Influence of Network Structure on the Crystallization Behavior in Chemically Crosslinked Hydrogels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of 8-arm PEG-Acrylate (8PEG)
2.3. Methods
2.4. 8PEG-UV Hydrogel Samples Preparation
2.5. 8PEG–NH3 Hydrogel Samples Preparation
2.6. 8PEG Macromonomer Film Preparation
3. Results and Discussion
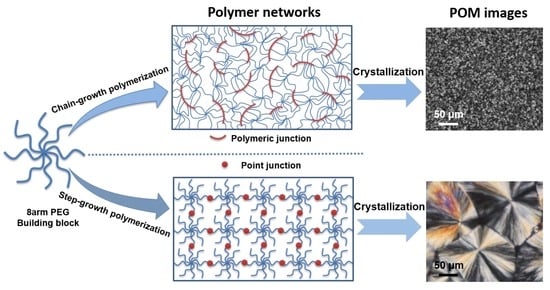
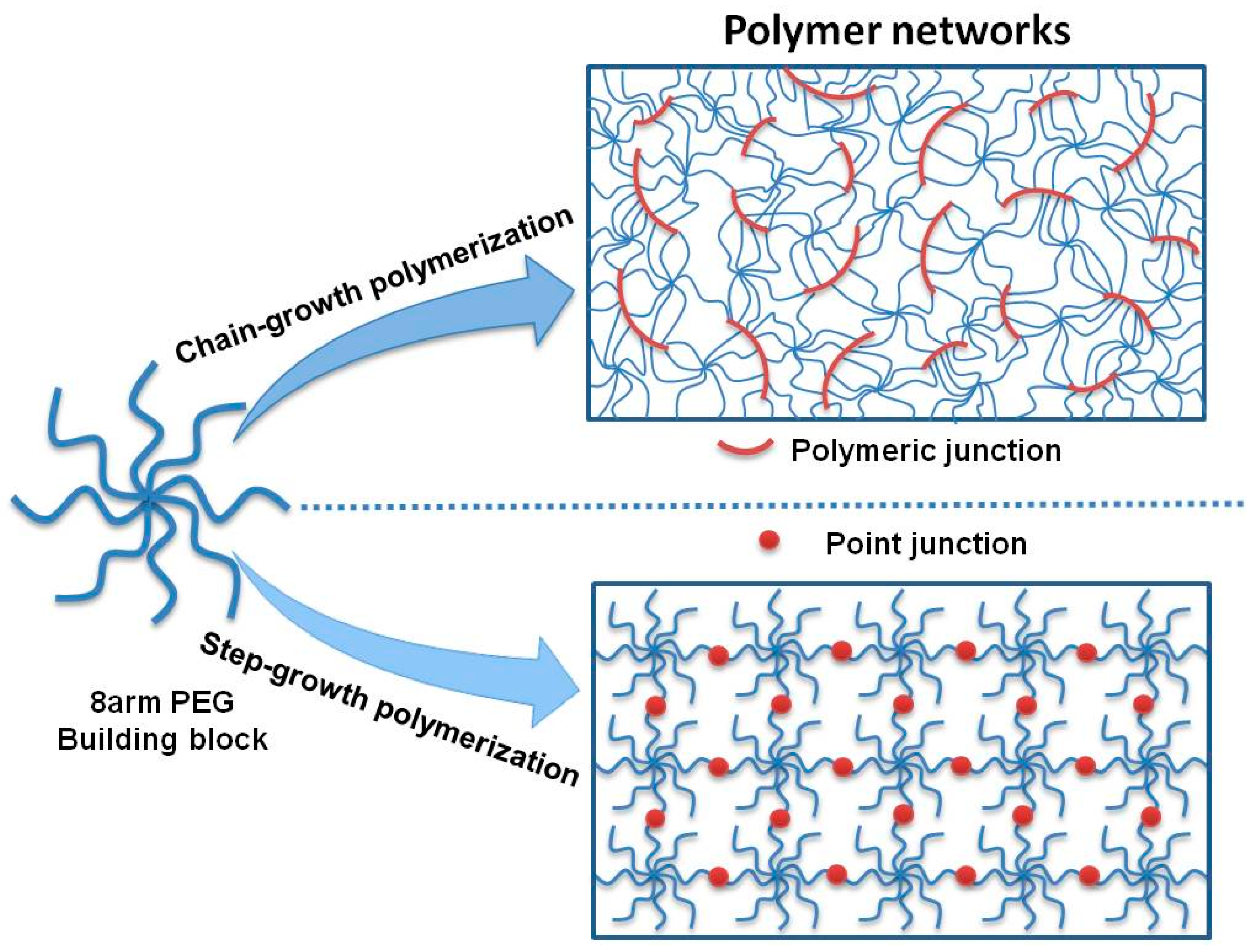
3.1. Hydrogel Formation and Network Structure
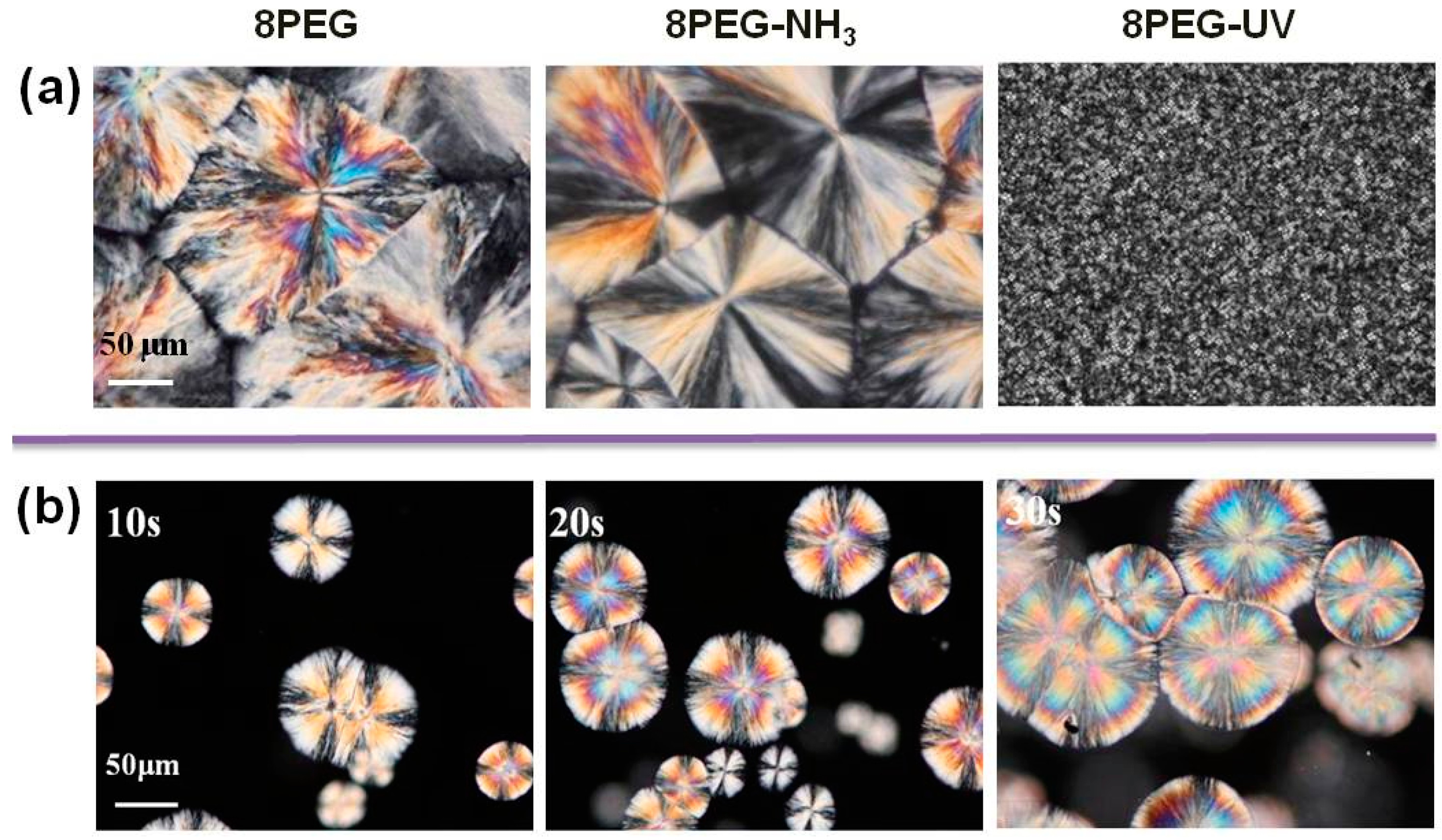
3.2. Spherulites Observation
3.3. Crystal Structure Characterization
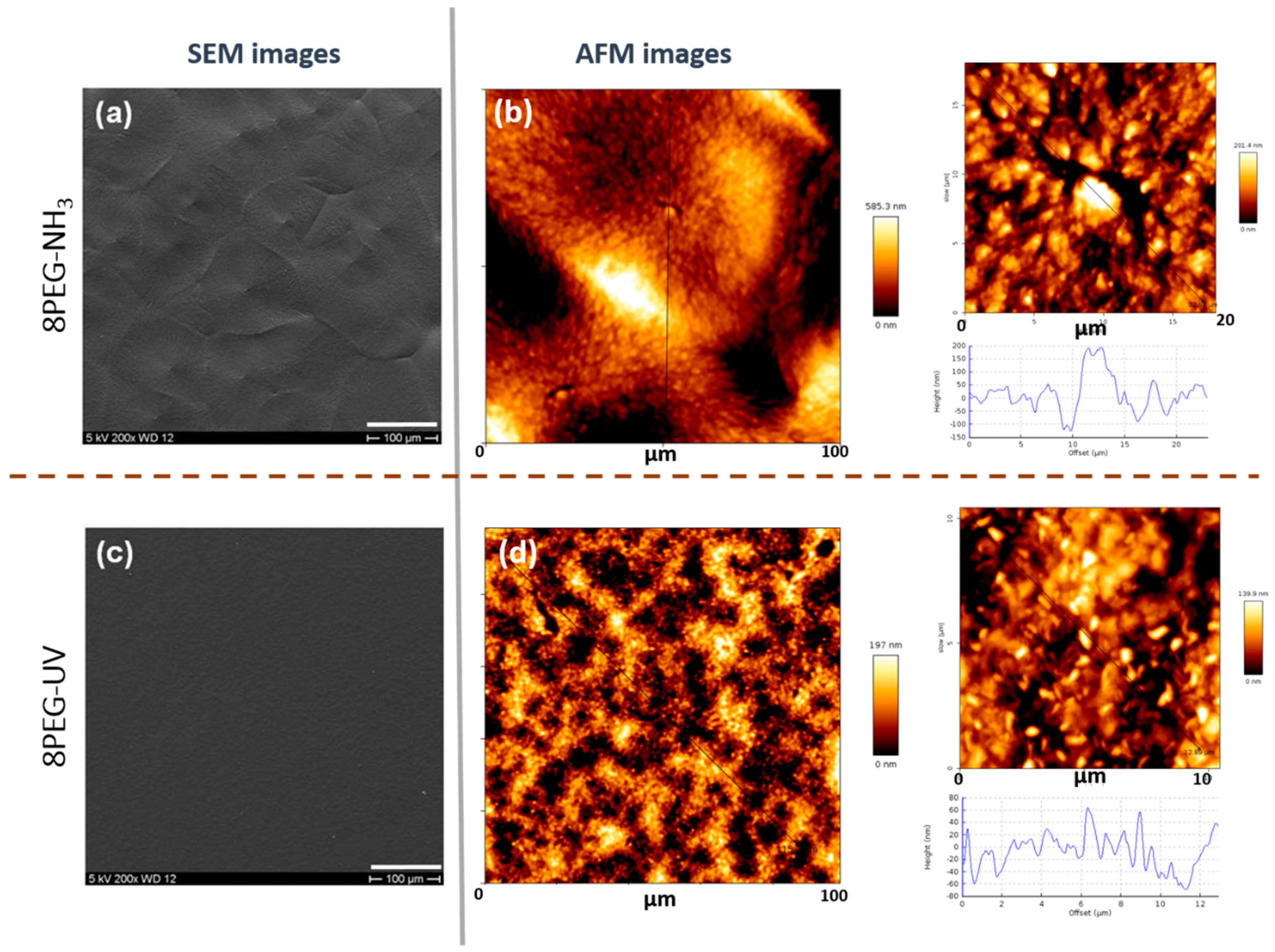
3.4. Investigation of the Surface Morphology
3.5. Hydration and Dehydration Dynamics
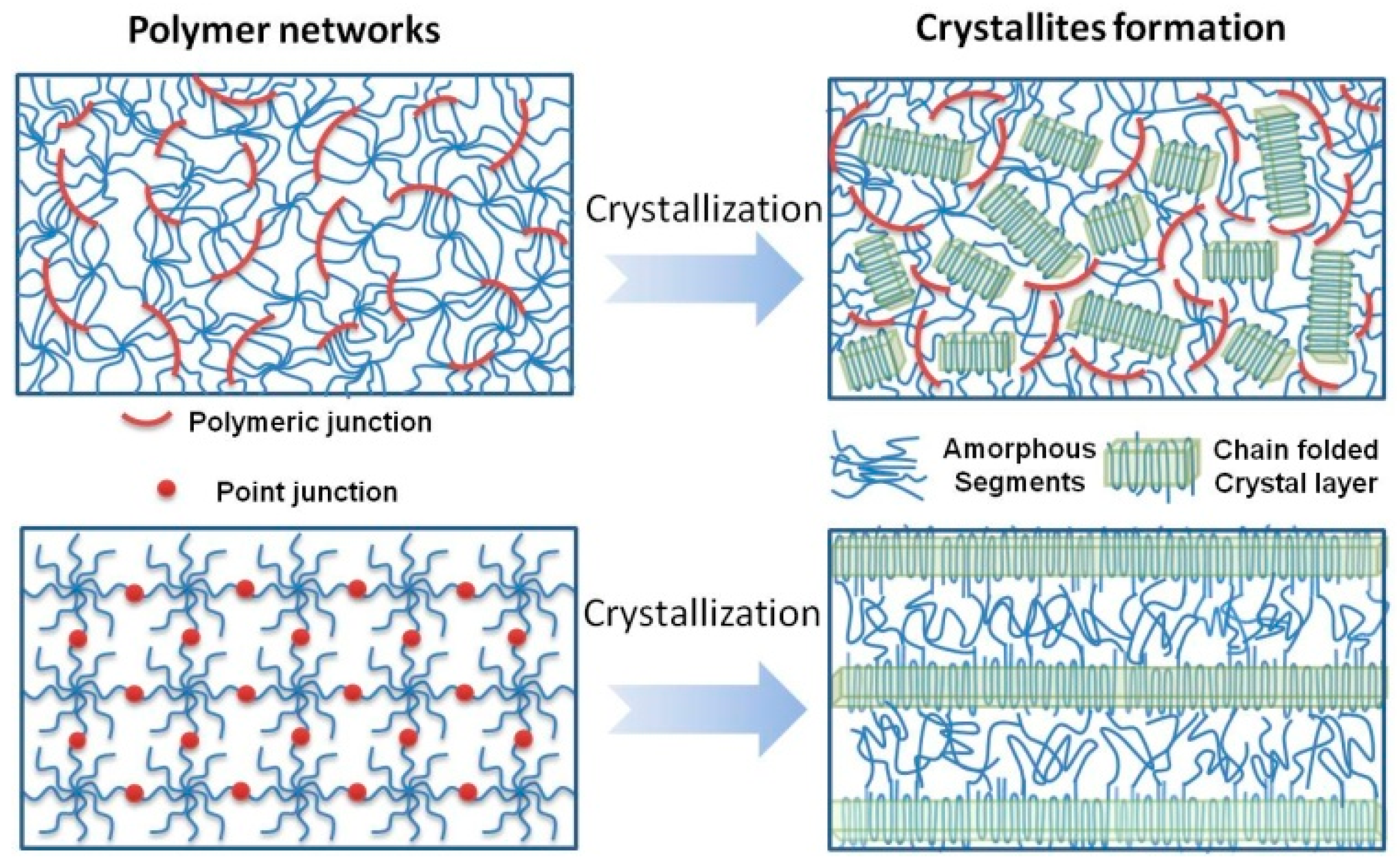
3.6. Proposed Crystallization Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hirst, A.R.; Escuder, B.; Miravet, J.F.; Smith, D.K. High-tech applications of self-assembling supramolecular nanostructured gel-phase materials: From regenerative medicine to electronic devices. Angew. Chem. Int. Ed. 2008, 47, 8002–8018. [Google Scholar] [CrossRef] [PubMed]
- Seliktar, D. Designing cell-compatible hydrogels for biomedical applications. Science 2012, 336, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, W.S.; Yu, X.Q.; Zhang, G.H.; Su, Z.Q. Synthesis and biomedical applications of fluorescent nanogels. Polym. Chem. 2016, 7, 5749–5762. [Google Scholar] [CrossRef]
