graphene oxide dispersions and the characteristics of their composite films. Carbon 2012, 50, 3184–3194. [Google Scholar] [CrossRef]
- Yasmin, A.; Luo, J.J.; Daniel, I.M. Processing of expanded graphite reinforced polymer nanocomposites. Compos. Sci. Technol. 2006, 66, 1182–1189. [Google Scholar] [CrossRef]
- Sullivan, E.M.; Gerhardt, R.A.; Wang, B.; Kalaitzidou, K. Effect of compounding method and processing conditions on the electrical response of exfoliated graphite nanoplatelet/polylactic acid nanocomposite films. J. Mater. Sci. 2015, 51, 2980–2990. [Google Scholar] [CrossRef]
- Xu, Z.; Niu, Y.; Yang, L.; Xie, W.; Li, H.; Gan, Z.; Wang, Z. Morphology, rheology and crystallization behavior of polylactide composites prepared through addition of five-armed star polylactide grafted multiwalled carbon nanotubes. Polymer 2010, 51, 730–737. [Google Scholar] [CrossRef]
- Bi, H.; Xu, M.; Ye, G.; Guo, R.; Cai, L.; Ren, Z. Mechanical, thermal, and shape memory properties of three-dimensional printing biomass composites. Polymers 2018, 10, 1234. [Google Scholar] [CrossRef] [PubMed]



| Nozzle Size | Layer Thickness | Nozzle Temperature | Print-Bed Temperature | Infill Density | Infill Pattern |
|---|---|---|---|---|---|
| 0.8 mm | 0.4 mm | 210 °C | 40 °C | 100% | Linear |
| Sample Codes | Te (N·m) | ΔT (°C) |
|---|---|---|
| rGO-0 | 0.76 | 7.29 |
| rGO-6 | 1.16 | 7.99 |
| rGO-9 | 1.44 | 8.67 |
| rGO-12 | 1.83 | 9.21 |
| Sample Codes | Tg1 (°C) | Tm2 (°C) | Tcc3 (°C) | Xc4 (%) | Tonset5 (°C) | Tmax6 (°C) | Residue at 210 °C (wt %) | Residue at 600 °C (wt %) | |
|---|---|---|---|---|---|---|---|---|---|
| TPLA | TTPU | ||||||||
| rGO-0 | 60.35 | 167.11 | 103.35 | 49.80 | 278.49 | 301.97 | 344.63 | 99.69 | 2.08 |
| rGO-6 | 60.05 | 167.21 | 102.22 | 44.48 | 274.73 | 299.67 | 365.95 | 99.39 | 7.46 |
| rGO-9 | 60.04 | 166.79 | 100.62 | 45.81 | 281.25 | 302.52 | 363.00 | 99.20 | 8.44 |
| rGO-12 | 59.72 | 166.82 | 100.75 | 47.77 | 289.52 | 310.06 | 360.53 | 99.49 | 10.17 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, R.; Ren, Z.; Jia, X.; Bi, H.; Yang, H.; Ji, T.; Xu, M.; Cai, L. Preparation and Characterization of 3D Printed PLA-Based Conductive Composites Using Carbonaceous Fillers by Masterbatch Melting Method. Polymers 2019, 11, 1589. https://doi.org/10.3390/polym11101589
Guo R, Ren Z, Jia X, Bi H, Yang H, Ji T, Xu M, Cai L. Preparation and Characterization of 3D Printed PLA-Based Conductive Composites Using Carbonaceous Fillers by Masterbatch Melting Method. Polymers. 2019; 11(10):1589. https://doi.org/10.3390/polym11101589
Chicago/Turabian StyleGuo, Rui, Zechun Ren, Xin Jia, Hongjie Bi, Haiying Yang, Tong Ji, Min Xu, and Liping Cai. 2019. "Preparation and Characterization of 3D Printed PLA-Based Conductive Composites Using Carbonaceous Fillers by Masterbatch Melting Method" Polymers 11, no. 10: 1589. https://doi.org/10.3390/polym11101589
APA StyleGuo, R., Ren, Z., Jia, X., Bi, H., Yang, H., Ji, T., Xu, M., & Cai, L. (2019). Preparation and Characterization of 3D Printed PLA-Based Conductive Composites Using Carbonaceous Fillers by Masterbatch Melting Method. Polymers, 11(10), 1589. https://doi.org/10.3390/polym11101589






