Droplet-Assisted Microfluidic Fabrication and Characterization of Multifunctional Polysaccharide Microgels Formed by Multicomponent Reactions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials for Microgel Synthesis
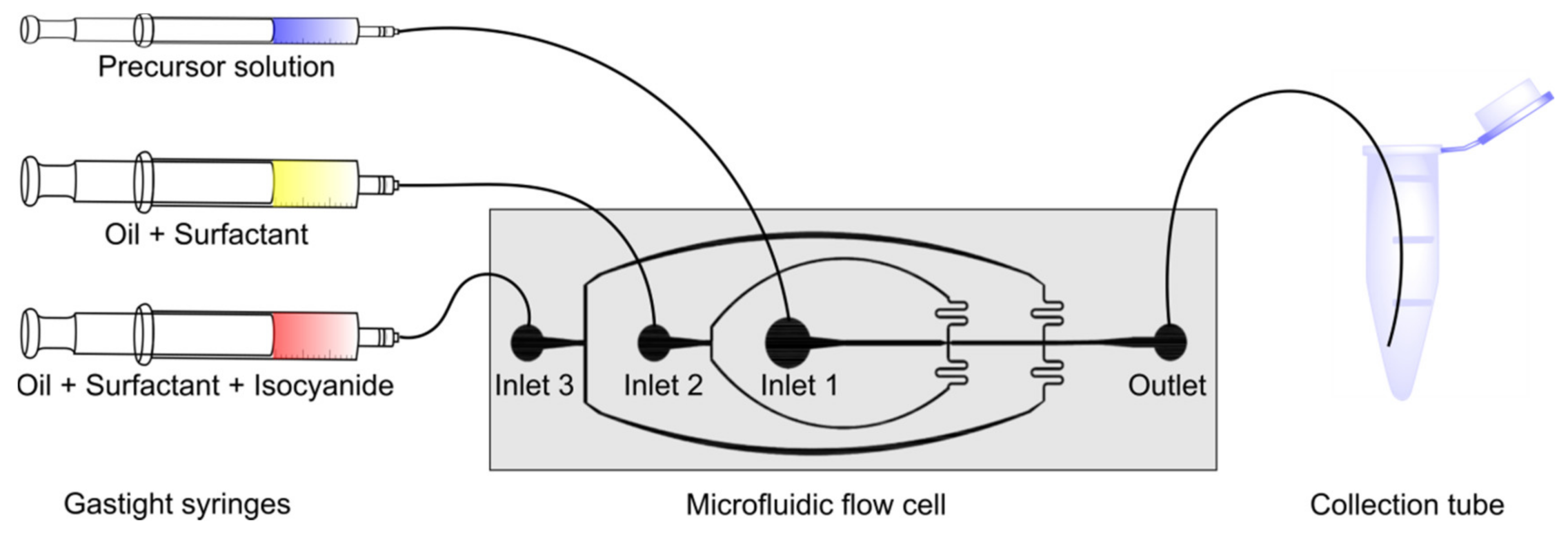
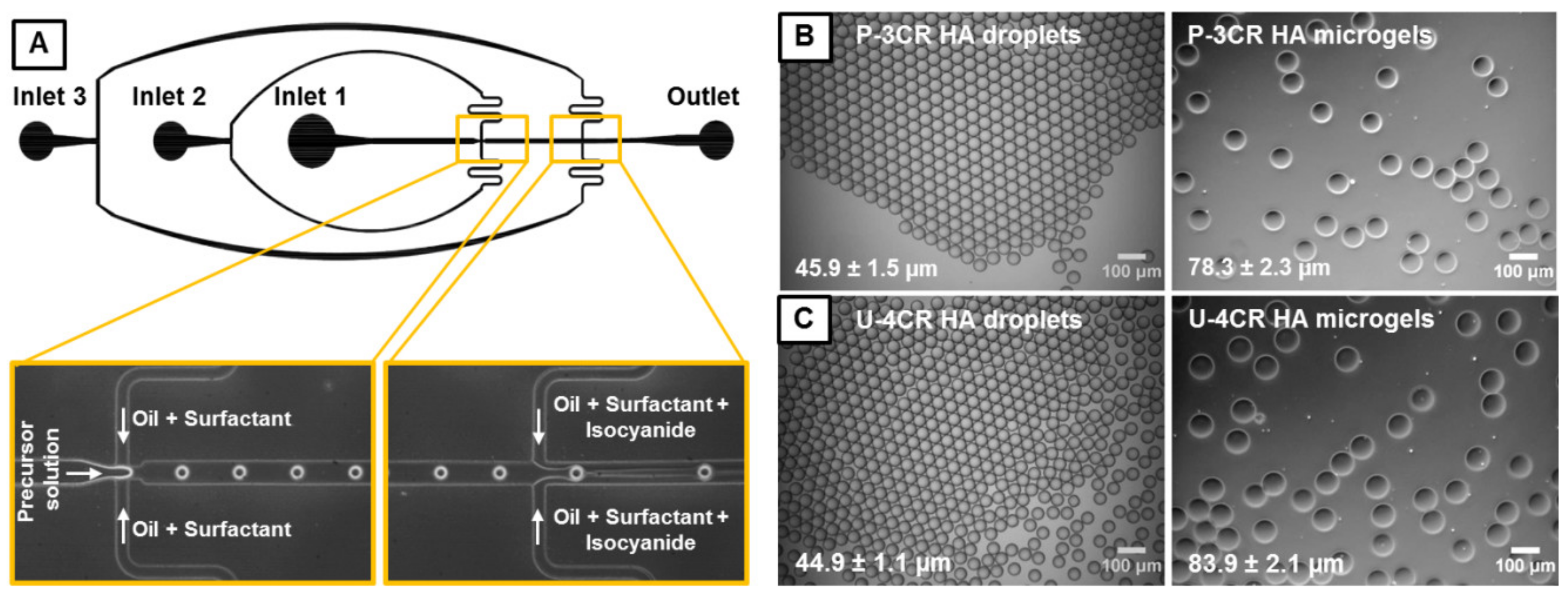
2.2. Microfluidic Device Fabrication and General Microfluidic Experimental Setup
2.3. Microfluidic Fabrication of P-3CR and U-4CR Microgels
2.3.1. General Procedure of Microgel Fabrication
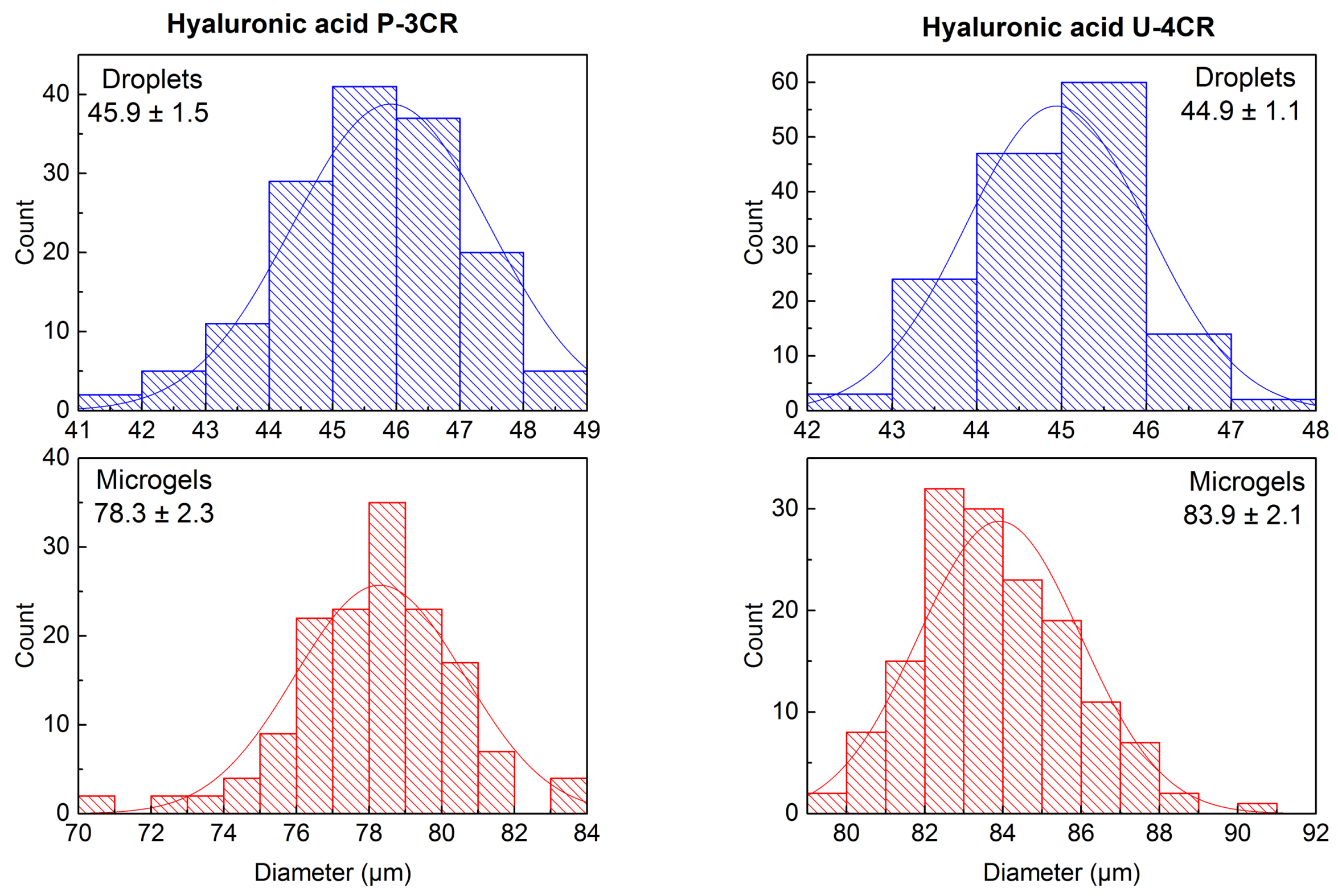
2.3.2. Hyaluronic Acid-Based P-3CR Microgels
2.3.3. Hyaluronic Acid-Based U-4CR Microgels
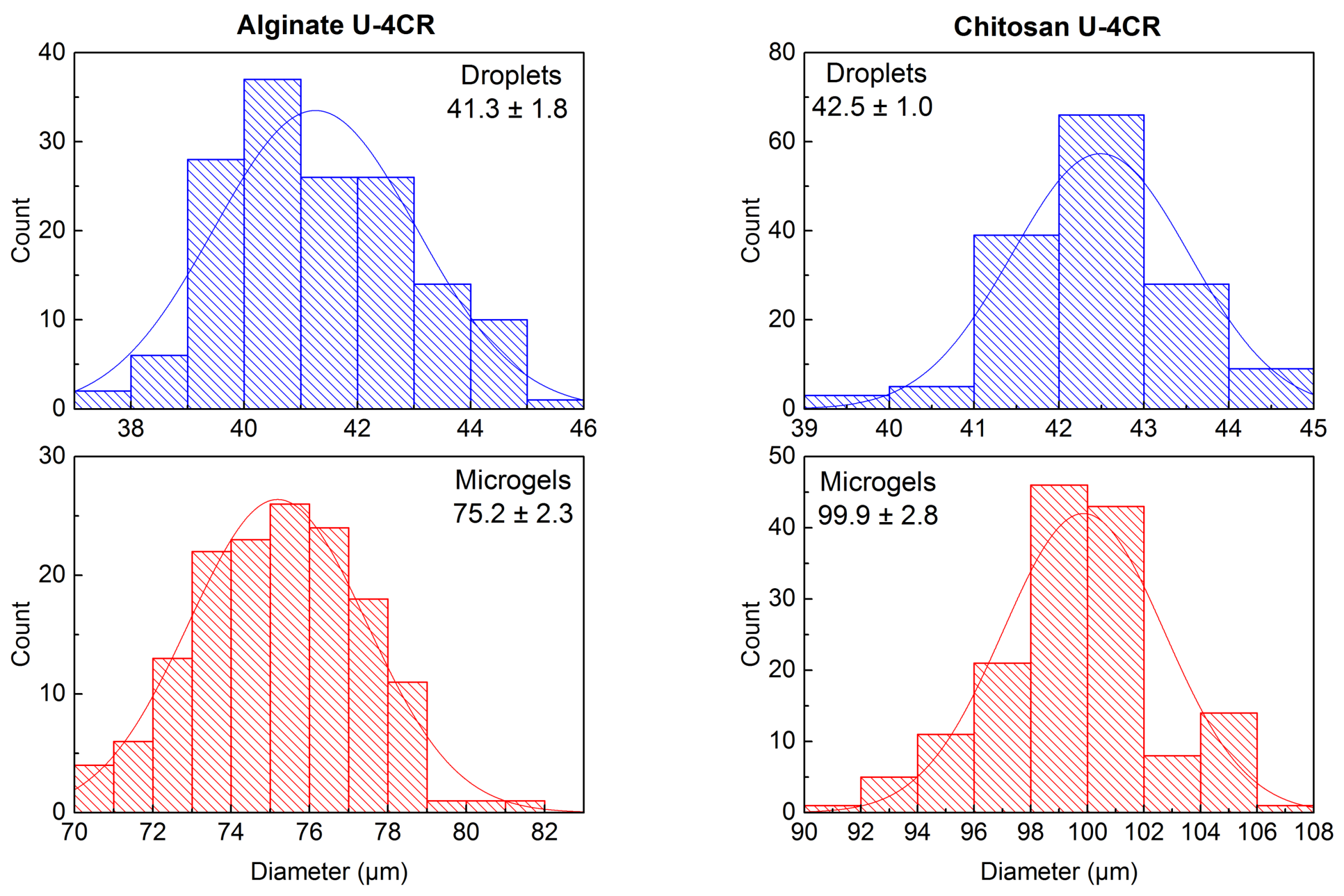
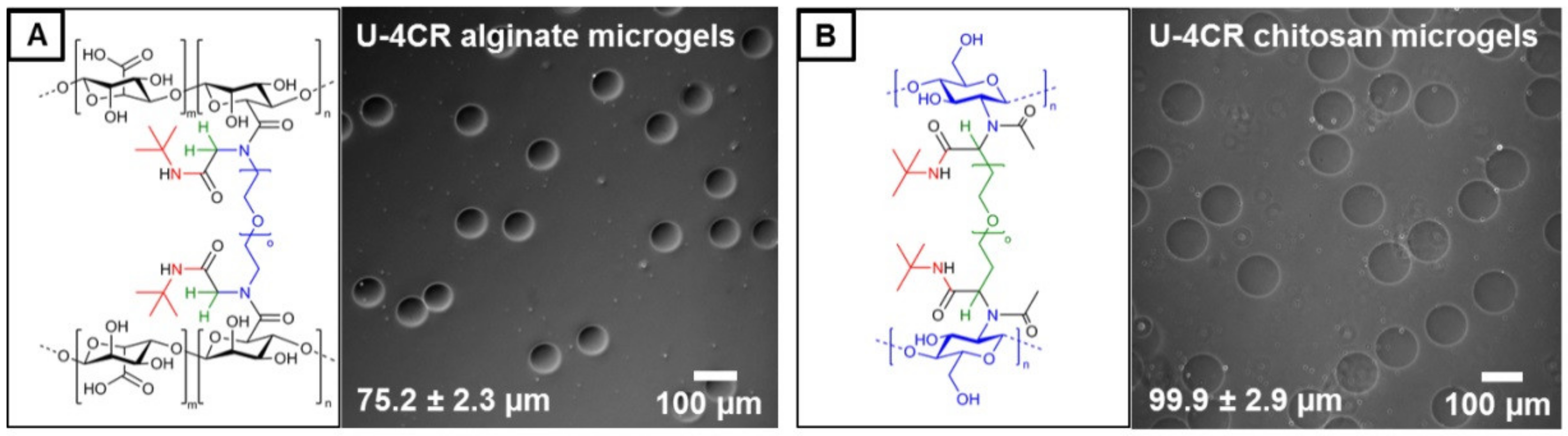
2.3.4. Alginate-Based U-4CR Microgels
2.3.5. Chitosan-Based U-4CR Microgels
2.3.6. Chitosan-Based, Biotin-Functionalized U-4CR Microgels
2.4. Immobilization and Activity Assay of Horseradish Peroxidase (HRP)
2.5. Colloidal Probe Atomic Force Microscopy (CP-AFM)
2.6. Confocal Brillouin Microscopy
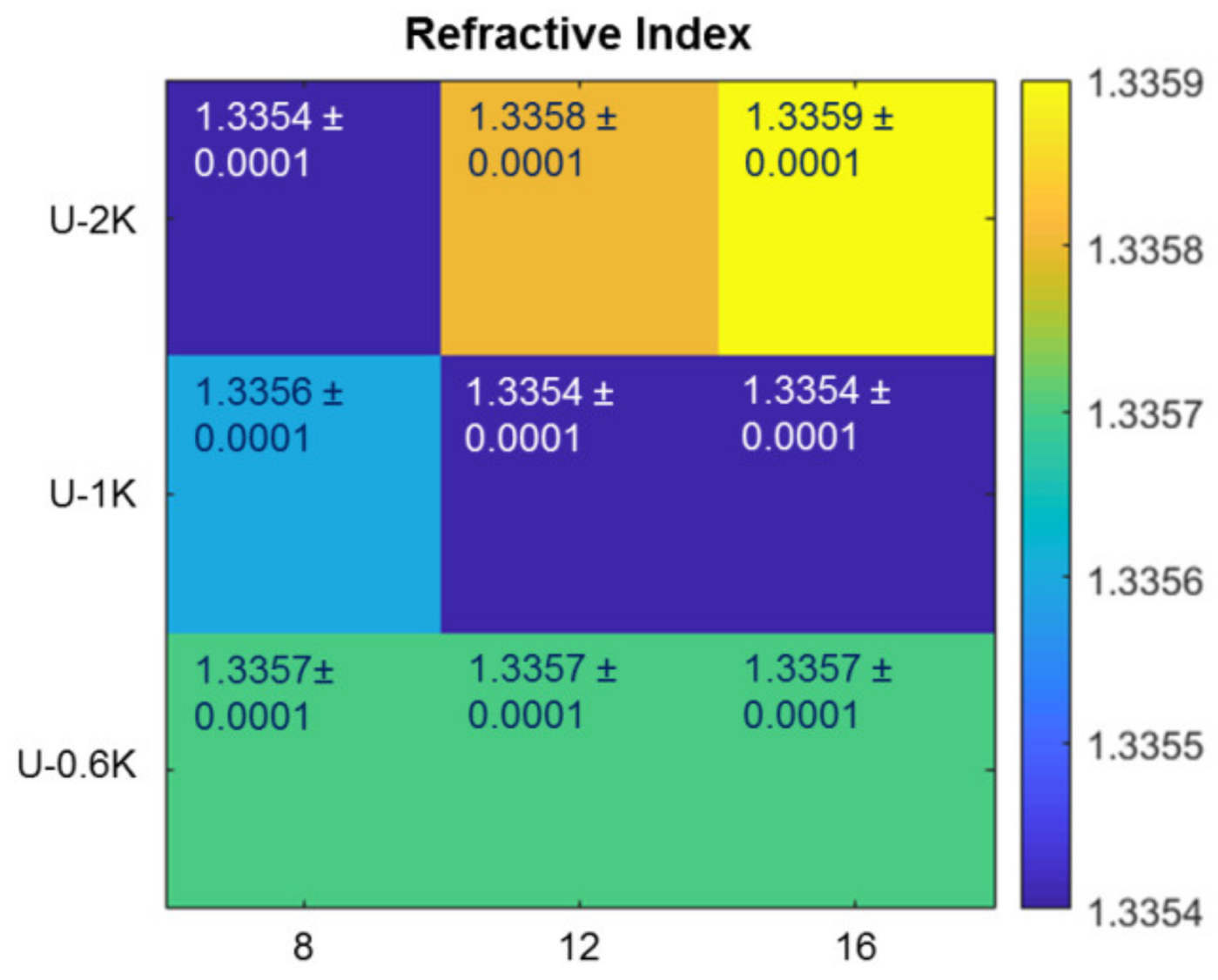
2.7. Refractive Index Measurement
2.8. Fluorescence Correlation Spectroscopy (FCS)
3. Results and Discussion
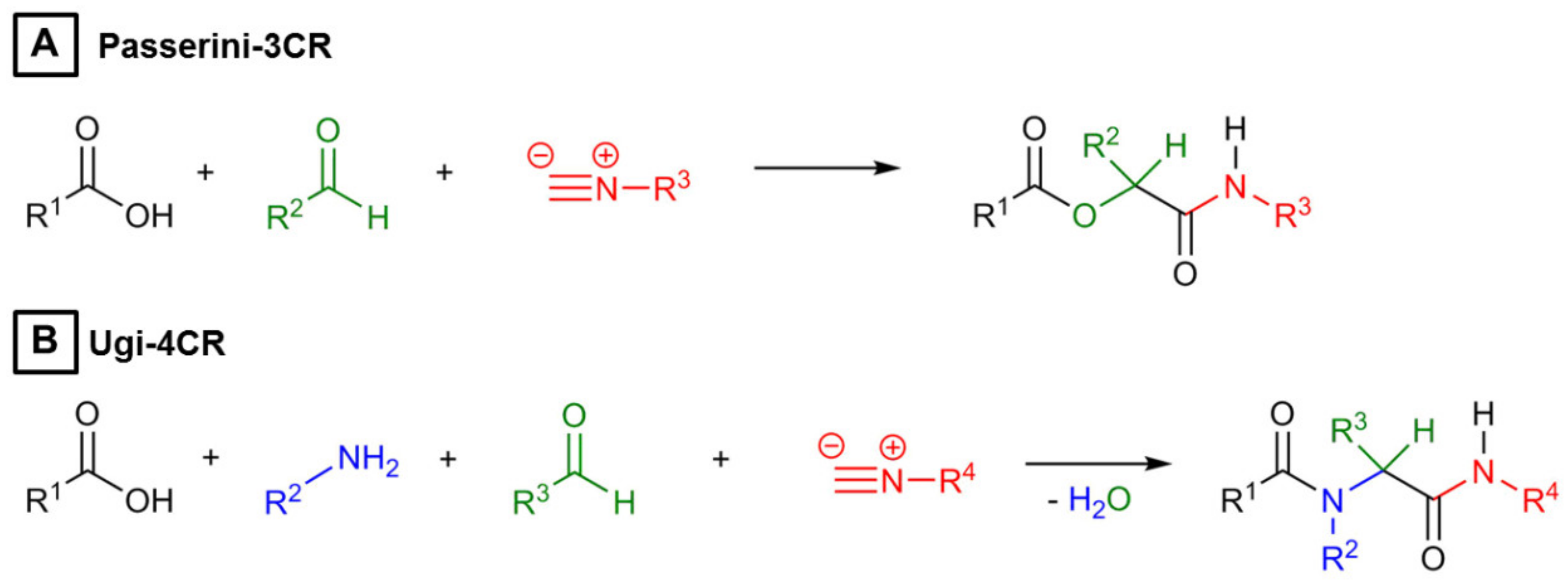
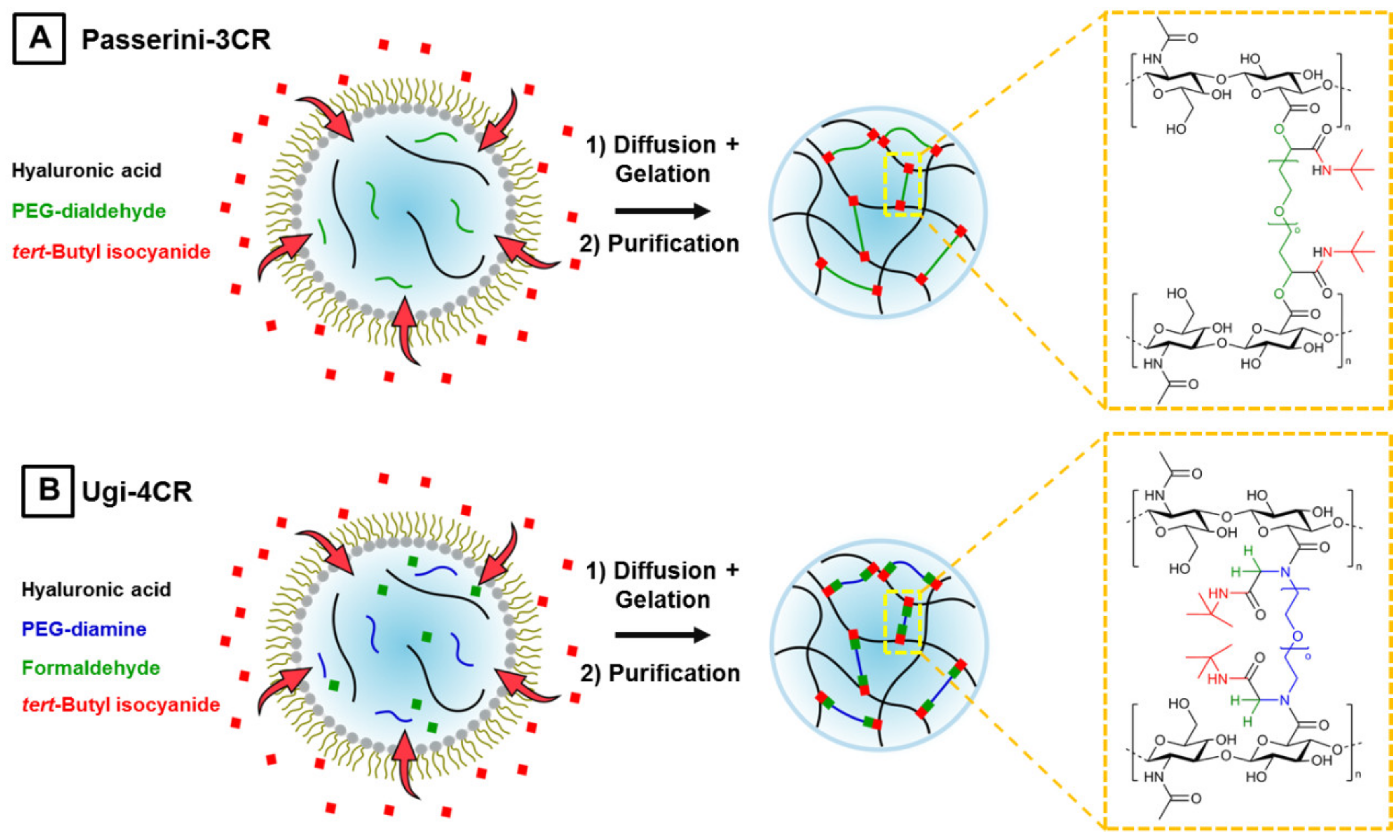
3.1. Microgel Preparation by Multicomponent Reactions via Droplet Microfluidics
3.2. In-Depth Analysis of Hyaluronic Acid-Based U-4CR Microgels
3.2.1. Screening Crosslinker Concentration and Molecular Weight in a Parameter Matrix
3.2.2. Young’s Moduli of Hyaluronic Acid-Based U-4CR Microgels Determined by CP-AFM
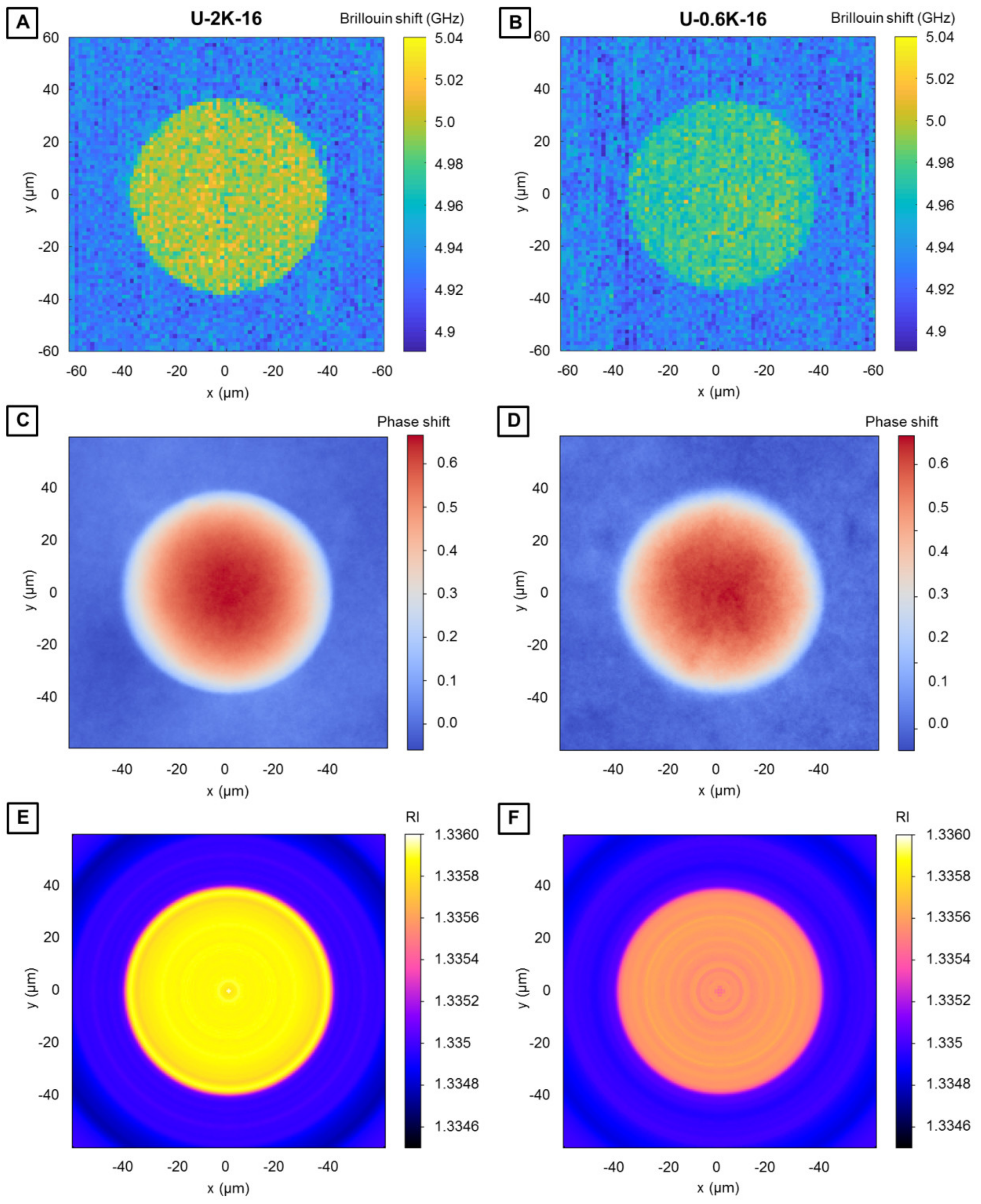
3.2.3. Analysis of Hyaluronic Acid-Based U-4CR Microgels by Optical Techniques
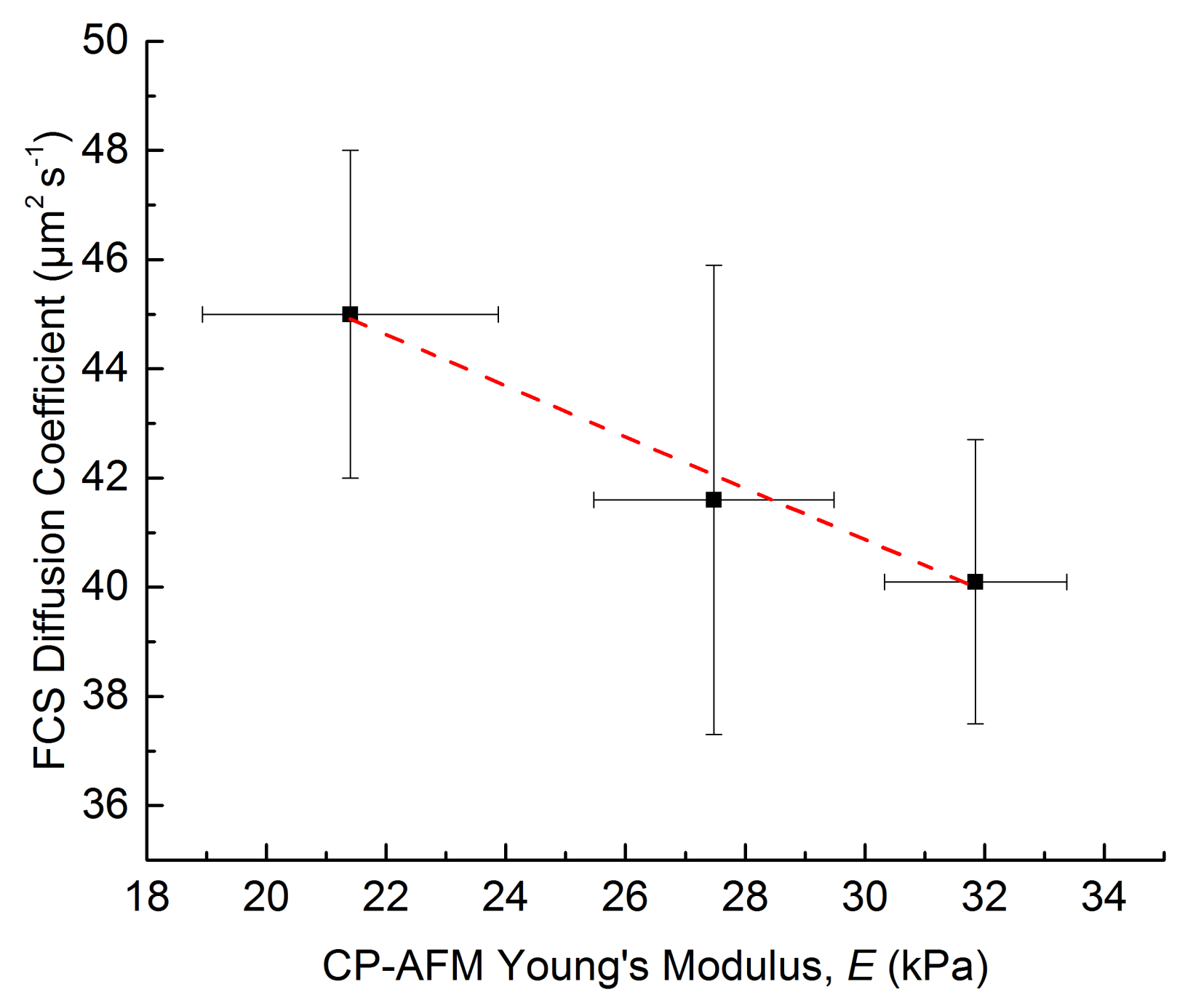
3.2.4. Diffusion Coefficients Determined by FCS and Conclusions on Microgel Network Structure
3.3. Introduction of Functionality in Chitosan-Based U-4CR Microgels
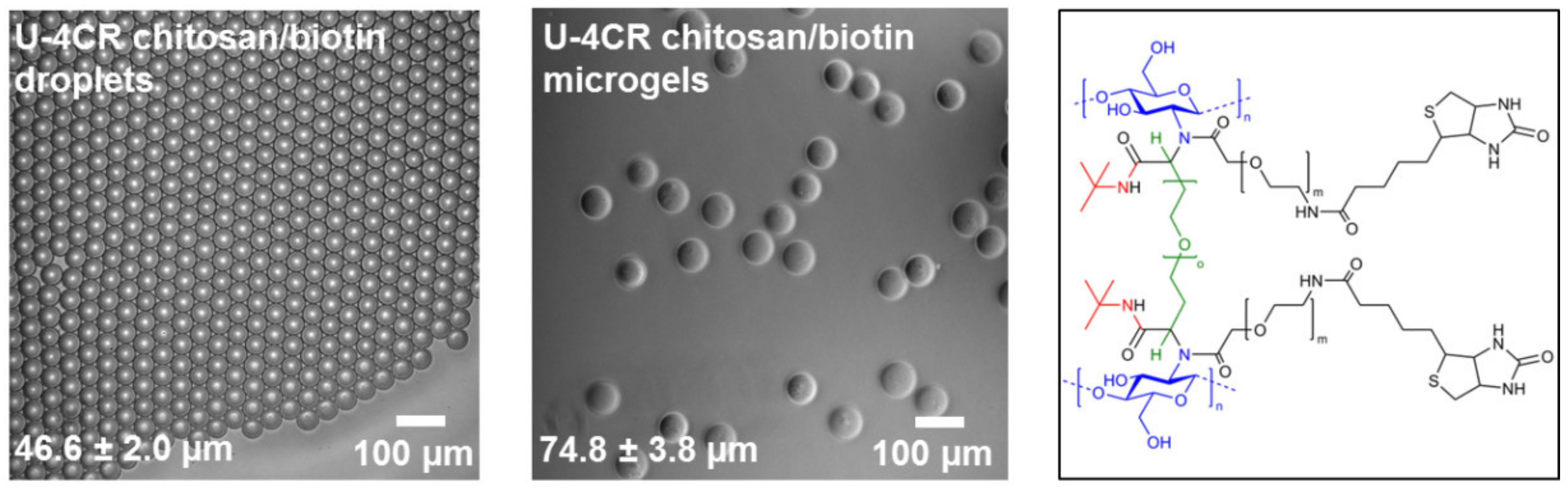
3.3.1. Biotin-Functionalized, Chitosan-Based U-4CR Microgels
3.3.2. Proof of Biotin Availability in Chitosan-Based Microgels by Streptavidin Conjugation
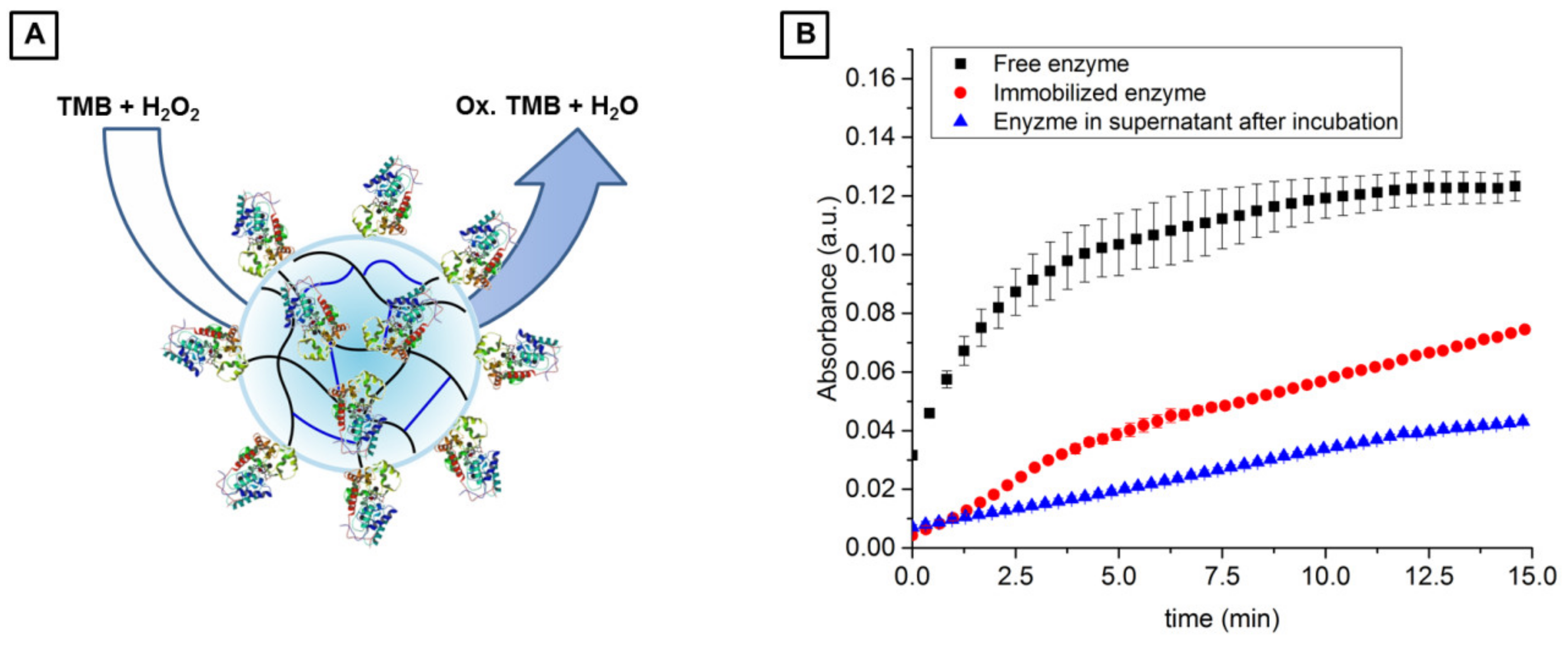
3.3.3. Application of Biotin-Functionalized, Chitosan-Based U-4CR Microgels for Enzyme Immobilization
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A
