PDDA-Montmorillonite Composites Loaded with Ru Nanoparticles: Synthesis, Characterization, and Catalytic Properties in Hydrogenation of 2-Butanone
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
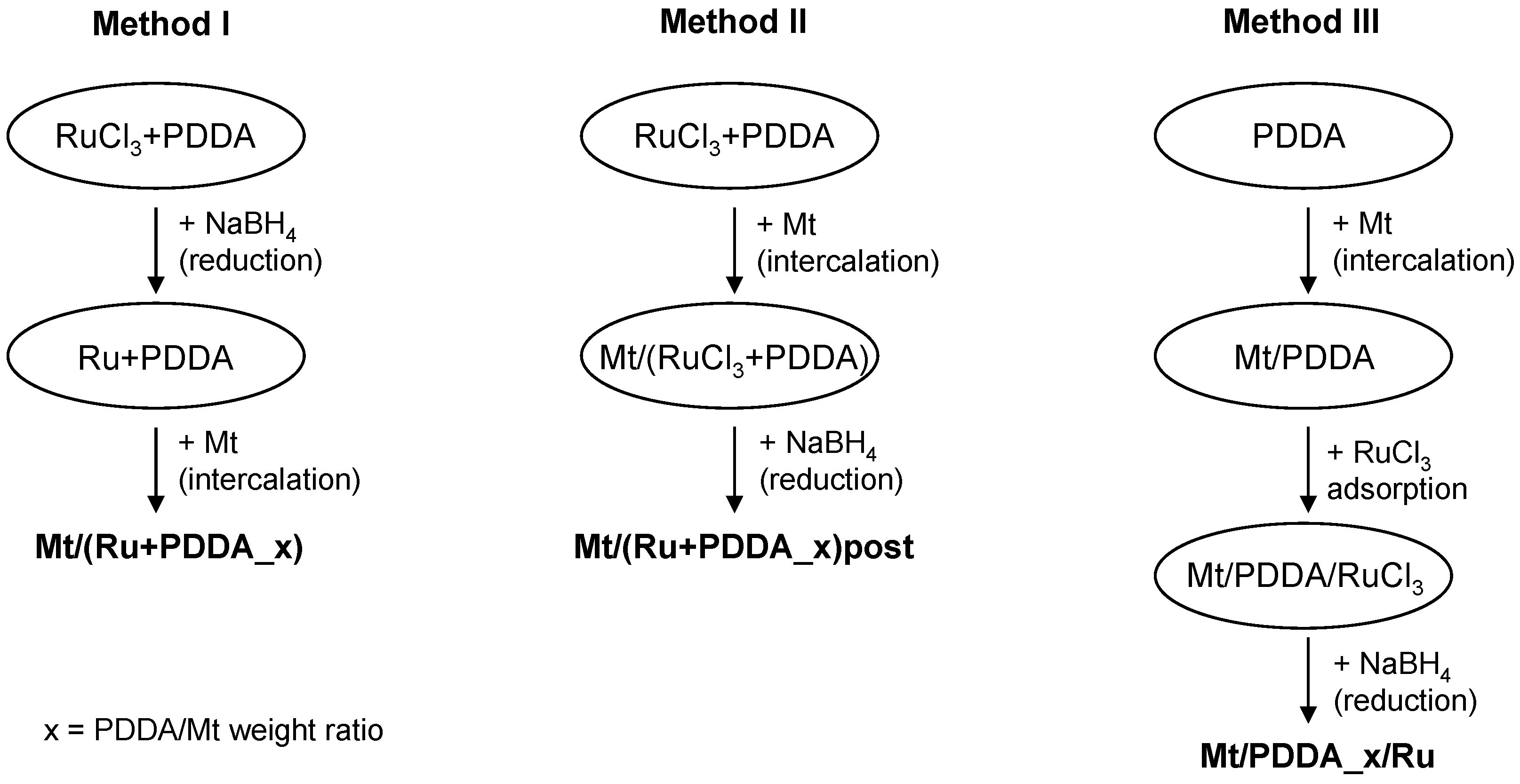
2.2. Methods
3. Results and Discussion
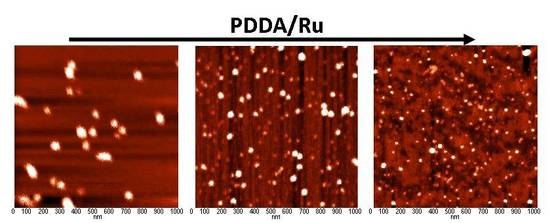
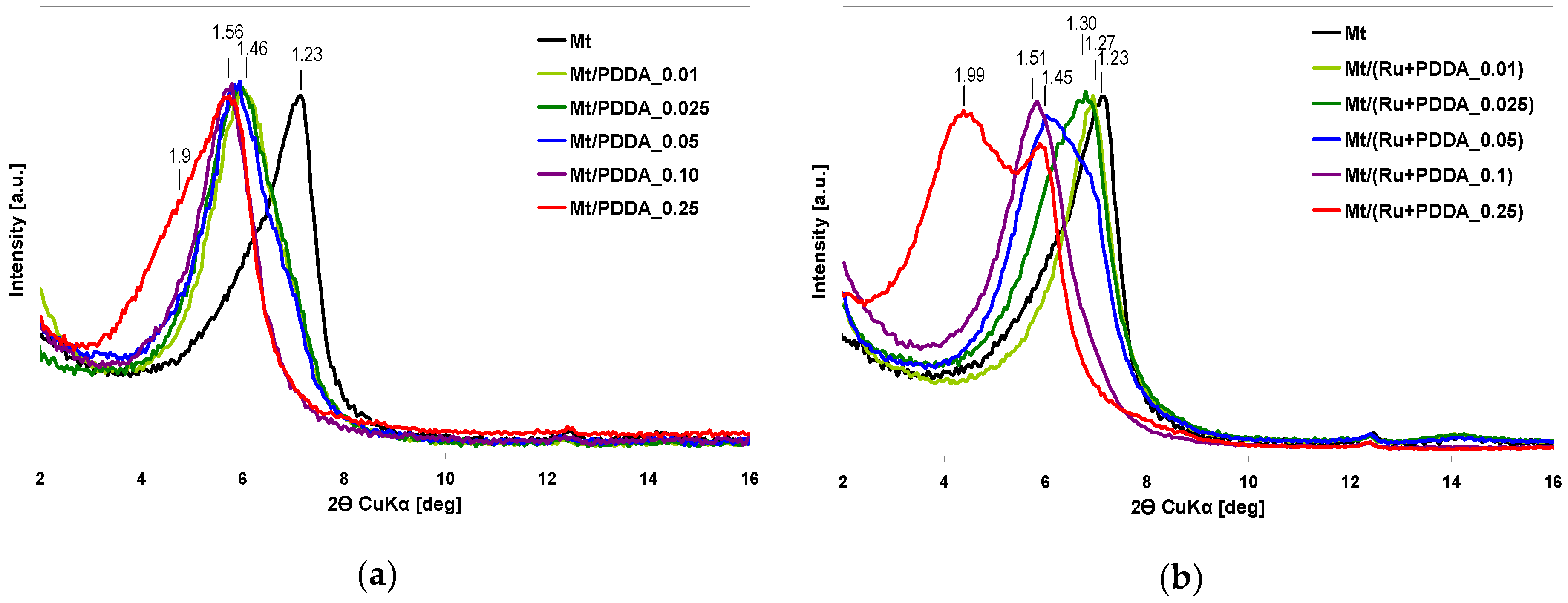
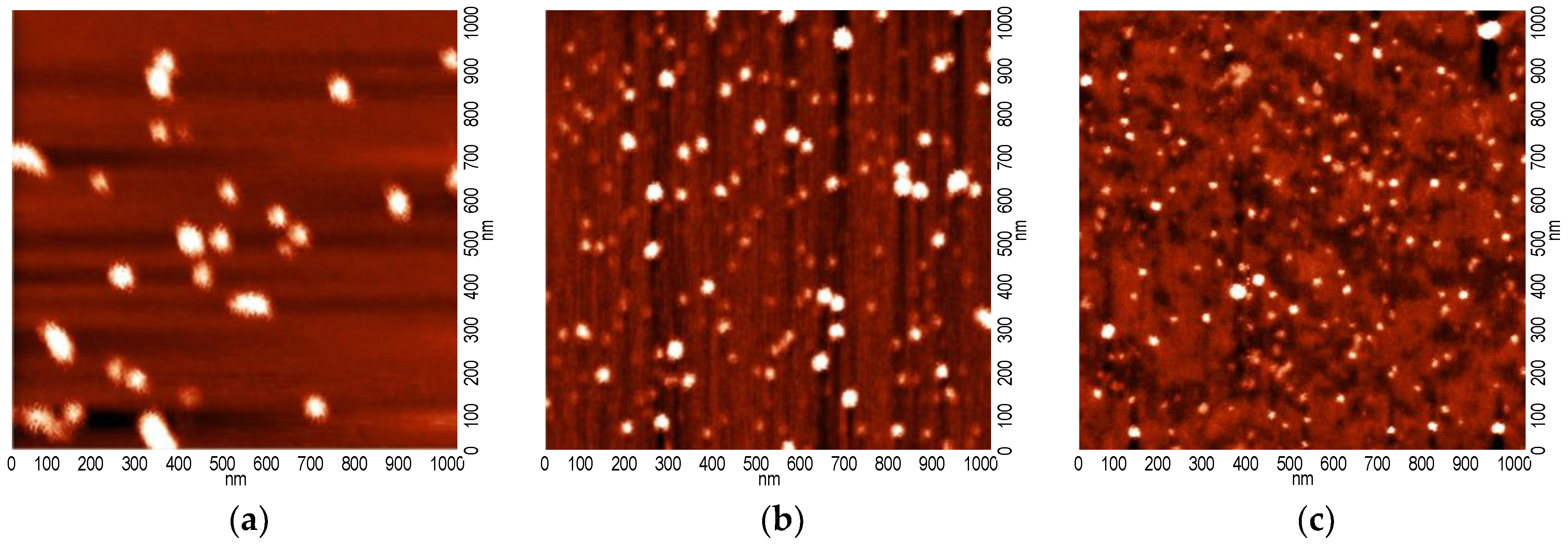
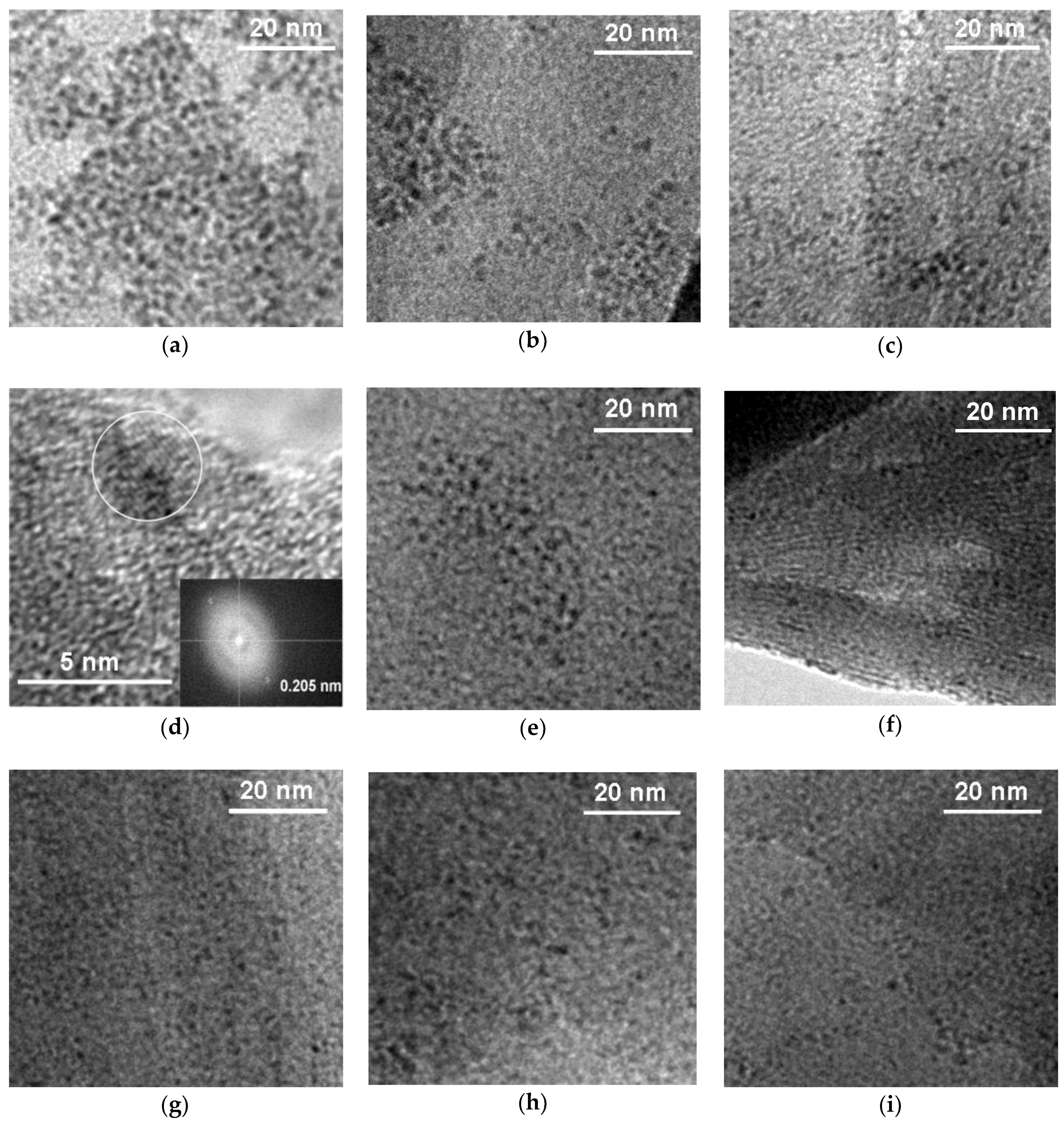
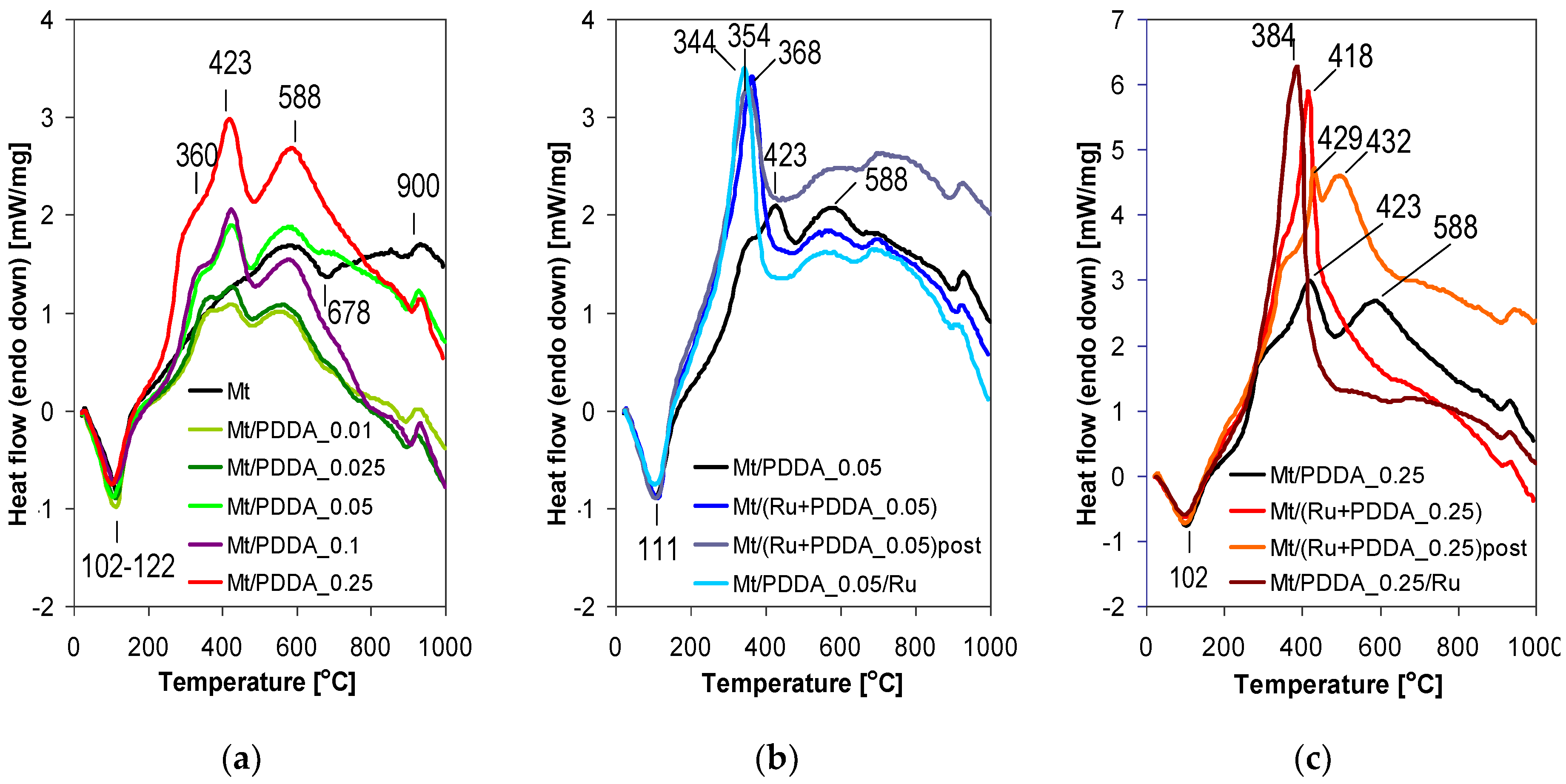
3.1. Physicochemical Characterization
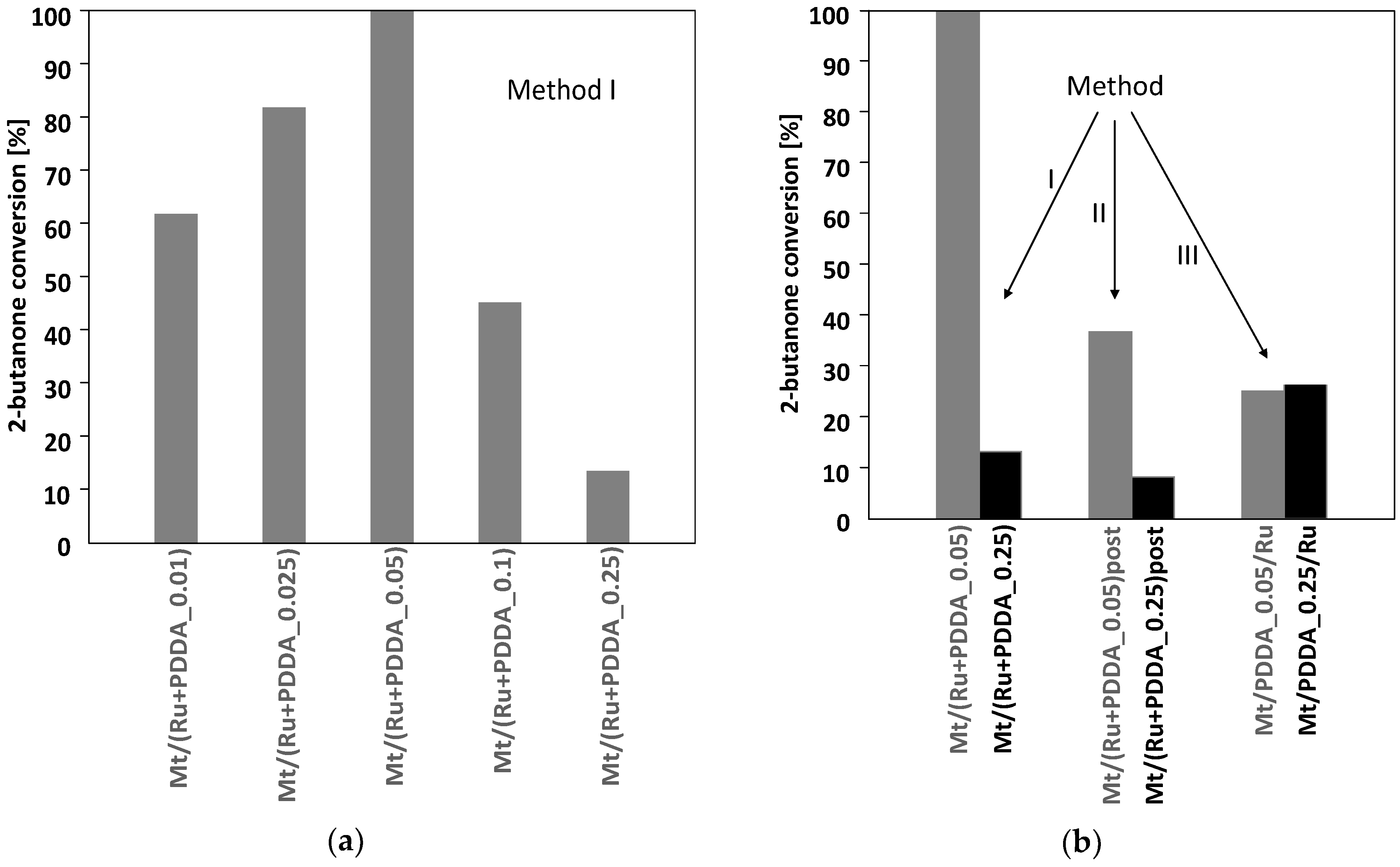
3.2. Catalytic Testing
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McKenna, K.P. Unique Bonding in Nanoparticles and Powders. In Nanoscale Materials in Chemistry, 2nd ed.; Klabunde, K.J., Richards, R.M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 15–36. ISBN 978-0-470-22270-6. [Google Scholar]
- Li, Y.; Boone, E.; El-Sayed, M.A. Size Effects of PVP-Pd Nanoparticles on the Catalytic Suzuki Reactions in Aqueous Solution. Langmuir 2002, 18, 4921–4925. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Kulkarni, G.U.; Thomas, P.J.; Edwards, P.P. Size-Dependent Chemistry: Properties of Nanocrystals. Chem. Eur. J. 2002, 8, 28–35. [Google Scholar] [CrossRef]
- Crooks, R.M.; Zhao, M.; Sun, L.; Chechik, V.; Lee, K.Y. Dendrimer-Encapsulated Metal Nanoparticles: Synthesis, Characterization, and Applications to Catalysis. Acc. Chem. Res. 2001, 34, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Bönnemann, H.; Richards, R.M. Nanoscopic Metal Particles—Synthetic Methods and Potential Applications. Eur. J. Inorg. Chem. 2001, 2455–2480. [Google Scholar] [CrossRef]
