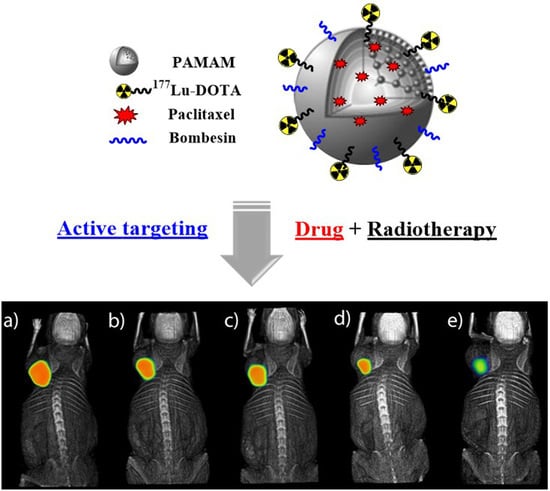
Synthesis and Evaluation of 177Lu-DOTA-DN(PTX)-BN for Selective and Concomitant Radio and Drug—Therapeutic Effect on Breast Cancer Cells
Abstract
1. Introduction
2. Materials
3. Methods
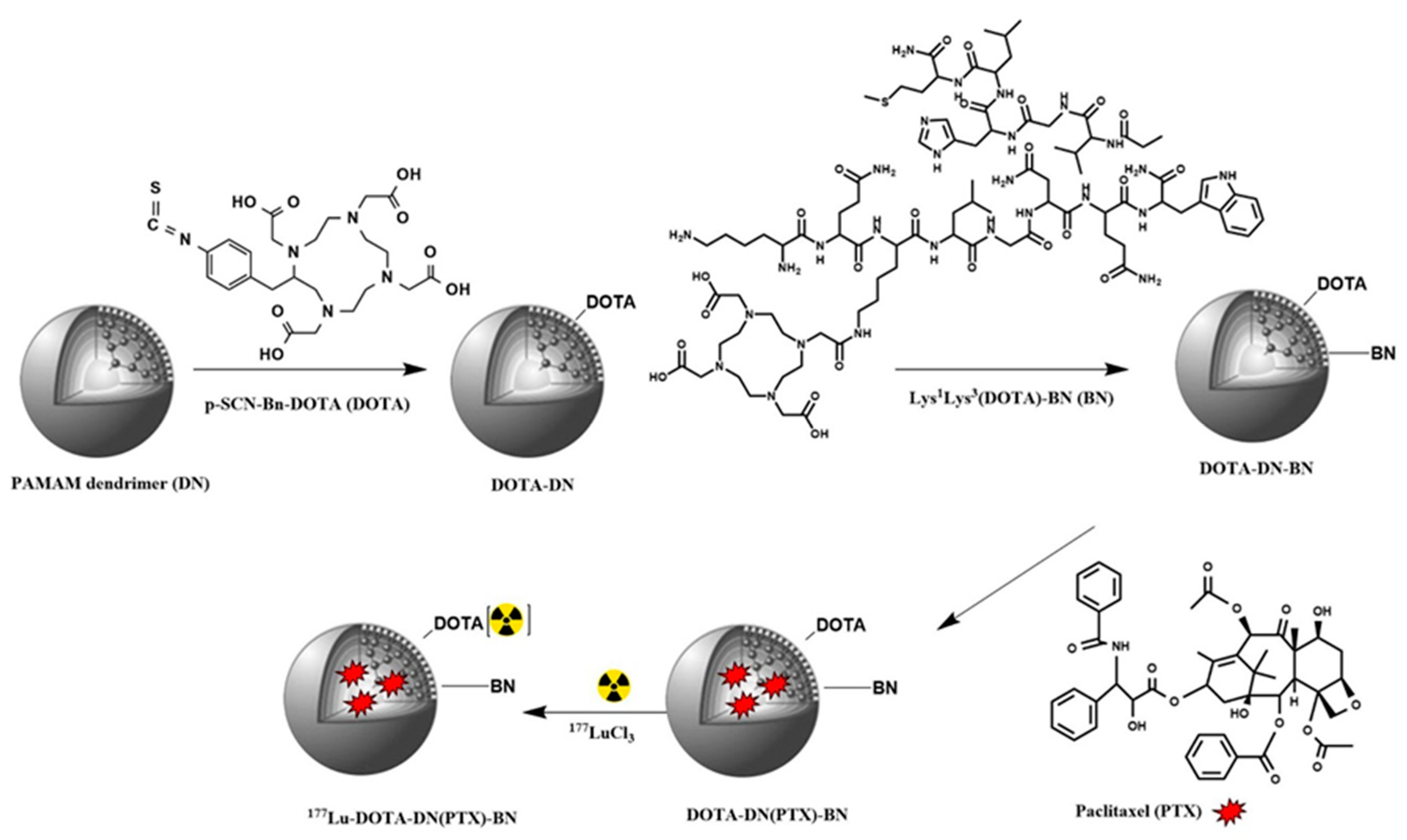
3.1. Synthesis of DOTA-DN and DOTA-DN-BN
3.2. Radiolabeling of Dendrimeric Systems
3.3. Physicochemical Characterization
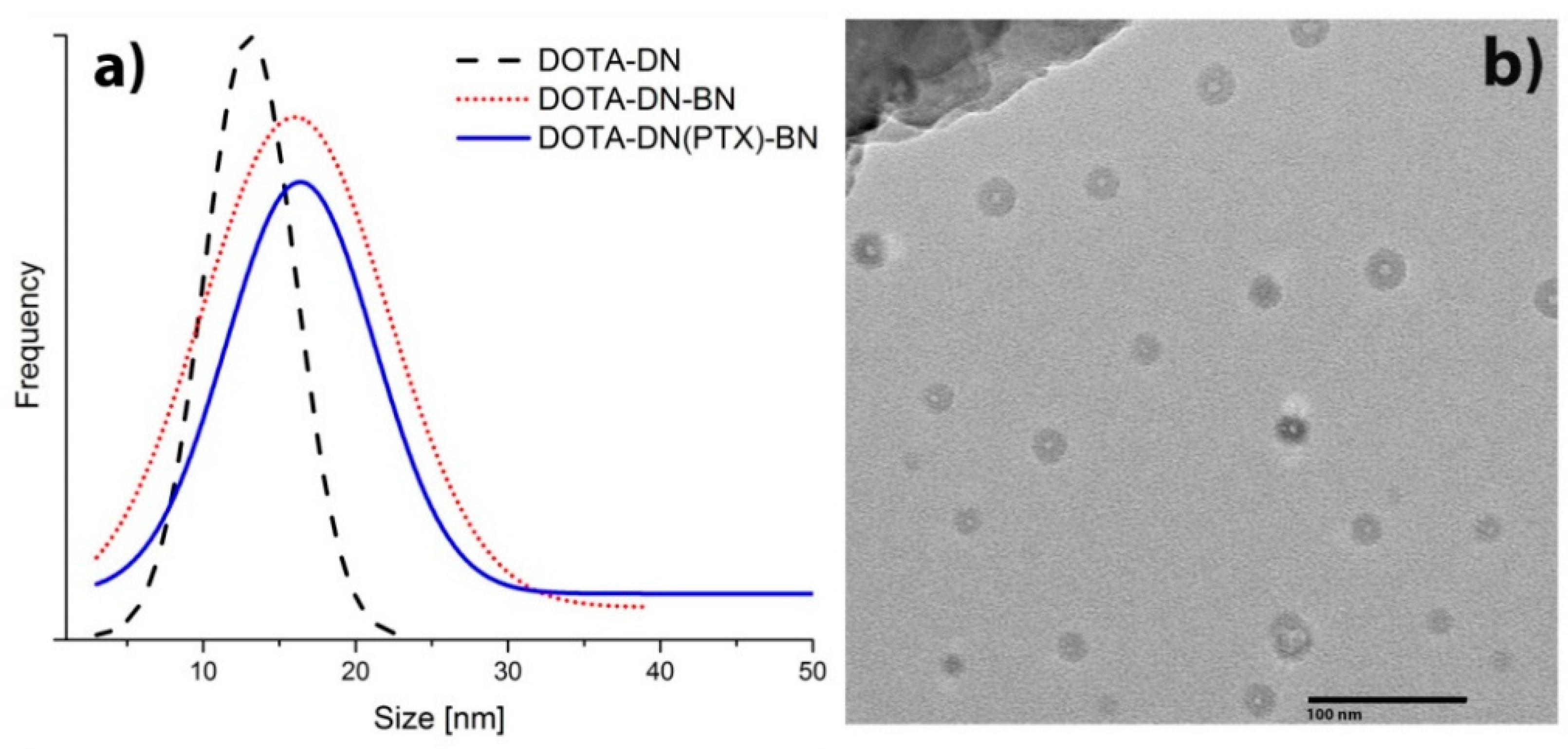
3.3.1. Transmission Electron Microscopy (TEM)
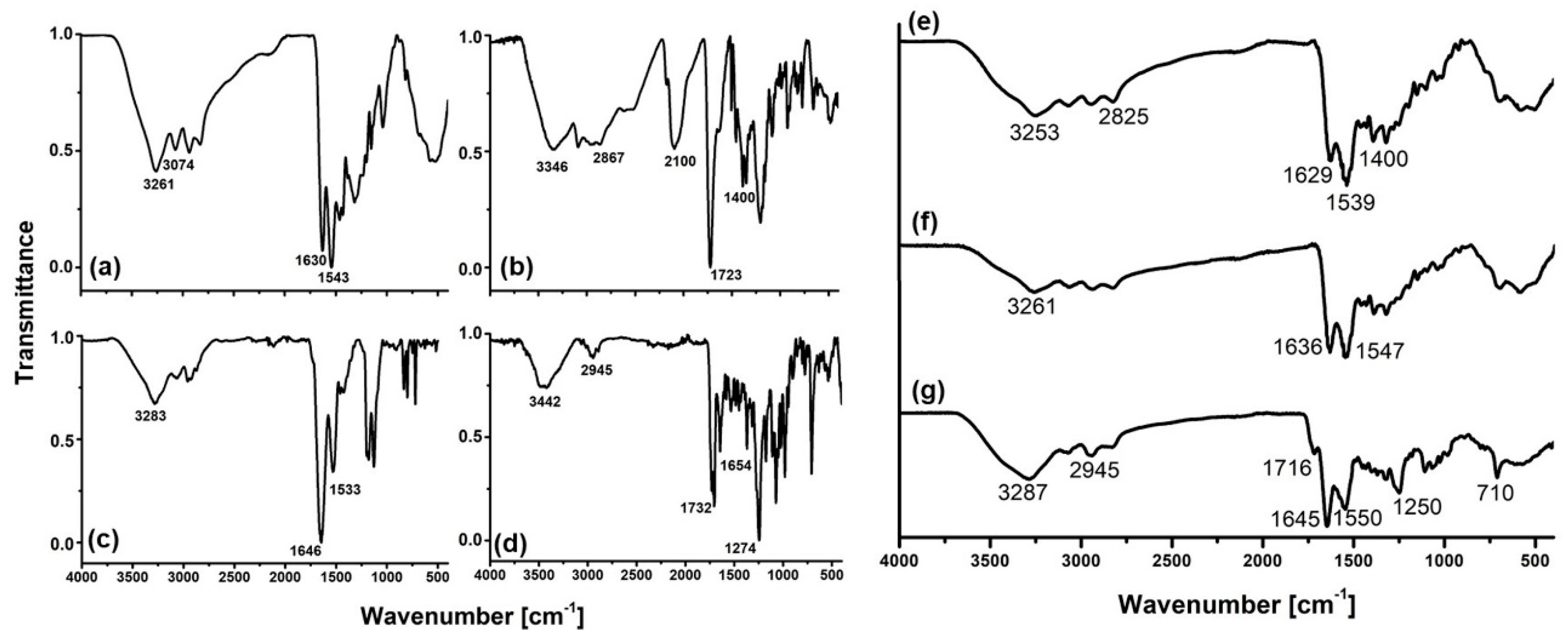
3.3.2. Fourier Transform Infrared Spectroscopy (FT-IR)
3.3.3. Entrapment Efficiency
3.3.4. Conjugation Efficiency of DOTA and Bombesin
3.3.5. In Vitro PTX Release
3.3.6. Radiolabeling Stability
3.4. In Vitro Studies
3.4.1. Cell Line
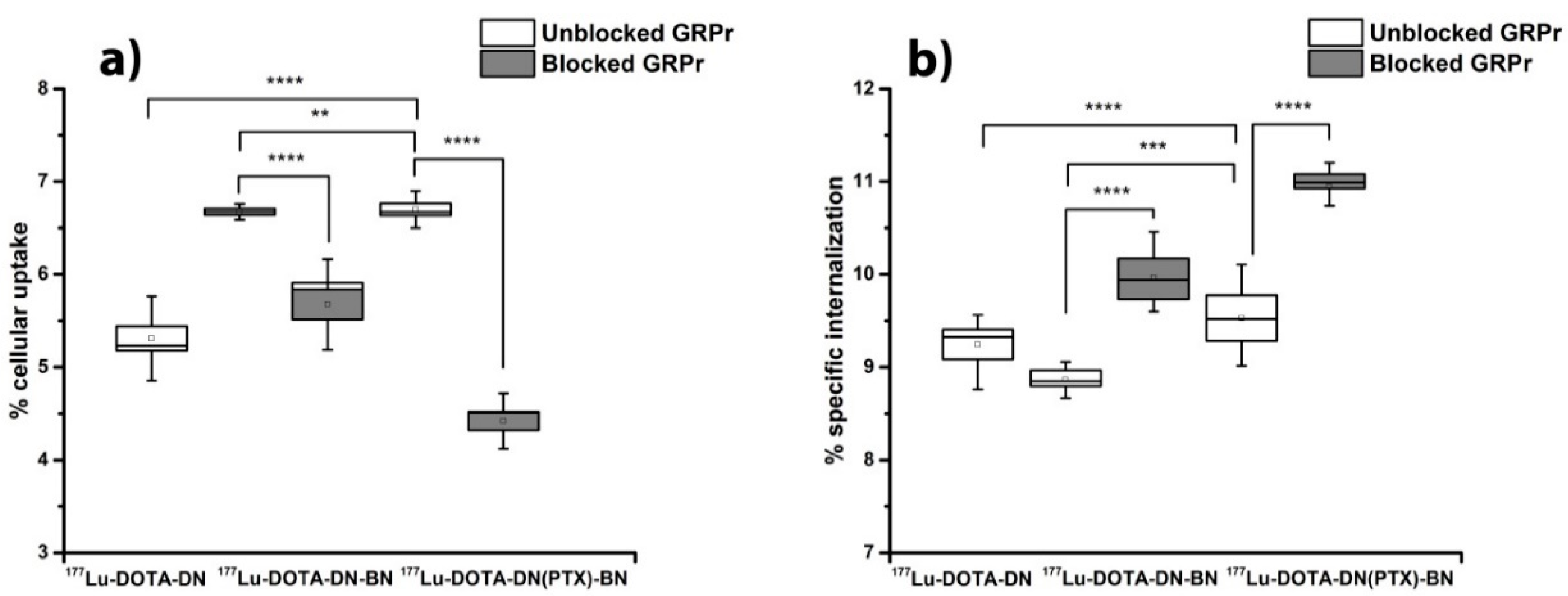
3.4.2. Cellular Uptake
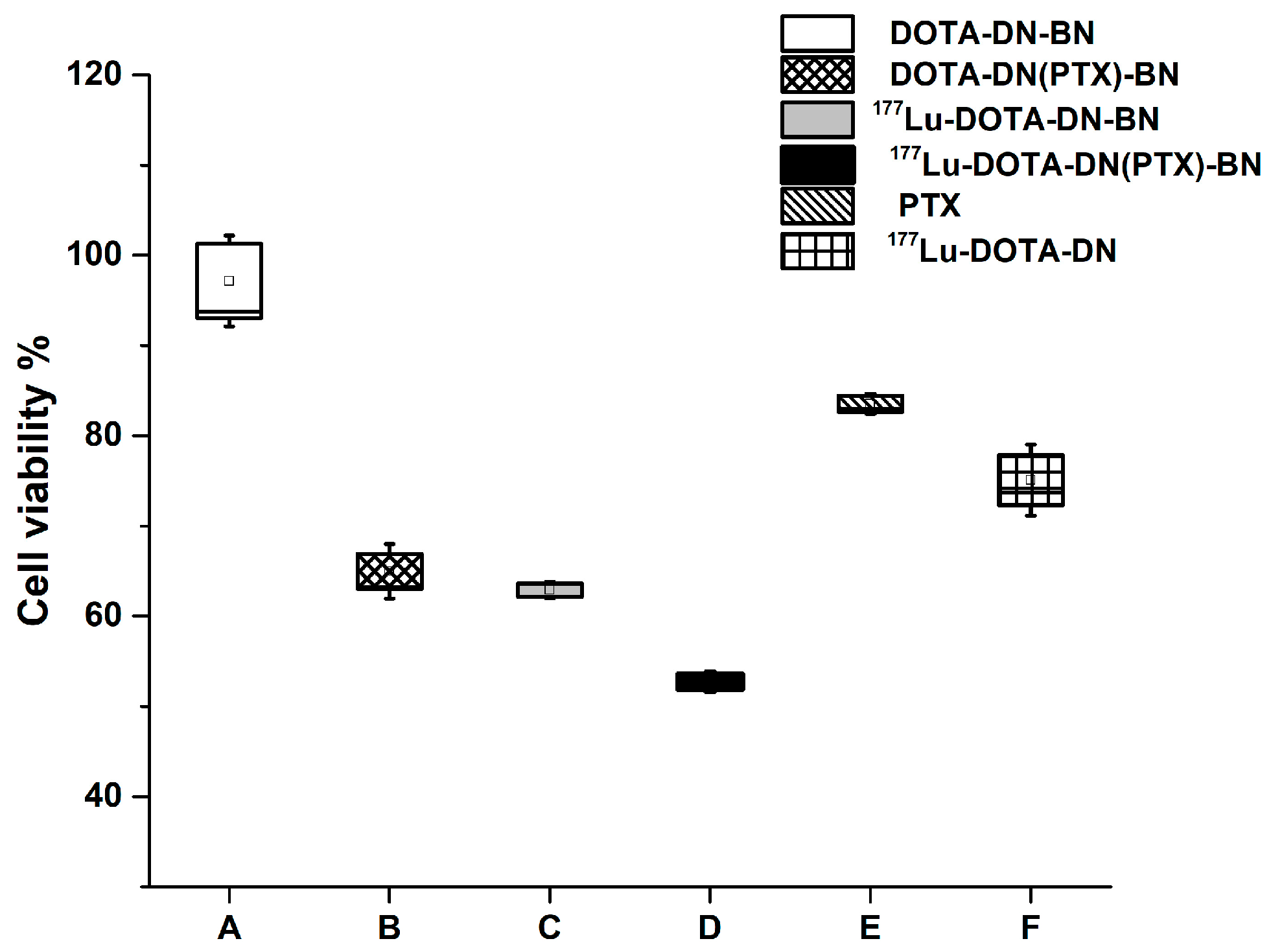
3.4.3. Cytotoxic Effect on T47D Cells
3.4.4. Radiation-Absorbed Doses to the T47D Cell Nucleus
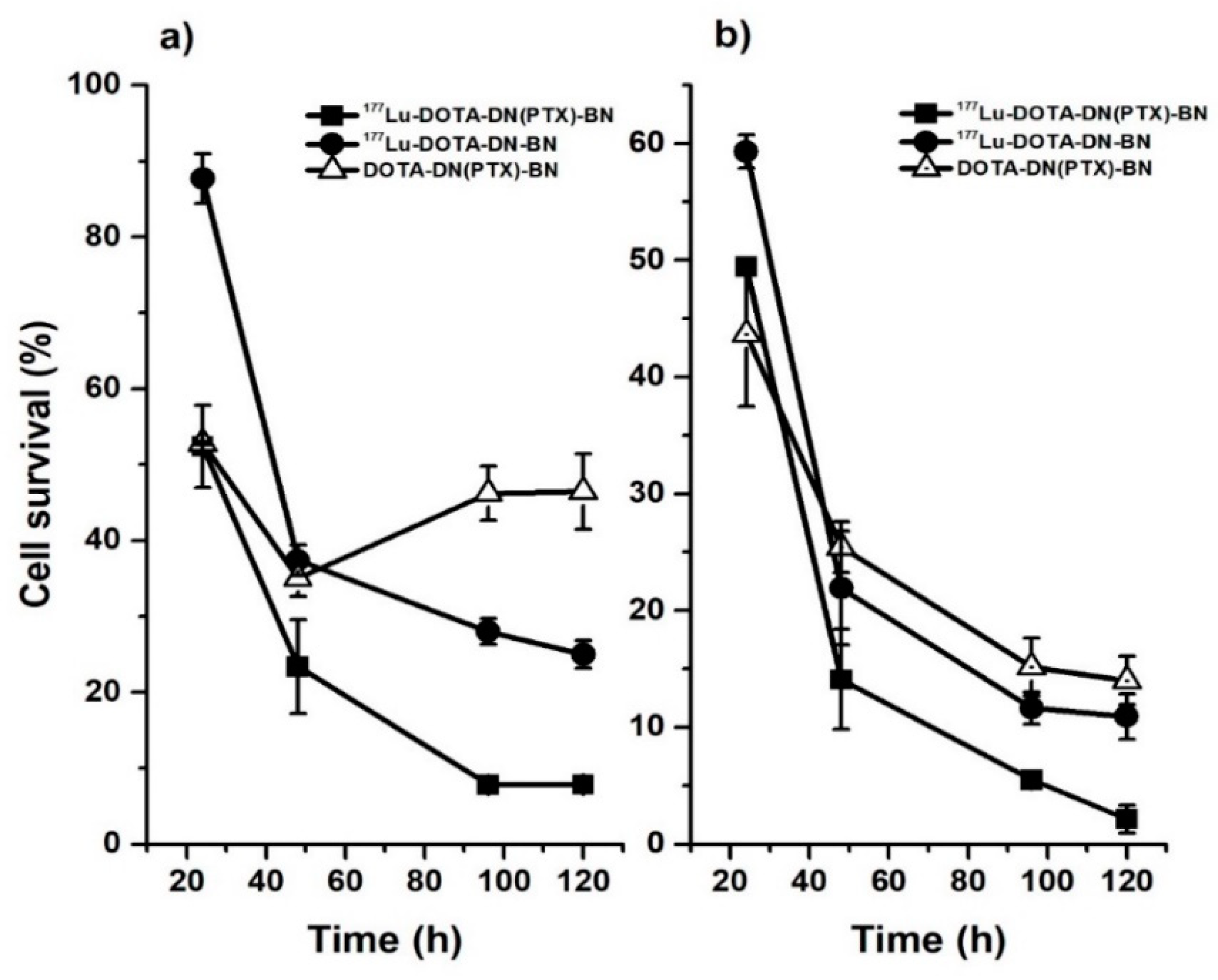
3.4.5. In-Vitro Cell Treatment
3.5. In Vivo Studies
3.5.1. SPECT-CT Imaging
3.5.2. Biodistribution
4. Results and Discussion
4.1. Infra-Red Spectroscopy
4.2. In Vitro Paclitaxel Release
4.3. Serum Stability
4.4. In Vitro Studies
4.4.1. Cell Uptake
4.4.2. Cell Viability
4.4.3. In Vivo Studies
5. Conclusions
Author Contributions
Funding Statement
