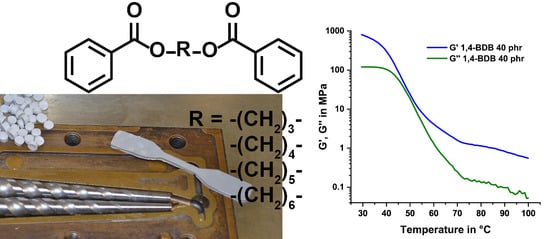
Designing Green Plasticizers: Linear Alkyl Diol Dibenzoate Plasticizers and a Thermally Reversible Plasticizer
Abstract
:1. Introduction
2. Methods and Materials
2.1. Synthesis
2.2. Extrusion of Poly(Vinyl Chloride)/Plasticizer Blends
2.3. Mechanical Test Bar Production
2.4. Differential Scanning Calorimetry (DSC)
2.5. Dynamic Mechanical Thermal Analysis (DMTA)
2.6. Tensile Testing
2.7. Surface Hardness by Nano-Indentation
2.8. Microscopy
2.9. Statistical Analysis
3. Results
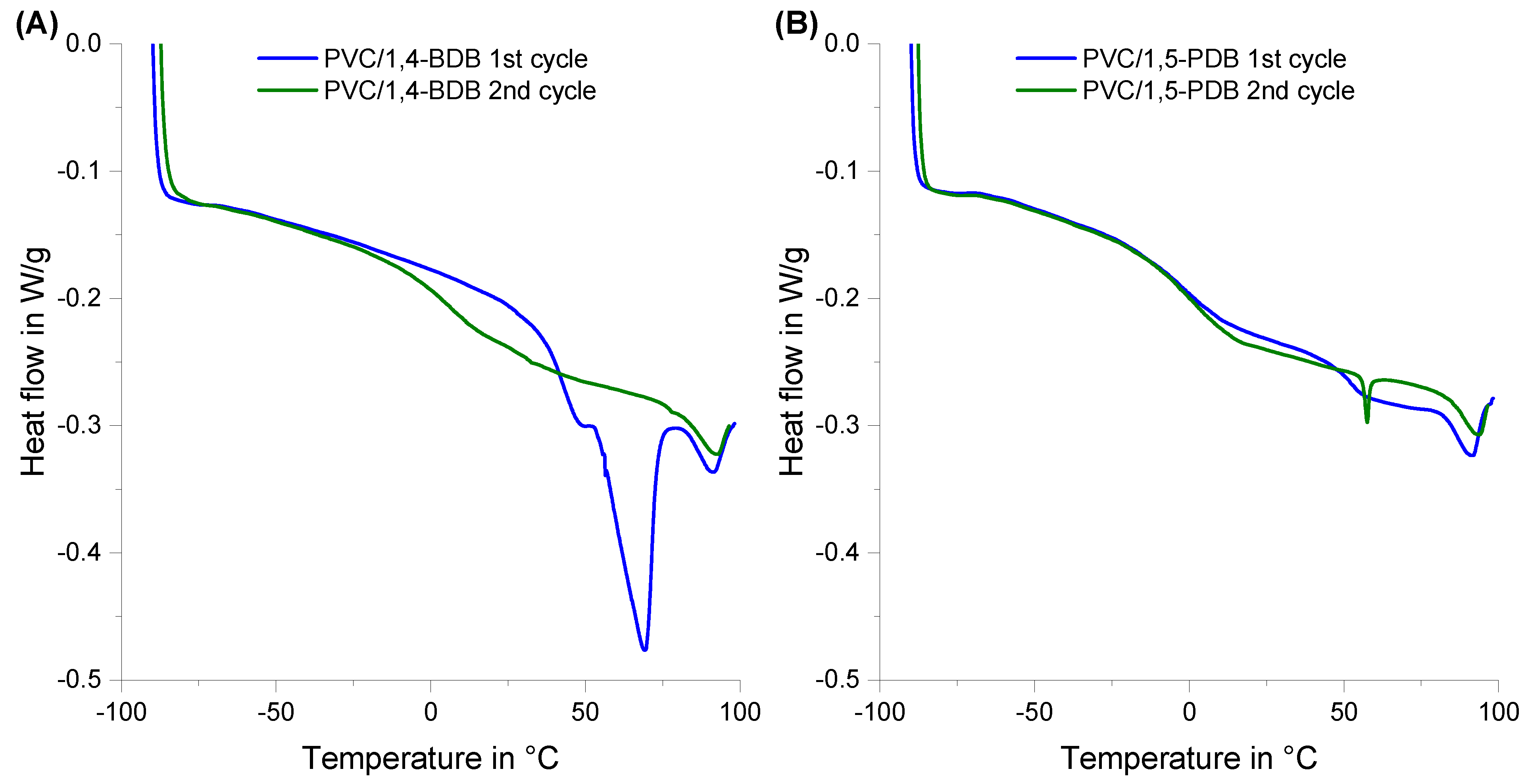
3.1. Glass Transition Temperature (Tg)
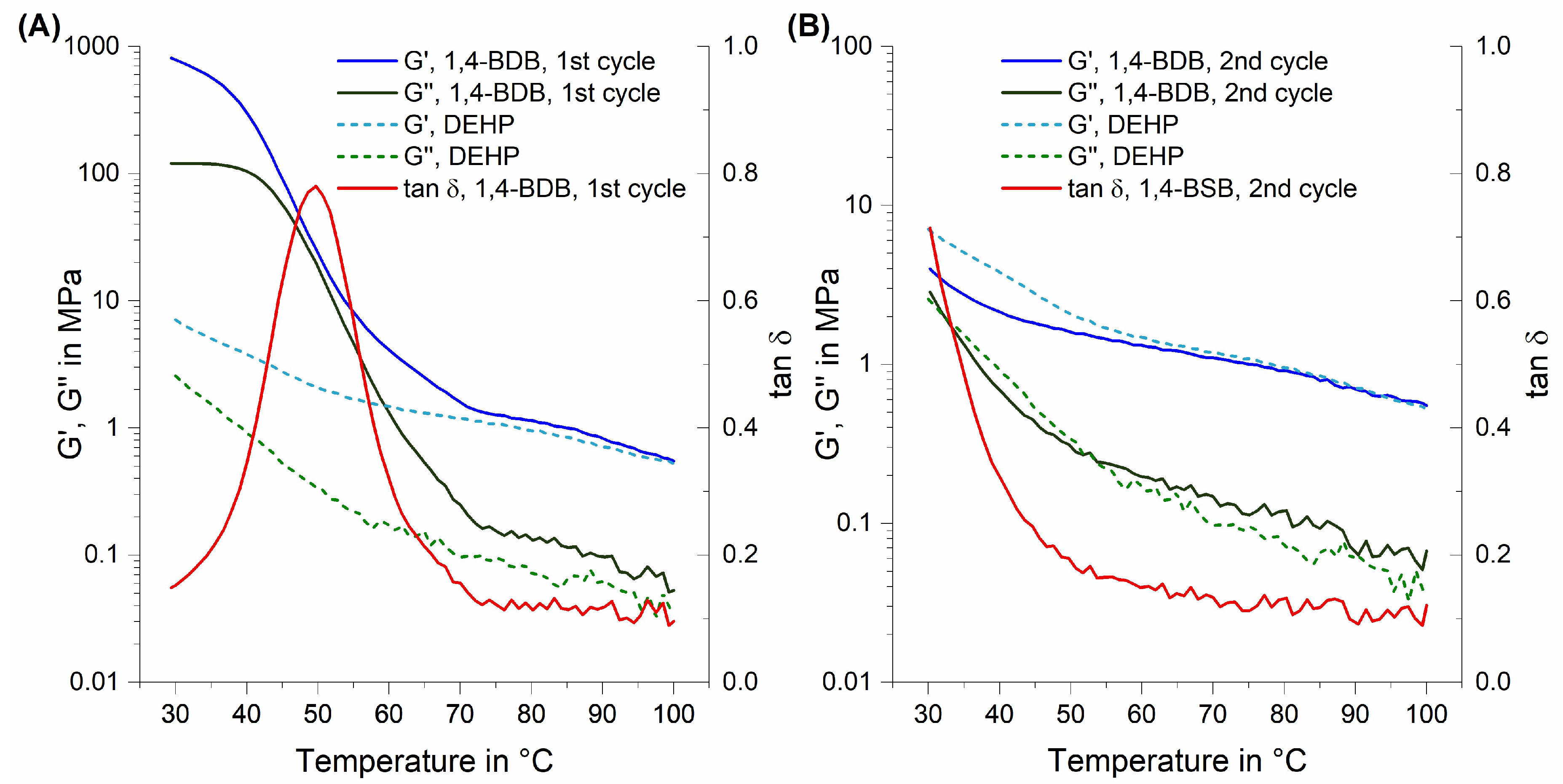
3.2. Dynamic Mechanical Thermal Analysis (DMTA) in Torsion Mode
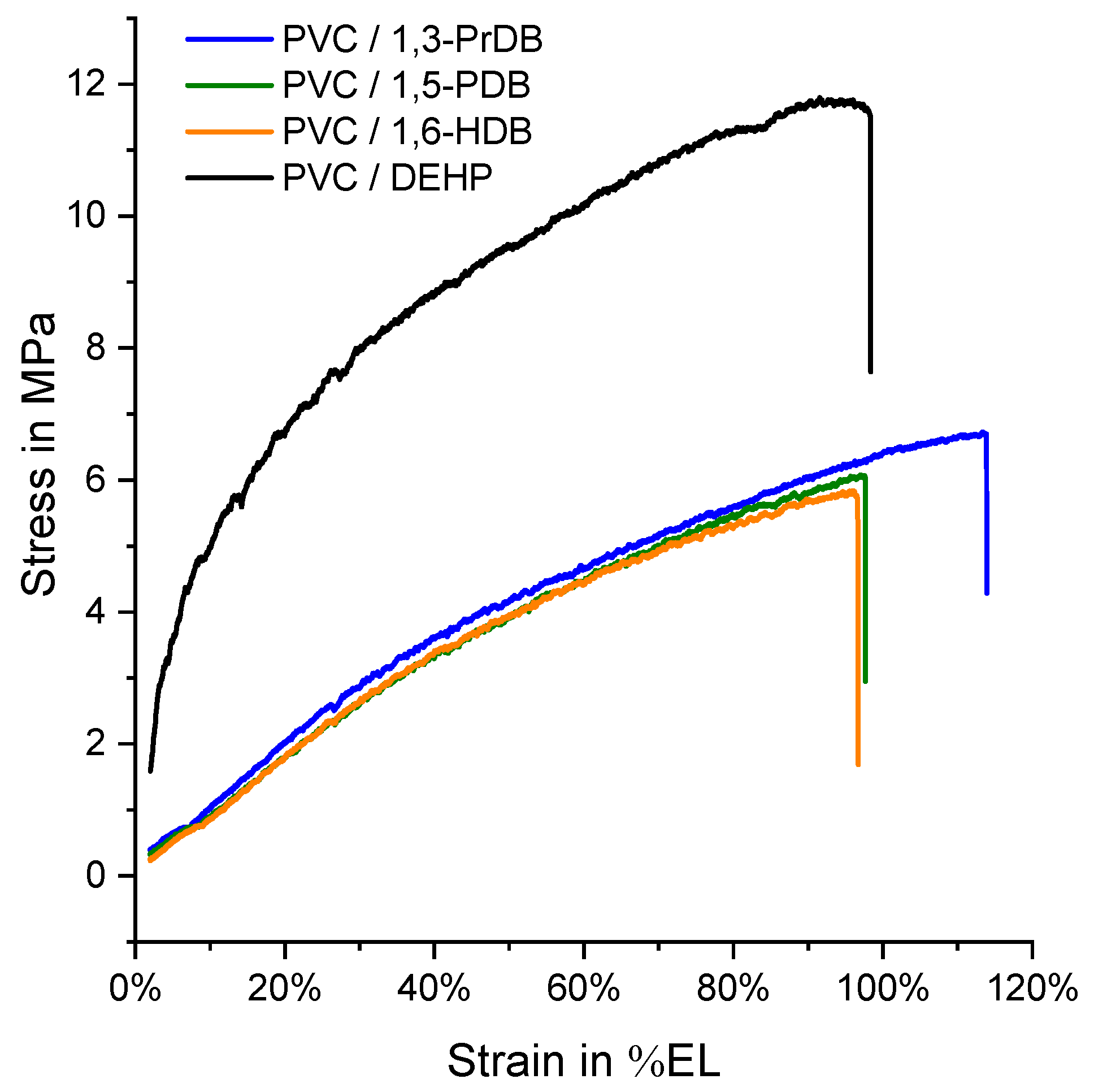
3.3. Tensile Testing
3.4. Surface Hardness by Nano-Indentation
3.5. Microscopy
4. Discussion
4.1. Dibenzoate Plasticizer Effectiveness in Poly(Vinyl Chloride)
4.2. The Special Case of 1,4-Butanediol Dibenzoate (1,4-BDB)
4.3. Diol Dibenzoate Safety
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Spevacek, J. A change is gonna come. Nat. Rev. Chem. 2017, 1, 8. [Google Scholar] [CrossRef]
- Fischer, I.; Schmitt, W.F.; Porth, H.C.; Allsopp, M.W.; Vianello, G. Poly(vinyl chloride). In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Erythropel, H.C.; Maric, M.; Nicell, J.A.; Leask, R.L.; Yargeau, V. Leaching of the plasticizer di(2-ethylhexyl)phthalate (DEHP) from plastic containers and the question of human exposure. Appl. Microbiol. Biotechnol. 2014, 98, 9967–9981. [Google Scholar] [CrossRef] [PubMed]
