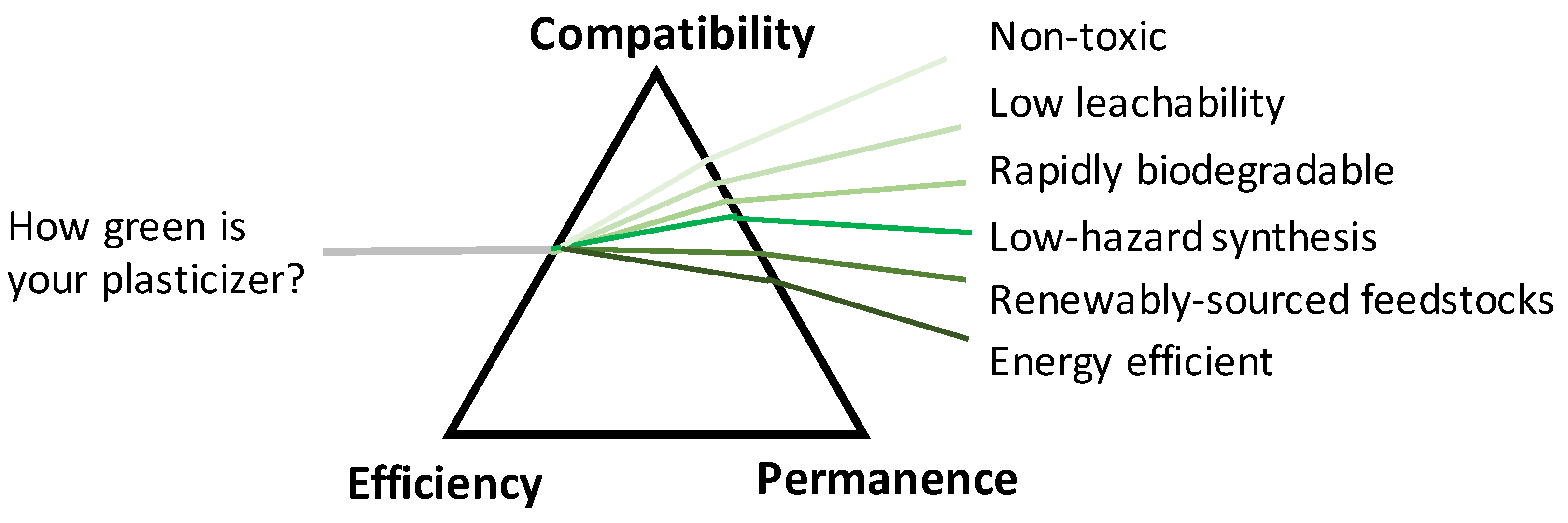
How Green is Your Plasticizer?
Abstract
1. Introduction
2. Historical Perspective
3. Designing Non-Toxic Chemicals
- In a one-generational study, a “hyperbranched polyglycerol” plasticizer was shown not to be acutely toxic [65].
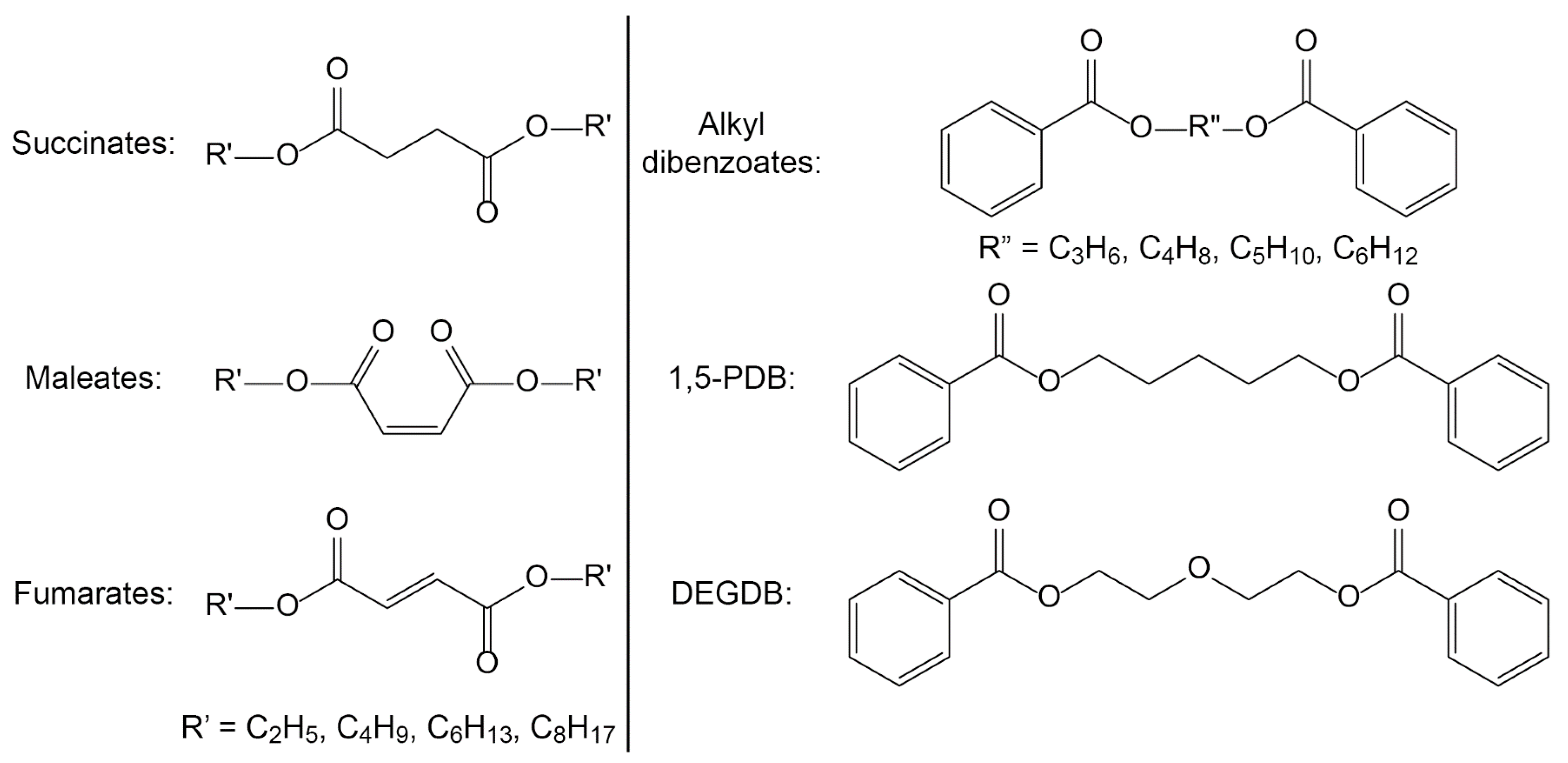
- In a two-generational study, two proposed green plasticizers, dioctyl succinate (DOS) and 1,4-butanediol dibenzoate (BDB), were both shown to exhibit no acute toxicity, and DOS also showed no reproductive toxicity, while BDB could produce “subtle but significant alterations of estrogen signaling in the adult testis” [34,66].
- In a two-generational study, commercially-available di(2-ethylhexyl) adipate (DEHA) was shown to have developmental toxicity at doses above 200 mg/kg/day as evidenced by increased postnatal deaths, yet no reproductive toxicity (antiandrogenic effects) was found [67].
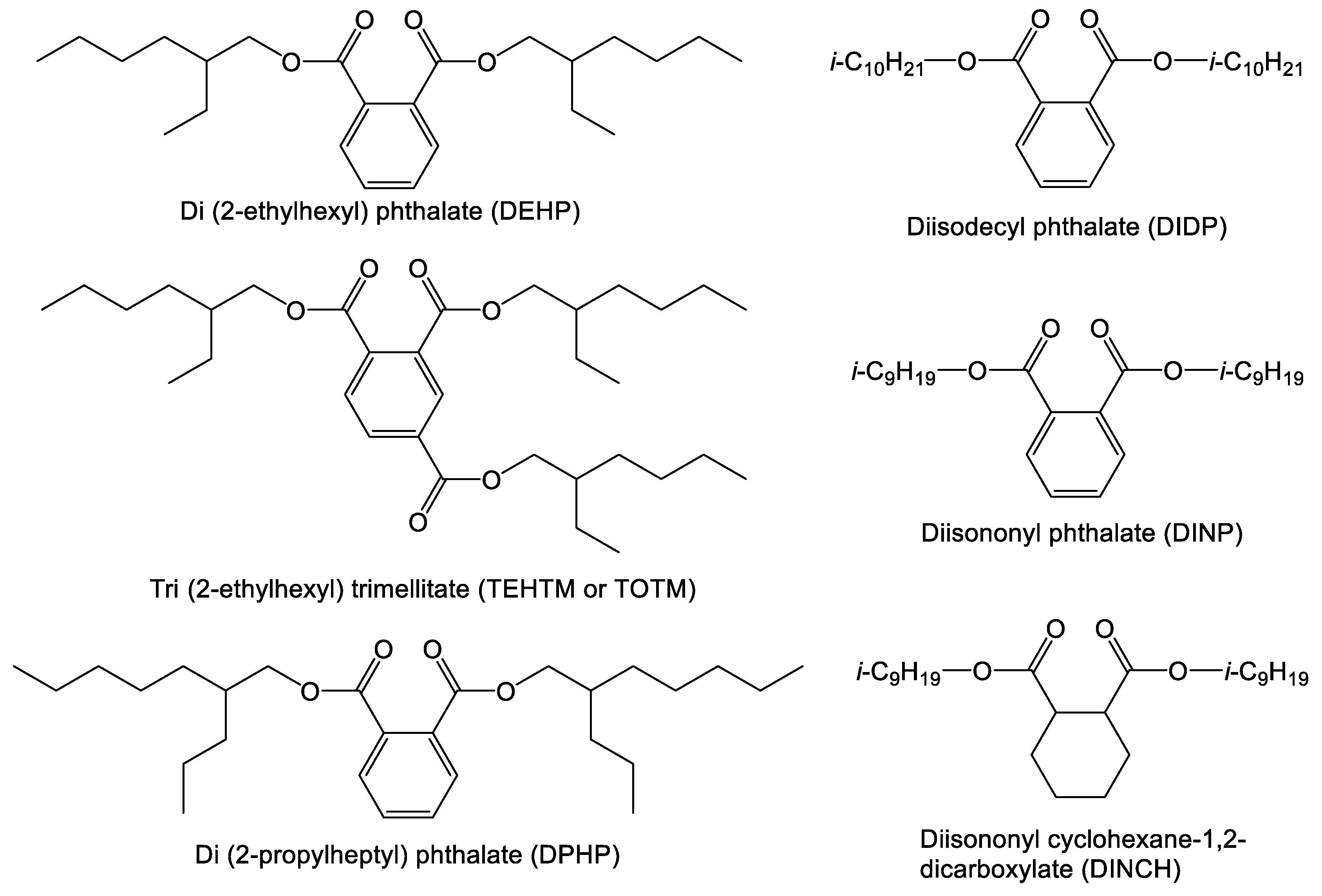
