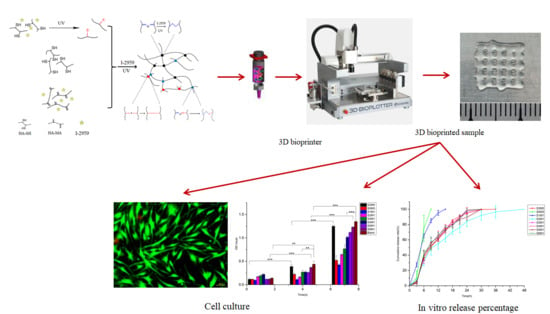
3D Bioprinting of the Sustained Drug Release Wound Dressing with Double-Crosslinked Hyaluronic-Acid-Based Hydrogels
Abstract
1. Introduction
2. Experimental
2.1. Materials
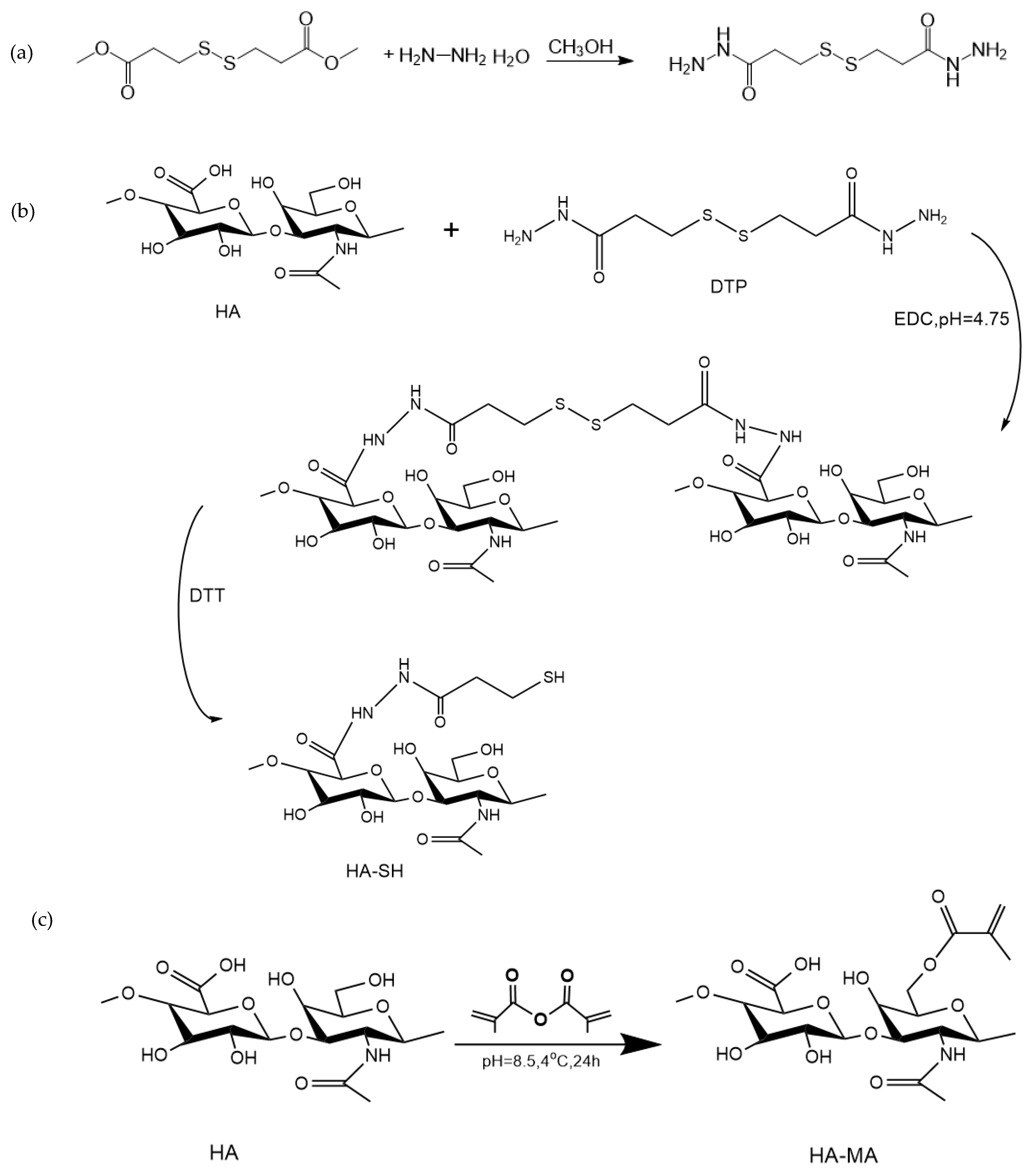
2.2. Synthesis of HA-SH
2.2.1. Preparation of 3,3′-Dithiobis(Propanoic Hydrazide) (DTP)
2.2.2. Synthesis of HA-SH
2.3. Synthesis of HA-MA
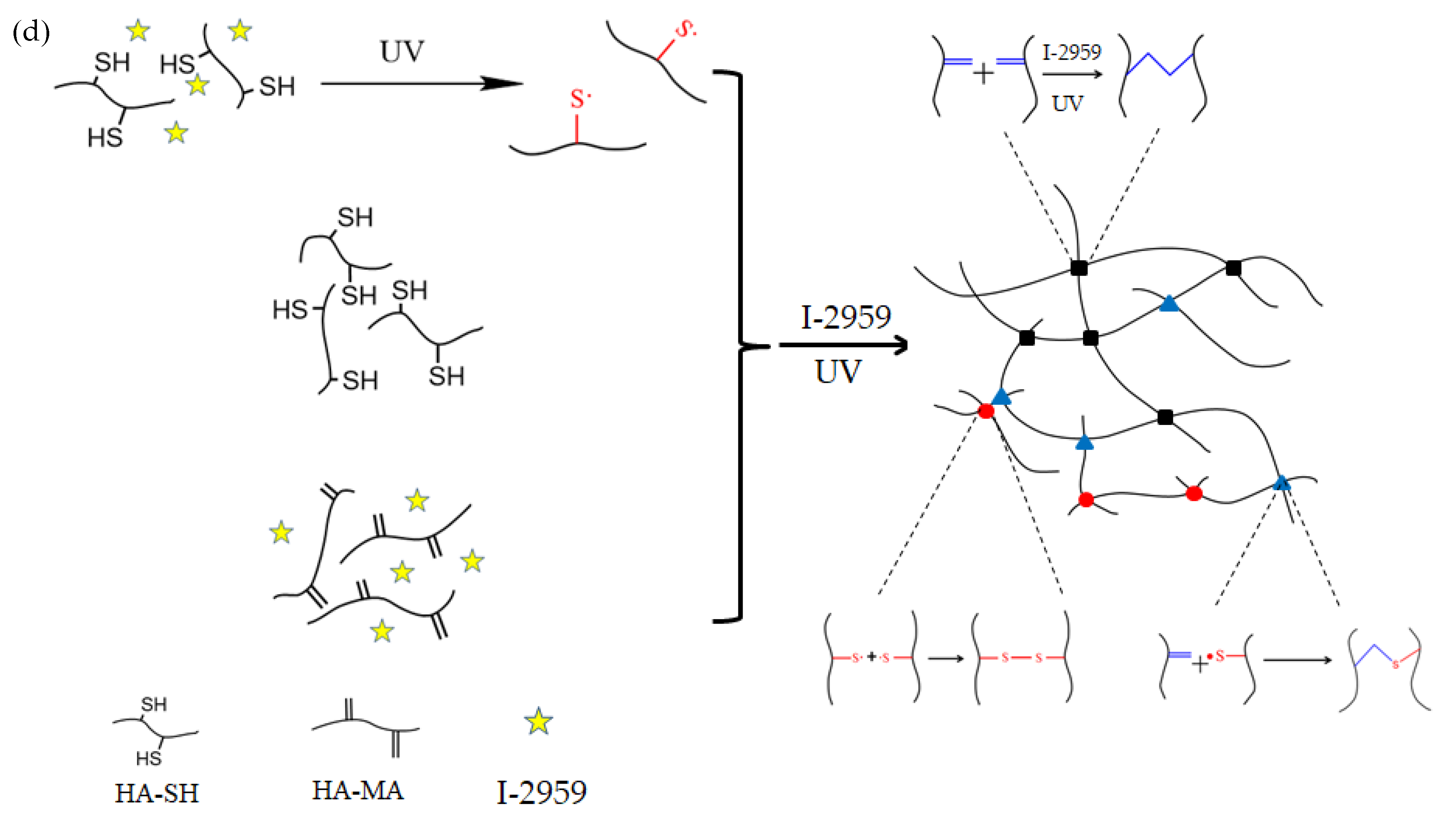
2.4. Preparation of the Double-Crosslinked HA-SH/HA-MA Hydrogels
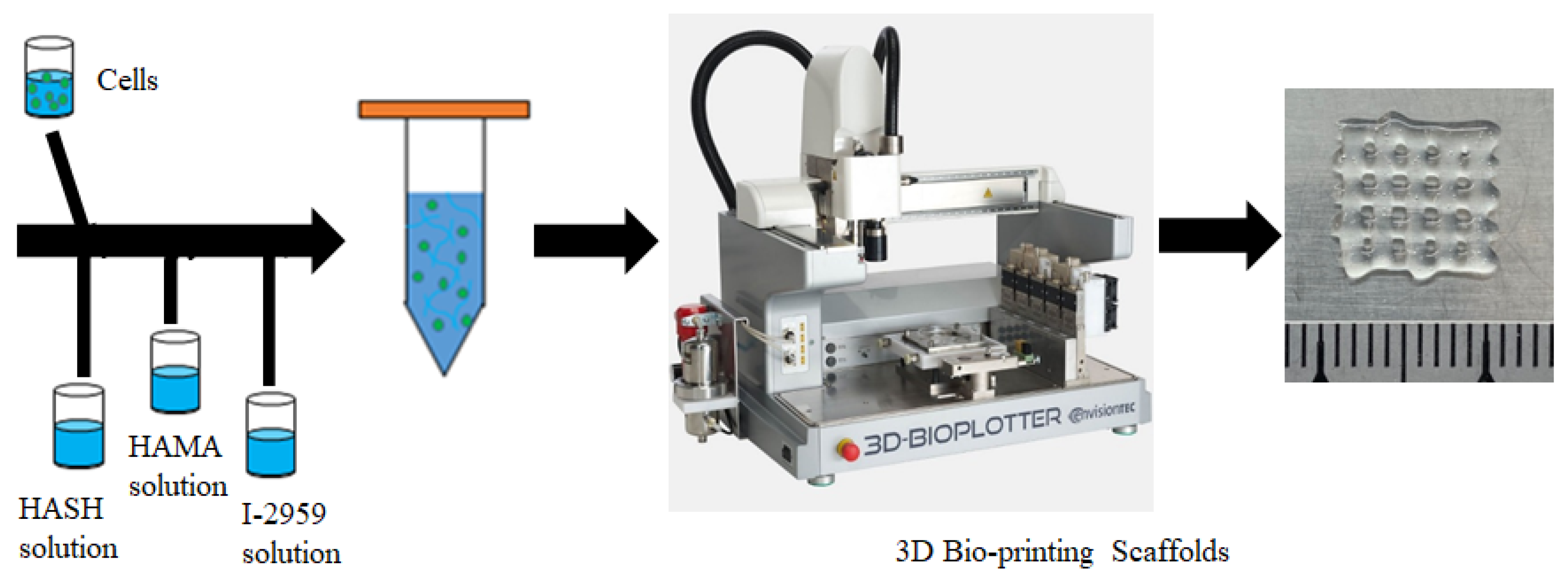
2.5. Preparation of 3D Bioink and 3D Extrusion Bioprinting Process
2.5.1. Preparation of the Bioink Encapsulating Cells
2.5.2. 3D Bioprinting Process
2.6. Characterization of the Hydrogel
2.7. Morphology of the Hydrogel
2.8. Swelling Test
2.9. In Vitro Degradation Test
2.10. Cytotoxicity Assays
2.10.1. Preparation of Cells
2.10.2. Preparation of Medium
2.10.3. CCK-8 Assay
2.10.4. Live/Dead Assay
2.11. In Vitro Drug Release Test
3. Results and Discussion
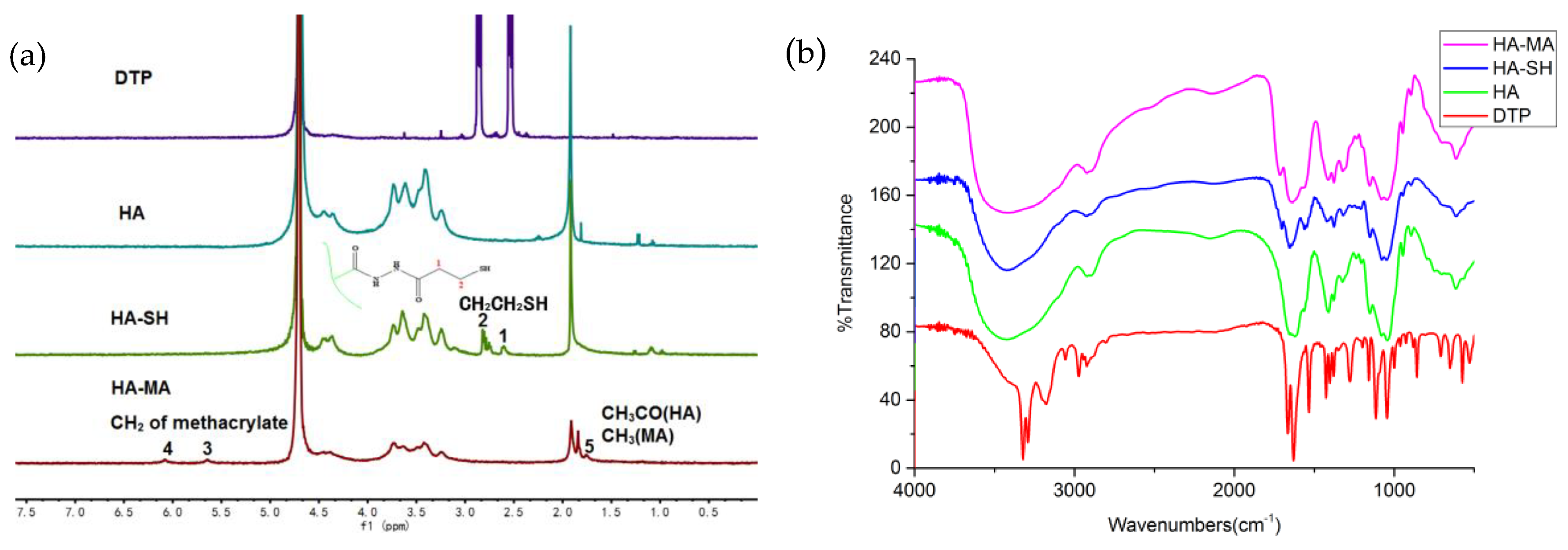
3.1. Synthesis of HA-SH and HA-MA
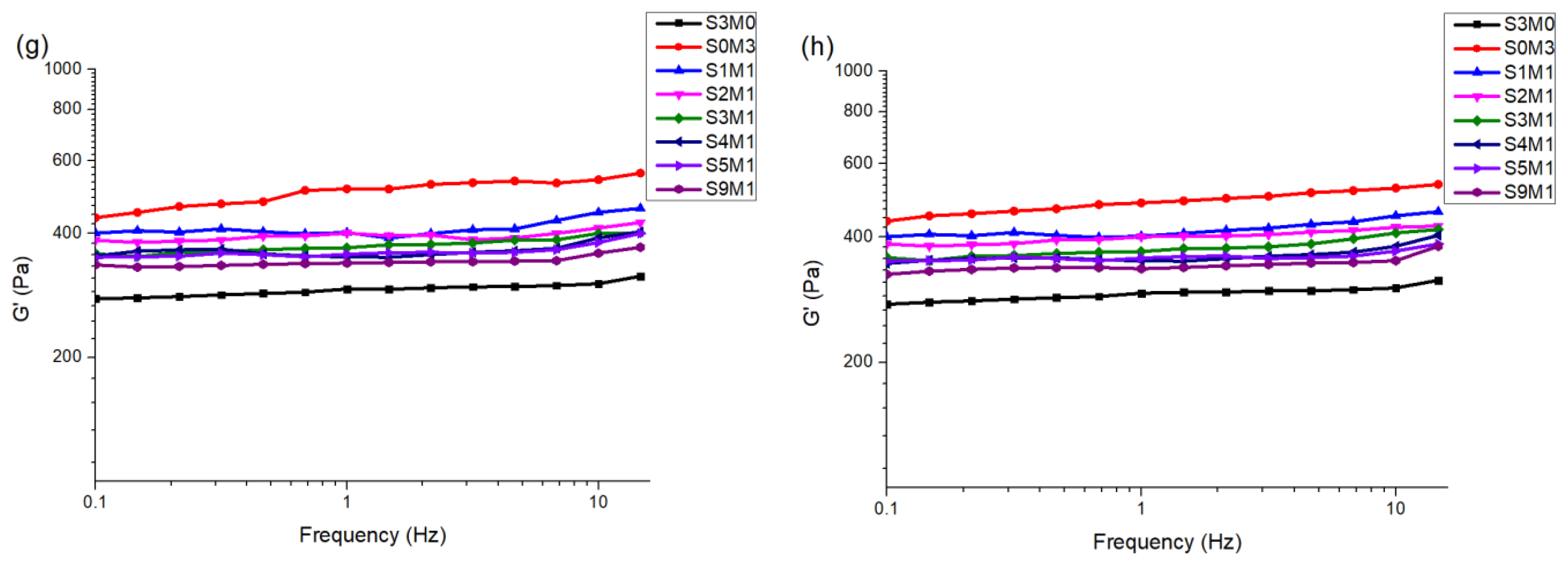
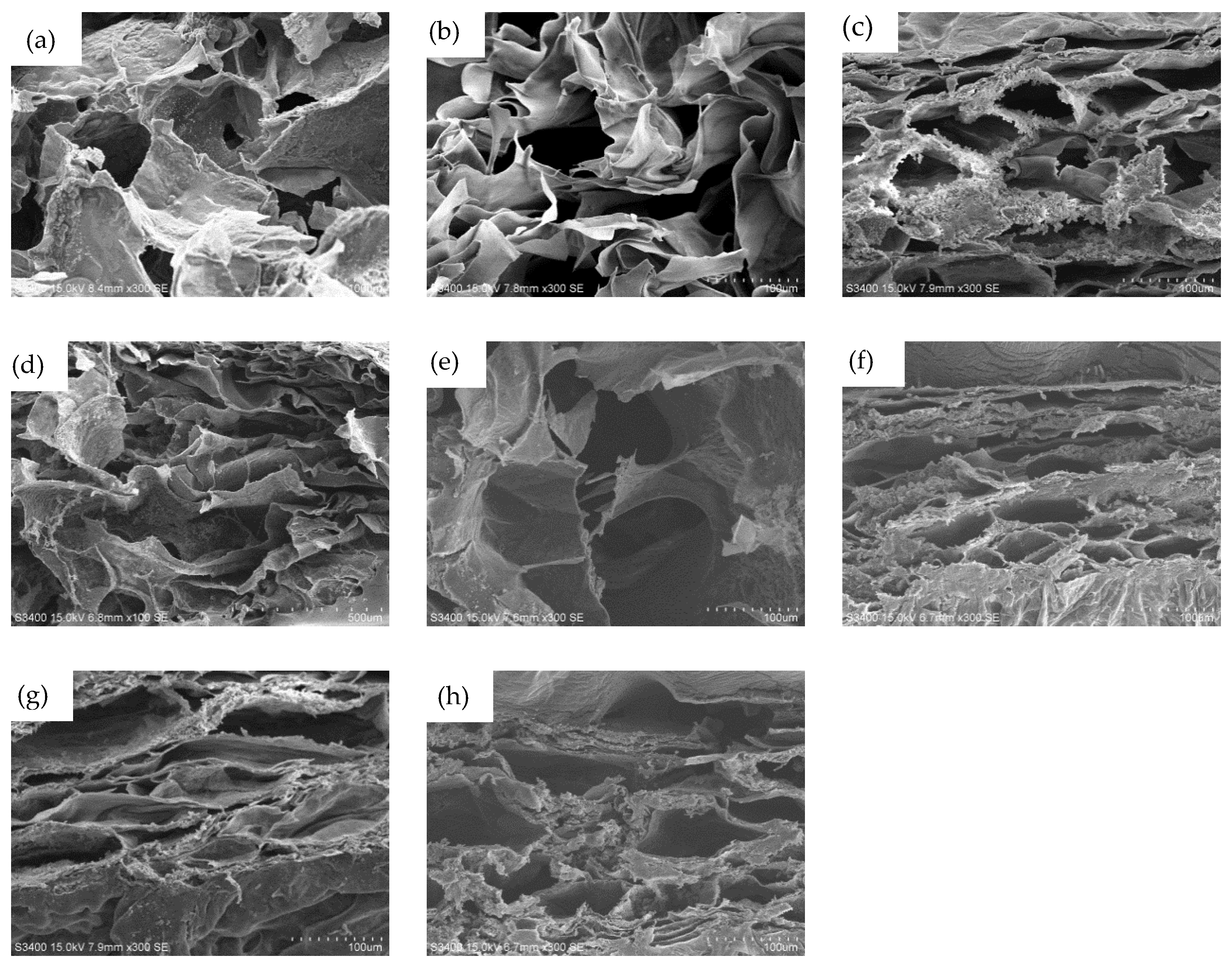
3.2. The Properties of the Hydrogels
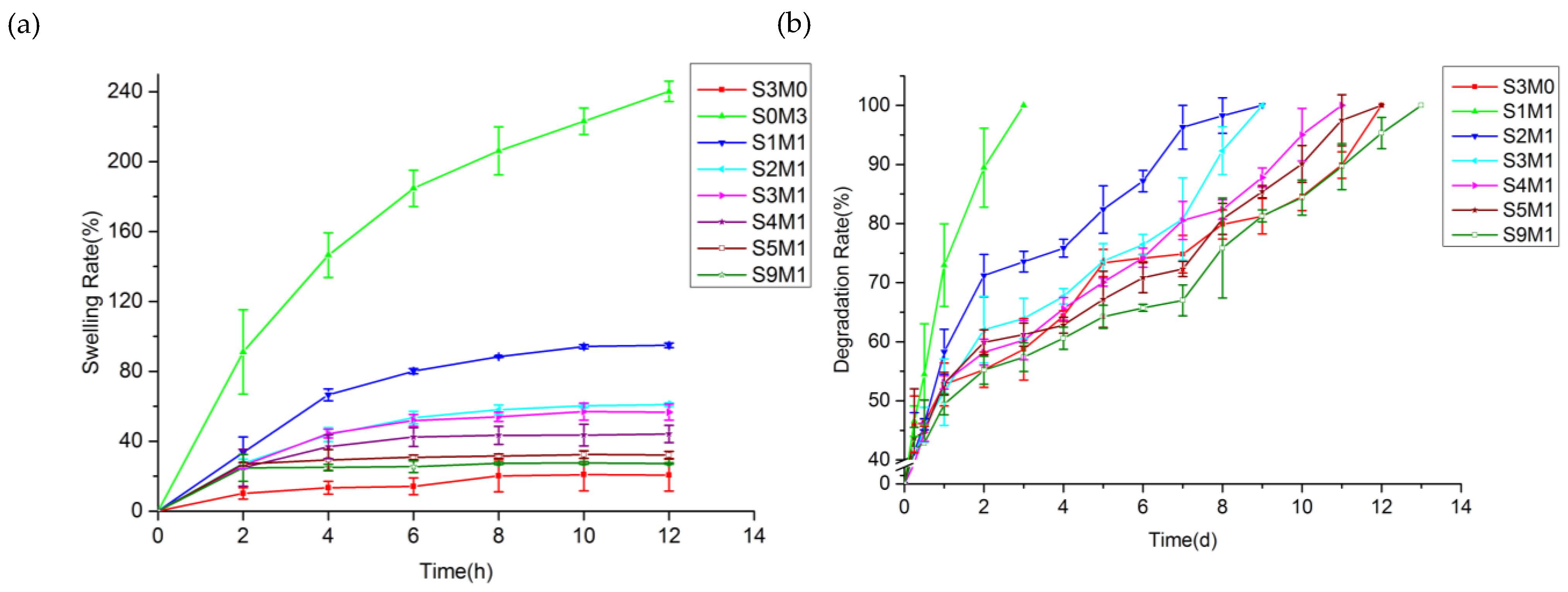
3.3. The Swelling Properties of the Hydrogel
3.4. In Vitro Degradation
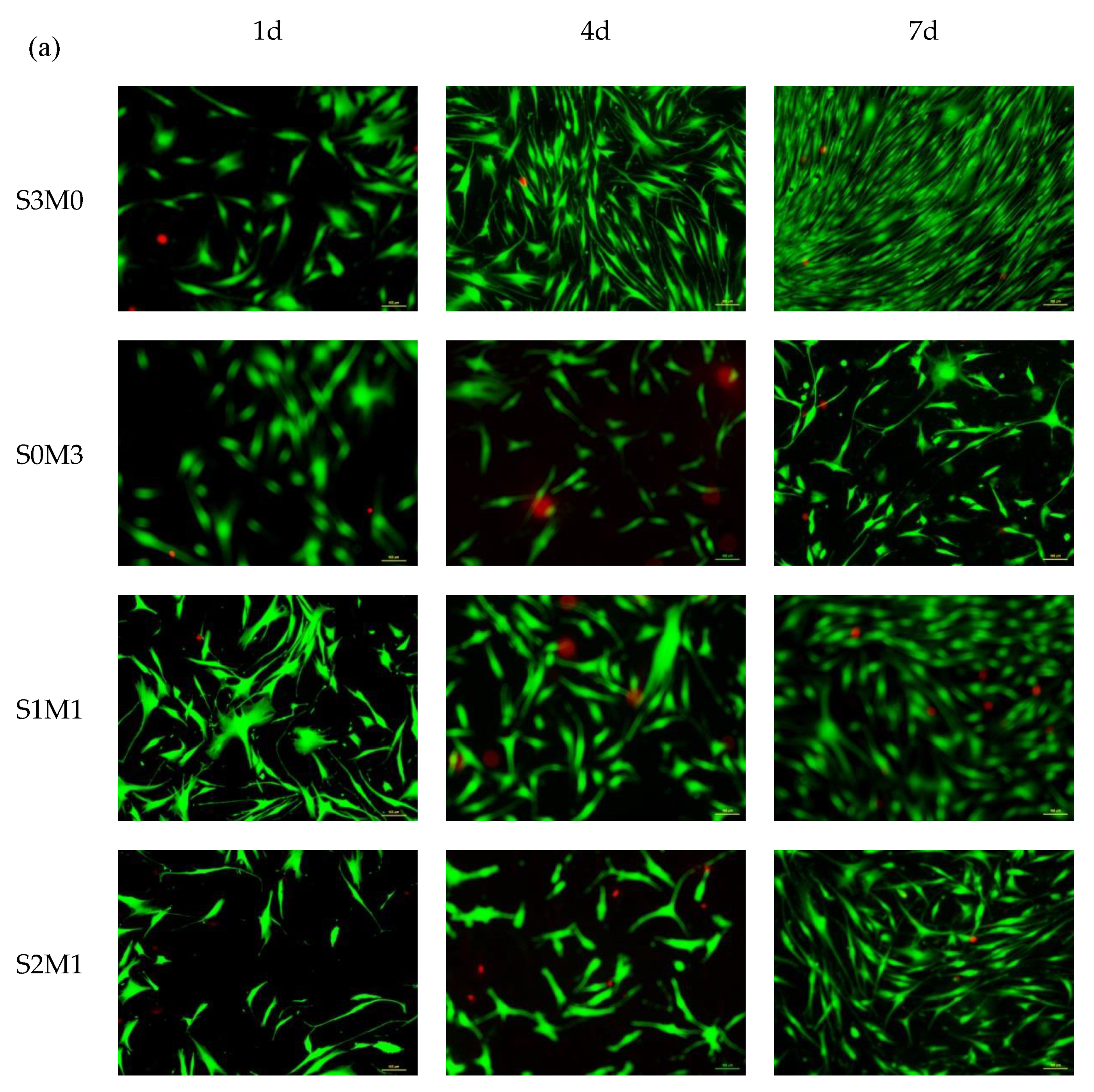
3.5. Cytocompatibility
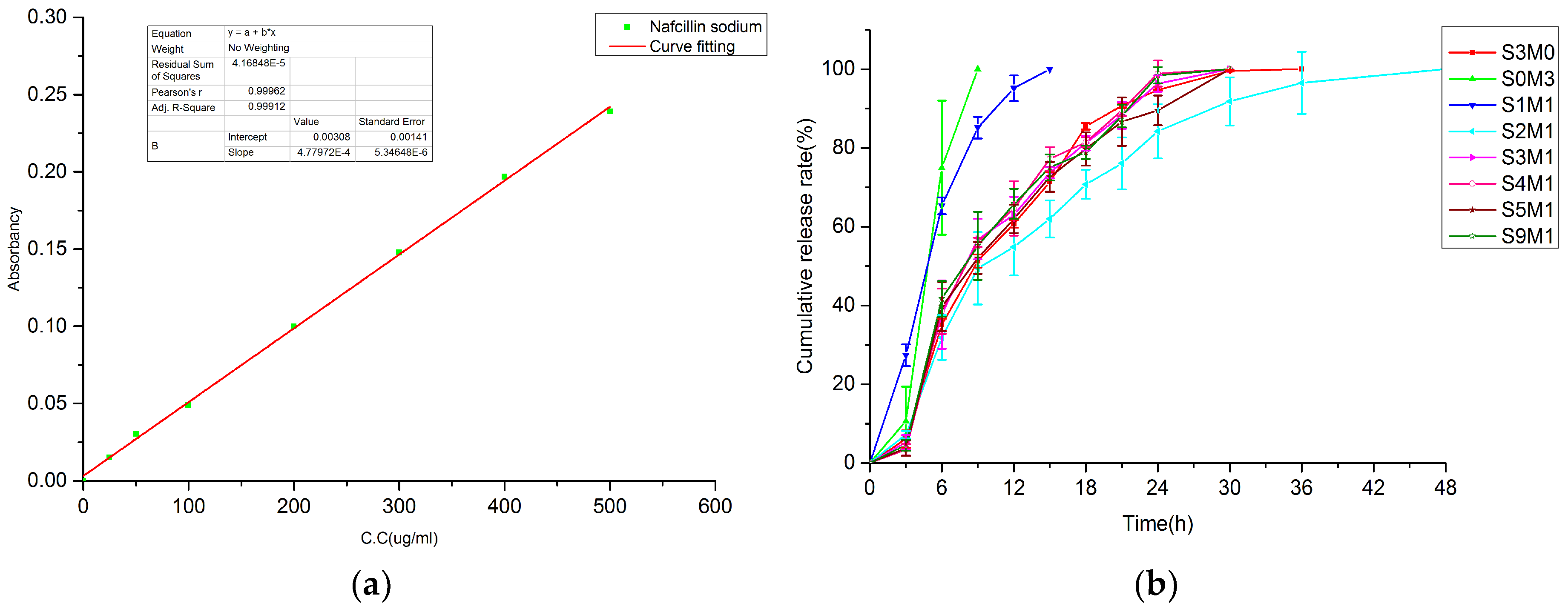
3.6. In Vitro Drug Release
3.7. 3D Bioprinting of HA-MA/HA-SH
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel Wound Dressings for Bioactive Treatment of Acute and Chronic Wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Beldon, P. Basic science of wound healing. Surgery (Oxford) 2010, 28, 409–412. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Kirsner, R. Pathophysiology of acute wound healing. Clin. Dermatol. 