- Mersiowsky, I.; Weller, M.; Ejlertsson, J. Fate of plasticised PVC products under landfill conditions: A laboratory-scale landfill simulation reactor study. Water Res. 2001, 35, 3063–3070. [Google Scholar] [CrossRef]
- Sears, J.K.; Darby, J.R. The Technology of Plasticizers; John Wiley: New York, NY, USA, 1982. [Google Scholar]
- IHS Markit. Chemical Economic Handbook—Plasticizers; IHS Markit: London, UK, 2015. [Google Scholar]
- Murphy, J. The Additives for Plastics Handbook, 2nd ed.; Elsevier: New York, NY, USA, 2001. [Google Scholar]
- AGPU. Everything about PVC—From Manufacturing to Recycling; AGPU: Bonn, Germany, 2016. [Google Scholar]
- Wams, T.J. Diethylhexylphthalate as an environmental contaminant—A review. Sci. Total Environ. 1987, 66, 1–16. [Google Scholar] [CrossRef]
- Piche, C.D.; Sauvageau, D.; Vanlian, M.; Erythropel, H.C.; Robaire, B.; Leask, R.L. Effects of di-(2-ethylhexyl) phthalate and four of its metabolites on steroidogenesis in MA-10 cells. Ecotoxicol. Environ. Saf. 2012, 79, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Traore, K.; Li, W.; Amri, H.; Huang, H.; Wu, C.; Chen, H.; Zirkin, B.; Papadopoulos, V. Molecular mechanisms mediating the effect of mono-(2-ethylhexyl) phthalate on hormone-stimulated steroidogenesis in MA-10 mouse tumor leydig cells. Endocrinology 2010, 151, 3348–3362. [Google Scholar] [CrossRef] [PubMed]