- Ford, W.T. Inorganic–Organic Composites. In Nanoscale Materials in Chemistry, 2nd ed.; Klabunde, K.J., Richards, R.M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 369–403. ISBN 978-0-470-22270-6. [Google Scholar]
- Li, Y.; El-Sayed, M.A. The Effect of Stabilizers on the Catalytic Activity and Stability of Pd Colloidal Nanoparticles in the Suzuki Reactions in Aqueous Solution. J. Phys. Chem. B 2001, 105, 8938–8943. [Google Scholar] [CrossRef]
- Králik, M.; Biffis, A.J. Catalysis by metal nanoparticles supported on functional organic polymers. Mol. Catal. A 2001, 177, 113–13810. [Google Scholar] [CrossRef]
- Liu, P. Polymer modified clay minerals: A review. Appl. Clay Sci. 2007, 38, 64–76. [Google Scholar] [CrossRef]
- Chiu, C.W.; Huang, T.K.; Wang, Y.C.; Alamani, B.G.; Lin, J.J. Intercalation strategies in clay/polymer hybrids. Prog. Polym. Sci. 2014, 39, 443–485. [Google Scholar] [CrossRef]
- Zhou, C.H. An overview on strategies towards clay-based designer catalysts for green and sustainable catalysis. Appl. Clay Sci. 2011, 53, 87–96. [Google Scholar] [CrossRef]
- Azeez, A.A.; Rhee, K.Y.; Park, S.J.; Hui, D. Epoxy clay nanocomposites – processing, properties and applications: A review. Compos Part B 2013, 45, 308–320. [Google Scholar] [CrossRef]
