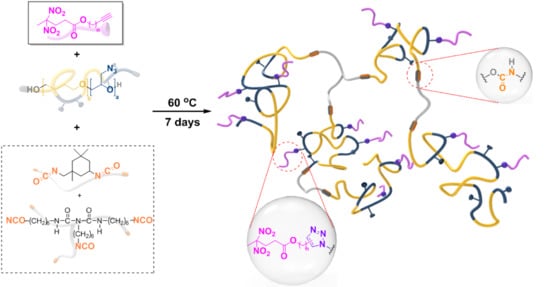
Reactive Energetic Plasticizers Utilizing Cu-Free Azide-Alkyne 1,3-Dipolar Cycloaddition for In-Situ Preparation of Poly(THF-co-GAP)-Based Polyurethane Energetic Binders
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Characterization
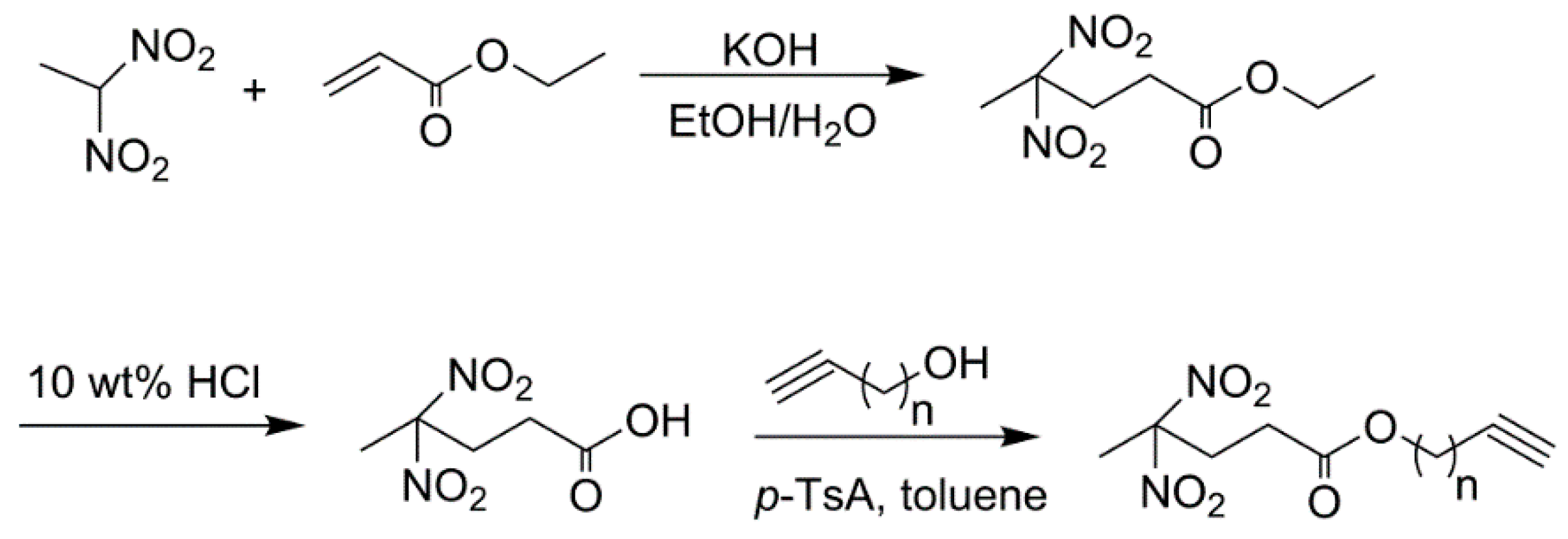
2.3. Synthesis of REPs
4,4-dinitro valeric ester (DNVE)
4,4-dinitro valeric acid (DNVA)
REPs: Prop-2-yn-1-yl-4,4-dinitropentanoate (PDNP) and but-3-yn-1-yl-4,4-dinitropentanoate (BDNP)
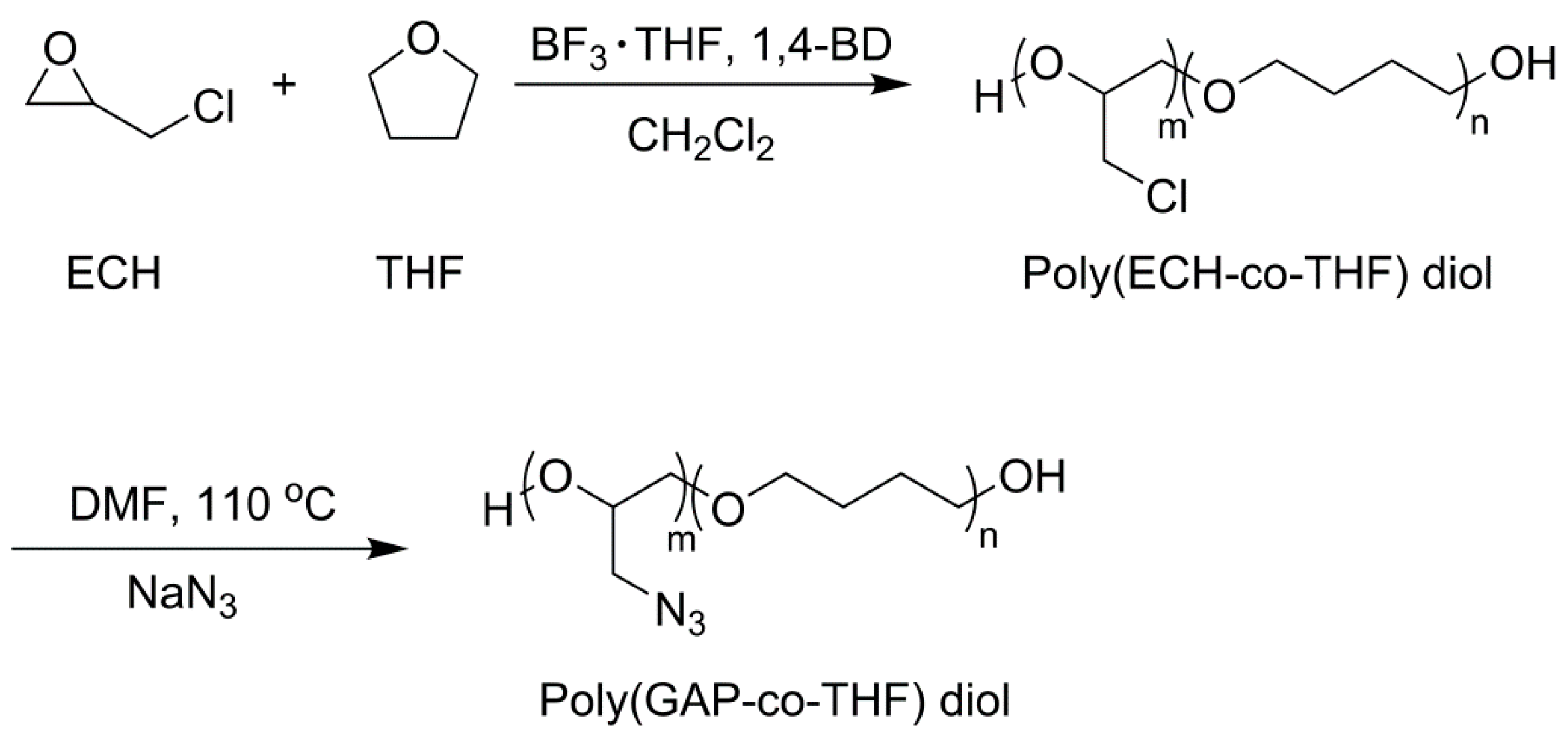
2.4. Synthesis of PGT Prepolymer
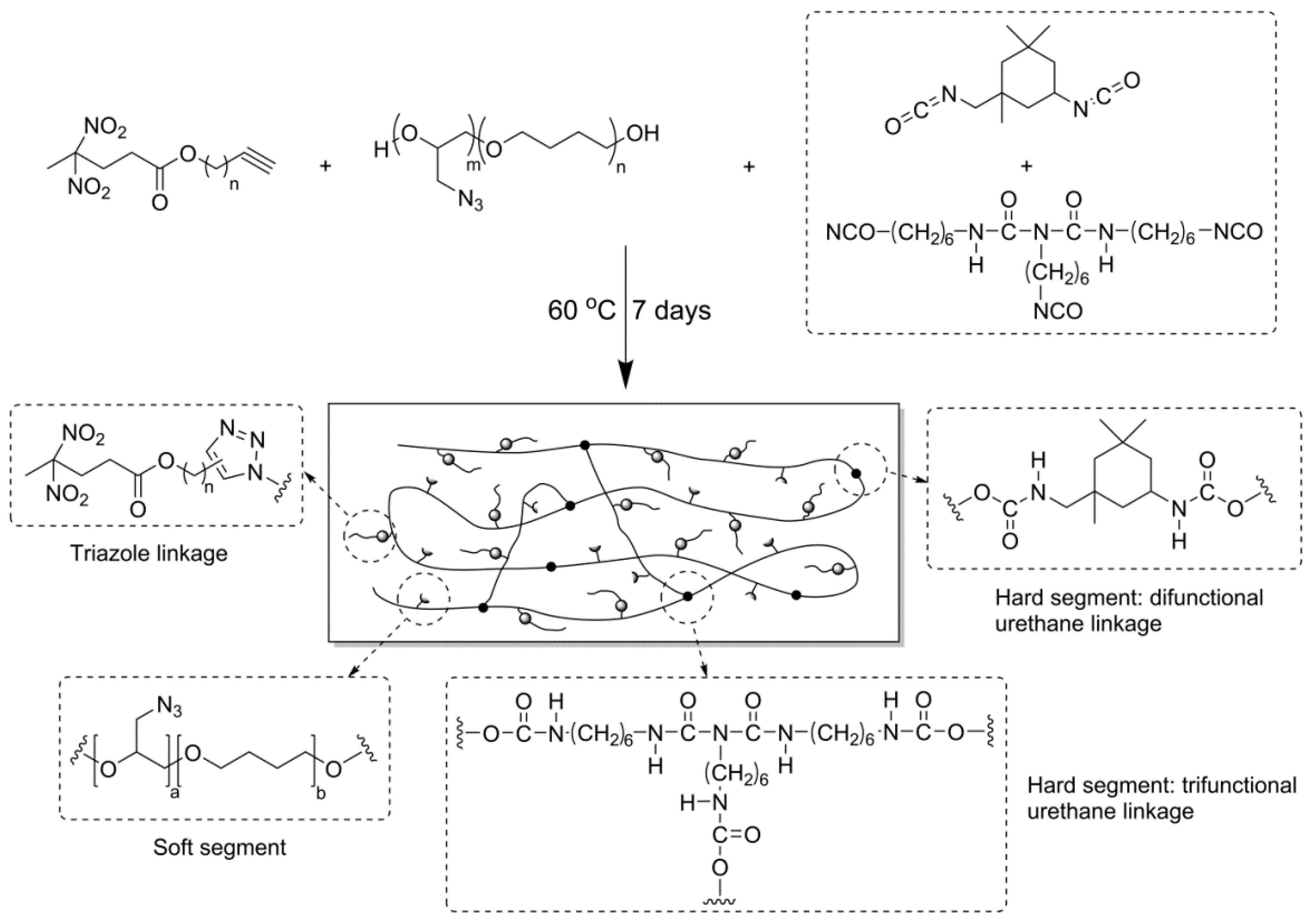
2.5. Preparation of the PGT-Based PUs with REPs
2.6. Cu-Free Huisgen 1,3-Dipolar Cycloaddition
3. Results and Discussion
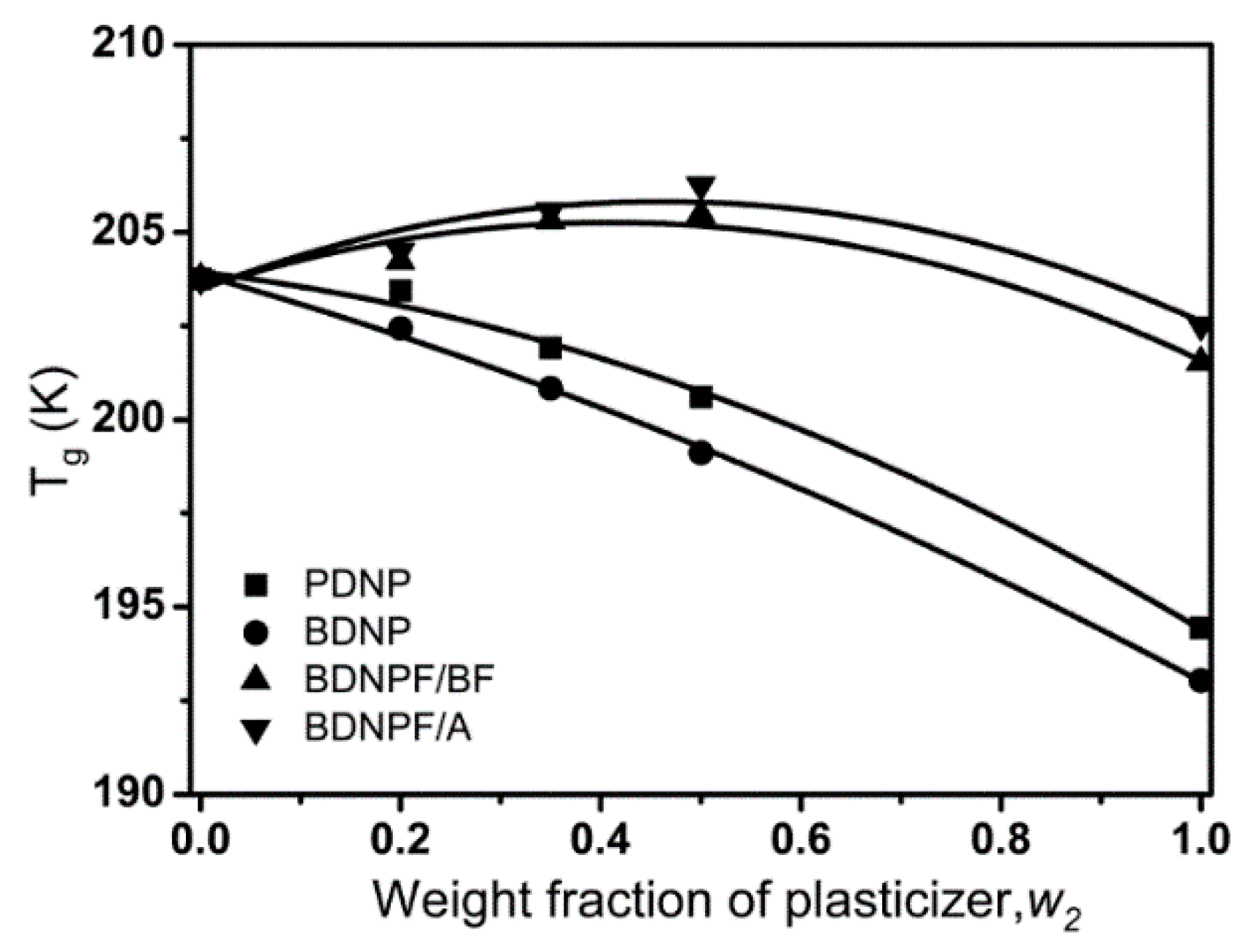
3.1. Plasticizing Performance of REPs
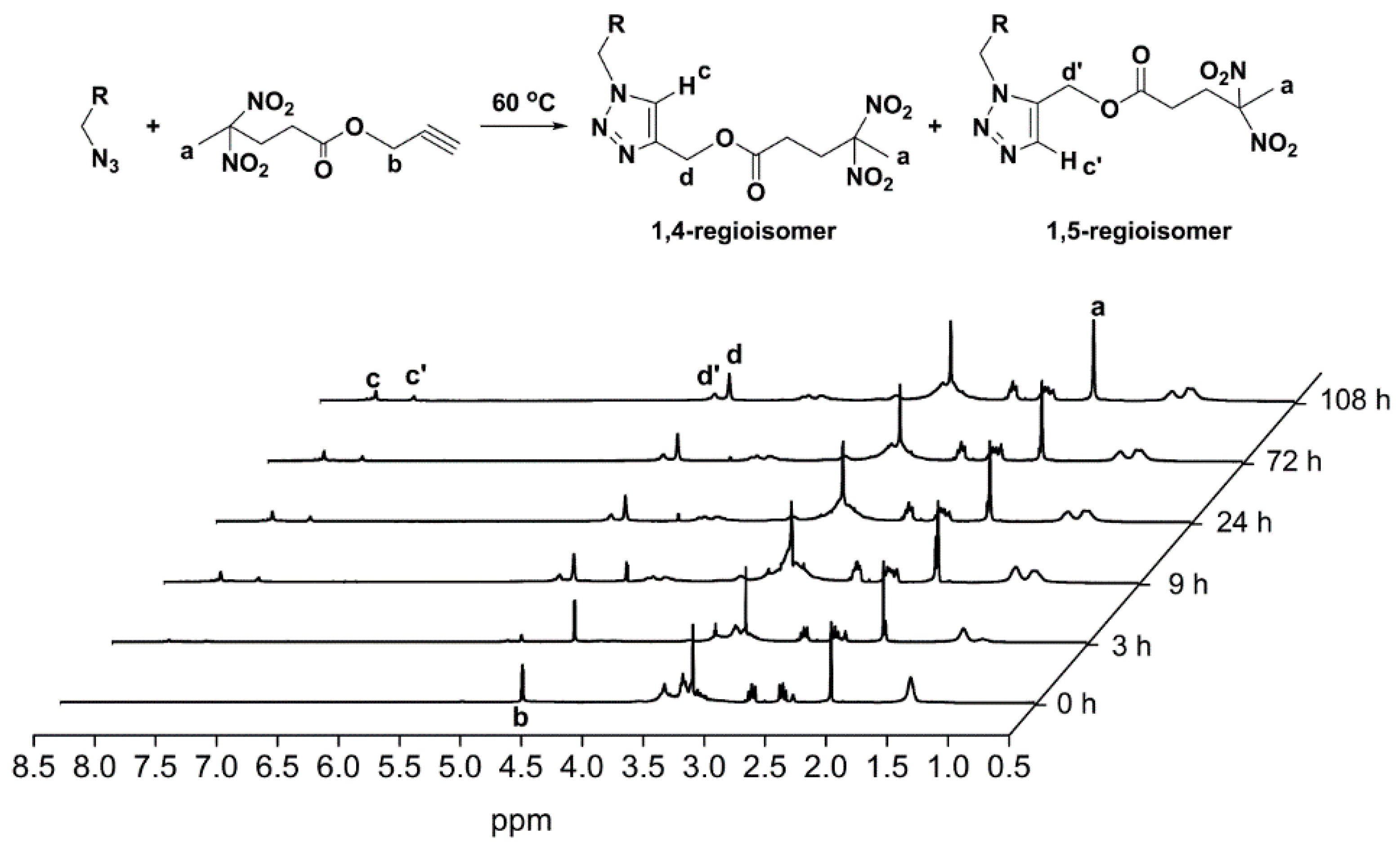
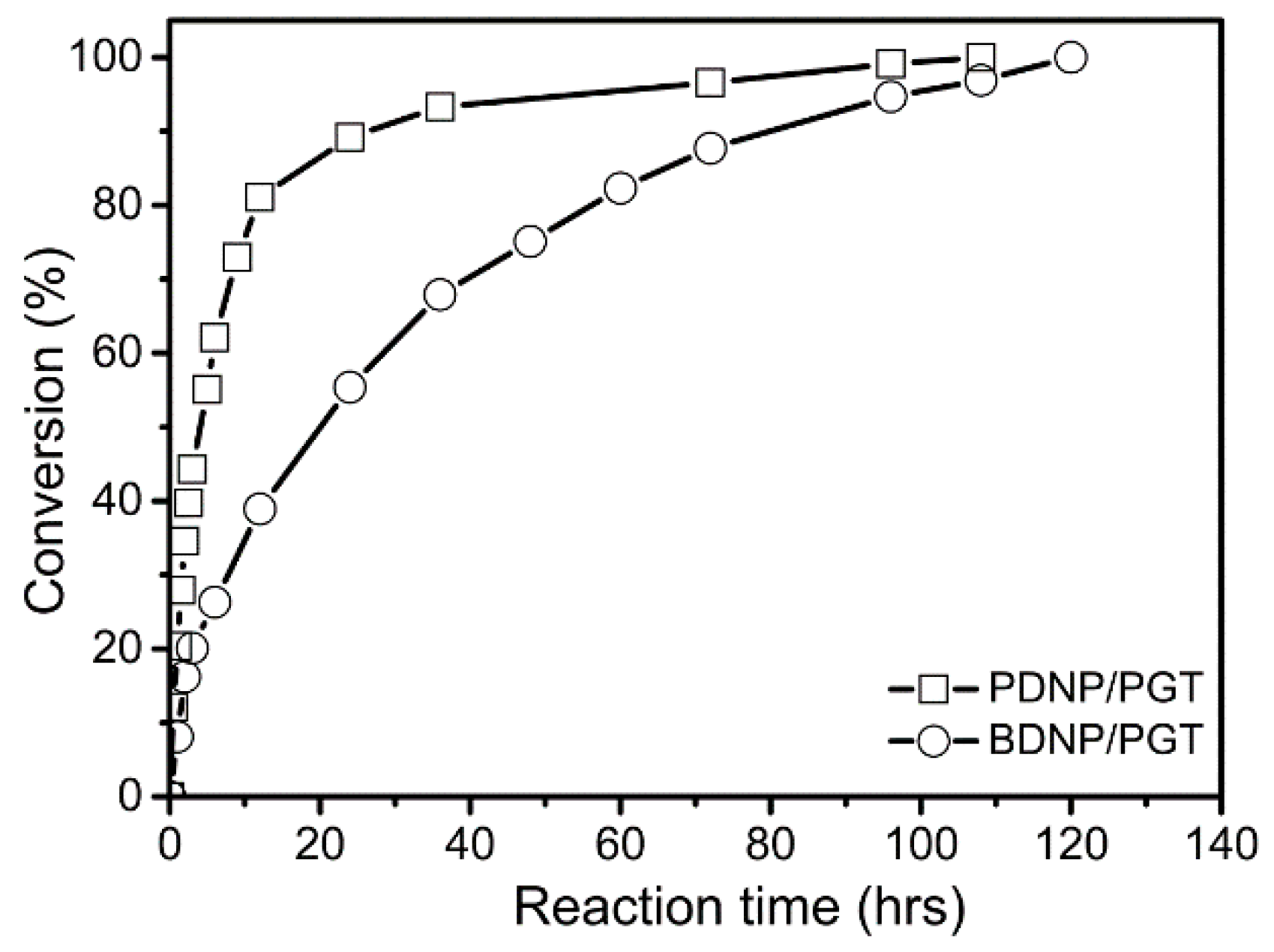
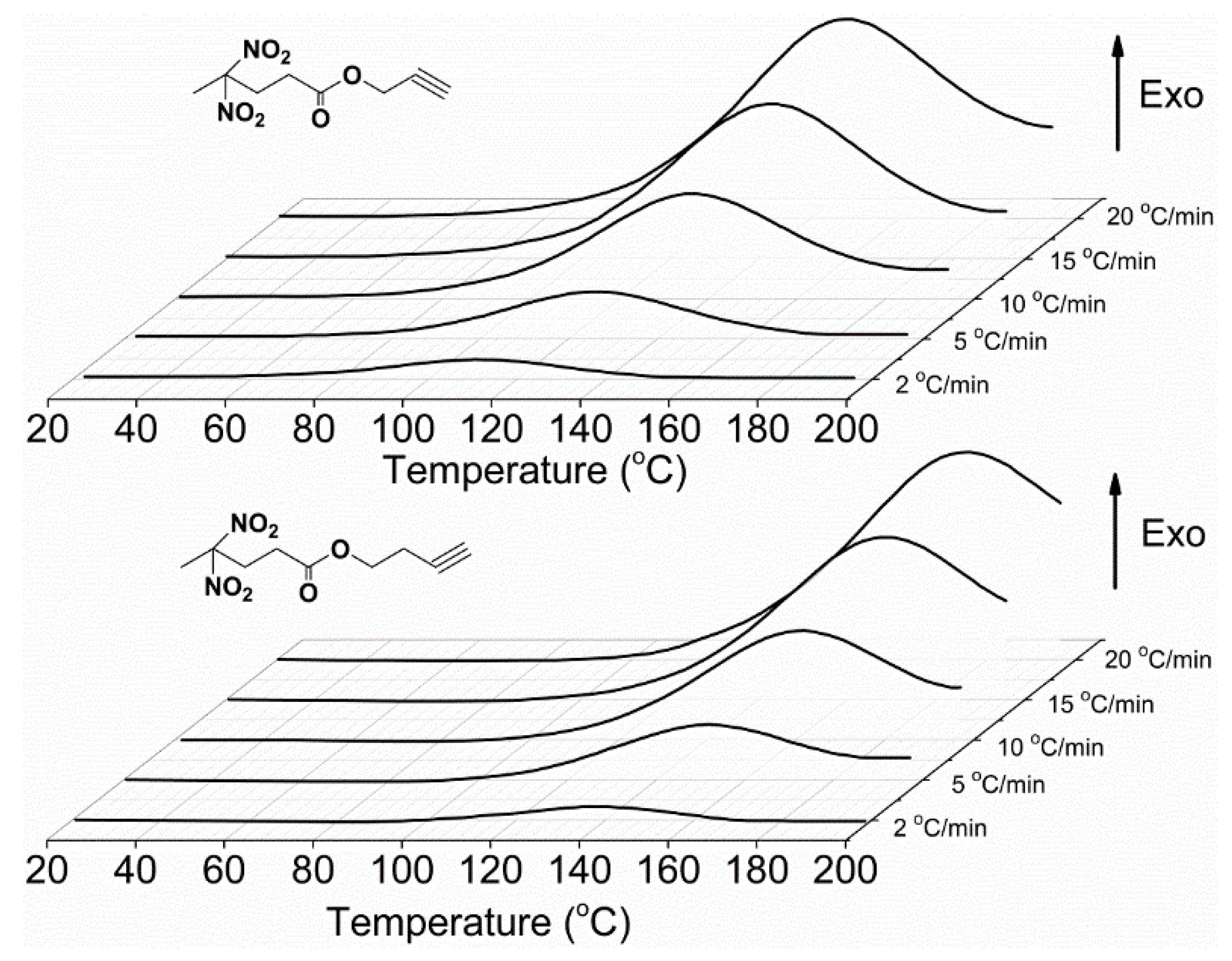
3.2. Huisgen 1,3-DPCA Reactivity
3.3. Properties of REP/PGT-Based PUs
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Colclough, M.E.; Desai, H.; Millar, R.W.; Paul, N.C.; Stewart, M.J.; Golding, P. Energetic polymers as binders in composite propellants and explosives. Polym. Adv. Technol. 1994, 5, 554–560. [Google Scholar] [CrossRef]