- Pant, N.; Shukla, M.; Patel, D.K.; Shukla, Y.; Mathur, N.; Gupta, Y.K.; Saxena, D.K. Correlation of phthalate exposures with semen quality. Toxicol. Appl. Pharmacol. 2008, 231, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Swan, S.H.; Main, K.M.; Liu, F.; Stewart, S.L.; Kruse, R.L.; Calafat, A.M.; Mao, C.S.; Redmon, J.B.; Ternand, C.L.; Sullivan, S.; et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ. Health Perspect. 2005, 113, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Lovekamp-Swan, T.; Davis, B.J. Mechanisms of phthalate ester toxicity in the female reproductive system. Environ. Health Perspect. 2003, 111, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Erythropel, H.C.; Shipley, S.; Bormann, A.; Nicell, J.A.; Maric, M.; Leask, R.L. Designing green plasticizers: Influence of molecule geometry and alkyl chain length on the plasticizing effectiveness of diester plasticizers in PVC blends. Polymer 2016, 89, 18–27. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Erythropel, H.C.; Zimmerman, J.B.; de Winter, T.M.; Petitjean, L.; Melnikov, F.; Lam, C.H.; Lounsbury, A.W.; Mellor, K.E.; Jankovic, N.Z.; Tu, Q.; et al. The green chemistree: 20 years after taking root with the 12 principles. Green Chem. 2018, 20, 1929–1961. [Google Scholar] [CrossRef]
- Arendt, W.D.; Lang, J. New benzoate plasticizers for polyvinyl chloride: Introduction and performance example. J. Vinyl Addit. Technol. 1998, 4, 184–188. [Google Scholar] [CrossRef]