2007, 25, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Monaco, J.L.; Lawrence, W.T. Acute wound healing: An overview. Clin. Plast. Surg. 2003, 30, 1–12. [Google Scholar] [CrossRef]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 2007, 341, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Rodero, M.P.; Khosrotehrani, K. Skin wound healing modulation by macrophages. Int. J. Clin. Exp. Pathol. 2010, 3, 643–653. [Google Scholar] [PubMed]
- Yin, R.; Wang, K.; Du, S.; Chen, L.; Nie, J.; Zhang, W. Design of genipin-crosslinked microgels from concanavalin A and glucosyloxyethyl acrylated chitosan for glucose-responsive insulin delivery. Carbohydr. Polym. 2014, 103, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, M.; Kumar, P.; Choonara, Y.E.; Du Toit, L.C.; Pillay, V. Artificial, Triple-Layered, Nanomembranous Wound Patch for Potential Diabetic Foot Ulcer Intervention. Materials 2018, 11, 2128. [Google Scholar] [CrossRef]
- Tsirogianni, A.K.; Moutsopoulos, N.M.; Moutsopoulos, H.M. Wound healing: Immunological aspects. Injury-Int. J. Care Inj. 2006, 37, S5–S12. [Google Scholar] [CrossRef]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef]
- Jia, Y.H.; Zhang, H.B.; Yang, S.B.; Xi, Z.H.; Tang, T.T.; Yin, R.X.; Zhang, W.J. Electrospun PLGA membrane incorporated with andrographolide-loaded mesoporous silica nanoparticles for sustained antibacterial wound dressing. Nanomedicine 2018, 13, 2881–2899. [Google Scholar] [CrossRef] [PubMed]
- Zecheru, T.; Rotariu, T.; Rusen, E.; Marculescu, B.; Miculescu, F.; Alexandrescu, L.; Antoniac, I.; Stancu, I.-C. Poly (2-hydroxyethyl methacrylate-co-dodecyl methacrylate-co-acrylic acid): Synthesis, physico-chemical characterisation and Nafcillin carrier. J. Mater. Sci. Mater. Med. 2010, 21, 2793–2804. [Google Scholar] [CrossRef] [PubMed]