- Akhavan, J.; Koh, E.; Waring, S.; Kron, E. Effect of UV and thermal radiation on polyNIMMO. Polymer 2001, 42, 7711–7718. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kwon, Y.; Kim, C.K. Thermal and mechanical properties of hydroxyl-terminated polybutadiene-based polyurethane/polyhedral oligomeric silsesquioxane nanocomposites plasticized with DOA. J. Nanosci. Nanotechnol. 2013, 13, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Kwon, Y.; Kim, C.K. Synthesis and properties of hydroxyl-terminated polybutadiene-based polyurethanes reinforced with polyhedral oligomeric silsesquioxanes. J. Nanosci. Nanotechnol. 2014, 14, 8671–8677. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Kim, C.K.; Kwon, Y. Ablation and fire-retardant properties of hydroxyl-terminated polybutadiene-based polyurethane-g-polyhedral oligomeric silsesquioxane composites. High Perform. Polym. 2015, 27, 749–757. [Google Scholar] [CrossRef]
- Haska, S.B.; Bayramli, E.; Pekel, F.; Özkar, S. Mechanical properties of HTPB-IPDI-based elastomers. J. Appl. Polym. Sci. 1996, 64, 2347–2354. [Google Scholar] [CrossRef]
- Patil, P.R.; Krishnamurthy, V.N.; Joshi, S.S. Differential scanning calorimetric study of HTPB based composite propellants in presence of nano ferric oxide. Propellants Explos. Pyrotech. 2006, 31, 442–446. [Google Scholar] [CrossRef]
- Kuwahara, T.; Kubota, N. Energetic solid fuels for ducted rockets. Int. Annu. Conf. 1990, 40, 1–10. [Google Scholar]
- Gaur, B.; Lochab, B.; Choudhary, V.; Varma, I.K. Azido polymers—Energetic binders for solid rocket propellants. J. Macromol. Sci. Part C Polym. Rev. 2003, 43, 505–545. [Google Scholar] [CrossRef]