- Arendt, W.D.; Joshi, M. Specialty plasticizers. In Handbook of Vinyl Formulating, 2nd ed.; Grossman, R.F., Ed.; Wiley: Hoboken, NJ, USA, 2008; pp. 239–286. [Google Scholar]
- Gartshore, J.; Cooper, D.G.; Nicell, J.A. Biodegradation of plasticizers by rhodotorula rubra. Environ. Toxicol. Chem. 2003, 22, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Firlotte, N.; Cooper, D.G.; Marić, M.; Nicell, J.A. Characterization of 1,5-pentanediol dibenzoate as a potential “green” plasticizer for poly(vinyl chloride). J. Vinyl Addit. Technol. 2009, 15, 99–107. [Google Scholar] [CrossRef]
- Boethling, R.S.; Sommer, E.; DiFiore, D. Designing small molecules for biodegradability. Chem. Rev. 2007, 107, 2207–2227. [Google Scholar] [CrossRef] [PubMed]
- Kermanshahi pour, A.; Cooper, D.G.; Mamer, O.A.; Maric, M.; Nicell, J.A. Mechanisms of biodegradation of dibenzoate plasticizers. Chemosphere 2009, 77, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Kermanshahi pour, A.; Roy, R.; Cooper, D.G.; Maric, M.; Nicell, J.A. Biodegradation kinetics of dibenzoate plasticizers and their metabolites. Biochem. Eng. J. 2013, 70, 35–45. [Google Scholar] [CrossRef]
- Segura, P.A.; Kaplan, P.; Erythropel, H.C.; Yargeau, V. Comparative rapid toxicity screening of commercial and potential “green” plasticizers using bioluminescent bacteria. Ind. Eng. Chem. Res. 2012, 51, 11555–11560. [Google Scholar] [CrossRef]
- Boisvert, A.; Jones, S.; Issop, L.; Erythropel, H.C.; Papadopoulos, V.; Culty, M. In vitro functional screening as a means to identify new plasticizers devoid of reproductive toxicity. Environ. Res. 2016, 150, 496–512. [Google Scholar] [CrossRef] [PubMed]
