Biocompatibility of Small-Diameter Vascular Grafts in Different Modes of RGD Modification
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Graft Fabrication
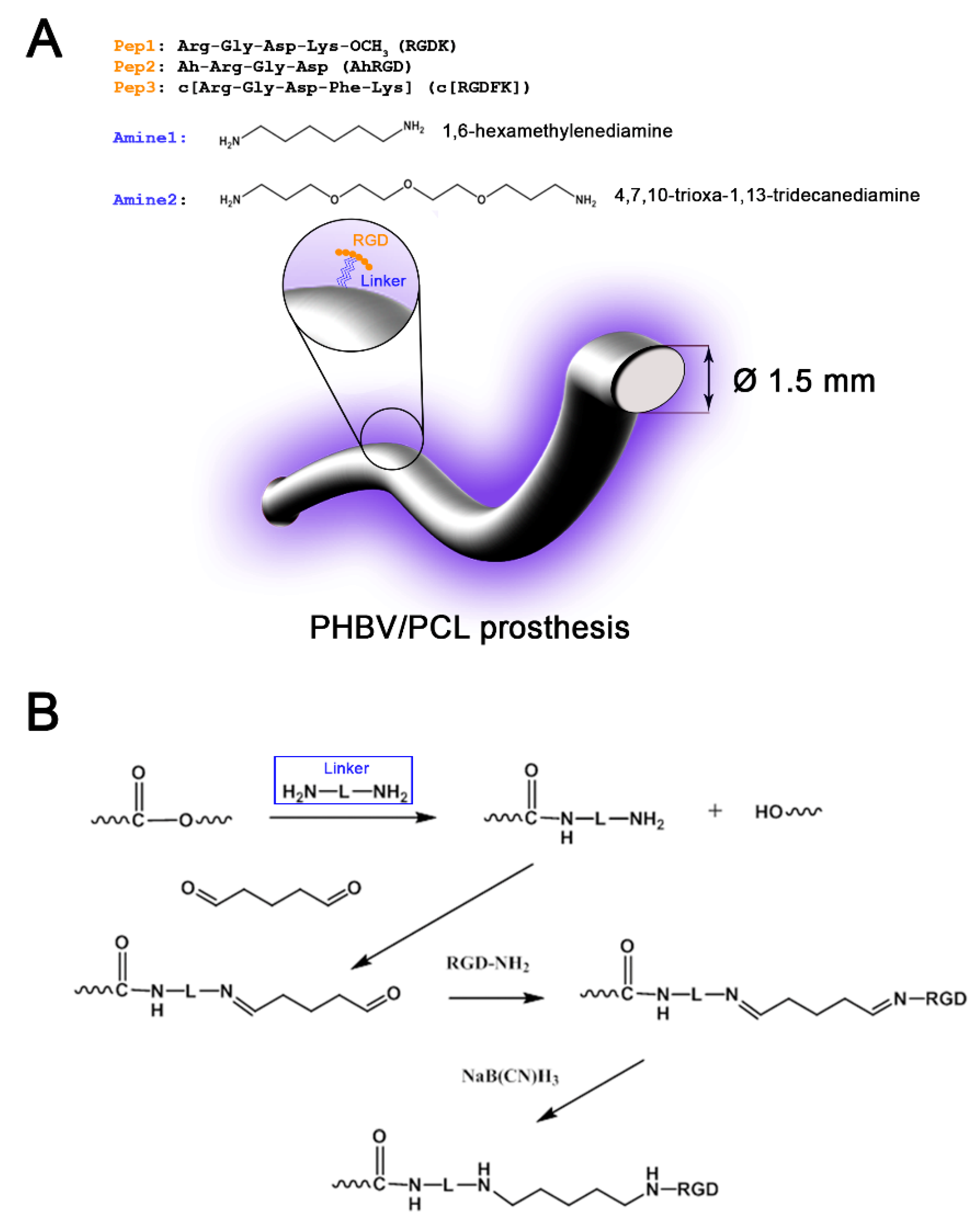
2.3. Graft Modification with Amine Linkers
2.4. Immobilization of RGD-Containing Peptides on Graft Luminal Surface
2.5. Arginine Positivity Test (Sakaguchi Test)
2.6. Tensile Testing
2.7. Haemolysis Testing
2.8. Platelet Aggregation Testing
2.9. Thrombogenicity Testing
2.10. Isolation and Characterization of Human ECFCs
- CD3, CD14, HLA-DR, CD45;
- CD34, KDR, CD146, CD133, CD31, CD45;
- vWF, CD146.