- Grant, L.R.; Flanagan, J.E.; Frankel, M.B. Historical development of glycidyl azide polymer. J. Propuls. Power 1992, 8, 560–563. [Google Scholar]
- Mohan, Y.M.; Raju, K.M. Synthesis and characterization of GAP-THF copolymers. Int. J. Polym. Mater. 2006, 55, 203–217. [Google Scholar] [CrossRef]
- Stacer, R.G.; Husband, D.M. Molecular structure of the ideal solid propellant binder. Propellants Explos. Pyrotech. 1991, 16, 167–176. [Google Scholar] [CrossRef]
- Zhai, J.; Shan, Z.; Li, J.; Li, X.; Guo, X.; Yang, R. Study on influence of terminal structure on mechanical properties of GAP elastomers. J. Appl. Polym. Sci. 2013, 128, 2319–2324. [Google Scholar] [CrossRef]
- Marcilla, A.; Garcia, S.; Garcia-Quesada, J.C. Migrability of PVC plasticizers. Polym. Test. 2008, 27, 221–233. [Google Scholar] [CrossRef]
- Provatas, A. Energetic plasticizer migration studies. J. Energ. Mater. 2003, 21, 237–245. [Google Scholar] [CrossRef]
- Yang, B.; Bai, Y.; Cao, Y. Effects of inorganic nano-particles on plasticizers migration of flexible PVC. J. Appl. Polym. Sci. 2010, 115, 2178–2182. [Google Scholar] [CrossRef]
- Ma, M.; Shen, Y.; Kwon, Y.; Chung, C.; Kim, J.S. Reactive energetic plasticizers for energetic polyurethane binders prepared via simultaneous Huisgen azide-alkyne cycloaddition and polyurethane reaction. Propellants Explos. Pyrotech. 2016, 41, 746–756. [Google Scholar] [CrossRef]