- Napruszewska, B.D.; Michalik-Zym, A.; Dula, R.; Bielanska, E.; Rojek, W.; Machej, T.; Socha, R.P.; Lityńska-Dobrzyńska, L.; Bahranowski, K.; Serwicka, E.M. Composites derived from exfoliated Laponite and Mn-Al hydrotalcite prepared in inverse microemulsion: A new strategy for design of robust VOCs combustion catalysts. Appl. Catal. B 2017, 211, 46–56. [Google Scholar] [CrossRef]
- Napruszewska, B.D.; Michalik-Zym, A.; Rogowska, M.; Bielańska, E.; Rojek, W.; Gaweł, A.; Wójcik-Bania, M.; Bahranowski, K.; Serwicka, E.M. Novel Montmorillonite/TiO2/MnAl-Mixed Oxide Composites Prepared from Inverse Microemulsions as Combustion Catalysts. Materials 2017, 10, 1326. [Google Scholar] [CrossRef] [PubMed]
- Papp, S.; Szücs, A.; Dékány, I. Preparation of Pd0 nanoparticles stabilized by polymers and layered silicate. Appl. Clay Sci. 2001, 19, 155–172. [Google Scholar] [CrossRef]
- Papp, S.; Dékány, I.; Szél, J.; Oszkó, A. Synthesis of Polymer-Stabilized Nanosized Rhodium Particles in the Interlayer Space of Layered Silicates. Chem. Mater. 2004, 16, 1674–1685. [Google Scholar] [CrossRef]
- Zhao, X.; Mai, Z.; Kang, X.; Zou, X. Direct electrochemistry and electrocatalysis of horseradish peroxidase based on clay–chitosan-gold nanoparticle nanocomposite. Biosens. Bioelectron. 2008, 23, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Jlassi, K.; Singh, A.; Aswal, D.K.; Losno, R.; Benna-Zayani, M.; Chehimi, M.M. Novel, ternary clay/polypyrrole/silver hybrid materials through in situ photopolymerization. Colloids Surf. A 2013, 439, 193–199. [Google Scholar] [CrossRef]