- In several one- and two-generational studies, the commercially available DINCH (hydrogenated DINP) showed no acute toxic effect [68], yet there were some indications that it might have an effect on the developing reproductive system of male rats as well as a similar effect as observed with BDB (see above) [30,34,66].
- In a one-generational study, a plasticizer candidate closely resembling DINCH (“DL9TH”) was shown to be safe for adult rats, with a further claim that the compound also showed no reproductive toxicity. This was based on tests with adult animals, not a two-generational study [69].
4. Designing for Biodegradation
- Biodegradation testing of poly(caprolactone)-based plasticizers by R. rhodochrous, which demonstrated rapid biodegradability and no build-up of stable metabolites [92].
- Biodegradation testing of DEHP and 15 diesters of varying side chain length based on succinic acid, maleic acid, and fumaric acid by R. rhodochrous, as discussed above. The experiments revealed the influence of both central structure as well as side chain length and its branching on biodegradation kinetics [45,82,83].
5. Designing for Permanence
- Leaching of the commercial plasticizers DEHP, DINCH, TOTM/TEHTM and di(2-ehtlyhexyl) terephthalate (DEHT) from hospital tubing into 50% ethanol in water [99].
- Leaching of several commercial plasticizers including phthalates and DEHA found in food packaging into aqueous acetic acid (3%), distilled water and ethanol (15% in water) [100].
- Leaching of alternative dibenzoate, succinate, maleate, and fumarate-based plasticizers from PVC disks at 29 wt % loading into reverse-osmosis purified water [4].
- Leaching of oligomeric ɛ-caprolactone in PVC disks at 39 wt % loading in n-hexane [92].
- Leaching of oligomeric poly(butylene adipate) in PVC films at 40 wt % loading into water [97].
- Leaching of curcumin-derived plasticizer candidates at 5, 15, 25, and 35 wt % in PVC into water and n-hexane [101].
- Leaching of tetra-esters based on pentaerythritol at several concentrations in PVC into distilled water, olive oil, ethanol (10% in water), acetic acid (30% in water), and petroleum ether [102].
- Leaching of DEHP from hemodialysis tubing, with and without polyurethane coating, into newborn calf serum [103].