- Landsem, E.; Jensen, T.L.; Kristensen, T.E.; Hansen, F.K.; Benneche, T.; Unneberg, E. Isocyanate-free and dual curing of smokeless composite rocket propellants. Propellants Explos. Pyrotech. 2013, 38, 75–86. [Google Scholar] [CrossRef]
- Hagen, T.H.; Jensen, T.L.; Unneberg, E.; Stenstrøm, Y.H.; Kristensen, T.E. Curing of Glycidyl Azide Polymer (GAP) diol using isocyanate, isocyanate-free, synchronous dual, and sequential dual curing systems. Propellants Explos. Pyrotech. 2015, 40, 275–284. [Google Scholar] [CrossRef]
- Min, B.S.; Jeon, H.B.; Jeong, T.U.; Kim, S.Y. Energetic polymeric networks prepared via a solvent- and catalyst-free thermal cycloaddition of azide-bearing polymers with alkynes and hydroxyl-isocyanate addition reactions. Polym. Chem. 2015, 6, 7913–7920. [Google Scholar] [CrossRef]
- Mohan, Y.M.; Mani, Y.; Raju, K.M. Synthesis of azido polymers as potential energetic propellant binders. Des. Monomers Polym. 2006, 9, 201–236. [Google Scholar] [CrossRef]
- Lee, B.J.; Bae, I.J.; Kim, J.S.; Kim, S.H. Method for Preparing a Difunctional Poly(GAP-co-THF) Diol for Preparation of Polyurethane Having Excellent Mechanical Properties. U.S. Patent 8,871,872, 28 October 2014. [Google Scholar]
- Choi, J.; Moon, D.S.; Jang, J.U.; Bin Yin, W.; Lee, B.; Lee, K.J. Synthesis of highly functionalized thermoplastic polyurethanes and their potential applications. Polymer 2017, 116, 287–294. [Google Scholar] [CrossRef]