- Xu, M.; Zhao, J.; Shu, G.; Liu, Q.; Zeng, M. Heterogeneous Catalytic Composites from Palladium Nanoparticles in Montmorillonite Intercalated with Poly (Vinyl Pyrrolidone) Chains. Polymers 2018, 10, 669. [Google Scholar] [CrossRef]
- Fan, M.; Wang, R.; Jia, S. Controllable synthesis of iron nanoparticles on polyethylenimine-modified montmorillonite: Dependence on the amine protonation extent. Appl. Clay Sci. 2018, 162, 418–427. [Google Scholar] [CrossRef]
- Brigatti, M.F.; Galán, E.; Theng, B.K.G. Structure and Mineralogy of Clay Minerals. In Handbook of Clay Science, 2nd ed.; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Part A; pp. 21–81. ISBN 978-0-08-098259-5. [Google Scholar]
- Dautzenberg, H.; Gornitz, E.; Jaeger, W. Synthesis and characterization of poly (diallyldimethylammonium chloride) in a broad range of molecular weight. Macromol. Chem. Phys. 1998, 199, 1561–1571. [Google Scholar] [CrossRef]
- Michel, C.; Gallezot, P. Why Is Ruthenium an Efficient Catalyst for the Aqueous-Phase Hydrogenation of Biosourced Carbonyl Compounds? ACS Catal. 2015, 5, 4130–4132. [Google Scholar] [CrossRef]
- Breen, J.P.; Burch, R.; Griffin, K.; Hardacre, C.; Hayes, M.; Huang, X.; O’Brien, S.D. Bimetallic effects in the liquid-phase hydrogenation of 2-butanone. J. Catal. 2005, 236, 270–281. [Google Scholar] [CrossRef]
- Breen, C.; Rawson, J.O.; Mann, B.E. Adsorption of polycations on clays: An in situ study using 133Cs solution-phase NMR. J. Mater. Chem. 1996, 6, 253–260. [Google Scholar] [CrossRef]