2.11. Assessment of Viability and Adhesion of ECFCs Seeded onto the Graft Surface
2.12. Assessment of Graft In Vivo Performance
2.13. Histological Examination
2.14. Evaluation of Graft Calcification
2.15. Immunofluorescence Staining
2.16. Statistical Analysis
3. Results
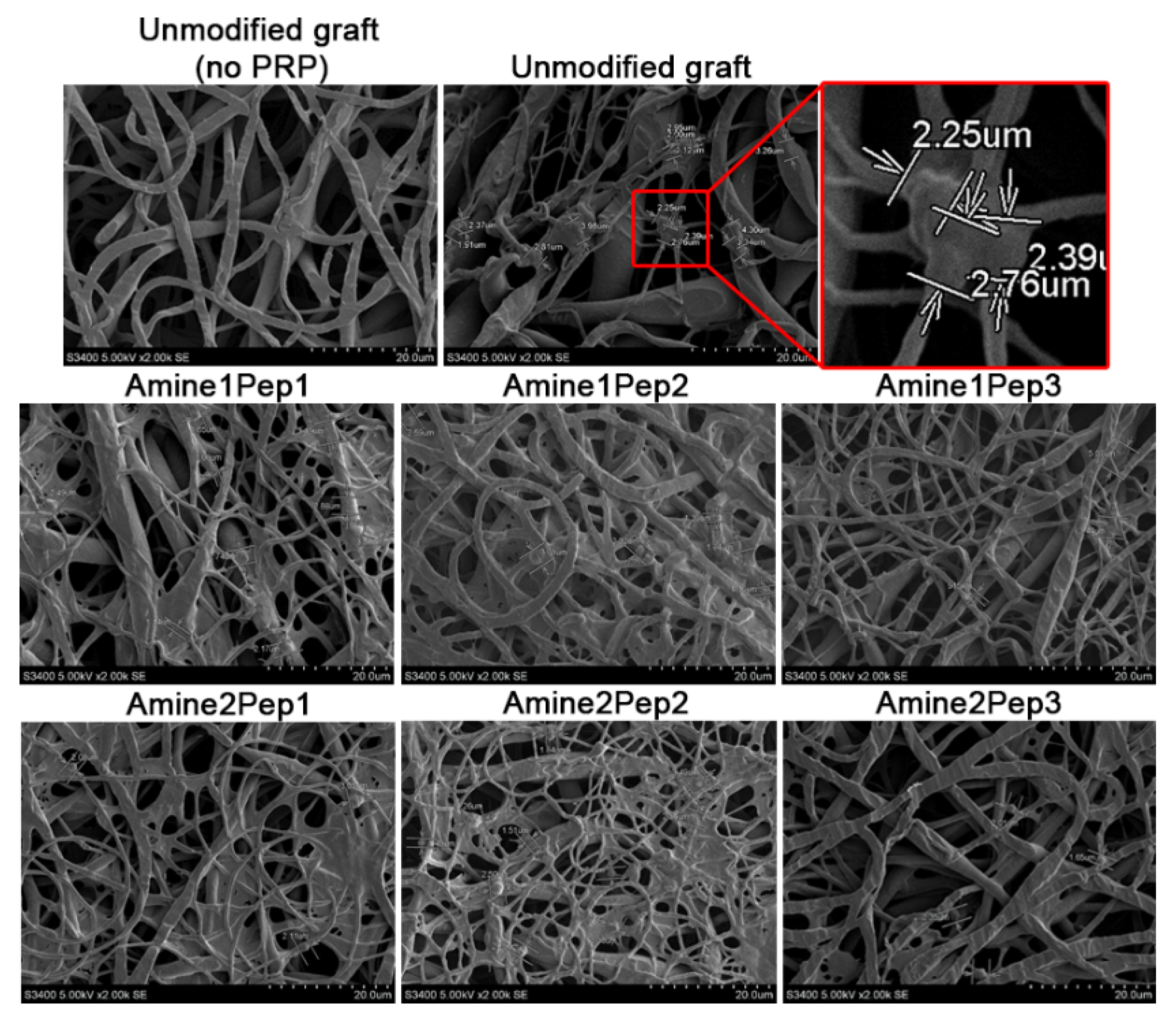
3.1. Fabrication of Small-Diameter Vascular Grafts in Different Modes of RGD Peptide Modification
3.2. Tensile Testing of RGD-Modified Grafts
3.3. Modification by All Types of RGD Peptides Negatively Affects Graft Hemocompatibility
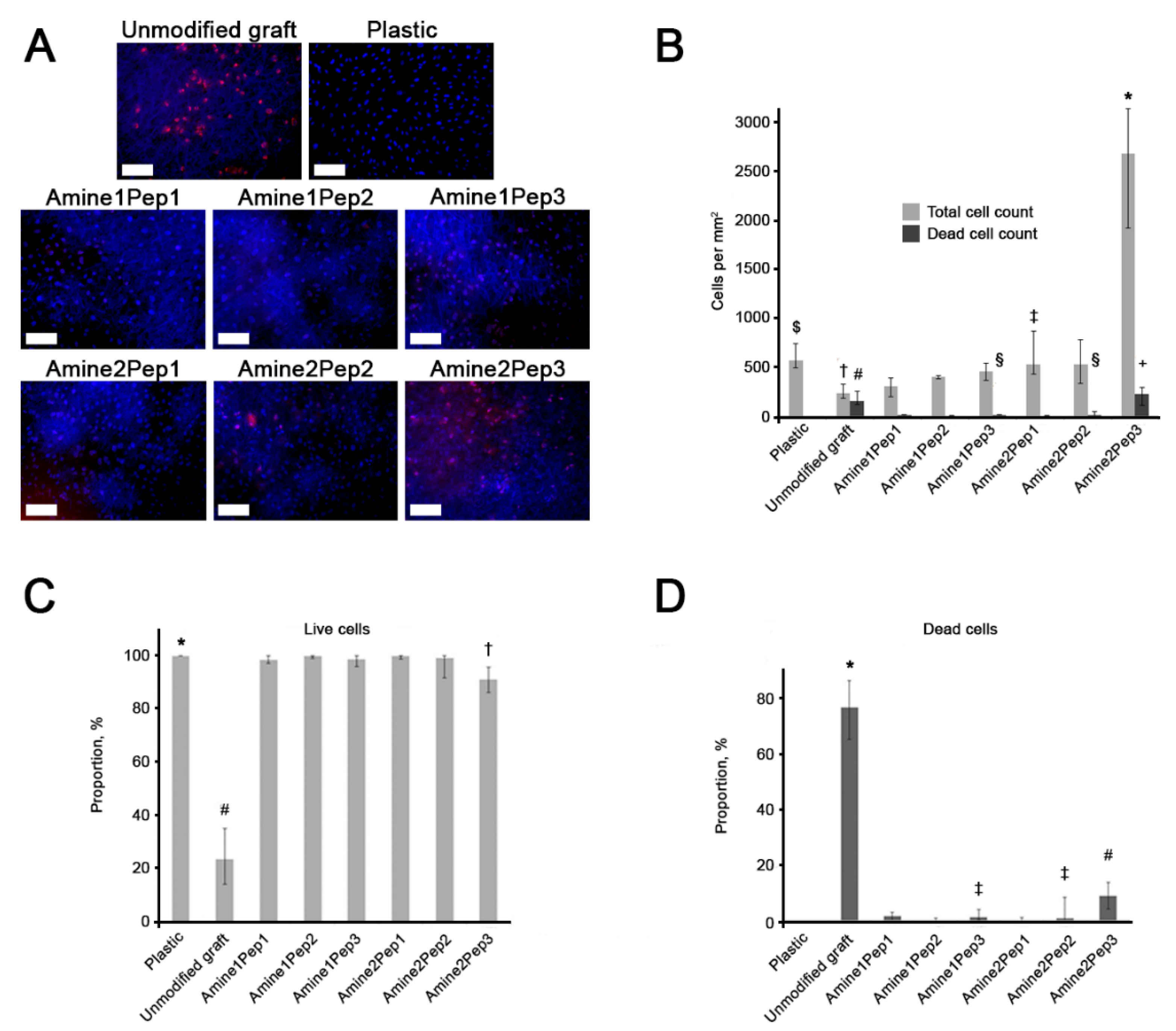
3.4. Graft Modification with Amine2Pep3 Improves Adhesion and Viability of Human ECFCs In Vitro
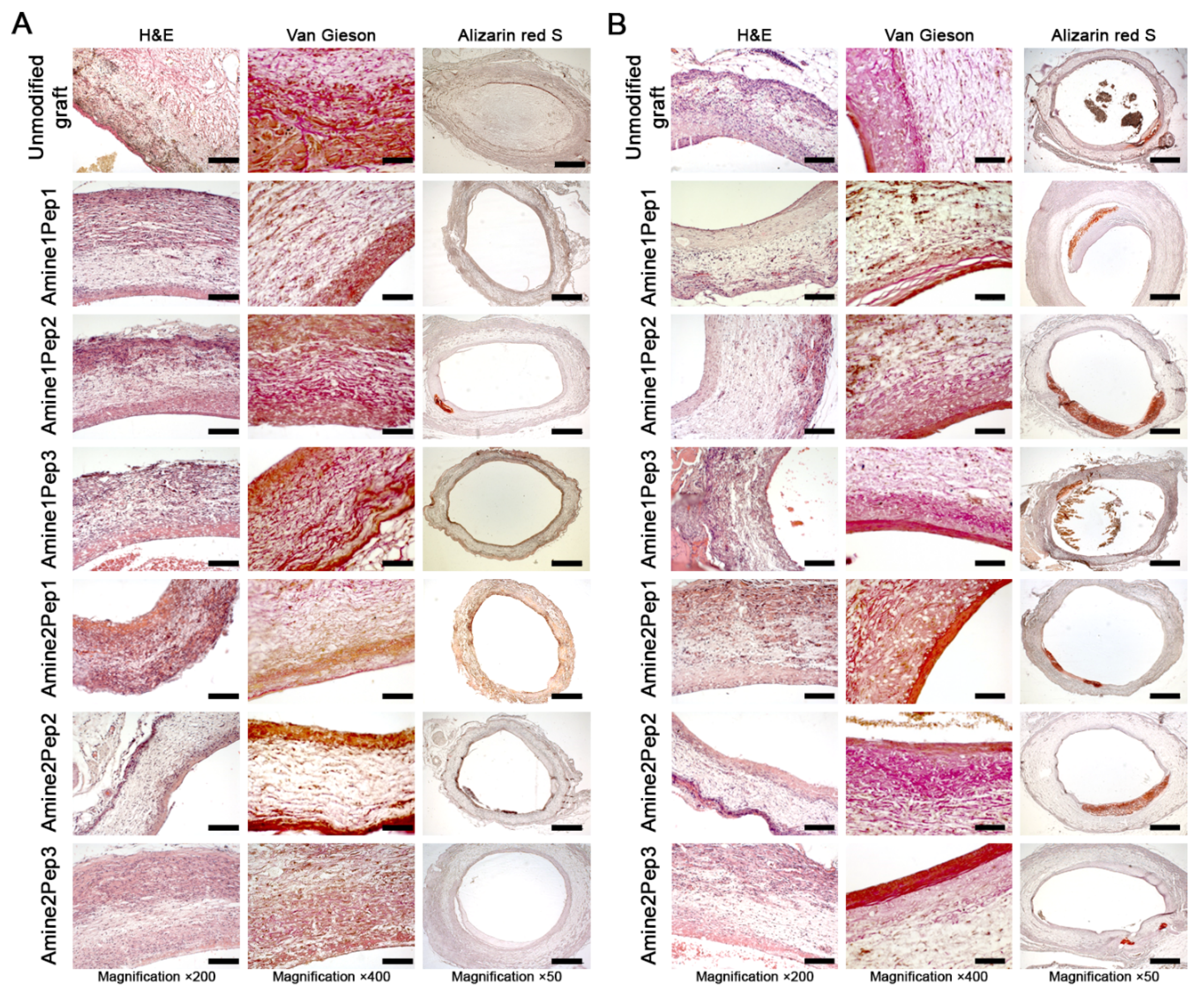
3.5. Modification with Amine2Pep3 Enhances Graft Biocompatibility In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Rocco, K.A.; Maxfield, M.W.; Best, C.A.; Dean, E.W.; Breuer, C.K. In Vivo Applications of Electrospun Tissue-Engineered Vascular Grafts: A Review. Tissue Eng. Part B. Rev. 2014, 20, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Taggart, D.P. Current Status of Arterial Grafts for Coronary Artery Bypass Grafting. Ann. Cardiothorac. Surg. 2013, 2, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Feng, Y.; Guo, J.; Wang, H.; Li, Q.; Yang, J.; Hao, X.; Lv, J.; Ma, N.; Li, W. Surface Modification and Endothelialization of Biomaterials as Potential Scaffolds for Vascular Tissue Engineering Applications. Chem. Soc. Rev. 2015, 44, 5680–5742. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Lee, B.L.-P.; Ning, X.; Murthy, N.; Dong, N.; Li, S. End-Point Immobilization of Heparin on Plasma-Treated Surface of Electrospun Polycarbonate-Urethane Vascular Graft. Acta Biomater. 2017, 51, 138–147. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, Z.; Wei, Z.; Liu, C.; Qiao, T.; Ran, F.; Bai, Y.; Jiang, X.; Ding, Y. Development and Validation of Small-Diameter Vascular Tissue from a Decellularized Scaffold Coated With Heparin and Vascular Endothelial Growth Factor. Artif. Organs 2009, 33, 230–239. [Google Scholar] [CrossRef]
