Effect of Cinnamon Extraction Oil (CEO) for Algae Biofilm Shelf-Life Prolongation
Abstract
1. Introduction
2. Materials and Methods
2.1. Solution Casting Method
2.2. Soil Burial Test
2.3. Tensile Test
2.4. Fourier Transform Infrared Spectrometer (FTIR)
2.5. Scanning Electron Microscopy (SEM)
3. Results and Discussions
3.1. Biofilm Thickness
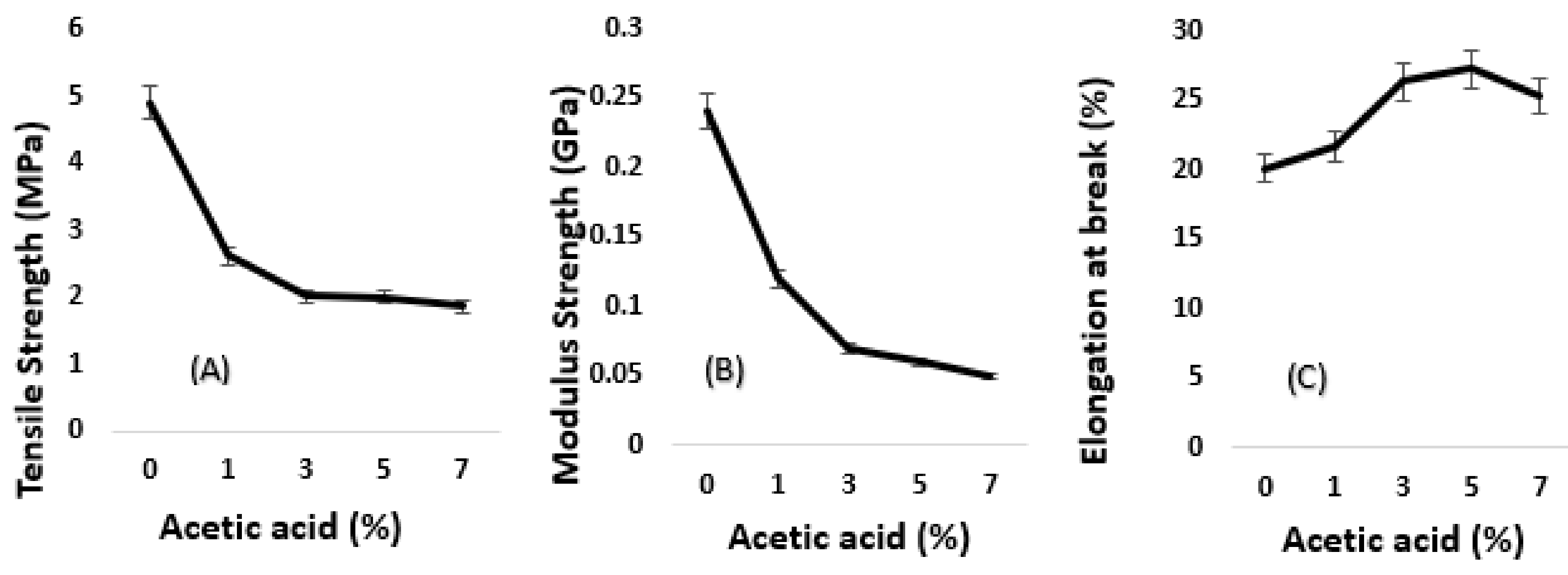
3.2. Algae-Based Biofilm with Acetic Acid
3.2.1. Tensile Data
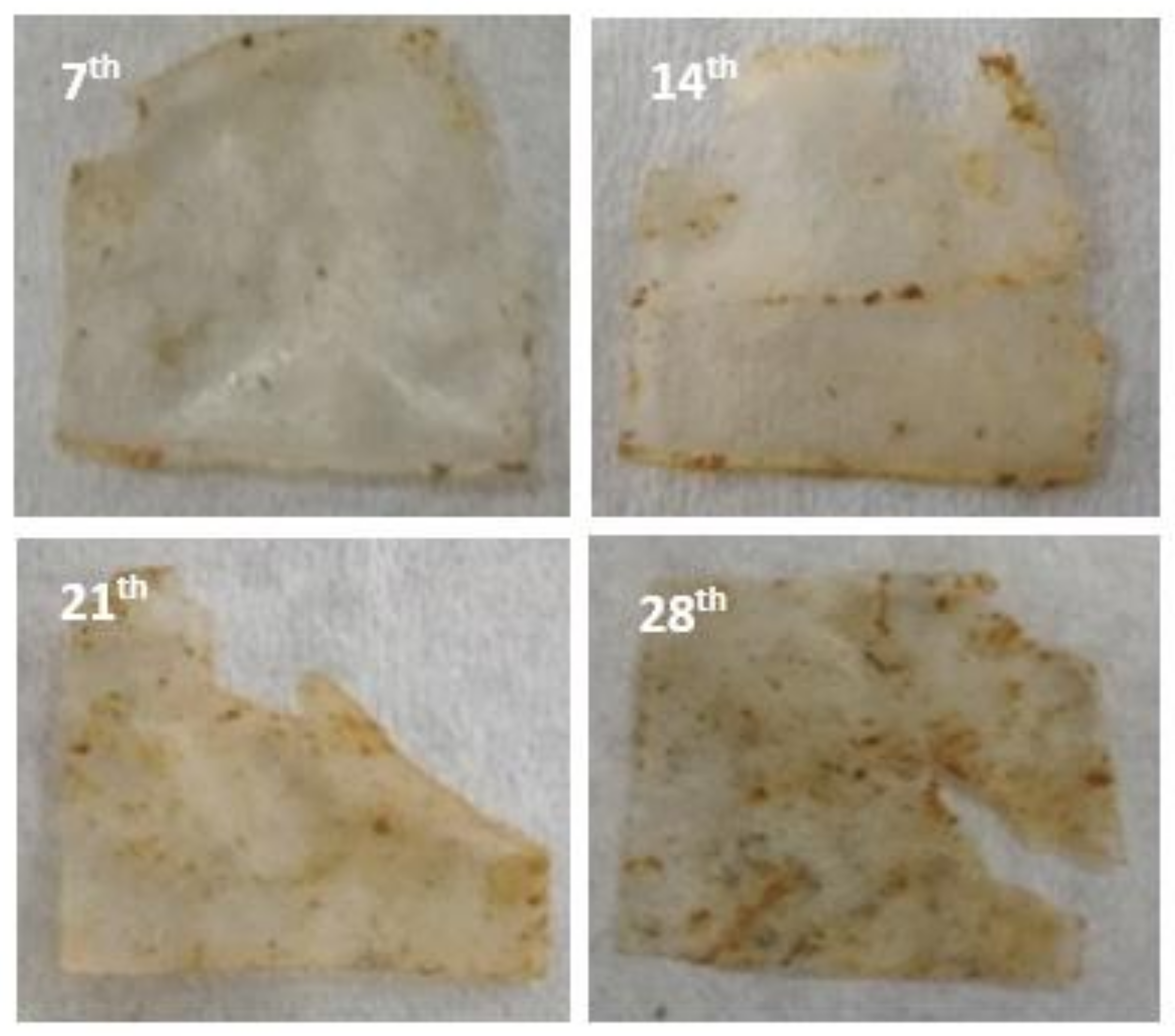
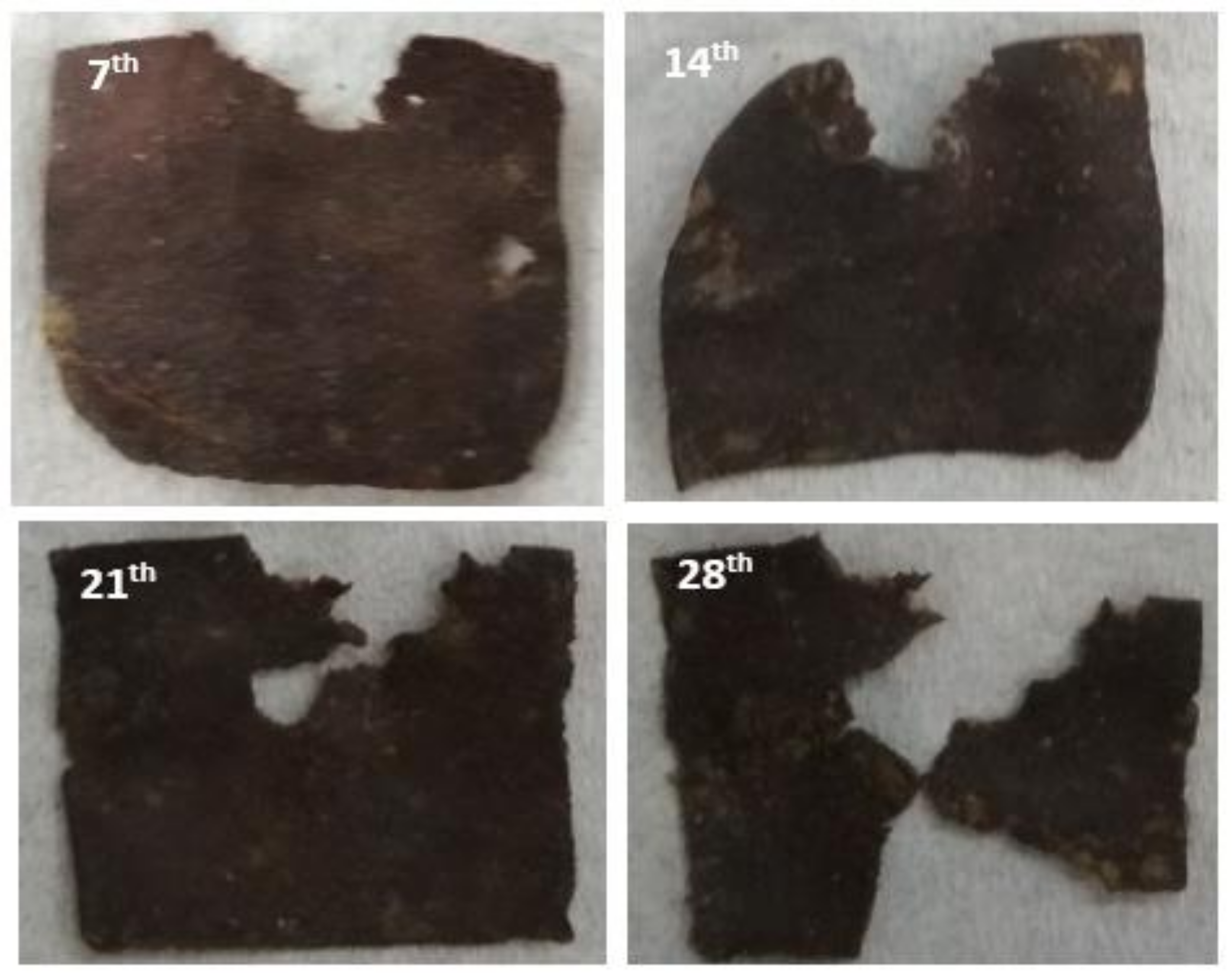
3.2.2. Soil Burial Test
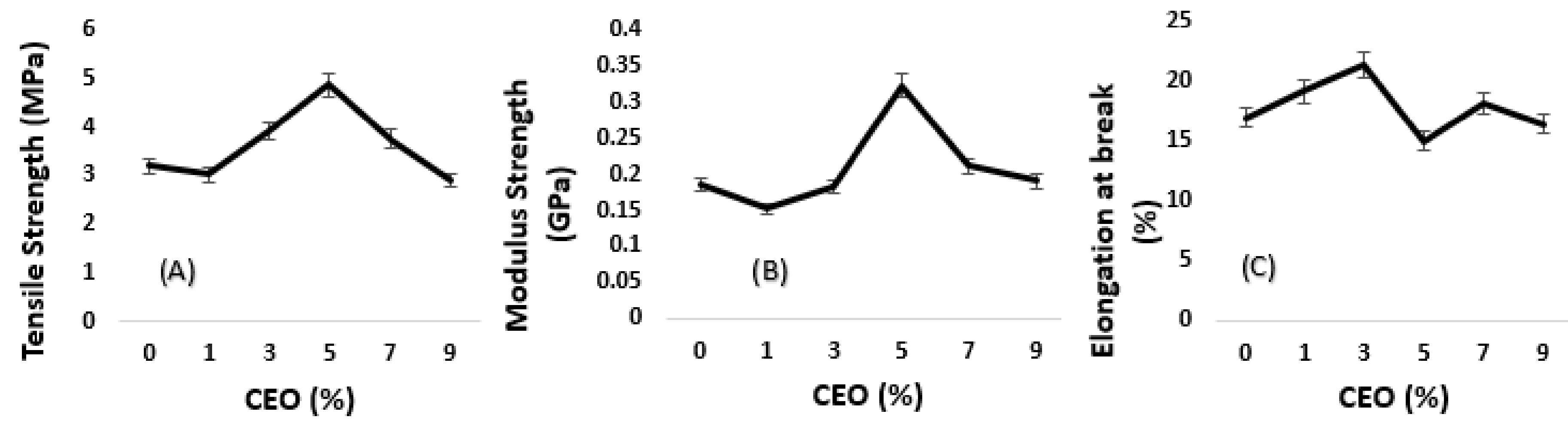
3.3. Algae-Based Biofilm with CEO
3.3.1. Tensile Data
3.3.2. Soil Burial Test
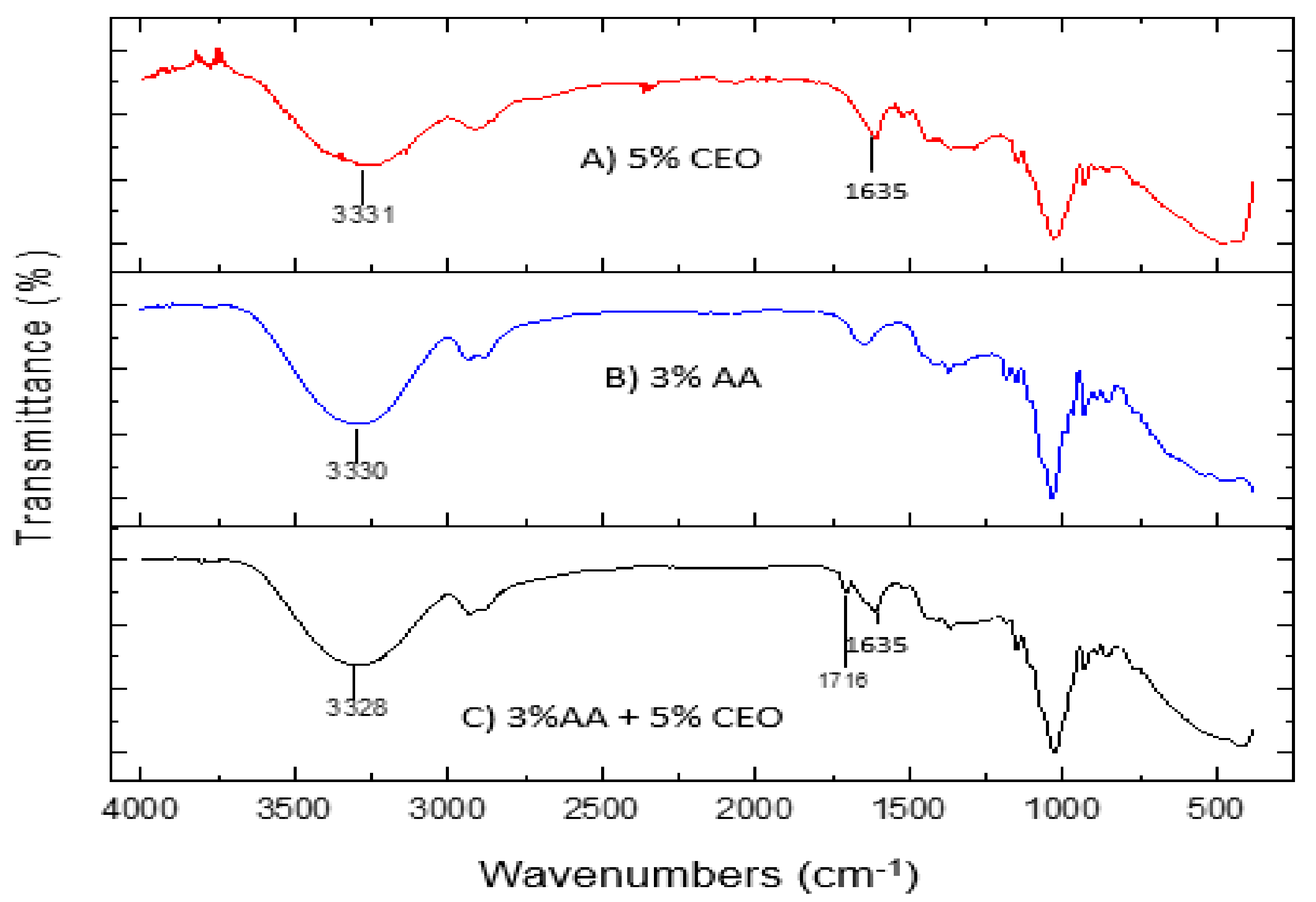
3.4. Fourier Transform Infra-Red (FTIR) Spectroscopy Data
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Webb, H.K.; Arnott, J.; Crawford, R.J.; Ivanova, E.P. Plastic degradation and its environmental implications with special reference to poly (ethylene terephthalate). Polymers 2013, 5, 1–18. [Google Scholar] [CrossRef]
- Ku, K.J.; Hong, Y.H.; Song, K.B. Mechanical properties of a Gelidium corneum edible film containing catechin and its application in sausages. J. Food Sci. 2008, 73, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.; Yang, Y. Citric acid cross-linking of starch films. Food Chem. 2010, 118, 702–711. [Google Scholar] [CrossRef]