- Churchman, G.J. Formation of complexes between bentonite and different cationic polyelectrolytes and their use as sorbents for non-ionic and anionic pollutants. Appl. Clay Sci. 2002, 21, 177–189. [Google Scholar] [CrossRef]
- Czímerová, A.; Jankovič, L.; Madejová, J.; Čeklovský, A. Unique Photoactive Nanocomposites Based on Rhodamine 6G/Polymer/Montmorillonite Hybrid Systems. J. Polym. Sci. B Polym. Phys. 2013, 51, 1672–1679. [Google Scholar] [CrossRef]
- Meunier, A. Clays; Springer-Verlag: Berlin, Heidelberg, Germany, 2005; pp. 196–198. ISBN 978–3-540-21667-4. [Google Scholar]
- Langier-Kuźniarowa, A. Thermal Analysis of Organoclay Complexes. In Organo Clay Complexes and Interactions; Yariv, S., Cross, H., Eds.; Marcel Dekker: New York, NY, USA, 2001; pp. 273–344. ISBN 0-8247-0586-6. [Google Scholar]
- Yariv, S. The role of charcoal on DTA curves of organo-clay complexes: an overview. Appl. Clay Sci. 2004, 24, 225–236. [Google Scholar] [CrossRef]
- Fajnor, V.Š.; Jesenák, K. Differential thermal analysis of montmorillonite. J. Therm. Anal. 1996, 46, 489–493. [Google Scholar] [CrossRef]
- Kamiuchi, N.; Mitsui, T.; Muroyama, H.; Matsui, T.; Kikuchi, R.; Eguchi, K. Catalytic combustion of ethyl acetate and nano-structural changes of ruthenium catalysts supported on tin oxide. Appl. Catal. B 2010, 97, 120–126. [Google Scholar] [CrossRef]