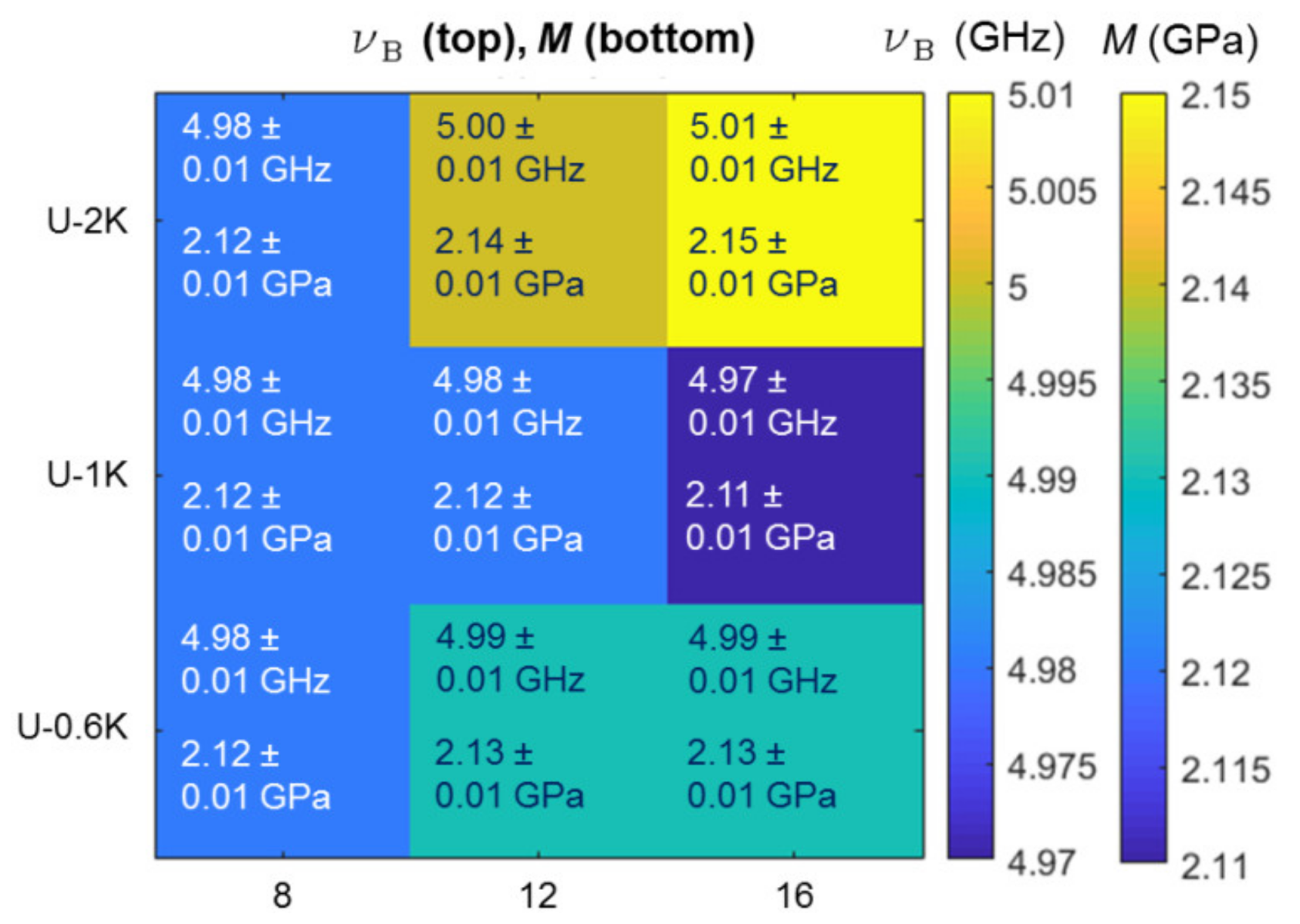
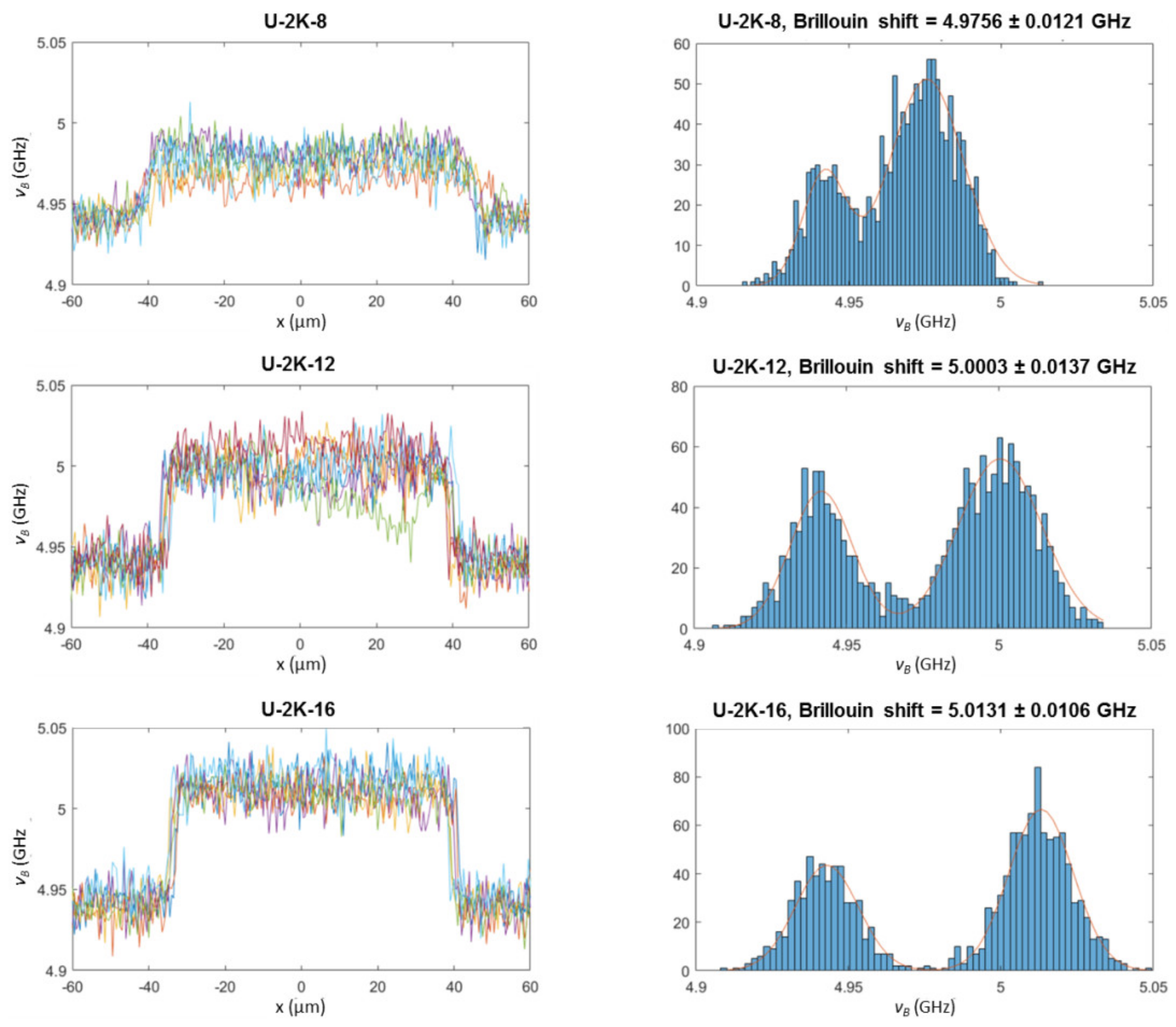
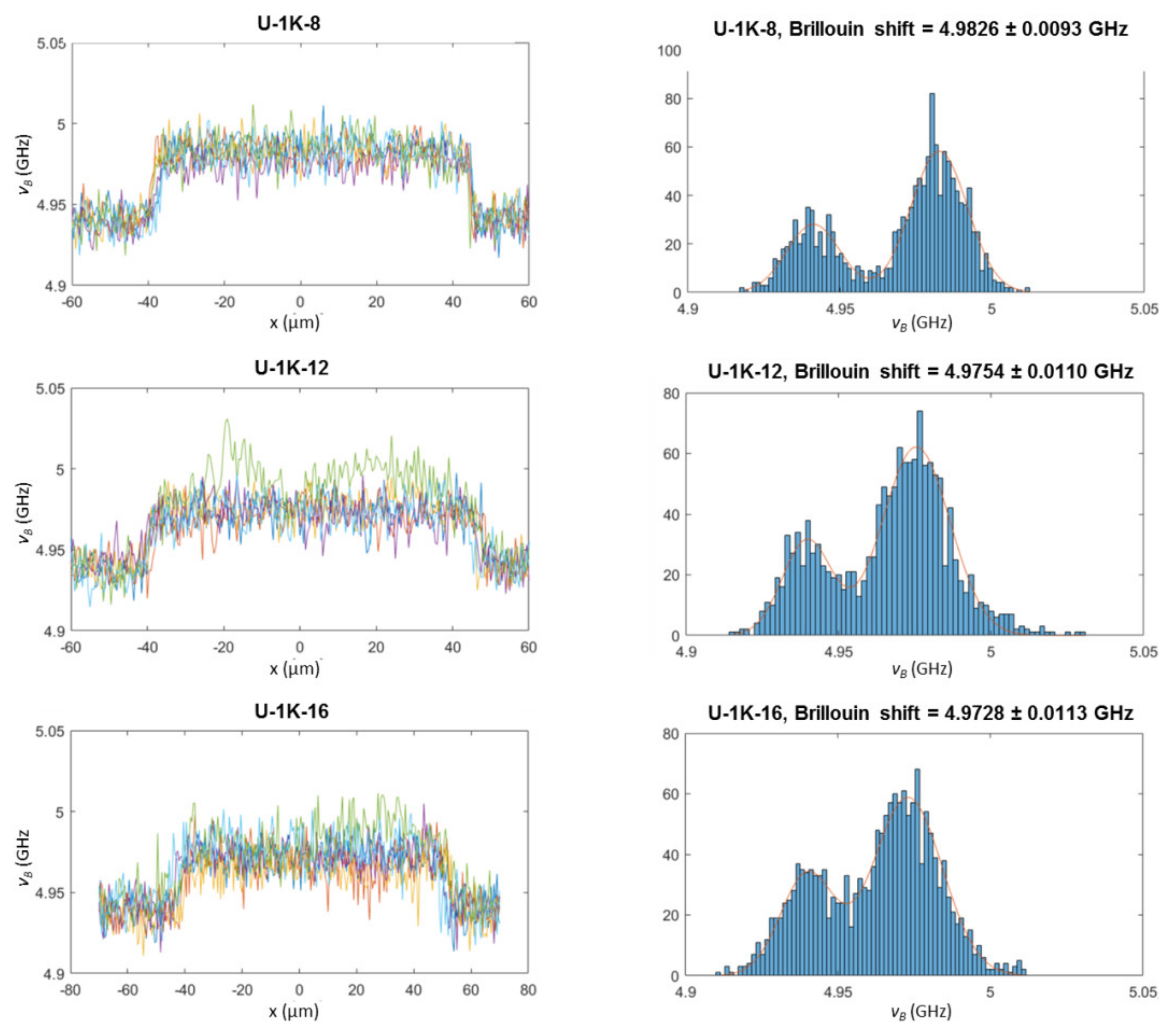
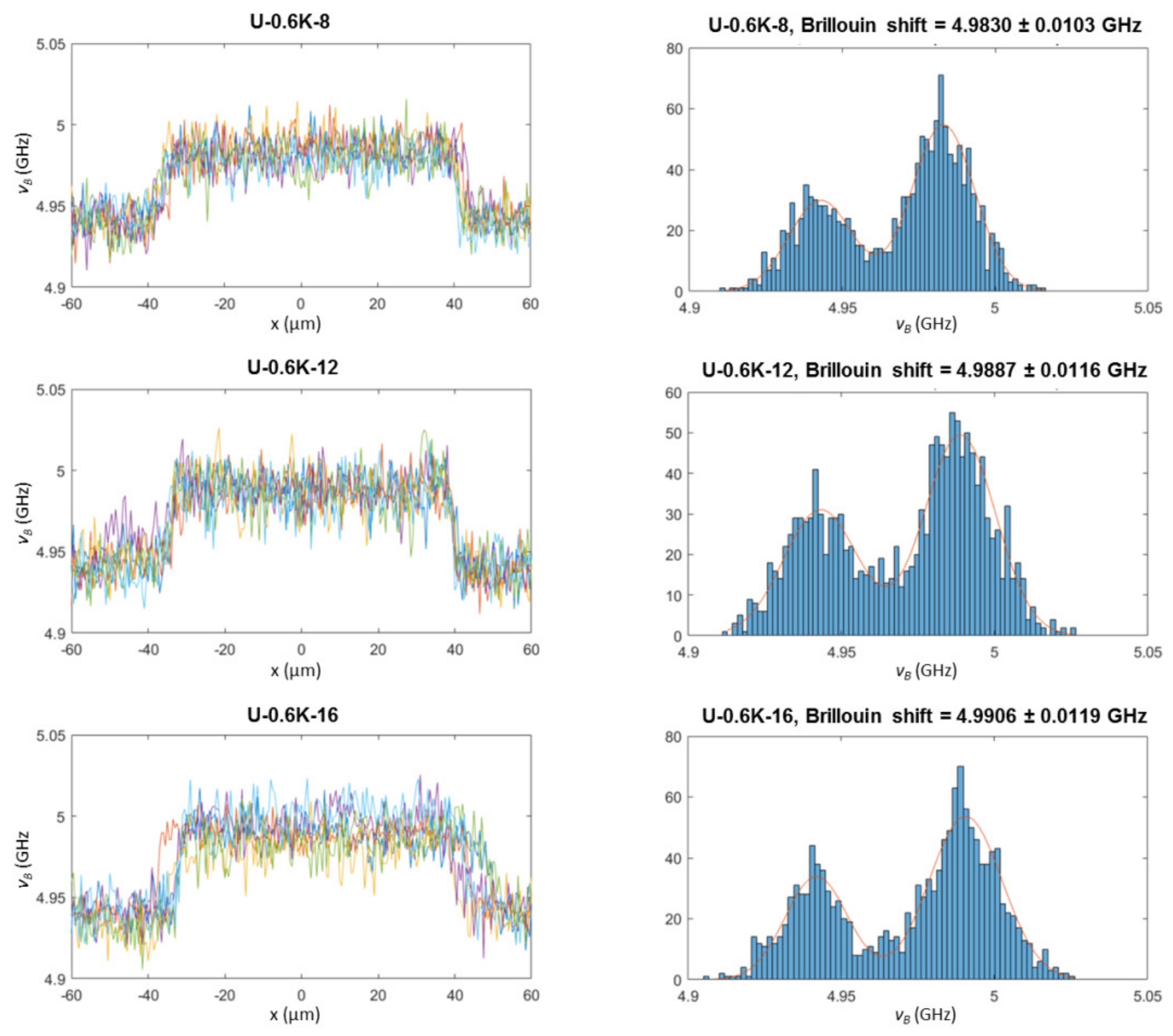
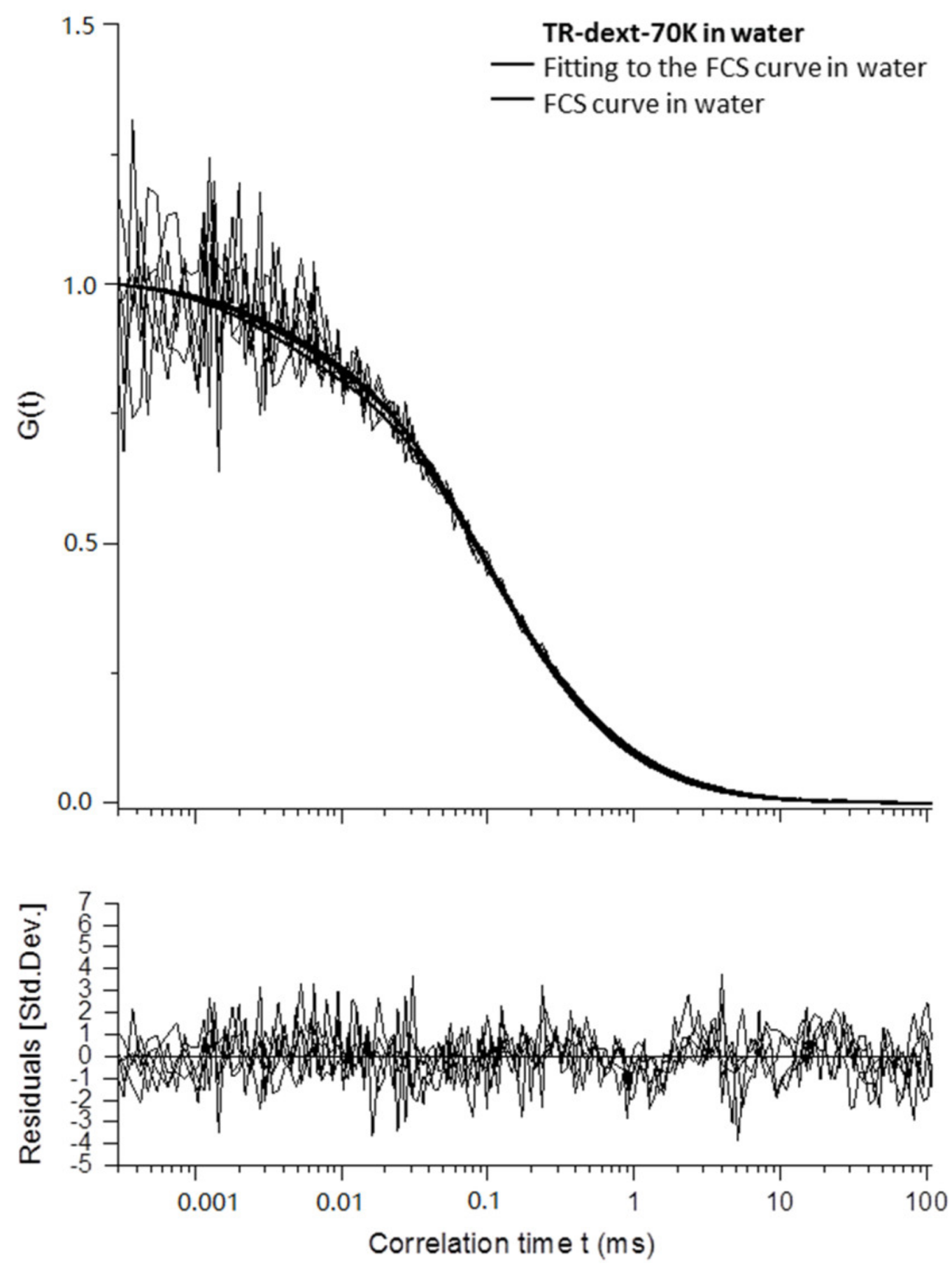
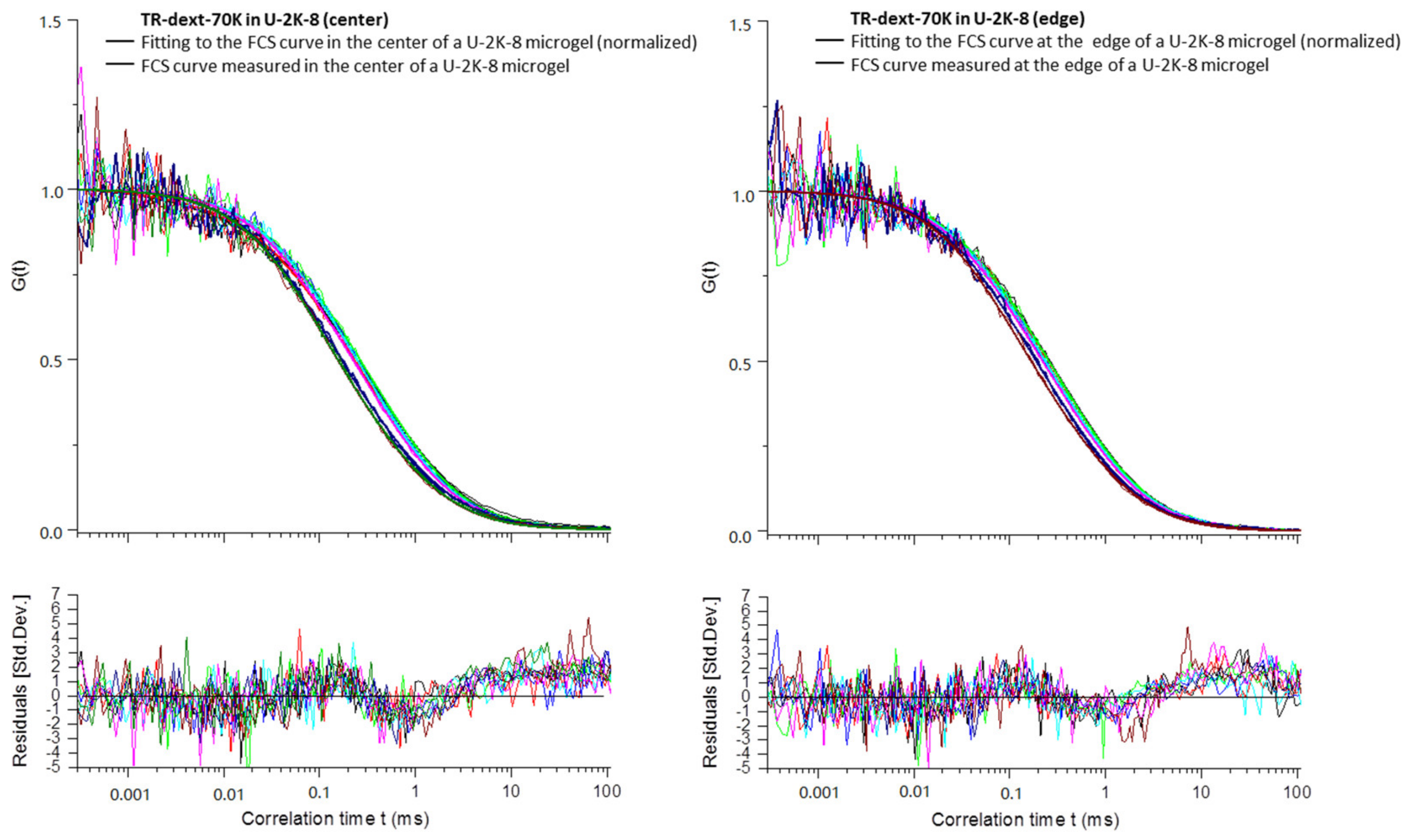
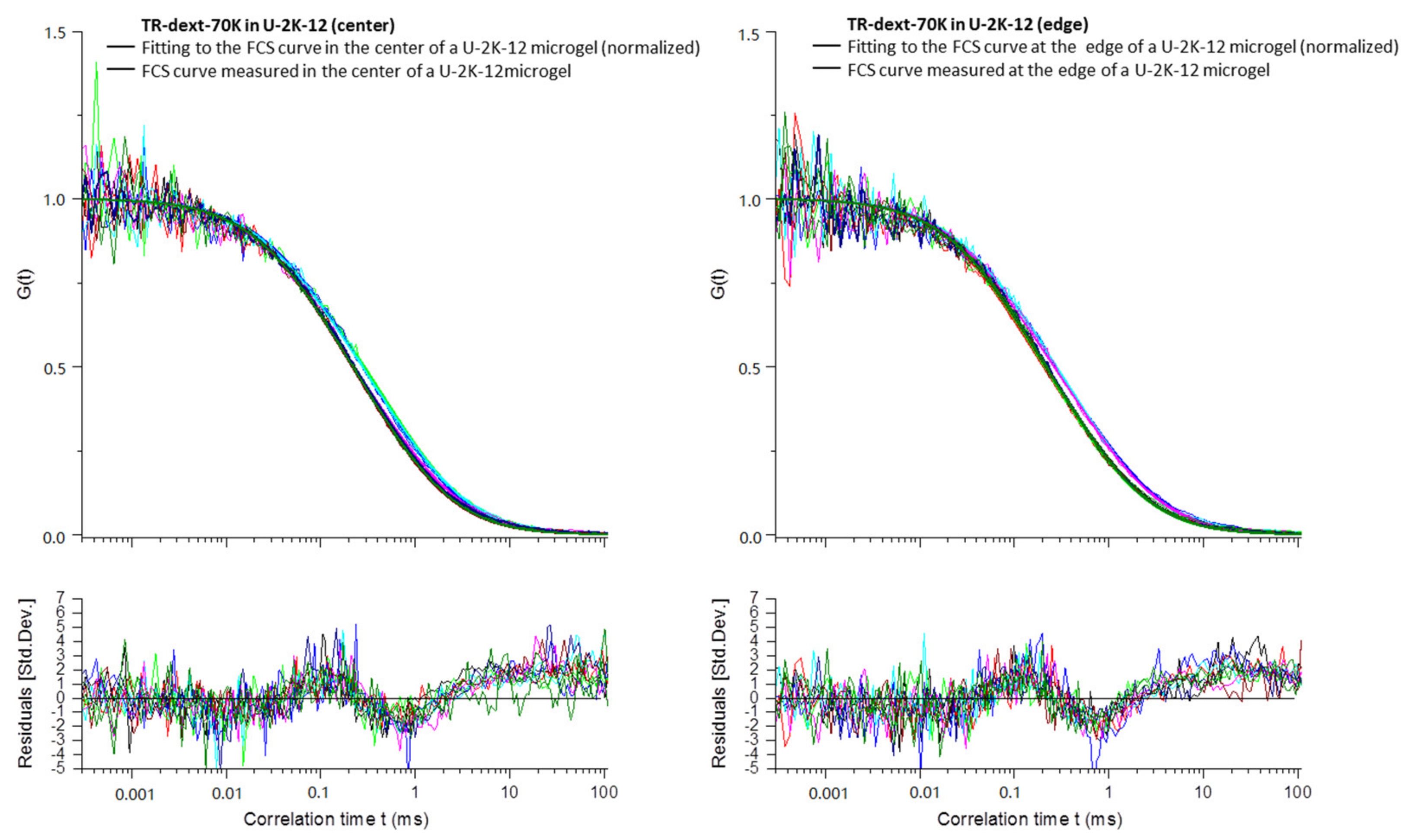
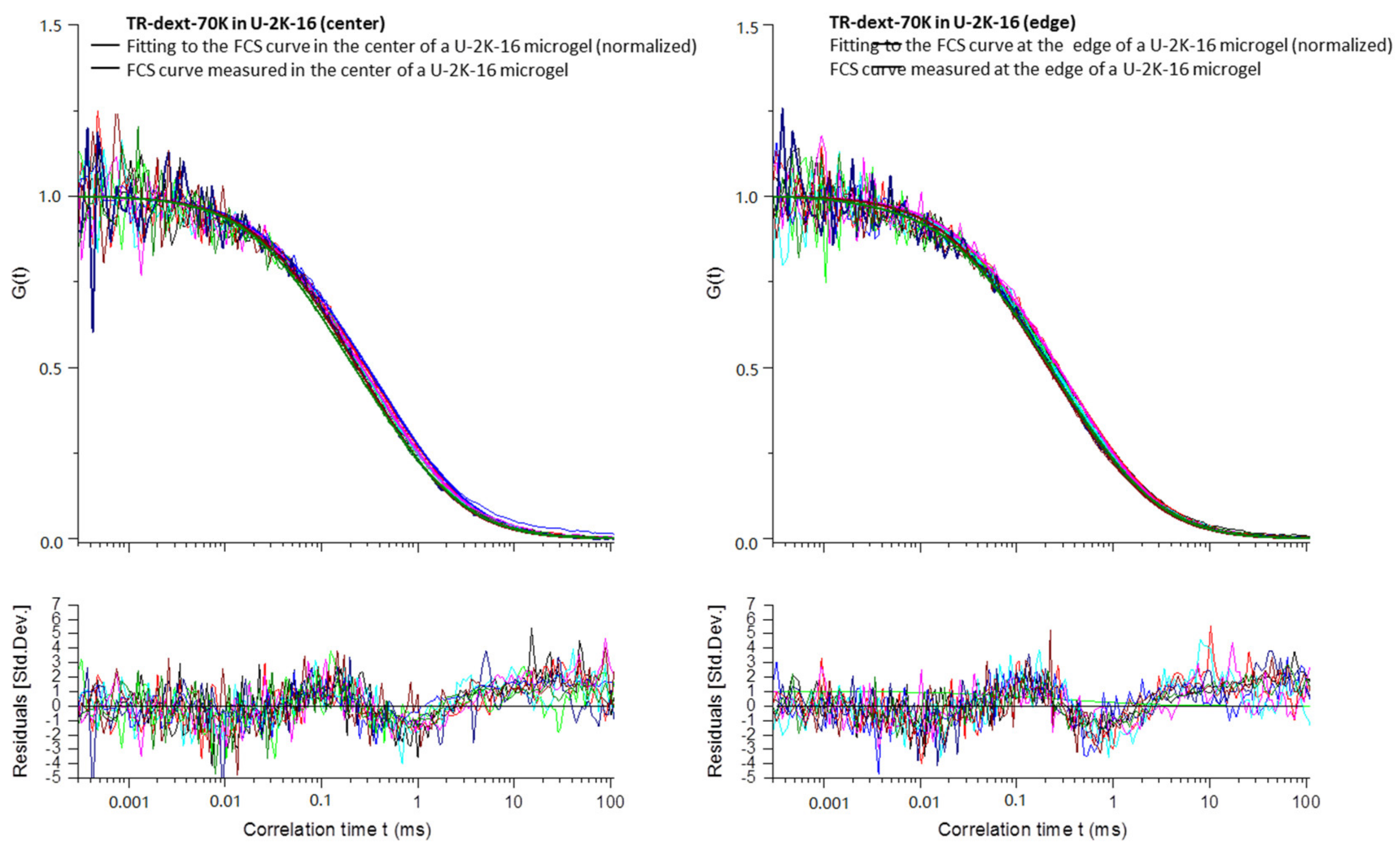
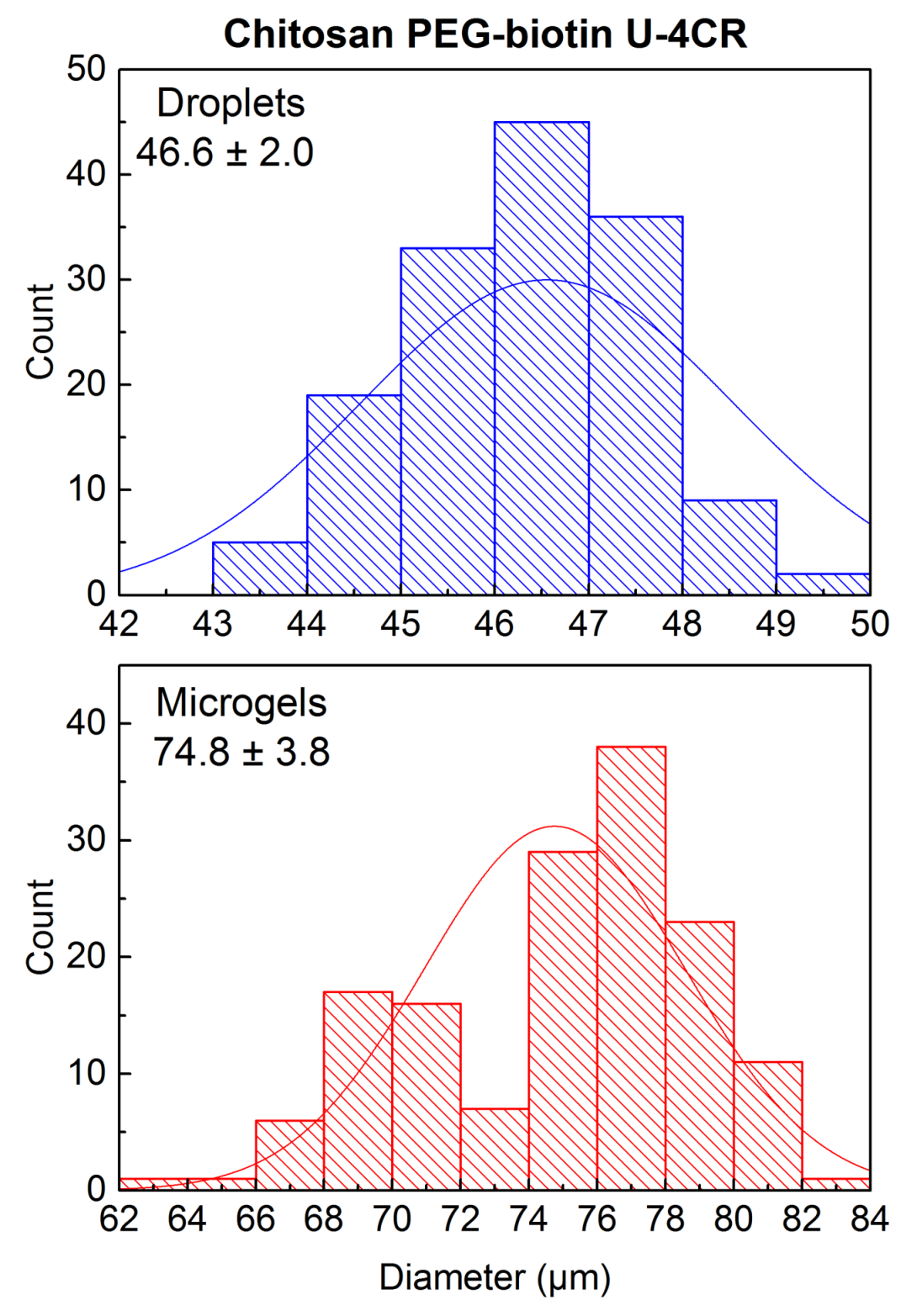
Appendix B
Calculation of Error Propagation of Size Increase during Microdroplet-to-Microgel Transition
References
- Alemán, J.; Chadwick, A.V.; He, J.; Hess, M.; Horie, K.; Jones, R.G.; Kratochvíl, P.; Meisel, I.; Mita, I.; Moad, G. Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic-organic hybrid materials (iupac recommendations 2007). Pure Appl. Chem. 2007, 79, 1801–1829. [Google Scholar] [CrossRef]
- Walta, S.; Pergushov, D.V.; Oppermann, A.; Steinschulte, A.A.; Geisel, K.; Sigolaeva, L.V.; Plamper, F.A.; Wöll, D.; Richtering, W. Microgels enable capacious uptake and controlled release of architecturally complex macromolecular species. Polymer 2017, 119, 50–58. [Google Scholar] [CrossRef]
- Jiang, W.; Li, M.; Chen, Z.; Leong, K.W. Cell-laden microfluidic microgels for tissue regeneration. Lab Chip 2016, 16, 4482–4506. [Google Scholar] [CrossRef] [PubMed]
- Thiele, J.; Ma, Y.; Foschepoth, D.; Hansen, M.M.; Steffen, C.; Heus, H.; Huck, W. DNA-functionalized hydrogels for confined membrane-free in vitro transcription/translation. Lab Chip 2014, 14, 2651–2656. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-H.; Lee, K.-Y.; Byun, J.-Y.; Kim, B.-G.; Kim, D.-M. On-bead expression of recombinant proteins in an agarose gel matrix coated on a glass slide. Lab Chip 2012, 12, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Krzek, M.; van Beek, H.L.; Permentier, H.P.; Bischoff, R.; Fraaije, M.W. Covalent immobilization of a flavoprotein monooxygenase via its flavin cofactor. Enzym. Microb. Technol. 2016, 82, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Kim, J.-C. Novel ph-sensitive microgels prepared using salt bridge. Int. J. Pharm. 2010, 388, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Neubauer, M.P.; Thiele, J.; Fery, A.; Huck, W. Artificial microniches for probing mesenchymal stem cell fate in 3d. Biomater. Sci. 2014, 2, 1661–1671. [Google Scholar] [CrossRef]