- Seol, Y.J.; Lee, H.; Copus, J.S.; Kang, H.W.; Cho, D.W.; Atala, A.; Lee, S.J.; Yoo, J.J. 3D bioprinted biomask for facial skin reconstruction. Bioprinting 2018, 10, e00028. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R. Skin bioprinting: A novel approach for creating artificial skin from synthetic and natural building blocks. Prog. Biomater. 2018, 7, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.L.; Qi, J.T.Z.; Yeong, W.Y.; Naing, M.W. Proof-of-concept: 3D bioprinting of pigmented human skin constructs. Biofabrication 2018, 10, 025005. [Google Scholar] [CrossRef] [PubMed]
- Pourchet, L.J.; Thepot, A.; Albouy, M.; Courtial, E.J.; Boher, A.; Blum, L.J.; Marquette, C.A. Human Skin 3D Bioprinting Using Scaffold-Free Approach. Adv. Healthc. Mater. 2017, 6, 1601101. [Google Scholar] [CrossRef]
- Lee, V.; Singh, G.; Trasatti, J.P.; Bjornsson, C.; Xu, X.; Tran, T.N.; Yoo, S.S.; Dai, G.; Karande, P. Design and Fabrication of Human Skin by Three-Dimensional Bioprinting. Tissue Eng. Part C 2013, 20, 473–484. [Google Scholar] [CrossRef]
- Kang, H.-W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef]
- Shi, Y.; Xing, T.L.; Yin, R.X.; Yang, S.M.; Wei, J.; Zhang, W.J.; Zhang, H.B.; Yong, S.; TianLong, X.; Ruixue, Y.; et al. Tyrosinase doped bioink for 3D bioprinting of living skin constructs. Biomed. Mater. 2018, 13, 035008. [Google Scholar] [CrossRef]
- Murphy, S.V.; Skardal, A.; Atala, A. Evaluation of hydrogels for bio-printing applications. J. Biomed. Mater. Res. Part A 2013, 101, 272–284. [Google Scholar] [CrossRef]
- Skardal, A.; Atala, A. Biomaterials for Integration with 3-D Bioprinting. Ann. Biomed. Eng. 2014, 43, 730–746. [Google Scholar] [CrossRef] [PubMed]
- Demirtaş, T.T.; Irmak, G.; Gümüşderelioğlu, M. A bioprintable form of chitosan hydrogel for bone tissue engineering. Biofabrication 2017, 9, 035003. [Google Scholar] [CrossRef] [PubMed]
- Compaan, A.M.; Song, K.; Huang, Y. Gellan Fluid Gel as a Versatile Support Bath Material for Fluid Extrusion Bioprinting. ACS Appl. Mater. Interfaces 2019, 11, 5714–5726. [Google Scholar] [CrossRef] [PubMed]