- Wingborg, N.; Eldsäter, C. 2,2-Dinitro-1,3-bis-nitrooxy-propane (NPN): A new energetic plasticizer. Propellants Explos. Pyrotech. 2002, 27, 314–319. [Google Scholar] [CrossRef]
- Wypych, G. Handbook of Plasticizers; ChemTec Publishing: Toronto, ON, Canada, 2004; p. 115. [Google Scholar]
- Ward, I.M.; Hadley, D.W. An Introduction to the Mechanical Properties of Solid Polymers; John Wiley & Sons Ltd.: Chichester, UK, 1993; pp. 16–196. [Google Scholar]
- Rahm, M.; Malmström, E.; Eldsäter, C. Design of an ammonium dinitramide compatible polymer matrix. J. Appl. Polym. Sci. 2011, 122, 1–11. [Google Scholar] [CrossRef]
- Gorman, I.E.; Willer, R.L.; Kemp, L.K.; Storey, R.F. Development of a triazole-cure resin system for composites: Evaluation of alkyne curatives. Polymer 2012, 53, 2548–2558. [Google Scholar] [CrossRef]
- Li, Z.; Seo, T.S.; Ju, J. 1, 3-Dipolar cycloaddition of azides with electron-deficient alkynes under mild condition in water. Tetrahedron Lett. 2004, 45, 3143–3146. [Google Scholar] [CrossRef]
- Chambers, R.D. Fluorine in Organic Chemistry; Blackwell Publishing Ltd.: Oxford, UK, 2004; p. 175. [Google Scholar]
- Nia, A.S.; Rana, S.; Döhler, D.; Osim, W.; Binder, W.H. Nanocomposites via a direct graphene-promoted “click”-reaction. Polymer 2015, 79, 21–28. [Google Scholar]