- Bray, L.J.; Binner, M.; Holzheu, A.; Friedrichs, J.; Freudenberg, U.; Hutmacher, D.W.; Werner, C. Multi-parametric hydrogels support 3d in vitro bioengineered microenvironment models of tumour angiogenesis. Biomaterials 2015, 53, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Crescenzi, V.; Cornelio, L.; Di Meo, C.; Nardecchia, S.; Lamanna, R. Novel hydrogels via click chemistry: Synthesis and potential biomedical applications. Biomacromolecules 2007, 8, 1844–1850. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Angeles, G.; Němcová, M.; Příkopová, E.; Šmejkalová, D.; Pravda, M.; Kučera, L.; Velebný, V. Reductive alkylation of hyaluronic acid for the synthesis of biocompatible hydrogels by click chemistry. Carbohydr. Polym. 2012, 90, 1704–1711. [Google Scholar] [CrossRef] [PubMed]
- Nimmo, C.M.; Owen, S.C.; Shoichet, M.S. Diels−alder click cross-linked hyaluronic acid hydrogels for tissue engineering. Biomacromolecules 2011, 12, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Gao, X.; Dong, H.; He, H.; Cao, X. High-throughput generation of hyaluronic acid microgels via microfluidics-assisted enzymatic crosslinking and/or diels–alder click chemistry for cell encapsulation and delivery. Appl. Mater. Today 2017, 9, 49–59. [Google Scholar] [CrossRef]
- Yu, Y.; Deng, C.; Meng, F.; Shi, Q.; Feijen, J.; Zhong, Z. Novel injectable biodegradable glycol chitosan-based hydrogels crosslinked by michael-type addition reaction with oligo (acryloyl carbonate)-b-poly (ethylene glycol)-b-oligo (acryloyl carbonate) copolymers. J. Biomed. Mater. Res. Part A 2011, 99, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Young, J.L.; Engler, A.J. Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. Biomaterials 2011, 32, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Nie, T.; Baldwin, A.; Yamaguchi, N.; Kiick, K.L. Production of heparin-functionalized hydrogels for the development of responsive and controlled growth factor delivery systems. J. Control. Release 2007, 122, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Cumpstey, I. Chemical modification of polysaccharides. ISRN Organ. Chem. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Kirschning, A.; Dibbert, N.; Dräger, G. Chemical functionalization of polysaccharides—Towards biocompatible hydrogels for biomedical applications. Chem. A Eur. J. 2018, 24, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Vasco, A.V.; Pérez, C.S.; Morales, F.E.; Garay, H.E.; Vasilev, D.; Gavin, J.A.; Wessjohann, L.A.; Rivera, D.G. Macrocyclization of peptide side chains by the ugi reaction: Achieving peptide folding and exocyclic n-functionalization in one shot. J. Organ. Chem. 2015, 80, 6697–6707. [Google Scholar] [CrossRef] [PubMed]
- Sehlinger, A.; Meier, M.A. Passerini and ugi multicomponent reactions in polymer science. In Multi-Component and Sequential Reactions in Polymer Synthesis; Springer: Cham, Switzerland, 2014; pp. 61–86. [Google Scholar]
- Crescenzi, V.; Francescangeli, A.; Capitani, D.; Mannina, L.; Renier, D.; Bellini, D. Hyaluronan networking via ugi’s condensation using lysine as cross-linker diamine. Carbohydr. Polym. 2003, 53, 311–316. [Google Scholar] [CrossRef]
- Crescenzi, V.; Francescangeli, A.; Segre, A.L.; Capitani, D.; Mannina, L.; Renier, D.; Bellini, D. Nmr structural study of hydrogels based on partially deacetylated hyaluronan. Macromol. Biosci. 2002, 2, 272–279. [Google Scholar] [CrossRef]
- Bu, H.; Kjøniksen, A.-L.; Knudsen, K.D.; Nyström, B. Rheological and structural properties of aqueous alginate during gelation via the ugi multicomponent condensation reaction. Biomacromolecules 2004, 5, 1470–1479. [Google Scholar] [CrossRef] [PubMed]
- Bu, H.; Kjøniksen, A.-L.; Nyström, B. Effects of ph on dynamics and rheology during association and gelation via the ugi reaction of aqueous alginate. Eur. Polym. J. 2005, 41, 1708–1717. [Google Scholar] [CrossRef]
- de Nooy, A.E.; Masci, G.; Crescenzi, V. Versatile synthesis of polysaccharide hydrogels using the passerini and ugi multicomponent condensations. Macromolecules 1999, 32, 1318–1320. [Google Scholar] [CrossRef]
- Maleki, A.; Kjøniksen, A.-L.; Nyström, B. Characterization of the chemical degradation of hyaluronic acid during chemical gelation in the presence of different cross-linker agents. Carbohydr. Res. 2007, 342, 2776–2792. [Google Scholar] [CrossRef] [PubMed]