- Maver, T.; Smrke, D.M.; Kurečič, M.; Gradišnik, L.; Maver, U.; Kleinschek, K.S. Combining 3D printing and electrospinning for preparation of pain-relieving wound-dressing materials. J. Sol-Gel Sci. Technol. 2018, 88, 33–48. [Google Scholar] [CrossRef]
- Fraser, J. Hyaluronan: Its nature, distribution functions and turnover. J. Intern. Med. 1997, 242, 17–33. [Google Scholar] [CrossRef]
- Da Silva, L.P.; Pirraco, R.P.; Santos, T.C.; Novoa-Carballal, R.; Cerqueira, M.T.; Reis, R.L.; Correlo, V.M.; Marques, A.P. Neovascularization Induced by the Hyaluronic Acid-Based Spongy-Like Hydrogels Degradation Products. ACS Appl. Mater. Interfaces 2016, 8, 33464–33474. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic Acid Hydrogels for Biomedical Applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-Network Hydrogels with Extremely High Mechanical Strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Haque, M.A.; Kurokawa, T.; Gong, J.P. Super tough double network hydrogels and their application as biomaterials. Polymer 2012, 53, 1805–1822. [Google Scholar] [CrossRef]
- Li, G.; Zhang, H.; Fortin, D.; Xia, H.; Zhao, Y. Poly (vinyl alcohol)-Poly (ethylene glycol) Double-Network Hydrogel: A General Approach to Shape Memory and Self-healing Functionalities. Langmuir 2015, 31, 11709–11716. [Google Scholar] [CrossRef]
- Tong, X.; Yang, F. Engineering interpenetrating network hydrogels as biomimetic cell niche with independently tunable biochemical and mechanical properties. Biomaterials 2014, 35, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Law, N.; Doney, B.; Glover, H.; Qin, Y.; Aman, Z.M.; Sercombe, T.B.; Liew, L.J.; Dilley, R.J.; Doyle, B.J. Characterisation of Hyaluronic Acid Methylcellulose Hydrogels for 3D Bioprinting. J. Mech. Behav. Biomed. Mater. 