- Hosseini, M.H.; Razavi, S.H.; Mousavi, M.A. Antimicrobial, physical and mechanical properties of chitosan-based films incorporated with thyme, clove and cinnamon essential oils. J. Food Process. Preserv. 2009, 33, 727–743. [Google Scholar] [CrossRef]
- Sung, S.Y.; Sin, L.T.; Tee, T.T.; Bee, S.T.; Rahmat, A.R.; Rahman, W.A.; Vikhraman, M. Antimicrobial agents for food packaging applications. Trends Food Sci. Technol. 2013, 33, 110–123. [Google Scholar] [CrossRef]
- Cui, H.Y.; Zhou, H.; Lin, L.; Zhao, C.T.; Zhang, X.J.; Xiao, Z.H.; Li, C.Z. Antibacterial activity and mechanism of cinnamon essential oil and its application in milk. J. Anim. Plant Sci. 2016, 26, 532–541. [Google Scholar]
- Du, W.X.; Olsen, C.W.; Avena-Bustillos, R.J.; McHugh, T.H.; Levin, C.E.; Friedman, M. Effects of allspice, cinnamon, and clove bud essential oils in edible apple films on physical properties and antimicrobial activities. J. Food Sci. 2009, 74, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Gautam, N.; Kaur, I. Soil burial biodegradation studies of starch grafted polyethylene and identification of Rhizobium meliloti therefrom. J. Environ. Chem. Ecotoxicol. 2013, 5, 147–158. [Google Scholar] [CrossRef]