- Rylander, P.N. Catalytic Hydrogenation in Organic Syntheses; Academic Press: New York, NY, USA, 1979; pp. 82–112. ISBN 978-0-12-605355-5. [Google Scholar]
- Gavnholt, J.; Schiøtz, J. Structure and reactivity of ruthenium nanoparticles. Phys. Rev. B 2008, 77, 035404-1–035404-10. [Google Scholar] [CrossRef]
- Karim, A.M.; Prasad, V.; Mpourmpakis, G.; Lonergan, W.W.; Frenkel, A.I.; Chen, J.G.; Vlachos, D.G. Correlating Particle Size and Shape of Supported Ru/γ-Al2O3 Catalysts with NH3 Decomposition Activity. J. Am. Chem. Soc. 2009, 131, 12230–12239. [Google Scholar] [CrossRef] [PubMed]
- Bu, J.; Liu, J.L.; Chen, X.Y.; Zhuang, J.H.; Yan, S.R.; Qiao, M.H.; He, H.Y.; Fan, K.N. Ru/SBA-15 catalysts for partial hydrogenation of benzene to cyclohexene: Tuning the Ru crystallite size by Ba. Catal. Commun. 2008, 9, 2612–2615. [Google Scholar] [CrossRef]
- Akpa, B.S.; D’Agostino, C.; Gladden, F.L.; Hindle, K.; Manyar, H.; McGregor, J.; Li, R.; Neurock, M.; Sinha, N.; Sitt, E.H; et al. Solvent effects in the hydrogenation of 2-butanone. J. Catal. 2012, 289, 30–41. [Google Scholar] [CrossRef]
- Wan, H.; Vitter, A.; Chaudhari, R.V.; Subramaniam, B. Kinetic investigations of unusual solvent effects during Ru/C catalyzed hydrogenation of model oxygenates. J. Catal. 2014, 309, 174–184. [Google Scholar] [CrossRef]
- Jones, D.R.; Iqbal, S.; Kondrat, S.A.; Lari, G.M.; Miedziak, P.J.; Morgan, D.J.; Parker, S.F.; Hutchings, G.J. An investigation of the effect of carbon support on ruthenium/carbon catalysts for lactic acid and butanone hydrogenation. Phys. Chem. Chem. Phys. 2016, 18, 17259–17264. [Google Scholar] [CrossRef] [PubMed]