- Nardelli, T.C.; Erythropel, H.C.; Robaire, B. Toxicogenomic screening of replacements for di(2-ethylhexyl) phthalate (DEHP) using the immortalized TM4 sertoli cell line. PLoS ONE 2015, 10, e0138421. [Google Scholar] [CrossRef] [PubMed]
- Albert, O.; Nardelli, T.C.; Lalancette, C.; Hales, B.F.; Robaire, B. Effects of in utero and lactational exposure to new generation green plasticizers on adult male rats: A comparative study with di(2-ethylhexyl) phthalate. Toxicol. Sci. 2018, kfy072. [Google Scholar] [CrossRef]
- Nardelli, T.C.; Albert, O.; Lalancette, C.; Culty, M.; Hales, B.F.; Robaire, B. In utero and lactational exposure study in rats to identify replacements for di(2-ethylhexyl) phthalate. Sci. Rep. 2017, 7, 3862. [Google Scholar] [CrossRef] [PubMed]
- Kermanshahi Pour, A.; Mamer, O.A.; Cooper, D.G.; Maric, M.; Nicell, J.A. Metabolites from the biodegradation of 1,6-hexanediol dibenzoate, a potential green plasticizer, by rhodococcus rhodochrous. J. Mass Spectrom. 2009, 44, 662–671. [Google Scholar] [CrossRef] [PubMed]
- ASTM D-638. Standard Test Method for Tensile Properties of Plastics; American Society for Testing and Materials: West Conshohocken, PA, USA, 2014. [Google Scholar]
- ASTM D-4065. Standard Practice for Plastics: Dynamic Mechanical Properties: Determination and Report of Procedures; American Society for Testing and Materials: West Conshohocken, PA, USA, 2012. [Google Scholar]