- Herwig, G. Plant Natural Products: Synthesis, Biological Functions and Practical Applications; Wiley: Hoboken, NJ, USA, 2014; pp. 19–21. [Google Scholar]
- Singh, G.; Maurya, S.; de Lampasona, M.P.; Catalan, C.A.N. A comparison of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oils, oleoresins and their constituents. Food Chem. Toxicol. 2007, 45, 1650–1661. [Google Scholar] [CrossRef]
- Leja, K.; Lewandowicz, G. Polymer biodegradation and biodegradable polymers—A review. Pol. J. Environ. Stud. 2010, 19, 255–266. [Google Scholar]
- Bahram, S.; Reaei, M.; Soltani, M.; Kamali, A.; Ojagh, S.M.; Abdollahi, M. Whey Protein Concentrate Edible Film Activated with Cinnamon Essential Oil. J. Food Process. Preserv. 2014, 38, 1251–1258. [Google Scholar] [CrossRef]
- Faisal ZH, T.; Amri, F.; Salmah, H.; Tahir, I. The Effect of Acetic Acid on Properties of Coconut Shell Filled Low Density Polyethylene Composites. Indones. J. Chem. 2010, 10, 334–340. [Google Scholar]
- Desai, M.A.; Kurve, V.; Smith, B.S.; Campano, S.G.; Soni, K.; Schilling, M.W. Utilization of buffered vinegar to increase the shelf life of chicken retail cuts packaged in carbon dioxide. Poult. Sci. 2014, 93, 1850–1854. [Google Scholar] [CrossRef] [PubMed]