6. Green Production
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Braun, D.; Cherdron, H.; Ritter, H. Polymer Synthesis: Theory and Practice: Fundamentals, Methods, Experiments; Springer: Heidelberg, Germany, 2001; pp. 105–110. [Google Scholar]
- Wilkes, C.E.; Summers, J.W.; Daniels, C.A.; Berard, M.T. PVC Handbook; Hanser: Cincinnati, OH, USA, 2005. [Google Scholar]
- Sears, J.K.; Darby, J.R. The Technology of Plasticizers; John Wiley: New York, NY, USA, 1982. [Google Scholar]
- Erythropel, H.C.; Maric, M.; Nicell, J.A.; Leask, R.L.; Yargeau, V. Leaching of the plasticizer di(2-ethylhexyl)phthalate (DEHP) from plastic containers and the question of human exposure. Appl. Microbiol. Biotechnol. 2014, 98, 9967–9981. [Google Scholar] [CrossRef] [PubMed]
- Kastner, J.; Cooper, D.G.; Marić, M.; Dodd, P.; Yargeau, V. Aqueous leaching of di-2-ethylhexyl phthalate and “green” plasticizers from poly(vinyl chloride). Sci. Total Environ. 2012, 432, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.L.; Stalling, D.L.; Johnson, J.L. Phthalate esters as environmental contaminants. Nature 1972, 238, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Wams, T.J. Diethylhexylphthalate as an environmental contaminant-a review. Sci. Total Environ. 1987, 66, 1–16. [Google Scholar] [CrossRef]
- Rudel, R.A.; Camann, D.E.; Spengler, J.D.; Korn, L.R.; Brody, J.G. Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environ. Sci. Technol. 2003, 37, 4543–4553. [Google Scholar] [CrossRef] [PubMed]
- Butte, W.; Heinzow, B. Pollutants in house dust as indicators of indoor contamination. Rev. Environ. Contam. Toxicol. 2002, 175, 1–46. [Google Scholar] [PubMed]
- Becker, K.; Seiwert, M.; Angerer, J.; Heger, W.; Koch, H.M.; Nagorka, R.; Rosskamp, E.; Schluter, C.; Seifert, B.; Ullrich, D. Dehp metabolites in urine of children and dehp in house dust. Int. J. Hyg. Environ. Health 2004, 207, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Fromme, H.; Schütze, A.; Lahrz, T.; Kraft, M.; Fembacher, L.; Siewering, S.; Burkardt, R.; Dietrich, S.; Koch, H.M.; Völkel, W. Non-phthalate plasticizers in german daycare centers and human biomonitoring of dinch metabolites in children attending the centers (lupe 3). Int. J. Hyg. Environ. Health 2016, 219, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, C.D.; Thompson, I.P.; Burns, R.G. Degradation and impact of phthalate plasticizers on soil microbial communities. Environ. Toxicol. Chem. 2000, 19, 1253–1261. [Google Scholar] [CrossRef]
- Piche, C.D.; Sauvageau, D.; Vanlian, M.; Erythropel, H.C.; Robaire, B.; Leask, R.L. Effects of di-(2-ethylhexyl) phthalate and four of its metabolites on steroidogenesis in ma-10 cells. Ecotoxicol. Environ. Safe 2012, 79, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Traore, K.; Li, W.; Amri, H.; Huang, H.; Wu, C.; Chen, H.; Zirkin, B.; Papadopoulos, V. Molecular mechanisms mediating the effect of mono-(2-ethylhexyl) phthalate on hormone-stimulated steroidogenesis in ma-10 mouse tumor leydig cells. Endocrinology 2010, 151, 3348–3362. [Google Scholar] [CrossRef] [PubMed]
- Pant, N.; Shukla, M.; Patel, D.K.; Shukla, Y.; Mathur, N.; Gupta, Y.K.; Saxena, D.K. Correlation of phthalate exposures with semen quality. Toxicol. Appl. Pharmacol. 2008, 231, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Richburg, J.H.; Boekelheide, K. Mono-(2-ethylhexyl) phthalate rapidly alters both sertoli cell vimentin filaments and germ cell apoptosis in young rat testes. Toxicol. Appl. Pharmacol. 1996, 137, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Swan, S.H.; Main, K.M.; Liu, F.; Stewart, S.L.; Kruse, R.L.; Calafat, A.M.; Mao, C.S.; Redmon, J.B.; Ternand, C.L.; Sullivan, S.; et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ. Health Perspect. 2005, 113, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Canada Consumer Product Safety Act: Phthalates Regulations. Available online: http://laws-lois.justice.gc.ca/eng/regulations/SOR-2016-188/page-1.html (accessed on 23 May 2018).
- United States Consumer Product Safety Improvement Act of 2008, Section 108. Available online: http://www.cpsc.gov/PageFiles/129663/cpsia.pdf (accessed on 14 August 2008).
- EU. 1999/0238(Cod): Child Health Protection: Phthalates, Dangerous Substances, Safety of Toys. Available online: http://www.europarl.europa.eu/oeil/popups/ficheprocedure.do?lang=en&reference=1999/0238 (accessed on 10 November 1999).
- Bureau Veritas. Revision of Phthalate Requirements in Toys under Japan Food Sanitation Law. Available online: http://www.toy-icti.org/PDFs/Jap-PhthReqRev.pdf (accessed on 6 September 2011).