Conflicts of Interest
References
- Ferro-Flores, G.; Arteaga de Murphy, C.; Melendez-Alafort, L. Third Generation Radiopharmaceuticals for Imaging and Targeted Therapy. Curr. Pharm. Anal. 2006, 2, 339–352. [Google Scholar] [CrossRef]
- de Ferreira, C.A.; Fuscaldi, L.L.; Townsend, D.M.; Rubello, D.; de Barros, A.L.B. Radiolabeled bombesin derivatives for preclinical oncological imaging. Biomed. Pharmacother. 2017, 87, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Brabander, T.; Teunisse, J.; Van Eijck, C.; Franssen, G.J.; Feelders, R.A.; de Herder, W.W.; Kwekkeboom, D.J. Peptide Receptor Radionuclide Therapy for Neuroendocrine Tumors. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Baratto, L.; Jadvar, H.; Iagaru, A. Prostate Cancer Theranostics Targeting Gastrin-Releasing Peptide Receptors. Mol. Imaging Biol. 2018, 20, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Cornelio, D.B.; Roesler, R.; Schwartsmann, G. Gastrin-releasing peptide receptor as a molecular target in experimental anticancer therapy. Ann. Oncol. 2007, 18, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Jaimes-Aguirre, L.; Gibbens-Bandala, B.V.; Morales-Avila, E.; Ocampo-García, B.E.; Seyedeh-Fatemeh, M.; Amirhosein, A. Polymer-Based Drug Delivery Systems, Development and Pre-Clinical Status. Curr. Pharm. Des. 2016, 22, 2886–2903. [Google Scholar] [CrossRef] [PubMed]