- Williams, S.K.; Kleinert, L.B.; Patula-Steinbrenner, V. Accelerated Neovascularization and Endothelialization of Vascular Grafts Promoted by Covalently Bound Laminin Type 1. J. Biomed. Mater. Res. Part A 2011, 99, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, Y.; Shen, Y.; Wang, A.; Wang, S.; Xie, T. The Functions and Applications of RGD in Tumor Therapy and Tissue Engineering. Int. J. Mol. Sci. 2013, 14, 13447–13462. [Google Scholar] [CrossRef]
- Harburger, D.S.; Calderwood, D.A. Integrin Signalling at a Glance. J. Cell Sci. 2009, 122, 159–163. [Google Scholar] [CrossRef]
- Gabriel, M.; van Nieuw Amerongen, G.P.; Van Hinsbergh, V.W.M.; Amerongen, A.V.V.N.; Zentner, A. Direct Grafting of RGD-Motif-Containing Peptide on the Surface of Polycaprolactone Films. J. Biomater. Sci. Polym. Ed. 2006, 17, 567–577. [Google Scholar] [CrossRef]
- Gabriel, M.; Nazmi, K.; Dahm, M.; Zentner, A.; Vahl, C.-F.; Strand, D. Covalent RGD Modification of the Inner Pore Surface of Polycaprolactone Scaffolds. J. Biomater. Sci. Polym. Ed. 2012, 23, 941–953. [Google Scholar] [CrossRef]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD Modified Polymers: Biomaterials for Stimulated Cell Adhesion and Beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, Y.J.; Lee, S.J.; Lee, S.K.; Lee, K.Y. The Effect of Spacer Arm Length of an Adhesion Ligand Coupled to an Alginate Gel on the Control of Fibroblast Phenotype. Biomaterials 2010, 31, 5545–5551. [Google Scholar] [CrossRef]
- Houseman, B.T.; Mrksich, M. The Microenvironment of Immobilized Arg-Gly-Asp Peptides Is an Important Determinant of Cell Adhesion. Biomaterials 2001, 22, 943–955. [Google Scholar] [CrossRef]
- Antonova, L.; Seifalian, A.; Kutikhin, A.; Sevostyanova, V.; Matveeva, V.; Velikanova, E.; Mironov, A.; Shabaev, A.; Glushkova, T.; Senokosova, E.; et al. Conjugation with RGD Peptides and Incorporation of Vascular Endothelial Growth Factor Are Equally Efficient for Biofunctionalization of Tissue-Engineered Vascular Grafts. Int. J. Mol. Sci. 2016, 17, 1920. [Google Scholar] [CrossRef] [PubMed]