- Boyer, R.F. Effect of plasticizers on some physical properties of polymers. TAPPI J. 1951, 34, 357–362. [Google Scholar]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Erythropel, H.C.; Zimmerman, J.B.; de Winter, T.M.; Petitjean, L.; Melnikov, F.; Lam, C.H.; Lounsbury, A.W.; Mellor, K.E.; Jankovic, N.Z.; Tu, Q.; et al. The green chemistree: 20 years after taking root with the 12 principles. Green Chem. 2018, 20, 1929–1961. [Google Scholar] [CrossRef]
- Wypych, G. Handbook of Plasticizers, 3rd ed.; Elsevier: Toronto, ON, Canada, 2017; pp. 159–162. [Google Scholar]
- Wilson, A.S. Plasticisers: Selection, Applications and Implications; Rapra Technology Limited: Shropshire, UK, 1996. [Google Scholar]
- Krauskopf, L.G. How about alternatives to phthalate plasticizers? J. Vinyl Addit. Technol. 2003, 9, 159–171. [Google Scholar] [CrossRef]
- Lankey, R.L.; Anastas, P.T. Life-cycle approaches for assessing green chemistry technologies. Ind. Eng. Chem. Res. 2002, 41, 4498–4502. [Google Scholar] [CrossRef]
- Kutz, M. Applied Plastics Engineering Handbook: Processing, Materials, and Applications; Elsevier Science: Oxford, UK, 2016; pp. 533–553. [Google Scholar]
- Seymour, R.B.; Deanin, R.D.; Meeting, A.C.S. History of Polymeric Composites; Taylor & Francis: Utrecht, The Netherlands, 1987. [Google Scholar]
- Bisig, M.D. Plasticizer Market Update. In Proceedings of the 20th Annual Vinyl Compounding Conference, Ponte Vedra Beach, FL, USA, 19–21 July 2009. [Google Scholar]
- Scientific Evaluation of Health Effects. Available online: https://www.acsh.org/Publications (accessed on 22 June 1999).
- Gangolli, S.D. Testicular effects of phthalate esters. Environ. Health Perspect. 1982, 45, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Tomita, I.; Nakamura, Y.; Aoki, N.; Inui, N. Mutagenic/carcinogenic potential of dehp and mehp. Environ. Health Perspect. 1982, 45, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Tuncak, B. Driving innovation: How stronger laws pull safer chemicals into the market. Sustain. Dev. Law Policy 2014, 14, 4. [Google Scholar]
- Wadey, B.L. An innovative plasticizer for sensitive applications. J. Vinyl Addit. Technol. 2003, 9, 172–176. [Google Scholar] [CrossRef]
- Biron, M. Thermoplastics and Thermoplastic Composites; William Andrew: Oxford, UK, 2013; pp. 205–215. [Google Scholar]
- AGPU. Everything about PVC-from Manufacturing to Recycling. Available online: www.renolit.com (accessed on 3 September 2014).
- Erythropel, H.C.; Börmann, A.; Nicell, J.A.; Leask, R.L.; Maric, M. Designing green plasticizers: Linear alkyl diol dibenzoate plasticizers and a thermally reversible plasticizer. Polymers 2018, 10, 646. [Google Scholar] [CrossRef]
- Bui, T.T.; Giovanoulis, G.; Cousins, A.P.; Magnér, J.; Cousins, I.T.; de Wit, C.A. Human exposure, hazard and risk of alternative plasticizers to phthalate esters. Sci. Total Environ. 2016, 541, 451–467. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-H.; Ma, L.; Hu, Y.-X.; Wang, D.-X.; Fang, L.; Li, X.-L.; Zhao, J.-C.; Yu, H.-R.; Ying, H.-Z.; Yu, C.-H. Transcriptome profiling and pathway analysis of hepatotoxicity induced by tris (2-ethylhexyl) trimellitate (TOTM) in mice. Environ. Toxicol. Pharmacol. 2016, 41, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Nardelli, T.C.; Albert, O.; Lalancette, C.; Culty, M.; Hales, B.F.; Robaire, B. In utero and lactational exposure study in rats to identify replacements for di(2-ethylhexyl) phthalate. Sci. Rep. 2017, 7, 3862. [Google Scholar] [CrossRef] [PubMed]