- de Nooy, A.E.; Capitani, D.; Masci, G.; Crescenzi, V. Ionic polysaccharide hydrogels via the passerini and ugi multicomponent condensations: Synthesis, behavior and solid-state nmr characterization. Biomacromolecules 2000, 1, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Shulepov, I.D.; Kozhikhova, K.V.; Panfilova, Y.S.; Ivantsova, M.N.; Mironov, M.A. One-pot synthesis of cross-linked sub-micron microgels from pure cellulose via the ugi reaction and their application as emulsifiers. Cellulose 2016, 23, 2549–2559. [Google Scholar] [CrossRef]
- Mironov, M.A.; Shulepov, I.D.; Ponomarev, V.S.; Bakulev, V.A. Synthesis of polyampholyte microgels from colloidal salts of pectinic acid and their application as ph-responsive emulsifiers. Colloid Polym. Sci. 2013, 291, 1683–1691. [Google Scholar] [CrossRef]
- Heida, T.; Neubauer, J.W.; Seuss, M.; Hauck, N.; Thiele, J.; Fery, A. Mechanically defined microgels by droplet microfluidics. Macromol. Chem. Phys. 2017, 218, 1600418. [Google Scholar] [CrossRef]
- Holtze, C.; Rowat, A.; Agresti, J.; Hutchison, J.; Angile, F.; Schmitz, C.; Köster, S.; Duan, H.; Humphry, K.; Scanga, R. Biocompatible surfactants for water-in-fluorocarbon emulsions. Lab Chip 2008, 8, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- Frey, A.; Meckelein, B.; Externest, D.; Schmidt, M.A. A stable and highly sensitive 3,3′,5,5′-tetramethylbenzidine-based substrate reagent for enzyme-linked immunosorbent assays. J. Immunol. Methods 2000, 233, 47–56. [Google Scholar] [CrossRef]
- Hutter, J.L.; Bechhoefer, J. Calibration of atomic-force microscope tips. Rev. Sci. Instrum. 1993, 64, 1868–1873. [Google Scholar] [CrossRef]
- Seuss, M.; Schmolke, W.; Drechsler, A.; Fery, A.; Seiffert, S. Core–shell microgels with switchable elasticity at constant interfacial interaction. ACS Appl. Mater. Interfaces 2016, 8, 16317–16327. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.L.; Kendall, K.; Roberts, A.D. Surface energy and the contact of elastic solids. Proc. R. Soc. Lond. A 1971, 324, 301–313. [Google Scholar] [CrossRef]
- Schlüßler, R.; Möllmert, S.; Abuhattum, S.; Cojoc, G.; Müller, P.; Kim, K.; Möckel, C.; Zimmermann, C.; Czarske, J.; Guck, J. Mechanical mapping of spinal cord growth and repair in living zebrafish larvae by brillouin imaging. Biophys. J. 2018, 115, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Scarcelli, G.; Yun, S.H. Confocal brillouin