- Lee, K.Y.; Yuk, S.H. Polymeric protein delivery systems. Prog. Polym. Sci. 2007, 32, 669–697. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, W.; Zhang, P.; Su, Z. Fabrication technologies and sensing applications of graphene-based composite films: Advances and challenges. Biosens. Bioelectron. 2017, 89, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, W.; Yu, X.; Wang, Z.; Wei, G.; Su, Z. When biomolecules meet graphene: From molecular level interactions to material design and applications. Nanoscale 2016, 8, 19491–19509. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, S.; Zhang, Z.; Löbus, A.; Strehmel, C.; Lensen, M.C. Blending PEG-based polymers and their use in surface micro-patterning by the FIMIC method to obtain topographically smooth patterns of elasticity. Biomater. Sci. 2014, 2, 410–418. [Google Scholar] [CrossRef]
- Wu, Z.L.; Gong, J.P. Hydrogels with self-assembling ordered structures and their functions. NPG Asia Mater. 2011, 3, 57–64. [Google Scholar] [CrossRef]
- Shibayama, M.; Norisuye, T. Gel formation analyses by dynamic light scattering. Bull. Chem. Soc. Jpn. 2002, 75, 641–659. [Google Scholar] [CrossRef]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 13–36. [Google Scholar] [CrossRef]
- Zalipsky, S.; Harris, J.M. Introduction to chemistry and biological applications of poly(ethylene glycol). In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1997; Volume 680, pp. 1–13. [Google Scholar]
- Cazalbou, S.; Combes, C.; Eichert, D.; Rey, C. Adaptative physico-chemistry of bio-related calcium phosphates. J. Mater. Chem. 2004, 14, 2148. [Google Scholar] [CrossRef]