2018, 77, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Stichler, S.; Böck, T.; Paxton, N.; Bertlein, S.; Levato, R.; Schill, V.; Smolan, W.; Malda, J.; Teßmar, J.; Blunk, T.; et al. Double printing of hyaluronic acid/poly (glycidol) hybrid hydrogels with poly (ε-caprolactone) for MSC chondrogenesis. Biofabrication 2017, 9, 044108. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, A.; Schreyer, D.J.; Chen, D.X. Use of the polycation polyethyleneimine to improve the physical properties of alginate–hyaluronic acid hydrogel during fabrication of tissue repair scaffolds. J. Biomater. Sci. Polym. Ed. 2015, 26, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Aljohani, W.; Ullah, M.W.; Zhang, X.; Yang, G. Bioprinting and its Applications in Tissue Engineering and Regenerative Medicine. Int. J. Biol. Macromol. 2017, 107, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi-Aghkand, F.; Gholizadeh-Ghaleh Aziz, S.; Panahi, Y.; Daraee, H.; Gorjikhah, F.; Gholizadeh-Ghaleh Aziz, S.; Hsanzadeh, A.; Akbarzadeh, A. Recent prospective of nanofiber scaffolds fabrication approaches for skin regeneration. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1635–1641. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Vercruysse, K.P.; Marecak, D.M.; Marecek, J.F.; Prestwich, G.D. Synthesis and in Vitro Degradation of New Polyvalent Hydrazide Cross-Linked Hydrogels of Hyaluronic Acid. Bioconjug. Chem. 1997, 8, 686–694. [Google Scholar] [CrossRef]
- Lovell, P.A.; El-Aasser, M.S. Emulsion Polymerization and Emulsion Polymers; John Wiley & Sons, Ltd.: New York, NY, USA, 1997. [Google Scholar]
- Landfester, K. Miniemulsions for Nanoparticle Synthesis. Top. Curr. Chem. 2003, 22, 75–123. [Google Scholar]
- Andrew, B.L. Thiol-ene “click” reactions and recent applications in polymer and materials synthesis. Polym. Chem. 2014, 5, 4820–4870. [Google Scholar]
- Katime, I.; Sáez, V.; Hernáez, E. Nafcillin release from poly (acrylic acid–co–methyl methacrylate) hydrogels. Polym. Bull. 2005, 55, 403–409. [Google Scholar] [CrossRef]