- Adinew, B. GC-MS and FT-IR analysis of constituents of essential oil from Cinnamon bark growing in South-west of Ethiopia. Int. J. Herb. Med. 2014, 1, 22–31. [Google Scholar]
- Reddy, N.; Li, Y.; Yang, Y. Alkali-catalyzed low temperature wet crosslinking of plant proteins using carboxylic acids. Biotechnol. Prog. 2009, 25, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Seligra, P.G.; Jaramillo, C.M.; Famá, L.; Goyanes, S. Biodegradable and non-retrogradable eco-films based on starch-glycerol with citric acid as crosslinking agent. Carbohydr. Polym. 2016, 138, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Sun, X.; Guo, X.; Ge, S.; Zhang, Q. Physicochemical properties, antimicrobial activity and oil release of fish gelatin films incorporated with cinnamon essential oil. Aquac. Fish. 2017, 4–11. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V.K. Cross-linking in Hydrogels—A Review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Q.; Critzer, F.; Davidson, P.M.; Zhong, Q. Physical and antibacterial properties of alginate films containing cinnamon bark oil and soybean oil. LWT Food Sci. Technol. 2015, 64, 423–430. [Google Scholar] [CrossRef]
- Awadhiya, A.; Kumar, D.; Verma, V. Crosslinking of agarose bioplastic using citric acid. Carbohydr. Polym. 2016, 151, 60–67. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.S.; Kim, E.A.; Jeon, Y.J. FTIR characterization and antioxidant activity of water soluble crude polysaccharides of Sri Lankan marine algae. Algae 2017, 32, 75–86. [Google Scholar] [CrossRef]
- Kwak, Y.S.; Kim, S.J.; Kim, H.Y. The antibacterial effect of Cinnamomum verum extract. Biomed. Res. 2017, 28, 6667–6670. [Google Scholar]
- Nowak, A.P.; Lisowska-Oleksiak, A. Red algae—An alternative source of carbon material for energy storage application. Int. J. Electrochem. Sci. 2014, 9, 3715–3724. [Google Scholar]
- Bunghez, F.; Socaci, C.; Zăgrean, F.; Fetea, F. Application of FT-MIR Spectroscopy for Fingerprinting Bioactive Molecules of Plant Ingredients and a New Formula with Antimicrobial Effect. Bull. UASVM Food Sci. Technol. 2013, 70, 64–65. [Google Scholar] [CrossRef]
- Dilek, Y.D.; Abel, U.U.; Tulay, B.O.; Aydin, A.; Ilkay, A.E.; Kazim, Y.; Deniz, G. Fourier transform infrared (FTIR) spectroscopy for identification of Chlorella vulgaris Beijerinck 1890 and Scenedesmus obliquus (Turpin) Kützing 1833. Afr. J. Biotechnol. 2012, 11, 3817–3824. [Google Scholar] [CrossRef]

| Sample (% Algae) | Biofilm without CEO Thickness (mm) | Biofilm with CEO Thickness (mm) |
|---|---|---|
| 0 | 0.2 ± 0.01 | 0.2 ± 0.01 |
| 1 | 0.2 ± 0.01 | 0.2 ± 0.01 |