- Ren, F.; Yesildag, C.; Zhang, Z.; Lensen, M.C. Surface Patterning of Gold Nanoparticles on PEG-Based Hydrogels to Control Cell Adhesion. Polymers 2017, 9, 154. [Google Scholar] [CrossRef]
- Armand, M.; Endres, F.; MacFarlane, D.R.; Ohno, H.; Scrosati, B. Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater. 2009, 8, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, S.; Jongerius, A.; Loebus, A.; Strehmel, C.; Zhang, Z.; Lensen, M.C. AFM Characterization of Elastically Micropatterned Surfaces Fabricated by Fill-Molding in Capillaries (FIMIC) and Investigation of the Topographical Influence on Cell Adhesion to the Patterns. Adv. Eng. Mater. 2012, 14, B56–B66. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, C.; Duan, J.; Wang, Y.; Liu, J.; Wang, L.; Kong, D. Poly(ethylene glycol) analogs grafted with low molecular weight poly(ethylene imine) as non-viral gene vectors. Acta Biomater. 2010, 6, 2650–2657. [Google Scholar] [CrossRef] [PubMed]
- Birnie, D.P.; Hau, S.; Kamber, D.; Kaz, D. Effect of ramping-up rate on film thickness for spin-on processing. J. Mater. Sci. Mater. Electron. 2005, 16, 715–720. [Google Scholar] [CrossRef]
- Birnie, D.P. Rational solvent selection strategies to combat striation formation during spin coating of thin films. J. Mater. Res. 2001, 16, 1145–1154. [Google Scholar] [CrossRef]
- Dalnoki-Veress, K.; Forrest, J.A.; Stevens, J.R.; Dutcher, J.R. Phase separation morphology of spin-coated polymer blend thin films. Phys. A Stat. Mech. Appl. 1997, 239, 87–94. [Google Scholar] [CrossRef]