microscopy for three-dimensional mechanical imaging. Nat. Photonics 2007, 2, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Scarcelli, G.; Polacheck, W.J.; Nia, H.T.; Patel, K.; Grodzinsky, A.J.; Kamm, R.D.; Yun, S.H. Noncontact three-dimensional mapping of intracellular hydromechanical properties by brillouin microscopy. Nat. Methods 2015, 12, 1132–1134. [Google Scholar] [CrossRef] [PubMed]
- Schürmann, M.; Cojoc, G.; Girardo, S.; Ulbricht, E.; Guck, J.; Müller, P. Three-dimensional correlative single-cell imaging utilizing fluorescence and refractive index tomography. J. Biophotonics 2018, 11, e201700145. [Google Scholar] [CrossRef] [PubMed]
- Schürmann, M.; Scholze, J.; Müller, P.; Guck, J.; Chan, C.J. Cell nuclei have lower refractive index and mass density than cytoplasm. J. Biophotonics 2016, 9, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Schürmann, M.; Guck, J. Odtbrain: A python library for full-view, dense diffraction tomography. BMC Bioinform. 2015, 16, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, C.M.; Košovan, P.; Richtering, W.; Wöll, D. Polymers in focus: Fluorescence correlation spectroscopy. Colloid Polym. Sci. 2014, 292, 2399–2411. [Google Scholar] [CrossRef]
- Lehmann, S.; Seiffert, S.; Richtering, W. Spatially resolved tracer diffusion in complex responsive hydrogels. J. Am. Chem. Soc. 2012, 134, 15963–15969. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Loman, A.; Pacheco, V.; Koberling, F.; Willbold, D.; Richtering, W.; Enderlein, J. Precise measurement of diffusion by multi-color dual-focus fluorescence correlation spectroscopy. EPL (Europhys. Lett.) 2008, 83, 46001. [Google Scholar] [CrossRef]
- Widengren, J.; Rigler, R.; Mets, Ü. Triplet-state monitoring by fluorescence correlation spectroscopy. J. Fluoresc. 1994, 4, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Abate, A.R.; Thiele, J.; Weitz, D.A. One-step formation of multiple emulsions in microfluidics. Lab Chip 2011, 11, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Ducker, W.A.; Senden, T.J.; Pashley, R.M. Direct measurement of colloidal forces using an atomic force microscope. Nature 1991, 353, 239–241. [Google Scholar] [CrossRef]