- Palmerston Mendes, L.; Pan, J.; Torchilin, V.P. Dendrimers as nanocarriers for nucleic acid and drug delivery in cancer therapy. Molecules 2017, 22, 1401. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Hu, J.; Xiao, J.; Cheng, Y. Dendrimer and cancer: A patent review (2006–present). Expert Opin. Ther. Pat. 2013, 23, 515–529. [Google Scholar] [CrossRef]
- Madaan, K.; Kumar, S.; Poonia, N.; Lather, V.; Pandita, D. Dendrimers in drug delivery and targeting: Drug-dendrimer interactions and toxicity issues. J. Pharm. Bioallied Sci. 2014, 6, 139–150. [Google Scholar] [CrossRef]
- Zhang, W.W.; Wang, Y.C.; Kan, X.M.; Wang, X.M.; Geng, D.M. Preparation and evaluation of peptide-dendrimer-paclitaxel conjugates for treatment of heterogeneous stage 1 non-small cell lung cancer in 293T and L132 cell lines. Trop. J. Pharm. Res. 2017, 16, 737–742. [Google Scholar] [CrossRef]
- Mendoza-Nava, H.; Ferro-Flores, G.; Ramírez, F.D.; Ocampo-García, B.; Santos-Cuevas, C.; Aranda-Lara, L.; Azorín-Vega, E.; Morales-Avila, E.; Isaac-Olivé, K. 177 Lu-Dendrimer Conjugated to Folate and Bombesin with Gold Nanoparticles in the Dendritic Cavity: A Potential Theranostic Radiopharmaceutical. J. Nanomater. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Escudero-Castellanos, A.; Ocampo-García, B.; Ferro-Flores, G.; Santos-Cuevas, C.; Morales-Ávila, E.; Luna-Gutiérrez, M.; Isaac-Olivé, K. Synthesis and preclinical evaluation of the 177 Lu-DOTA-PSMA(inhibitor)-Lys 3 -bombesin heterodimer designed as a radiotheranostic probe for prostate cancer. Nuclear Med. Commun. 2019, 40, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Goddu, S.M.; Budinger, T.F. MIRD Cellular, S. Values: Self-Absorbed Dose Per Unit Cumulated Activity for Select Radionuclides and Monoenergetic Electron and Alpha Particle Emitters Incorporated into Different Cell Compartments; Society of Nuclear Medicine: Reston, VA, USA, 1997. [Google Scholar]
- Haviland, J.S.; Mannino, M.; Griffin, C.; Porta, N.; Sydenham, M.; Bliss, J.M.; Yarnold, J.R. Late normal tissue effects in the arm and shoulder following lymphatic radiotherapy: Results from the UK START (Standardisation of Breast Radiotherapy) trials. Radiother Oncol. 2018, 126, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Tekade, R.K.; Tekade, M.; Kumar, M.; Chauhan, A.S. Dendrimer-stabilized smart-nanoparticle (DSSN) platform for targeted delivery of hydrophobic antitumor therapeutics. Pharm. Res. 2015, 32, 910–928. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yao, H. Dendrimer-paclitaxel complexes for efficient treatment in ovarian cancer: Study on OVCAR-3 and HEK293T cells. Acta Biochim. Pol. 2018, 65, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Devarakonda, B.; Judefeind, A.; Chigurupati, S.; Thomas, S.; Shah, G.V.; Otto, D.P.; de Villiers, M.M. The effect of polyamidoamine dendrimers on the in vitro cytotoxicity of paclitaxel in cultured prostate cancer (PC-3M) cells. J. Biomed. Nanotechnol. 2007, 3, 384–393. [Google Scholar] [CrossRef]
- Rompicharla, S.V.K.; Kumari, P.; Ghosh, B.; Biswas, S. Octa-arginine modified poly(amidoamine) dendrimers for improved delivery and cytotoxic effect of paclitaxel in cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 2), 847–859. [Google Scholar] [CrossRef] [PubMed]
- Cline, E.N.; Li, M.H.; Choi, S.K.; Herbstman, J.F.; Kaul, N.; Meyhöfer, E.; Skiniotis, G.; Baker, J.R.; Larson, R.G.; Walter, N.G. Paclitaxel-Conjugated PAMAM Dendrimers Adversely Affect Microtubule Structure through Two Independent Modes of Action. Biomacromolecules 2013, 14, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Teow, H.M.; Zhou, Z.; Najlah, M.; Yusof, S.R.; Abbott, N.J.; D’Emanuele, A. Delivery of paclitaxel across cellular barriers using a dendrimer-based nanocarrier. Int. J. Pharm. 2013, 441, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.P.; Keservani, R.K.; Gautam, T.; Gupta, A.K.; Sharma, A.K. An Alternative Approach for Acetylation of Amine Terminated Poly- amidoamine (PAMAM) Dendrimer. Ars Pharm. 