- Dalnoki-Veress, K.; Forrest, J.A.; Stevens, J.R.; Dutcher, J.R. Phase separation morphology of thin films of polystyrene/polyisoprene blends. J. Polym. Sci. Part. B Polym. Phys. 1996, 34, 3017–3024. [Google Scholar] [CrossRef]
- Hobbs, J.K.; Vasilev, C.; Humphris, A.D. Real time observation of crystallization in polyethylene oxide with video rate atomic force microscopy. Polymer 2005, 46, 10226–10236. [Google Scholar] [CrossRef]
- Alfonso, J.C.; Russell, T.P. Kinetics of crystallization in semicrystalline/amorphous polymer mixtures. Macromolecules 1986, 19, 1143–1152. [Google Scholar] [CrossRef]
- Cheng, S.Z.D.; Wu, S.S.; Chen, J.; Zhuo, Q.; Quirk, R.P.; Meerwall, E.D.V.; Hsiao, B.S.; Habenschuss, A.; Zschack, P.R. Isothermal thickening and thinning processes in low-molecular-weight poly(ethylene oxide) fractions crystallized from the melt. 4. End-group dependence. Macromolecules 1993, 26, 5105–5117. [Google Scholar] [CrossRef]
- Schoenherr, H.; Frank, C.W. Ultrathin Films of Poly(ethylene oxides) on Oxidized Silicon. 1. Spectroscopic Characterization of Film Structure and Crystallization Kinetics. Macromolecules 2003, 36, 1188–1198. [Google Scholar] [CrossRef]
- Mya, K.Y.; Pramoda, K.P.; He, C.B. Crystallization behavior of star-shaped poly(ethylene oxide) with cubic silsesquioxane (CSSQ) core. Polymer 2006, 47, 5035–5043. [Google Scholar] [CrossRef]
- Toolan, D.T.W.; Isakova, A.; Hodgkinson, R.; Reeves-Mclaren, N.; Hammond, O.S.; Edler, K.J.; Briscoe, W.H.; Arnold, T.; Gough, T.; Topham, P.D.; et al. Insights into the Influence of Solvent Polarity on the Crystallization of Poly(ethylene oxide) Spin-Coated Thin Films via in Situ Grazing Incidence Wide-Angle X-ray Scattering. Macromolecules 2016, 49, 4579–4586. [Google Scholar] [CrossRef]