- Nardelli, T.C.; Erythropel, H.C.; Robaire, B. Toxicogenomic screening of replacements for di(2-ethylhexyl) phthalate (DEHP) using the immortalized tm4 sertoli cell line. PLoS ONE 2015, 10, e0138421. [Google Scholar] [CrossRef] [PubMed]
- Campioli, E.; Lee, S.; Lau, M.; Marques, L.; Papadopoulos, V. Effect of prenatal dinch plasticizer exposure on rat offspring testicular function and metabolism. Sci. Rep. 2017, 7, 11072. [Google Scholar] [CrossRef] [PubMed]
- Boberg, J.; Christiansen, S.; Axelstad, M.; Kledal, T.S.; Vinggaard, A.M.; Dalgaard, M.; Nellemann, C.; Hass, U. Reproductive and behavioral effects of diisononyl phthalate (DINP) in perinatally exposed rats. Reprod. Toxicol. 2011, 31, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Schultz, T.W.; Cronin, M.T.D.; Walker, J.D.; Aptula, A.O. Quantitative structure-activity relationships (QSARs) in toxicology: A historical perspective. J. Mol. Struct. THEOCHEM 2003, 622, 1–22. [Google Scholar] [CrossRef]
- Voutchkova, A.M.; Osimitz, T.G.; Anastas, P.T. Toward a comprehensive molecular design framework for reduced hazard. Chem. Rev. 2010, 110, 5845–5882. [Google Scholar] [CrossRef] [PubMed]
- Coish, P.; Brooks, B.W.; Gallagher, E.P.; Kavanagh, T.J.; Voutchkova-Kostal, A.; Zimmerman, J.B.; Anastas, P.T. Current status and future challenges in molecular design for reduced hazard. ACS Sustain. Chem. Eng. 2016, 4, 5900–5906. [Google Scholar] [CrossRef]
- Coish, P.; Brooks, B.W.; Gallagher, E.P.; Mills, M.; Kavanagh, T.J.; Simcox, N.; Lasker, G.A.; Botta, D.; Schmuck, S.C.; Voutchkova-Kostal, A.; et al. The molecular design research network. Toxicol. Sci. 2018, 161, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Segura, P.A.; Kaplan, P.; Erythropel, H.C.; Yargeau, V. Comparative rapid toxicity screening of commercial and potential “green” plasticizers using bioluminescent bacteria. Ind. Eng. Chem. Res. 2012, 51, 11555–11560. [Google Scholar] [CrossRef]
- Nalli, S.; Cooper, D.G.; Nicell, J.A. Biodegradation of plasticizers by Rhodococcus rhodochrous. Biodegradation 2002, 13, 343–352. [Google Scholar] [CrossRef]
- Gartshore, J.; Cooper, D.G.; Nicell, J.A. Biodegradation of plasticizers by Rhodotorula rubra. Environ. Toxicol. Chem. 2003, 22, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Kermanshahi Pour, A.; Mamer, O.A.; Cooper, D.G.; Maric, M.; Nicell, J.A. Metabolites from the biodegradation of 1,6-hexanediol dibenzoate, a potential green plasticizer, by Rhodococcus rhodochrous. J. Mass Spectrom. 2009, 44, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Erythropel, H.C.; Maric, M.; Cooper, D.G. Designing green plasticizers: Influence of molecular geometry on biodegradation and plasticization properties. Chemosphere 2012, 86, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Surhio, M.A.; Talpur, F.N.; Nizamani, S.M.; Talpur, M.K.; Afridi, H.I.; Khaskheli, A.A.; Bhurgri, S.; Surhio, J.A. Leaching of phthalate esters from different drinking stuffs and their subsequent biodegradation. Environ. Sci. Pollut. Res. 2017, 24, 18663–18671. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, S.; Pradeep, S.; Sarath Josh, M.; Kumar, S.; Masai, E. A monograph on the remediation of hazardous phthalates. J. Hazard. Mater. 2015, 298, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.A.; Henttu, P.; Parker, M.G.; Sumpter, J.P. The estrogenic activity of phthalate esters in vitro. Environ. Health Perspect. 