- Duraczyńska, D.; Michalik-Zym, A.; Napruszewska, B.D.; Dula, R.; Socha, R.P.; Lityńska-Dobrzyńska, L.; Gaweł, A.; Bahranowski, K.; Serwicka, E.M. Efficient and Versatile Ru/SBA-15 Catalysts for Liquid-hase Hydrogenation of the C=C and C=O Bonds under Mild Conditions. ChemistrySelect 2016, 1, 2148–2155. [Google Scholar] [CrossRef]



| Sample | d001 (nm) | Na/Si | Ru (wt %) | ΔTG200-1000 (wt %) | 2-Butanone Conversion (%) |
|---|---|---|---|---|---|
| Mt | 1.25 | 0.092 | - | 5.6 | 0 |
| Mt/PDDA_0.01 | 1.46 | 0.021 | - | 7.3 (6.3) 1 | n.d 2 |
| Mt/PDDA_0.025 | 1.49 | 0.012 | - | 8.5 (7.4) | n.d. |
| Mt/PDDA_0.05 | 1.50 | 0.009 | - | 9.7 (9.2) | 0 |
| Mt/PDDA_0.1 | 1.56 | 0.003 | - | 12.3 (12.5) | n.d. |
| Mt/PDDA_0.25 | 1.53 | 0.001 | - | 15.9 (21.3, 15.0 *) | n.d. |
| Mt/(Ru + PDDA_0.01) | 1.27 | 0.080 | 1.92 | 6.2 (6.2) | 63 |
| Mt/(Ru + PDDA_0.025) | 1.30 | 0.062 | 1.85 | 6.8 (7.3) | 82 |
| Mt/(Ru + PDDA_0.05) | 1.45 (1.30) | 0.044 | 1.95 | 8.2 (9.0) | 100 |
| Mt/(Ru + PDDA_0.1) | 1.51 | 0.028 | 1.81 | 12.0 (12.4) | 45 |
| Mt/(Ru + PDDA_0.25) | 1.99 (1.50) | 0.008 | 1.67 | 19.8 (21.3, 14.7 *) | 13 |
| Mt/(Ru + PDDA_0.05)post | 1.49 | 0.046 | 1.74 | 9.6 (9.0) | 36 |
| Mt/(Ru + PDDA_0.25)post | 1.97 (1.56) | 0.002 | 1.61 | 18.9 (21.3, 14.7 *) | 7 |
| Mt/PDDA_0.05/Ru | 1.51 | 0.048 | 1.99 | 9.4 (9.0) | 26 |