2015, 56, 155–159. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Li, L.; Guo, Z.; Xue, Z.; Lu, X. An electrochemical paracetamol sensor based on layer-by-layer covalent attachment of MWCNTs. Anal. Methods 2016, 8, 2218–2225. [Google Scholar] [CrossRef]
- Shadrack, D.M.; Mubofu, E.B.; Nyandoro, S.S. Synthesis of Polyamidoamine Dendrimer for Encapsulating Tetramethylscutellarein for Potential Bioactivity Enhancement. Int. J. Mol. Sci. 2015, 16, 26363–26377. [Google Scholar] [CrossRef] [PubMed]
- Narmani, A.; Yavari, K.; Mohammadnejad, J. Imaging, biodistribution and in vitro study of smart 99mTc-PAMAM G4 dendrimer as novel nano-complex. Colloids Surf. B Biointerfaces 2017, 159, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Lara, L.; Ferro-Flores, G.; Azorín-Vega, E.; de María Ramírez, F.; Jiménez-Mancilla, N.; Ocampo-García, B.; Santos-Cuevas, C.; Isaac-Olivé, K. Synthesis and evaluation of Lys 1 (α,γ-Folate)Lys 3 (177 Lu-DOTA)-Bombesin(1-14) as a potential theranostic radiopharmaceutical for breast cancer. Appl. Radiat. Isot. 2016, 107, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Hiremath, J.G.; Khamar, N.S.; Palavalli, S.G.; Rudani, C.G.; Aitha, R.; Mura, P. Paclitaxel loaded carrier based biodegradable