1997, 105, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Soto, A.M.; Sonnenschein, C.; Chung, K.L.; Fernandez, M.F.; Olea, N.; Olea Serrano, F. The e-screen assay as a tool to identify estrogens: An update on estrogenic environmental pollutants. Environ. Health Perspect. 1995, 103, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Dreier, D.A.; Connors, K.A.; Brooks, B.W. Comparative endpoint sensitivity of in vitro estrogen agonist assays. Regul. Toxicol. Pharm. 2015, 72, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Van Meerloo, J.; Kaspers, G.J.; Cloos, J. Cell sensitivity assay. In Cancer Cell Culture; Humana Press: Totowa, NJ, USA, 2011; pp. 237–245. [Google Scholar]
- Boisvert, A.; Jones, S.; Issop, L.; Erythropel, H.C.; Papadopoulos, V.; Culty, M. In vitro functional screening as a means to identify new plasticizers devoid of reproductive toxicity. Environ. Res. 2016, 150, 496–512. [Google Scholar] [CrossRef] [PubMed]
- Ekwall, B.; Nordensten, C.; Albanus, L. Toxicity of 29 plasticizers to hela cells in the mit-24 system. Toxicology 1982, 24, 199–210. [Google Scholar] [CrossRef]
- Eljezi, T.; Pinta, P.; Richard, D.; Pinguet, J.; Chezal, J.-M.; Chagnon, M.-C.; Sautou, V.; Grimandi, G.; Moreau, E. In vitro cytotoxic effects of dehp-alternative plasticizers and their primary metabolites on a l929 cell line. Chemosphere 2017, 173, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Albaladejo, E.; Fernandes, D.; Lacorte, S.; Porte, C. Comparative toxicity, oxidative stress and endocrine disruption potential of plasticizers in jeg-3 human placental cells. Toxicol. Vitro 2017, 38, 41–48. [Google Scholar]
- Sipes, N.S.; Martin, M.T.; Kothiya, P.; Reif, D.M.; Judson, R.S.; Richard, A.M.; Houck, K.A.; Dix, D.J.; Kavlock, R.J.; Knudsen, T.B. Profiling 976 toxcast chemicals across 331 enzymatic and receptor signaling assays. Chem. Res. Toxicol. 2013, 26, 878–895. [Google Scholar] [CrossRef] [PubMed]
- Attene-Ramos, M.S.; Miller, N.; Huang, R.; Michael, S.; Itkin, M.; Kavlock, R.J.; Austin, C.P.; Shinn, P.; Simeonov, A.; Tice, R.R.; et al. The tox21 robotic platform for the assessment of environmental chemicals–from vision to reality. Drug Discov. Today 2013, 18, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.-H.; Sedykh, A.; Huang, R.; Xia, M.; Tice, R.R. A data analysis pipeline accounting for artifacts in tox21 quantitative high-throughput screening assays. J. Biomol. Screen. 2015, 20, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.L.E.; Ostby, J.; Furr, J.; Price, M.; Veeramachaneni, D.N.R.; Parks, L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol. Sci. 2000, 58, 350–365. [Google Scholar] [CrossRef] [PubMed]
- Foster, P.M.D.; Mylchreest, E.; Gaido, K.W.; Sar, M. Effects of phthalate esters on the developing reproductive tract of male rats. Hum. Reprod. Update 2001, 7, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Borch, J.; Ladefoged, O.; Hass, U.; Vinggaard, A.M. Steroidogenesis in fetal male rats is reduced by DEHP and DINP, but endocrine effects of DEHP are not modulated by DEHA in fetal, prepubertal and adult male rats. Reprod. Toxicol. 2004, 18, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.J.M.; Grande, S.W.; Talsness, C.E.; Gericke, C.; Grote, K.; Golombiewski, A.; Sterner-Kock, A.; Chahoud, I. A dose response study following in utero and lactational exposure to di-(2-ethylhexyl) phthalate (DEHP): Reproductive effects on adult male offspring rats. Toxicology 2006, 228, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Lyche, J.L.; Gutleb, A.C.; Bergman, Å.; Eriksen, G.S.; Murk, A.J.; Ropstad, E.; Saunders, M.; Skaare, J.U. Reproductive and developmental toxicity of phthalates. J. Toxicol. Environ. Health B 2009, 12, 225–249. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. “The Red book”: Risk Assessment in the Federal Government: Managing the Process; The National Academies Press: Washington, DC, USA, 1983; p. 191. [Google Scholar]