- Shu, X.Z.; Liu, Y.; Luo, Y.; Roberts, M.C.; Prestwich, G.D. Disulfide cross-linked hyaluronan hydrogels. Biomacromolecules 2002, 3, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhu, H.; Wang, J.; Fang, Q.; Peng, Z. Enzymatically Disulfide Crosslinked Chitosan/Hyaluronic Acid Layer-by-Layer Self-Assembled Microcapsules for Redox-Responsive Controlled Release of Protein. ACS Appl. Mater. Interfaces 2018, 10, 33493–33506. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Yang, J.; Lu, J.; Xiao, Y.; Fan, Y.; Zhang, X. Preparation and characterization of a novel hyaluronic acid–icariin conjugate hydrogel. Mater. Lett. 2014, 136, 41–44. [Google Scholar] [CrossRef]
- Yu, S.; Duan, Y.; Zuo, X.; Chen, X.; Mao, Z.; Gao, C. Mediating the invasion of smooth muscle cells into a cell-responsive hydrogel under the existence of immune cells. Biomaterials 2018, 180, 193–205. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Sui, J.; Chen, Y.; Bian, S.; Cui, Y.; Zhou, C.; Sun, Y.; Liang, J.; Fan, Y.; Zhang, X. Localized Multidrug Co-delivery by Injectable Self-crosslinking Hydrogel for Synergistic Combinational Chemotherapy. J. Mater. Chem. B 2017, 5, 4852–4862. [Google Scholar] [CrossRef]
- Huang, S.; Wang, C.; Xu, J.; Ma, L.; Gao, C. In situ assembly of fibrinogen/hyaluronic acid hydrogel via knob-hole interaction for 3D cellular engineering. Bioact. Mater. 2017, 2, 253–259. [Google Scholar] [CrossRef]
- Watanabe, I.; Hoshi, H.; Sato, M.; Suzuki, K. Rheological and Adhesive Properties to Identify Cohesive and Dispersive Ophthalmic Viscosurgical Devices. Chem. Pharm. Bull. 2019, 67, 277–283. [Google Scholar] [CrossRef]
- Mousavi Nejad, Z.; Torabinejad, B.; Davachi, S.M.; Zamanian, A.; Saeedi Garakani, S.; Najafi, F.; Nezafati, N. Synthesis, physicochemical, rheological and in-vitro characterization of double-crosslinked hyaluronic acid hydrogels containing dexamethasone and PLGA/dexamethasone nanoparticles as hybrid systems for specific medical applications. Int. J. Biol. Macromol. 2019, 126, 193–208. [Google Scholar] [CrossRef]