- Jung, F.; Braune, S.; Lendlein, A. Haemocompatibility Testing of Biomaterials Using Human Platelets. Clin. Hemorheol. Microcirc. 2013, 53, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Arimura, S.; Kawahara, K.; Biswas, K.K.; Abeyama, K.; Tabata, M.; Shimoda, T.; Ogomi, D.; Matsusaki, M.; Kato, S.; Ito, T.; et al. Hydroxyapatite Formed on/in Agarose Gel Induces Activation of Blood Coagulation and Platelets Aggregation. J. Biomed. Mater. Res. B. Appl. Biomater. 2007, 81, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Su, F.; Dong, J.; Fan, Z.; Duan, Y.; Li, S. In Vitro Biocompatibility Evaluation of Bioresorbable Copolymers Prepared from L-Lactide, 1, 3-Trimethylene Carbonate and Glycolide for Cardiovascular Applications. J. Biomater. Sci. Polym. Ed. 2015, 26, 497–514. [Google Scholar] [CrossRef]
- Matveeva, V.; Khanova, M.; Sardin, E.; Antonova, L.; Barbarash, O. Endovascular Interventions Permit Isolation of Endothelial Colony-Forming Cells from Peripheral Blood. Int. J. Mol. Sci. 2018, 19, 3453. [Google Scholar] [CrossRef]
- Hur, J.; Yoon, C.-H.; Kim, H.-S.; Choi, J.-H.; Kang, H.-J.; Hwang, K.-K.; Oh, B.-H.; Lee, M.-M.; Park, Y.-B. Characterization of Two Types of Endothelial Progenitor Cells and Their Different Contributions to Neovasculogenesis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 288–293. [Google Scholar] [CrossRef]
- Medina, R.J.; O’Neill, C.L.; Sweeney, M.; Guduric-Fuchs, J.; Gardiner, T.A.; Simpson, D.A.; Stitt, A.W. Molecular Analysis of Endothelial Progenitor Cell (EPC) Subtypes Reveals Two Distinct Cell Populations with Different Identities. BMC Med. Genom. 2010, 3, 18. [Google Scholar] [CrossRef]
- O’Neill, C.L.; McLoughlin, K.J.; Chambers, S.E.J.; Guduric-Fuchs, J.; Stitt, A.W.; Medina, R.J. The Vasoreparative Potential of Endothelial Colony Forming Cells: A Journey Through Pre-Clinical Studies. Front. Med. 2018, 5, 273. [Google Scholar] [CrossRef] [PubMed]
- Reinisch, A.; Hofmann, N.A.; Obenauf, A.C.; Kashofer, K.; Rohde, E.; Schallmoser, K.; Flicker, K.; Lanzer, G.; Linkesch, W.; Speicher, M.R.; et al. Humanized Large-Scale Expanded Endothelial Colony-Forming Cells Function in Vitro and in Vivo. Blood 2009, 113, 6716–6725. [Google Scholar] [CrossRef] [PubMed]