- Keicher, T.; Kuglstatter, W.; Eisele, S.; Wetzel, T.; Krause, H. Isocyanate-free curing of glycidyl azide polymer (GAP) with bis-propargyl-succinate (II). Propellants Explos. Pyrotech. 2009, 34, 210–217. [Google Scholar] [CrossRef]
- Chen, Y.; Kwon, Y.; Kim, J.S. Synthesis and characterization of bis(2,2-dinitropropyl ethylene) formal plasticizer for energetic binders. J. Ind. Eng. Chem. 2012, 18, 1069–1075. [Google Scholar] [CrossRef]
- Sivabalan, R.; Anniyappan, M.; Pawar, S.J.; Talawar, M.B.; Gore, G.M.; Venugopalan, S.; Gandhe, B.R. Synthesis, characterization and thermolysis studies on triazole and tetrazole based high nitrogen content high energy materials. J. Hazard. Mater. 2006, 137, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Ammon, H.L. Updated atom/functional group and atom_code volume additivity parameters for the calculation of crystal densities of single molecules, organic salts, and multi-fragment materials containing H, C, B, N, O, F, S, P, Cl, Br, and I. Propellants Explos. Pyrotech. 2008, 33, 92–102. [Google Scholar] [CrossRef]
- Sućeska, M. Calculation of the detonation properties of C-H-N-O explosives. Propellants Explos. Pyrotech. 1991, 16, 197–202. [Google Scholar] [CrossRef]

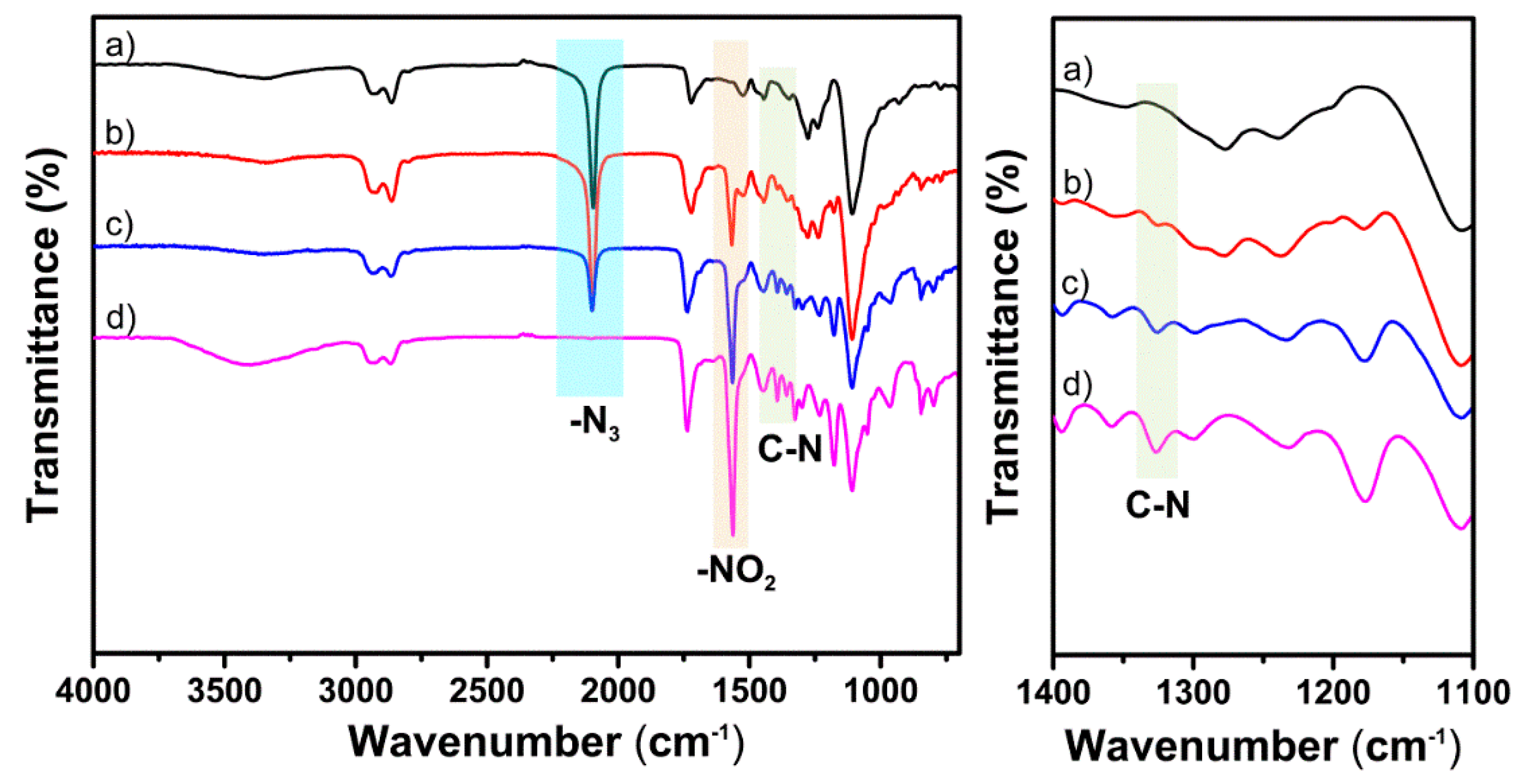

| I Value (K) | Viscosity (cP) a | ||
|---|---|---|---|
| 30 °C | 60 °C | ||
| PGT: REPs | |||
| PGT:PDNP PGT:BDNP | 6.31 ± 0.34 3.01 ± 0.33 | 450 350 | 140 114 |
| PGT: Conventional EPs | |||