- Liu, Y.; Shu, X.Z.; Prestwich, G.D. Biocompatibility and stability of disulfide-crosslinked hyaluronan films. Biomaterials 2005, 26, 4737–4746. [Google Scholar] [CrossRef]
- Li, B.; Wang, L.; Xu, F.; Gang, X.; Demirci, U.; Wei, D.; Li, Y.; Feng, Y.; Jia, D.; Zhou, Y. Hydrosoluble, UV-crosslinkable and injectable chitosan for patterned cell-laden microgel and rapid transdermal curing hydrogel in vivo. Acta Biomater. 2015, 22, 59–69. [Google Scholar] [CrossRef]
- West, D.; Hampson, I.; Arnold, F.; Kumar, S. Angiogenesis induced by degradation products of hyaluronic acid. Science 1985, 228, 1324–1326. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.G.; Malik, A.N.; Kim, T.K.; Manson, P.N.; Elisseeff, J.H. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials 2005, 26, 1211–1218. [Google Scholar] [CrossRef]
- Bryant, S.J.; Nuttelman, C.R.; Anseth, K.S. Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J. Biomater. Sci. Polym. Ed. 2000, 11, 439–457. [Google Scholar] [CrossRef] [PubMed]
- Broguiere, N.; Isenmann, L.; Zenobi-Wong, M. Novel enzymatically cross-linked hyaluronan hydrogels support the formation of 3D neuronal networks. Biomaterials 2016, 99, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; van Luttervelt, C.A. Towards a Resilient Manufacturing System. Ann. CIRP 2011, 60, 469–472. [Google Scholar] [CrossRef]
- Zhang, W.J.; Lin, Y. Principles of Design of Resilient Systems and its Application to Enterprise Information Systems. Enterp. Inf. Syst. 2010, 4, 99–110. [Google Scholar] [CrossRef]
- Zhang, H.B.; Zhou, L.; Zhang, W.J. Control of Scaffold Degradation in Tissue Engineering: A Review. Tissue Eng. Part B 2014, 10, 492–502. [Google Scholar] [CrossRef]



| Sample No. | HA-SH Conc. (w/v %) | HA-MA Conc. (w/v %) | Sol Conc. (w/v %) |
|---|---|---|---|
| S3M0 | 3 | 0 | 3 |