- ASTM E-2546. Standard Practice for Instrumented Indentation Testing; American Society for Testing and Materials: West Conshohocken, PA, USA, 2007. [Google Scholar]
- ATSDR. Toxicological Profile for Di(2-Ethylhexyl)phthalate (DEHP); U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2002.
- Albert, O.; Nardelli, T.C.; Hales, B.F.; Robaire, B. Identifying greener and safer plasticizers: A 4-step approach. Toxicol. Sci. 2018, 161, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Jamarani, R.; Erythropel, H.C.; Burkat, D.; Nicell, J.A.; Leask, R.L.; Maric, M. Rheology of green plasticizer/poly(vinyl chloride) blends via time-temperature superposition. Processes 2017, 5, 43. [Google Scholar] [CrossRef]
- Voss, A.; Stark, R.W.; Dietz, C. Surface versus volume properties on the nanoscale: Elastomeric polypropylene. Macromolecules 2014, 47, 5236–5245. [Google Scholar]
- Oxtoby, D.W.; Gillis, H.P.; Nachtrieb, N.H. Principles of Modern Chemistry, 4th ed.; Saunders College Publishing: Orlando, FL, USA, 1999. [Google Scholar]
- Sasanuma, Y.; Wagai, Y.; Suzuki, N.; Abe, D. Conformational characteristics and configurational properties of poly(butylene terephthalate) and structure—Property relationships of aromatic polyesters. Polymer 2013, 54, 3904–3913. [Google Scholar] [CrossRef]
- Dodge, R.W.; Mattice, W.L. Conformation of the ground-state dimer in poly(ethylene terephthalate). J. Polym. Sci. Part B Polym. Lett. 1993, 31, 207–212. [Google Scholar] [CrossRef]