- Carrel, T.; Winkler, B. Current Trends in Selection of Conduits for Coronary Artery Bypass Grafting. Gen. Thorac. Cardiovasc. Surg. 2017, 65, 549–556. [Google Scholar] [CrossRef]
- Mi, H.-Y.; Jing, X.; Thomsom, J.A.; Turng, L.-S. Promoting Endothelial Cell Affinity and Antithrombogenicity of Polytetrafluoroethylene (PTFE) by Mussel-Inspired Modification and RGD/Heparin Grafting. J. Mater. Chem. B 2018, 6, 3475–3485. [Google Scholar] [CrossRef]
- Noel, S.; Fortier, C.; Murschel, F.; Belzil, A.; Gaudet, G.; Jolicoeur, M.; De Crescenzo, G. Co-Immobilization of Adhesive Peptides and VEGF within a Dextran-Based Coating for Vascular Applications. Acta Biomater. 2016, 37, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Bastijanic, J.M.; Marchant, R.E.; Kligman, F.; Allemang, M.T.; Lakin, R.O.; Kendrick, D.; Kashyap, V.S.; Kottke-Marchant, K. In Vivo Evaluation of Biomimetic Fluorosurfactant Polymer-Coated Expanded Polytetrafluoroethylene Vascular Grafts in a Porcine Carotid Artery Bypass Model. J. Vasc. Surg. 2016, 63, 1620–1630.e4. [Google Scholar] [CrossRef] [PubMed]
- Delforge, D.; Gillon, B.; Art, M.; Dewelle, J.; Raes, M.; Remacle, J. Design of a Synthetic Adhesion Protein by Grafting RGD Tailed Cyclic Peptides on Bovine Serum Albumin. Lett. Pept. Sci. 1998, 5, 87–91. [Google Scholar] [CrossRef]
- Ho, C.Y.; Shanahan, C.M. Medial Arterial Calcification. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1475–1482. [Google Scholar] [CrossRef]
- Glushkova, T.V.; Ovcharenko, E.A.; Sevostyanova, V.V.; Klyshnikov, K.Y. [Calcification Patterns of the Components of the Cardiovascular System and Their Substitutes: Composition, Stucture and Localization of Calcific Deposits]. Kardiologiia 2018, 5, 72–81. [Google Scholar] [CrossRef]
- Mehta, R.I.; Mukherjee, A.K.; Patterson, T.D.; Fishbein, M.C. Pathology of Explanted Polytetrafluoroethylene Vascular Grafts. Cardiovasc. Pathol. 2011, 20, 213–221. [Google Scholar] [CrossRef]
- Shin, H.; Zygourakis, K.; Farach-Carson, M.C.; Yaszemski, M.J.; Mikos, A.G. Modulation of Differentiation and Mineralization of Marrow Stromal Cells Cultured on Biomimetic Hydrogels Modified with Arg-Gly-Asp Containing Peptides. J. Biomed. Mater. Res. A 2004, 69, 535–543. [Google Scholar] [CrossRef] [PubMed]