- Pulst, M.; Samiullah, M.H.; Baumeister, U.; Prehm, M.; Balko, J.; Thurn-Albrecht, T.; Busse, K.; Golitsyn, Y.; Reichert, D.; Kressler, J. Crystallization of Poly(ethylene oxide) with a Well-Defined Point Defect in the Middle of the Polymer Chain. Macromolecules 2016, 49, 6609–6620. [Google Scholar] [CrossRef]
- Kaneko, T.; Yamaoka, K.; Gong, J.P.; Osada, Y. Liquid-Crystalline Hydrogels. 1. Enhanced Effects of Incorporation of Acrylic Acid Units on the Liquid-Crystalline Ordering. Macromolecules 2000, 33, 412–418. [Google Scholar] [CrossRef]
- Kaneko, T.; Yamaoka, K.; Osada, Y.; Gong, J.P. Thermoresponsive shrinkage triggered by mesophase transition in liquid crystalline physical hydrogels. Macromolecules 2004, 37, 5385–5388. [Google Scholar] [CrossRef]
- Kato, T.; Hirai, Y.; Nakaso, S.; Moriyama, M. Liquid-crystalline physical gels. Chem. Soc. Rev. 2007, 36, 1857–1867. [Google Scholar] [CrossRef] [PubMed]
- Lin-Gibson, S.; Jones, R.L.; Washburn, N.R.; Horkay, F. Structure-property relationships of photopolymerizable poly(ethylene glycol) dimethacrylate hydrogels. Macromolecules 2005, 38, 2897–2902. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J. Statistical mechanics of cross-linked polymer networks I. Rubberlike elasticity. J. Chem. Phys. 1943, 11, 512–520. [Google Scholar] [CrossRef]