- Mendicuti, F.; Patel, B.; Viswanadhan, V.N.; Mattice, W.L. Identification of conformations conducive to intramolecular excimer formation in polyesters with different numbers of methylene units between aromatic rings. Polymer 1988, 29, 1669–1674. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Patel, M.K. Plastics derived from biological sources: Present and future: A technical and environmental review. Chem. Rev. 2012, 112, 2082–2099. [Google Scholar] [CrossRef] [PubMed]
- Beach, E.S.; Weeks, B.R.; Stern, R.; Anastas, P.T. Plastics additives and green chemistry. Pure Appl. Chem. 2013, 85, 1611–1624. [Google Scholar] [CrossRef]
- Snead, O.C., III; Gibson, K.M. Γ-hydroxybutyric acid. N. Engl. J. Med. 2005, 352, 2721–2732. [Google Scholar] [CrossRef] [PubMed]

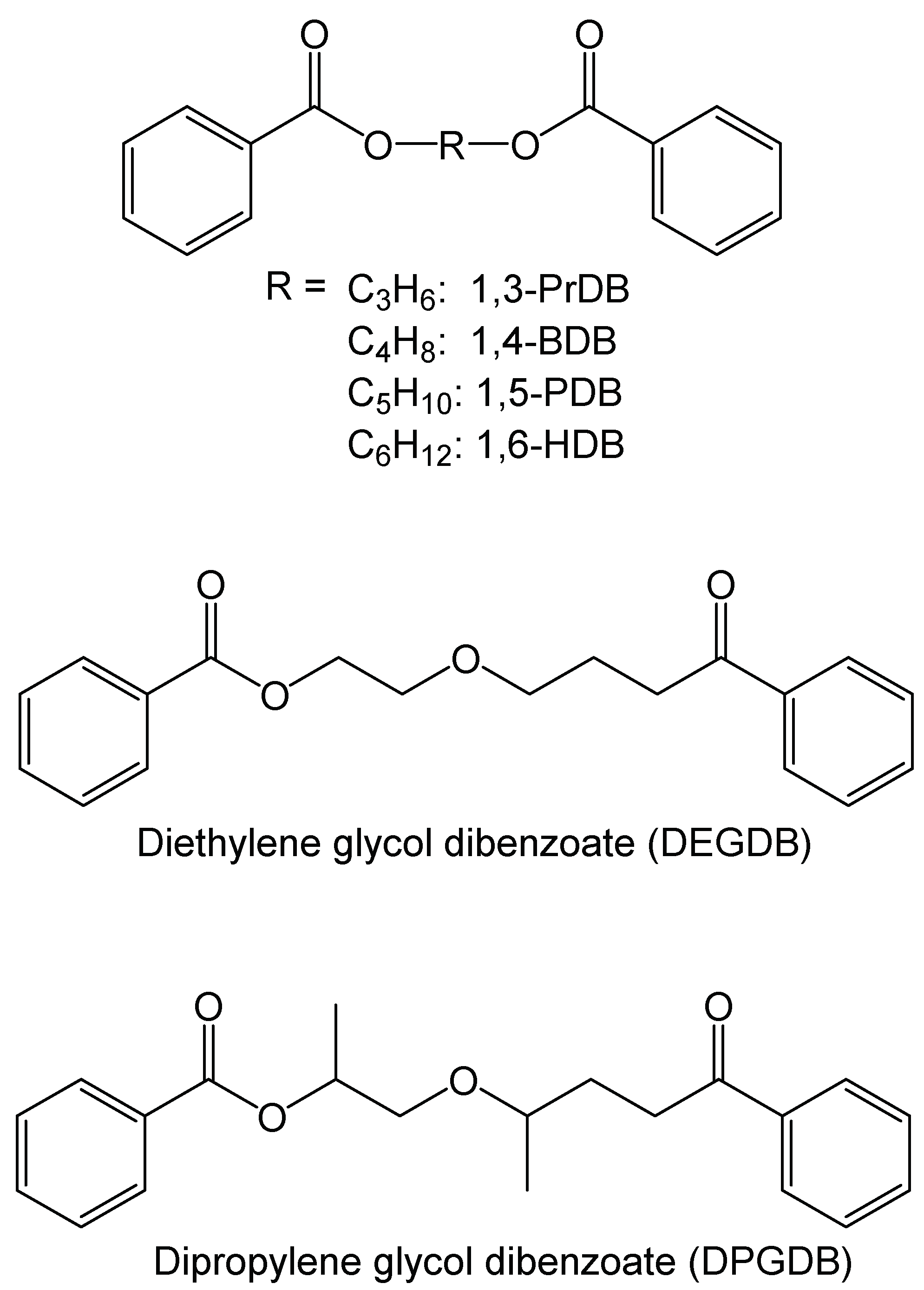


| Dibenzoate Compound | Melting Point (°C) | |
|---|---|---|
| By DSC | By Gallenkamp | |
| 1,3-propanediol DB | 57–59 | 55–57 |
| 1,4-butanediol DB | 80–81 | 78–80 |
| 1,5-pentanediol DB | 25 | 20 < m.p. < 37 (body T) |
| 1,6-hexanediol DB | 54–55 | 54–55 |
| Candidate Plasticizer | Tg (°C) by DSC (n = 3) | DMTA Torsion (at 25°C) (n = 1) | Tensile Testing (at 20°C) (n = 4) | Hardness (at 20°C) (n = 3) | |||
|---|---|---|---|---|---|---|---|
| G’ at 1 Hz (MPa) | G” at 1 Hz (MPa) | Elongation at Break (%EL) | Max. Stress (MPa) | Apparent Mod. at 25%EL (MPa) | Surface Hardness (MPa) | ||