| Type | Platelet Features |
|---|---|
| I | Disc-shaped (no deformation) |
| II | Platelet is increased in size with protrusion-like pseudopodia sticking out |
| III | Platelet is substantially increased in size, irregularly shaped, with pronounced pseudopodia, multiple platelets aggregate together |
| IV | Spreading of the platelet, cytoplasm expands among pseudopodia |
| V | Platelet in the form of a spot with granules, pseudopodia cannot be identified due to cytoplasm spreading |
| Time, min | The Number of Amino Groups *, mol/cm2 | |
|---|---|---|
| Amine1 (1,6-hexamethylenediamine) | Amine2 (4,7,10-trioxa-1,13-tridecanediamine) | |
| 10 | 5.1 × 10−9 | 5.8 × 10−9 |
| 30 | 6.3 × 10−9 | 8.6 × 10−9 |
| 60 | 8.9 × 10−9 | 8.1 × 10−9 |
| Linker | Peptide 1 (RGDK) | Peptide 2 (AhRGD) | Peptide 3 (c[RGDFK]) |
|---|---|---|---|
| Amine1 (1,6-hexamethylenediamine) | Amine1Pep1 | Amine1Pep2 | Amine1Pep3 |
| Amine2 (4,7,10-trioxa-1,13-tridecanediamine) | Amine2Pep1 | Amine2Pep2 | Amine2Pep3 |
| Sample | n | Ultimate Tensile Strength, MPa Median (25th and 75th Quartiles) Range | Elongation at Break, % Median (25th and 75th Quartiles) Range | Young’s modulus, MPa Median (25th and 75th Quartiles) Range | Thickness, mm Median (25th and 75th Quartiles) Range |
|---|---|---|---|---|---|
| Internal Mammary Artery | 6 | 2.48 (1.36–3.25) 1.07–6.52 | 29.72 (23.51–39.62) 22.0–50.88 | 2.42 (1.87–3.19) 1.53–3.34 | 0.27 (0.24–0.30) 0.21–0.90 |
| Unmodified Grafts | 6 | 3.85 (2.88–4.54) 2.38–4.62 | 102.7 (79.37–106.3) 74.92–119.23 | 21.8 (19.2–25.2) 18.2–27.5 | 0.38 (0.35–0.46) 0.35–0.47 |
| Amine1Pep1 | 6 | 1.29 (0.65–1.42) 0.51–1.58 * | 127.39 (31.5–135.52) 17.23–182.52 | 21.4 (10.2–24.8) 8.46–25.3 | 0.55 (0.49–0.55) 0.49–0.56 * |
| Amine1Pep2 | 6 | 0.81 (0.77–1.1) 0.68–1.2 * | 83.76 (81.2–132.8) 31.47–154.3 | 11.8 (11.1–17.5) 9.64–18.3 * | 0.45 (0.45–0.5) 0.41–0.56 |
| Amine1Pep3 | 6 | 0.72 (0.45–0.89) 0.3–1.0 * | 84.0 (17.26–99.83) 9.19–148.9 | 8.11 (6.86–12.4) 5.05–12.7 * | 0.45 (0.44–0.47) 0.42–0.5 |