- Jeon, O.; Song, S.J.; Lee, K.-J.; Park, M.H.; Lee, S.-H.; Hahn, S.K.; Kim, S.; Kim, B.-S. Mechanical properties and degradation behaviors of hyaluronic acid hydrogels cross-linked at various cross-linking densities. Carbohydr. Polym. 2007, 70, 251–257. [Google Scholar] [CrossRef]
- Yazici, I.; Okay, O. Spatial inhomogeneity in poly (acrylic acid) hydrogels. Polymer 2005, 46, 2595–2602. [Google Scholar] [CrossRef]
- Girardo, S.; Traeber, N.; Wagner, K.; Cojoc, G.; Herold, C.; Goswami, R.; Schluessler, R.; Abuhattum, S.; Taubenberger, A.; Reichel, F. Standardized microgel beads as elastic cell mechanical probes. J. Mater. Chem. B 2018. [Google Scholar] [CrossRef]
- Zhang, Z.; Nadezhina, E.; Wilkinson, K.J. Quantifying diffusion in a biofilm of streptococcus mutans. Antimicro. Agents Chemother. 2011, 55, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Green, N.M. [5] avidin and streptavidin. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1990; Volume 184, pp. 51–67. [Google Scholar]
- Veitch, N.C. Horseradish peroxidase: A modern view of a classic enzyme. Phytochemistry 2004, 65, 249–259. [Google Scholar] [CrossRef] [PubMed]
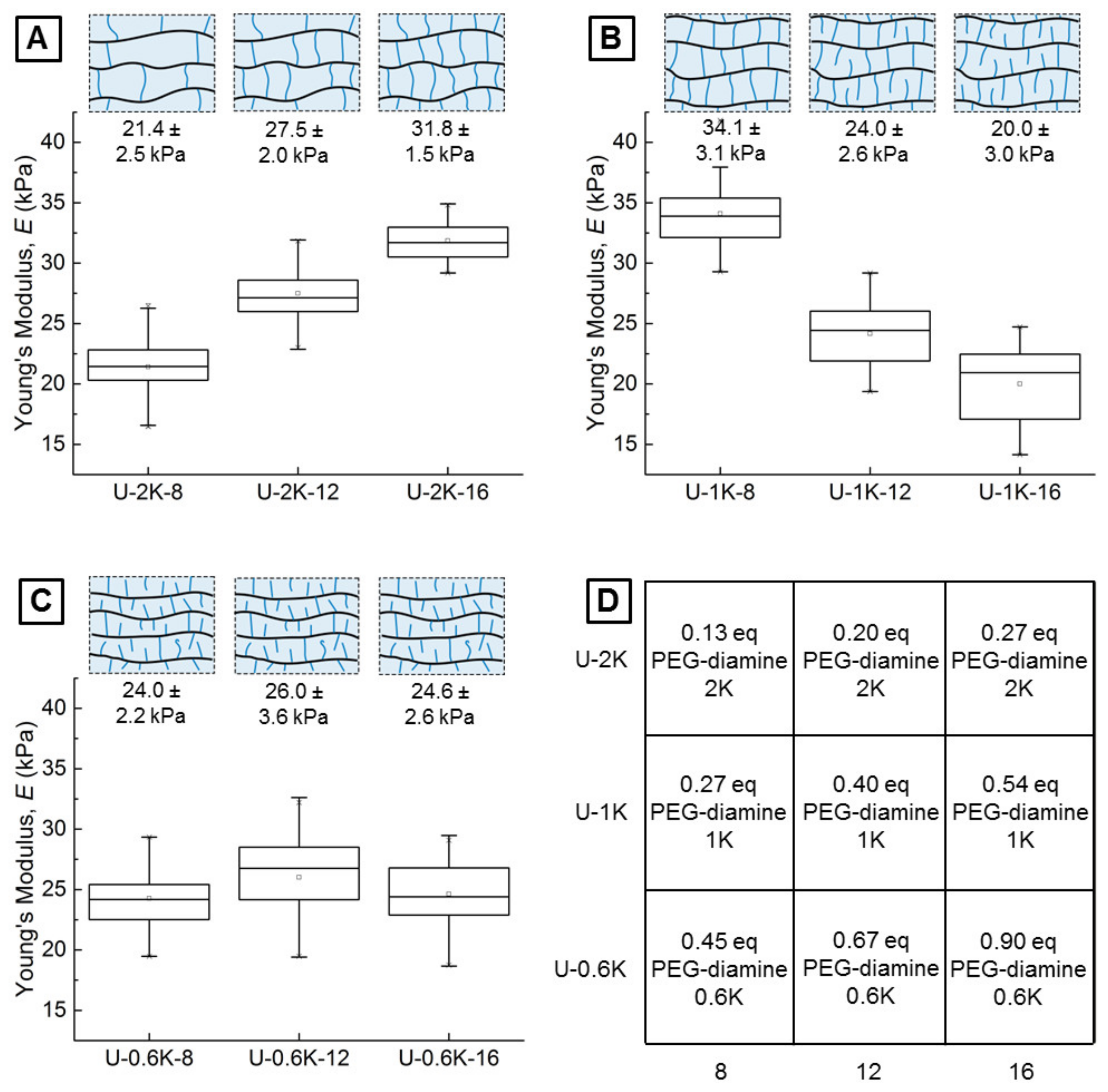

| Sample | PEG-Diamine (MW: 2000 g mol−1) | Formaldehyde 37% |
|---|---|---|
| U-2K-8 | 8 mg; 0.13 eq | 0.9 µL; 0.4 eq |
| U-2K-12 | 12 mg; 0.20 eq | 1.4 µL; 0.6 eq |
| U-2K-16 | 16 mg; 0.27 eq | 1.8 µL; 0.81 eq |
| Sample | PEG-Diamine (MW: 1000 g mol−1) | Formaldehyde 37% |
|---|---|---|
| U-1K-8 | 8 mg; 0.27 eq | 1.8 µL; 0.81 eq |
| U-1K-12 | 12 mg; 0.40 eq | 2.7 µL; 1.21 eq |
| U-1K-16 | 16 mg; 0.54 eq | 3.6 µL; 1.61 eq |
| Sample | PEG-Diamine (MW: 600 g mol−1) | Formaldehyde 37% |
|---|---|---|
| U-600-8 | 8 mg; 0.45 eq | 3.0 µL; 1,34 eq |
| U-600-12 | 12 mg; 0.67 eq | 4.5 µL; 2.02 eq |
| U-600-16 | 16 mg; 0.90 eq | 6.0 µL; 2.69 eq |
| Crosslinker Length | 8 mg | Crosslinker Amount 12 mg | 16 mg |
|---|---|---|---|
| 2000 g mol−1 | U-2K-8 | U-2K-12 | U-2K-16 |
| 1000 g mol−1 | U-1K-8 | U-1K-12 | U-1K-16 |
| 600 g mol−1 | U-0.6K-8 | U-0.6K-12 | U-0.6K-16 |
| Sample | Droplet Diameter | Microgel Diameter | Size Increase a |
|---|---|---|---|
| U-2K-8 | 44.5 ± 1.2 µm | 89.2 ± 4.2 µm | 2.0 ± 0.1 |
| U-2K-12 | 42.6 ± 1.2 µm | 72.9 ± 2.1 µm | 1.7 ± 0.1 |
| U-2K-16 | 40.6 ± 1.3 µm | 74.7 ± 1.8 µm | 1.8 ± 0.1 |