| 1,3-propanediol DB | 2.9 ± 1.1 | 53.4 | 20.3 | 119 ± 7 | 6.8 ± 0.3 | 8.3 ± 0.3 | 0.41 ± 0.03 |
| 1,4-butanediol DB | n.o./2.5 ± 3.1 a | - | - | - | - | - | - |
| 1,5-pentanediol DB | −2.3 ± 0.5 | 7.07 | 4.68 | 95 ± 7 b | 6.1 ± 0.3 b | 8.5 ± 0.3 b | 0.39 ± 0.02 |
| 1,6-hexanediol DB | −3.5 ± 2.3 | 26.6 | 9.26 | 98 ± 10 | 5.8 ± 0.2 | 7.9 ± 0.5 | 0.77 ± 0.11 |
| DEHP | −5.4 ± 0.5 | 10.5 | 4.27 | 96 ± 4 | 11.8 ± 0.1 | 12.1 ± 0.6 | 0.44 ± 0.05 b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erythropel, H.C.; Börmann, A.; Nicell, J.A.; Leask, R.L.; Maric, M. Designing Green Plasticizers: Linear Alkyl Diol Dibenzoate Plasticizers and a Thermally Reversible Plasticizer. Polymers 2018, 10, 646. https://doi.org/10.3390/polym10060646
Erythropel HC, Börmann A, Nicell JA, Leask RL, Maric M. Designing Green Plasticizers: Linear Alkyl Diol Dibenzoate Plasticizers and a Thermally Reversible Plasticizer. Polymers. 2018; 10(6):646. https://doi.org/10.3390/polym10060646
Chicago/Turabian StyleErythropel, Hanno C., Aurélie Börmann, Jim A. Nicell, Richard L. Leask, and Milan Maric. 2018. "Designing Green Plasticizers: Linear Alkyl Diol Dibenzoate Plasticizers and a Thermally Reversible Plasticizer" Polymers 10, no. 6: 646. https://doi.org/10.3390/polym10060646
APA StyleErythropel, H. C., Börmann, A., Nicell, J. A., Leask, R. L., & Maric, M. (2018). Designing Green Plasticizers: Linear Alkyl Diol Dibenzoate Plasticizers and a Thermally Reversible Plasticizer. Polymers, 10(6), 646. https://doi.org/10.3390/polym10060646