| Amine2Pep1 | 6 | 1.2 (1.14–1.22) 1.1–1.56 * | 65.73 (65.03–121.46) 27.53–155.63 | 22.1 (21.5–24.6) 20.7–24.9 | 0.54 (0.53–0.54) 0.53–0.58 * |
| Amine2Pep2 | 6 | 1.21 (1.21–1.23) 1.14–1.26 * | 109.93 (93.28–127.23) 86.47–132.54 | 22.9 (21.1–23.1) 21.0–23.3 | 0.49 (0.49–0.53) 0.49–0.55 * |
| Amine2Pep3 | 6 | 1.18 (1.03–1.6) 1.02–1.79 * | 136.74 (84.43–181.15) 76.32–198.09 | 19.4 (18.6–24.5) 17.9–27.6 | 0.52 (0.49–0.53) 0.49–0.53 * |
| Sample | Haemolysis, % Median (25th and 75th Quartiles) Range | Maximum Platelet Aggregation, % Median (25th and 75th Quartiles) Range |
|---|---|---|
| Pure Platelet-rich Plasma (Baseline Platelet Aggregation) | - | 14.61 (13.63–17.72) 9.43–20.64 |
| Unmodified Grafts | 0 (0–0) 0–0 | 17.25 (16.3–17.96) 15.89–18.63 |
| Amine1Pep1 | 0.36 (0–0.72) 0–0.72 | 24.09 (24.09–25.65) 23.44–26.28 * |
| Amine1Pep2 | 0.36 (0.36–0.72) 0.36–0.72 | 20.12 (19.56–21.19) 15.34–23.44 |
| Amine1Pep3 | 0.36 (0–0.36) 0–0.36 | 22.54 (22.49–23.74) 18.04–31.32 * |
| Amine2Pep1 | 0.36 (0–0.72) 0–0.72 | 21.58 (21.44–24.35) 21.19–24.39 * |
| Amine2Pep2 | 0.72 (0–1.08) 0–1.08 | 20.74 (19.95–23.59) 19.24–23.94 * |
| Amine2Pep3 | 0.72 (0–0.72) 0–0.72 | 22.24 (22.10–24.27) 22.04–25.54 * |
| Sample | Platelet Type Ratio, % | Platelet Deformation Index Median (25th and 75th Quartiles) | ||||
|---|---|---|---|---|---|---|
| I | II | III | IV | V | ||
| Unmodified Grafts | 0 | 15.4 | 73.1 | 9.6 | 1.9 | 2.7 (1.0–3.0) |
| Amine1Pep1 | 5.6 | 36.1 | 25 | 30.6 | 2.8 | 2.5 (2.0–3.0) |
| Amine1Pep2 | 1.5 | 11.4 | 50 | 31.4 | 5.7 | 3.31 (3.0–3.7) * |
| Amine1Pep3 | 0 | 10.8 | 43.1 | 30.7 | 15.4 | 3.7 (3.4–4.5) * |
| Amine2Pep1 | 3.1 | 31.3 | 28.1 | 15.6 | 21.9 | 2.6 (1.0–3.7) |
| Amine2Pep2 | 0 | 65.5 | 24.2 | 3.4 | 6.9 | 1.3 (0.0–2.2) |
| Amine2Pep3 | 4.8 | 28.6 | 40.5 | 7.1 | 19.0 | 2.9 (2.5–4.0) |
| Markers | CD31, % Range | vWF, % Range | KDR, % Range | CD146, % Range | CD34, % Range | CD133, % Range | CD3, % Range | CD14, % Range | CD45, % Range | HLA-DR, % Range |
|---|---|---|---|---|---|---|---|---|---|---|
| 3rd passage human ECFCs | 99.8 (99.1–100) | 94.1 (91.6–96.2) | 60.2 (48.4–68.0) | 99.5 (98.7–99.8) | 2.2 (0.1–8.9) | 0 (0–0.9) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Sample | Primary Patency | Thrombosis | Calcium Deposition | |||