| S0M3 | 0 | 3 | 3 |
| S1M1 | 1.5 | 1.5 | 3 |
| S2M1 | 2 | 1 | 3 |
| S3M1 | 2.25 | 0.75 | 3 |
| S4M1 | 2.4 | 0.6 | 3 |
| S5M1 | 2.5 | 0.5 | 3 |
| S9M1 | 2.7 | 0.3 | 3 |
| Sample | Zero-order | First-order | Higuchi | Korsmeyer–Pepass | ||
|---|---|---|---|---|---|---|
| R2 | R2 | R2 | K | n | R2 | |
| S3M0 | 0.83945 | 0.97477 | 0.93179 | 0.13402 | 0.5962 | 0.93053 |
| S0M3 | 0.89787 | 0.89929 | 0.67555 | 0.15434 | 0.54812 | 0.91531 |
| S1M1 | 0.89829 | 0.98126 | 0.94682 | 0.18979 | 0.63876 | 0.95857 |
| S2M1 | 0.81459 | 0.98675 | 0.94703 | 0.14115 | 0.5348 | 0.94361 |
| S3M1 | 0.88806 | 0.84777 | 0.93902 | 0.11691 | 0.65692 | 0.94717 |
| S4M1 | 0.87714 | 0.83995 | 0.92952 | 0.11745 | 0.65996 | 0.93701 |
| S5M1 | 0.89047 | 0.85235 | 0.93814 | 0.11196 | 0.66259 | 0.9471 |
| S9M1 | 0.87855 | 0.83368 | 0.93513 | 0.12182 | 0.6452 | 0.94155 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, H.; Xing, T.; Ding, Y.; Zhang, H.; Yin, R.; Zhang, W. 3D Bioprinting of the Sustained Drug Release Wound Dressing with Double-Crosslinked Hyaluronic-Acid-Based Hydrogels. Polymers 2019, 11, 1584. https://doi.org/10.3390/polym11101584
Si H, Xing T, Ding Y, Zhang H, Yin R, Zhang W. 3D Bioprinting of the Sustained Drug Release Wound Dressing with Double-Crosslinked Hyaluronic-Acid-Based Hydrogels. Polymers. 2019; 11(10):1584. https://doi.org/10.3390/polym11101584
Chicago/Turabian StyleSi, Haopeng, Tianlong Xing, Yulong Ding, Hongbo Zhang, Ruixue Yin, and Wenjun Zhang. 2019. "3D Bioprinting of the Sustained Drug Release Wound Dressing with Double-Crosslinked Hyaluronic-Acid-Based Hydrogels" Polymers 11, no. 10: 1584. https://doi.org/10.3390/polym11101584
APA StyleSi, H., Xing, T., Ding, Y., Zhang, H., Yin, R., & Zhang, W. (2019). 3D Bioprinting of the Sustained Drug Release Wound Dressing with Double-Crosslinked Hyaluronic-Acid-Based Hydrogels. Polymers, 11(10), 1584. https://doi.org/10.3390/polym11101584