polymeric implants: Preparation and in vitro characterization. Saudi Pharm. J. 2013, 21, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Mao, Y.; Ye, T.; Xia, S.; Wang, S.; Wang, S. Study on enhanced lymphatic exposure of polyamidoamin-alkali blue dendrimer for paclitaxel delivery and influence of the osmotic pressure on the lymphatic targeting. Drug Deliv. 2016, 23, 2617–2629. [Google Scholar] [CrossRef][Green Version]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Naha, P.C.; Mukherjee, S.P.; Byrne, H.J. Toxicology of Engineered Nanoparticles: Focus on Poly (amidoamine) Dendrimers. Int. J. Environ. Res. Public Health 2018, 15, 338. [Google Scholar] [CrossRef]
- Ischia, J.; Patel, O.; Bolton, D.; Shulkes, A.; Baldwin, G.S. Expression and function of Gastrin-releasing peptide (GRP) in normal and cancerous urological tissues. BJU Int. 2014, 113 (Suppl. 2), 40–47. [Google Scholar] [CrossRef]



| 177Lu- DOTA-DN-(PTX)-BN Cellular Location | Biokinetic Model A(t) | Dose (Gy/Bq) | Total Dose to Cell Nuclei (Gy/Bq) | |
|---|---|---|---|---|
| 4.23 | ||||
| Membrane | 15012 | 1.12 | ||
| Cytoplasm | 20700 | 3.11 |
| Biokinetic Model A(t) | Dose (Gy/MBq) | |
|---|---|---|