| 2 | 0.2 ± 0.01 | - |
| 3 | 0.2 ± 0.01 | 0.2 ± 0.02 |
| 4 | 0.2 ± 0.02 | - |
| 5 | 0.2 ± 0.01 | 0.2 ± 0.03 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Othman, M.; Rashid, H.; Jamal, N.A.; Shaharuddin, S.I.S.; Sulaiman, S.; Hairil, H.S.; Khalid, K.; Zakaria, M.N. Effect of Cinnamon Extraction Oil (CEO) for Algae Biofilm Shelf-Life Prolongation. Polymers 2019, 11, 4. https://doi.org/10.3390/polym11010004
Othman M, Rashid H, Jamal NA, Shaharuddin SIS, Sulaiman S, Hairil HS, Khalid K, Zakaria MN. Effect of Cinnamon Extraction Oil (CEO) for Algae Biofilm Shelf-Life Prolongation. Polymers. 2019; 11(1):4. https://doi.org/10.3390/polym11010004
Chicago/Turabian StyleOthman, Maizatulnisa, Haziq Rashid, Nur Ayuni Jamal, Sharifah Imihezri Syed Shaharuddin, Sarina Sulaiman, H. Saffiyah Hairil, Khalisanni Khalid, and Mohd Nazarudin Zakaria. 2019. "Effect of Cinnamon Extraction Oil (CEO) for Algae Biofilm Shelf-Life Prolongation" Polymers 11, no. 1: 4. https://doi.org/10.3390/polym11010004
APA StyleOthman, M., Rashid, H., Jamal, N. A., Shaharuddin, S. I. S., Sulaiman, S., Hairil, H. S., Khalid, K., & Zakaria, M. N. (2019). Effect of Cinnamon Extraction Oil (CEO) for Algae Biofilm Shelf-Life Prolongation. Polymers, 11(1), 4. https://doi.org/10.3390/polym11010004