| PGT:BDNPF/A b PGT:BDNPF/BF c | 11.04 ± 0.56 10.52 ± 0.50 | 692 648 | 160 142 |
| PU Binders | Mole Ratio of [C≡C]/[N3] | TGA in N2 | Mechanical Properties | |||
|---|---|---|---|---|---|---|
| Td,5wt% (°C) | Td,max (°C) | Tensile Strength (MPa) | Elongation at Break (%) | Modulus (MPa) | ||
| PGT PU | 0/0.5 | 227 | 237.1 | 0.40 ± 0.04 | 325 ± 40 | 0.21 ± 0.01 |
| PDNP (n = 1) PGT-based PU | 0.1/0.5 | 221.7 | 234 | 0.87 ± 0.06 | 246 ± 17 | 0.76 ± 0.01 |
| 0.3/0.5 | 222 | 233.3 | 1.45 ± 0.18 | 347 ± 23 | 0.82 ± 0.02 | |
| 0.5/0.5 | 227 | 229.7 | 5.23 ± 0.33 | 713 ± 34 | 6.71 ± 0.73 | |
| BDNP (n = 2) PGT-based PU | 0.1/0.5 | 220.3 | 236 | 0.88 ± 0.06 | 324 ± 24 | 0.59 ± 0.02 |
| 0.3/0.5 | 221 | 229.7 | 0.99 ± 0.05 | 280 ± 16 | 0.72 ± 0.01 | |
| 0.5/0.5 | 230 | 229.7 | 2.22 ± 0.03 | 432 ± 19 | 0.96 ± 0.07 | |
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Structure |  | 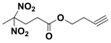 | 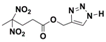 |  |
| Formula | C8H10N2O6 | C9H12N2O6 | C8H11N5O6 | C9H13N5O6 |
| FW (g mol−1) | 230.18 | 244.20 | 273.20 | 287.23 |
| Na (%) | 12.17 | 11.47 | 25.63 | 24.38 |
| Ob (%) | 41.70 | 39.31 | 35.14 | 33.42 |
| Td,onsetc (°C) | 191.0 | 199.5 | − | − |
| Tg (°C) | −78.71 | −80.21 | − | − |
| (g cm−3) | 1.2945 d | 1.2691 d | 1.4892 e | 1.4497 e |
| ff (kJ mol−1) | −161.8 | −202.2 | 18.1 | −16.2 |
| fg (kJ g−1) | −0.70 | −0.83 | 0.07 | −0.06 |