| Mt/PDDA_0.25/Ru | 1.57 | 0.001 | 1.74 | 16.3 (21.3, 14.7 *) | 27 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serwicka, E.M.; Zimowska, M.; Duraczyńska, D.; Napruszewska, B.D.; Nattich-Rak, M.; Mordarski, G.; Lityńska-Dobrzyńska, L.; Palkova, H. PDDA-Montmorillonite Composites Loaded with Ru Nanoparticles: Synthesis, Characterization, and Catalytic Properties in Hydrogenation of 2-Butanone. Polymers 2018, 10, 865. https://doi.org/10.3390/polym10080865
Serwicka EM, Zimowska M, Duraczyńska D, Napruszewska BD, Nattich-Rak M, Mordarski G, Lityńska-Dobrzyńska L, Palkova H. PDDA-Montmorillonite Composites Loaded with Ru Nanoparticles: Synthesis, Characterization, and Catalytic Properties in Hydrogenation of 2-Butanone. Polymers. 2018; 10(8):865. https://doi.org/10.3390/polym10080865
Chicago/Turabian StyleSerwicka, Ewa M., Małgorzata Zimowska, Dorota Duraczyńska, Bogna D. Napruszewska, Małgorzata Nattich-Rak, Grzegorz Mordarski, Lidia Lityńska-Dobrzyńska, and Helena Palkova. 2018. "PDDA-Montmorillonite Composites Loaded with Ru Nanoparticles: Synthesis, Characterization, and Catalytic Properties in Hydrogenation of 2-Butanone" Polymers 10, no. 8: 865. https://doi.org/10.3390/polym10080865
APA StyleSerwicka, E. M., Zimowska, M., Duraczyńska, D., Napruszewska, B. D., Nattich-Rak, M., Mordarski, G., Lityńska-Dobrzyńska, L., & Palkova, H. (2018). PDDA-Montmorillonite Composites Loaded with Ru Nanoparticles: Synthesis, Characterization, and Catalytic Properties in Hydrogenation of 2-Butanone. Polymers, 10(8), 865. https://doi.org/10.3390/polym10080865