- Zhang, Z.; Loebus, A.; de Vicente, G.; Ren, F.; Arafeh, M.; Ouyang, Z.; Lensen, M.C. Synthesis of Poly(ethylene glycol)-based Hydrogels via Amine-Michael Type Addition with Tunable Stiffness and Postgelation Chemical Functionality. Chem. Mater. 2014, 26, 3624–3630. [Google Scholar] [CrossRef]
- Ren, F.; Yesildag, C.; Zhang, Z.; Lensen, M.C. Functional PEG-Hydrogels Convey Gold Nanoparticles from Silicon and Aid Cell Adhesion onto the Nanocomposites. Chem. Mater. 2017, 29, 2008–2015. [Google Scholar] [CrossRef]
- Chassé, W.; Schlögl, S.; Riess, G.; Saalwächter, K. Inhomogeneities and local chain stretching in partially swollen networks. Soft Matter 2013, 9, 6943–6954. [Google Scholar] [CrossRef]
- Weiss, N.; Van Vliet, T.T.; Silberberg, A. Influence of polymerization initiation rate on permeability of aqueous polyacrylamide gels. J. Polym. Sci. Polym. Phys. Ed. 1981, 19, 1505–1512. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Hubbell, J.A. Synthesis and physicochemical characterization of end-linked poly(ethylene glycol)-co-peptide hydrogels formed by Michael-type addition. Biomacromolecules 2003, 4, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Hild, G. Model networks based on “endlinking” processes: Synthesis, structure and properties. Prog. Polym. Sci. 1998, 23, 1019–1149. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Ouyang, Z.F.; Schulze, R.; Keller, T.F.; Jandt, K.D.; Su, Z. Pathway mediated microstructures and phase morphologies of asymmetric double crystalline co-oligomers. RSC Adv. 2014, 4, 7900–7910. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Schulze, R.; Zhang, P.P.; Lüdecke, C.; Zhang, X.Q.; Su, Z.; Jandt, K.D. How different mesophases affect the interactive crystallisation of a block co-oligomer. Polymer 2014, 55, 1893–1900. [Google Scholar] [CrossRef]