| IS h (J) | 54.6 | 54.6 | − | − |
| Explo5 V6.02 values | ||||
| EU0 i (kJ kg−1) | −4930 | −4764 | −5046 | −4888 |
| TEj (K) | 3143 | 2952 | 3200 | 3034 |
| pCJk (GPa) | 11.7 | 10.6 | 17.0 | 15.4 |
| VDetl (m s−1) | 6020 | 5815 | 6979 | 6751 |
| Gas vol. m (L kg−1) | 580 | 594 | 514 | 529 |
| Isn (s) | 208 | 204 | 213 | 208 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, M.; Kwon, Y. Reactive Energetic Plasticizers Utilizing Cu-Free Azide-Alkyne 1,3-Dipolar Cycloaddition for In-Situ Preparation of Poly(THF-co-GAP)-Based Polyurethane Energetic Binders. Polymers 2018, 10, 516. https://doi.org/10.3390/polym10050516
Ma M, Kwon Y. Reactive Energetic Plasticizers Utilizing Cu-Free Azide-Alkyne 1,3-Dipolar Cycloaddition for In-Situ Preparation of Poly(THF-co-GAP)-Based Polyurethane Energetic Binders. Polymers. 2018; 10(5):516. https://doi.org/10.3390/polym10050516
Chicago/Turabian StyleMa, Mingyang, and Younghwan Kwon. 2018. "Reactive Energetic Plasticizers Utilizing Cu-Free Azide-Alkyne 1,3-Dipolar Cycloaddition for In-Situ Preparation of Poly(THF-co-GAP)-Based Polyurethane Energetic Binders" Polymers 10, no. 5: 516. https://doi.org/10.3390/polym10050516
APA StyleMa, M., & Kwon, Y. (2018). Reactive Energetic Plasticizers Utilizing Cu-Free Azide-Alkyne 1,3-Dipolar Cycloaddition for In-Situ Preparation of Poly(THF-co-GAP)-Based Polyurethane Energetic Binders. Polymers, 10(5), 516. https://doi.org/10.3390/polym10050516