|---|---|---|---|---|---|---|
| 1 Month | 3 Months | 1 Month | 3 Months | 1 Month | 3 Months | |
| Unmodified Grafts | 4/6 | 4/6 | 2/6 | 2/6 | 0/6 | 3/6 |
| Amine1Pep1 | 4/6 | 4/6 | 2/6 | 2/6 | 2/6 | 2/6 |
| Amine1Pep2 | 6/6 | 6/6 | 0/6 | 0/6 | 1/6 | 3/6 |
| Amine1Pep3 | 6/6 | 6/6 | 0/6 | 0/6 | 0/6 | 3/6 |
| Amine2Pep1 | 6/6 | 6/6 | 0/6 | 0/6 | 0/6 | 2/6 |
| Amine2Pep2 | 6/6 | 6/6 | 0/6 | 0/6 | 3/6 | 3/6 |
| Amine2Pep3 | 6/6 | 6/6 | 0/6 | 0/6 | 0/6 | 2/6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonova, L.V.; Silnikov, V.N.; Sevostyanova, V.V.; Yuzhalin, A.E.; Koroleva, L.S.; Velikanova, E.A.; Mironov, A.V.; Godovikova, T.S.; Kutikhin, A.G.; Glushkova, T.V.; et al. Biocompatibility of Small-Diameter Vascular Grafts in Different Modes of RGD Modification. Polymers 2019, 11, 174. https://doi.org/10.3390/polym11010174
Antonova LV, Silnikov VN, Sevostyanova VV, Yuzhalin AE, Koroleva LS, Velikanova EA, Mironov AV, Godovikova TS, Kutikhin AG, Glushkova TV, et al. Biocompatibility of Small-Diameter Vascular Grafts in Different Modes of RGD Modification. Polymers. 2019; 11(1):174. https://doi.org/10.3390/polym11010174
Chicago/Turabian StyleAntonova, Larisa V., Vladimir N. Silnikov, Victoria V. Sevostyanova, Arseniy E. Yuzhalin, Lyudmila S. Koroleva, Elena A. Velikanova, Andrey V. Mironov, Tatyana S. Godovikova, Anton G. Kutikhin, Tatiana V. Glushkova, and et al. 2019. "Biocompatibility of Small-Diameter Vascular Grafts in Different Modes of RGD Modification" Polymers 11, no. 1: 174. https://doi.org/10.3390/polym11010174
APA StyleAntonova, L. V., Silnikov, V. N., Sevostyanova, V. V., Yuzhalin, A. E., Koroleva, L. S., Velikanova, E. A., Mironov, A. V., Godovikova, T. S., Kutikhin, A. G., Glushkova, T. V., Serpokrylova, I. Y., Senokosova, E. A., Matveeva, V. G., Khanova, M. Y., Akentyeva, T. N., Krivkina, E. O., Kudryavtseva, Y. A., & Barbarash, L. S. (2019). Biocompatibility of Small-Diameter Vascular Grafts in Different Modes of RGD Modification. Polymers, 11(1), 174. https://doi.org/10.3390/polym11010174