- Tadokoro, H.; Chatani, Y.; Yoshimura, T.; Tahara, S.; Murahashi, S. Structural studies on polyethers, [-(CH2)m-O-]n. II. Molecular structure of polyethylene oxide. Makromol. Chem. 1964, 73, 109–127. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Li, Q.; Yesildag, C.; Bartsch, C.; Zhang, X.; Liu, W.; Loebus, A.; Su, Z.; Lensen, M.C. Influence of Network Structure on the Crystallization Behavior in Chemically Crosslinked Hydrogels. Polymers 2018, 10, 970. https://doi.org/10.3390/polym10090970
Zhang Z, Li Q, Yesildag C, Bartsch C, Zhang X, Liu W, Loebus A, Su Z, Lensen MC. Influence of Network Structure on the Crystallization Behavior in Chemically Crosslinked Hydrogels. Polymers. 2018; 10(9):970. https://doi.org/10.3390/polym10090970
Chicago/Turabian StyleZhang, Zhenfang, Qian Li, Cigdem Yesildag, Christoph Bartsch, Xiaoyuan Zhang, Wei Liu, Axel Loebus, Zhiqiang Su, and Marga C. Lensen. 2018. "Influence of Network Structure on the Crystallization Behavior in Chemically Crosslinked Hydrogels" Polymers 10, no. 9: 970. https://doi.org/10.3390/polym10090970
APA StyleZhang, Z., Li, Q., Yesildag, C., Bartsch, C., Zhang, X., Liu, W., Loebus, A., Su, Z., & Lensen, M. C. (2018). Influence of Network Structure on the Crystallization Behavior in Chemically Crosslinked Hydrogels. Polymers, 10(9), 970. https://doi.org/10.3390/polym10090970