| 11.73 | 3.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gibbens-Bandala, B.; Morales-Avila, E.; Ferro-Flores, G.; Santos-Cuevas, C.; Luna-Gutiérrez, M.; Ramírez-Nava, G.; Ocampo-García, B. Synthesis and Evaluation of 177Lu-DOTA-DN(PTX)-BN for Selective and Concomitant Radio and Drug—Therapeutic Effect on Breast Cancer Cells. Polymers 2019, 11, 1572. https://doi.org/10.3390/polym11101572
Gibbens-Bandala B, Morales-Avila E, Ferro-Flores G, Santos-Cuevas C, Luna-Gutiérrez M, Ramírez-Nava G, Ocampo-García B. Synthesis and Evaluation of 177Lu-DOTA-DN(PTX)-BN for Selective and Concomitant Radio and Drug—Therapeutic Effect on Breast Cancer Cells. Polymers. 2019; 11(10):1572. https://doi.org/10.3390/polym11101572
Chicago/Turabian StyleGibbens-Bandala, Brenda, Enrique Morales-Avila, Guillermina Ferro-Flores, Clara Santos-Cuevas, Myrna Luna-Gutiérrez, Gerardo Ramírez-Nava, and Blanca Ocampo-García. 2019. "Synthesis and Evaluation of 177Lu-DOTA-DN(PTX)-BN for Selective and Concomitant Radio and Drug—Therapeutic Effect on Breast Cancer Cells" Polymers 11, no. 10: 1572. https://doi.org/10.3390/polym11101572
APA StyleGibbens-Bandala, B., Morales-Avila, E., Ferro-Flores, G., Santos-Cuevas, C., Luna-Gutiérrez, M., Ramírez-Nava, G., & Ocampo-García, B. (2019). Synthesis and Evaluation of 177Lu-DOTA-DN(PTX)-BN for Selective and Concomitant Radio and Drug—Therapeutic Effect on Breast Cancer Cells. Polymers, 11(10), 1572. https://doi.org/10.3390/polym11101572