| U-1K-8 | 44.7 ± 2.7 µm | 79.7 ± 2.7 µm | 1.8 ± 0.1 |
| U-1K-12 | 44.9 ± 1.1 µm | 83.9 ± 2.1 µm | 1.9 ± 0.1 |
| U-1K-16 | 46.3 ± 1.1 µm | 90.8 ± 1.9 µm | 2.0 ± 0.1 |
| U-0.6K-8 | 47.5 ± 1.9 µm | 78.7 ± 4.1 µm | 1.7 ± 0.1 |
| U-0.6K-12 | 45.7 ± 1.0 µm | 75.7 ± 2.5 µm | 1.7 ± 0.1 |
| U-0.6K-16 | 44.0 ± 1.0 µm | 75.2 ± 4.1 µm | 1.7 ± 0.1 |
| Exp.a | Type | D1 (µm2 s−1) | D2 (µm2 s−1) | D1/D1sol | D2/D2sol | t1 (ms) | t2 (ms) |
|---|---|---|---|---|---|---|---|
| Mean | U-2K-8 | 45.0 ± 3.0 | 363 c | 0.85 | 1.00 | 0.66 ± 0.04 | 0.082 c |
| Mean | U-2K-12 | 41.6 ± 4.3 | 363 c | 0.78 | 1.00 | 0.73 ± 0.08 | 0.082 c |
| Mean | U-2K-16 | 40.1 ± 2.6 | 363 c | 0.76 | 1.00 | 0.75 ± 0.05 | 0.082 c |
| Mean | solution | 53.0 ± 12.0 | 366 ± 24 | - | - | 0.58 ± 0.16 | 0.082 c |
| Lit.b | solution | 37.0 ± 6.6 | 412 ± 18 | - | - | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hauck, N.; Seixas, N.; Centeno, S.P.; Schlüßler, R.; Cojoc, G.; Müller, P.; Guck, J.; Wöll, D.; Wessjohann, L.A.; Thiele, J. Droplet-Assisted Microfluidic Fabrication and Characterization of Multifunctional Polysaccharide Microgels Formed by Multicomponent Reactions. Polymers 2018, 10, 1055. https://doi.org/10.3390/polym10101055
Hauck N, Seixas N, Centeno SP, Schlüßler R, Cojoc G, Müller P, Guck J, Wöll D, Wessjohann LA, Thiele J. Droplet-Assisted Microfluidic Fabrication and Characterization of Multifunctional Polysaccharide Microgels Formed by Multicomponent Reactions. Polymers. 2018; 10(10):1055. https://doi.org/10.3390/polym10101055
Chicago/Turabian StyleHauck, Nicolas, Nalin Seixas, Silvia P. Centeno, Raimund Schlüßler, Gheorghe Cojoc, Paul Müller, Jochen Guck, Dominik Wöll, Ludger A. Wessjohann, and Julian Thiele. 2018. "Droplet-Assisted Microfluidic Fabrication and Characterization of Multifunctional Polysaccharide Microgels Formed by Multicomponent Reactions" Polymers 10, no. 10: 1055. https://doi.org/10.3390/polym10101055
APA StyleHauck, N., Seixas, N., Centeno, S. P., Schlüßler, R., Cojoc, G., Müller, P., Guck, J., Wöll, D., Wessjohann, L. A., & Thiele, J. (2018). Droplet-Assisted Microfluidic Fabrication and Characterization of Multifunctional Polysaccharide Microgels Formed by Multicomponent Reactions. Polymers, 10(10), 1055. https://doi.org/10.3390/polym10101055