- Daughton, C.G. “Emerging” chemicals as pollutants in the environment: A 21st century perspective. Renew. Resour. J. 2005, 23, 6–23. [Google Scholar]
- Francisco Sánchez-Bayo, P.J.B.R.M.M. Ecological Impacts of Toxic Chemicals; Bentham Science Publishers Limited: Houston, TX, USA, 2012. [Google Scholar]
- Boethling, R.S.; Sommer, E.; DiFiore, D. Designing small molecules for biodegradability. Chem. Rev. 2007, 107, 2207–2227. [Google Scholar] [CrossRef] [PubMed]
- Beek, B. Biodegradation and Persistence; Springer: New York, NY, USA, 2006; pp. 264–268. [Google Scholar]
- Kowalczyk, A.; Martin, T.J.; Price, O.R.; Snape, J.R.; van Egmond, R.A.; Finnegan, C.J.; Schäfer, H.; Davenport, R.J.; Bending, G.D. Refinement of biodegradation tests methodologies and the proposed utility of new microbial ecology techniques. Ecotoxicol. Environ. Safe 2015, 111, 9–22. [Google Scholar] [CrossRef] [PubMed]
- De Vivo, B.; Grasemann, B.; Stüwe, K. Geology-Volume III; Eolss Publ.: Oxford, UK, 2009. [Google Scholar]
- OECD. OECD Guidelines for the Testing of Chemicals, Section 3: Degradation and Accumulation; OECD Publishing: Paris, France, 1981–2014. [Google Scholar]
- Friedrich, J.; Längin, A.; Kümmerer, K. Comparison of an electrochemical and luminescence-based oxygen measuring system for use in the biodegradability testing according to closed bottle test (OECD 301d). Clean Soil Air Water 2012, 41, 251–257. [Google Scholar] [CrossRef]
- Kermanshahi pour, A.; Cooper, D.G.; Mamer, O.A.; Maric, M.; Nicell, J.A. Mechanisms of biodegradation of dibenzoate plasticizers. Chemosphere 2009, 77, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Kermanshahi pour, A.; Roy, R.; Cooper, D.G.; Maric, M.; Nicell, J.A. Biodegradation kinetics of dibenzoate plasticizers and their metabolites. Biochem. Eng. J. 2013, 70, 35–45. [Google Scholar] [CrossRef]
- Erythropel, H.C.; Brown, T.; Maric, M.; Nicell, J.A.; Cooper, D.G.; Leask, R.L. Designing greener plasticizers: Effects of alkyl chain length and branching on the biodegradation of maleate based plasticizers. Chemosphere 2015, 134, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Erythropel, H.C.; Dodd, P.; Leask, R.L.; Maric, M.; Cooper, D.G. Designing green plasticizers: Influence of alkyl chain length on biodegradation and plasticization properties of succinate based plasticizers. Chemosphere 2013, 91, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Nalli, S.; Cooper, D.G.; Nicell, J.A. Metabolites from the biodegradation of di-ester plasticizers by Rhodococcus rhodochrous. Sci. Total Environ. 2006, 366, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Nalli, S.; Cooper, D.G.; Nicell, J.A. Interaction of metabolites with R. Rhodochrous during the biodegradation of di-ester plasticizers. Chemosphere 2006, 65, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
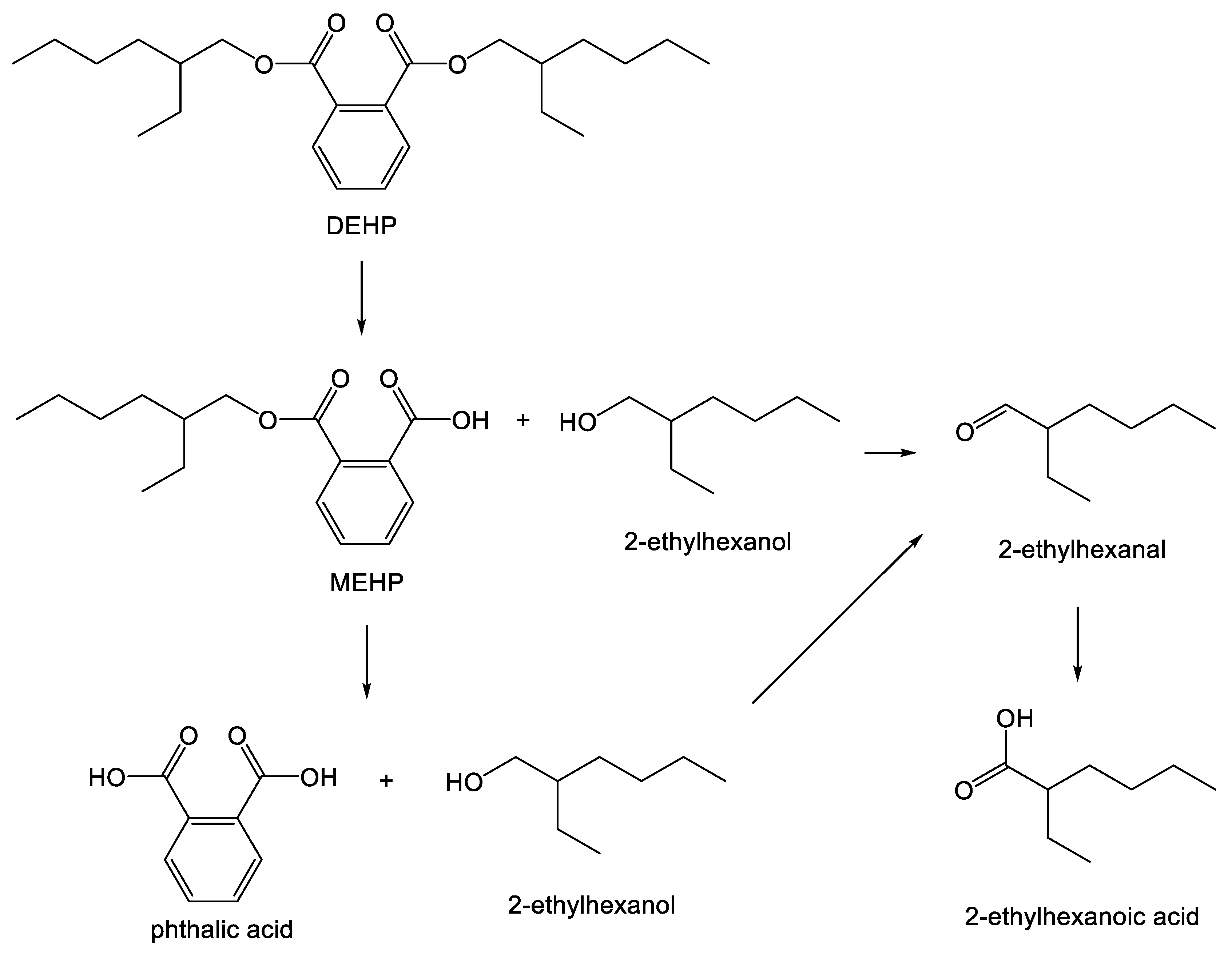
- Nalli, S.; Horn, O.J.; Grochowalski, A.R.; Cooper, D.G.; Nicell, J.A. Ori gin of 2-ethylhexanol as a VOC. Environ. Pollut. 2006, 140, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Horn, O.; Nalli, S.; Cooper, D.; Nicell, J. Plasticizer metabolites in the environment. Water Res. 2004, 38, 3693–3698. [Google Scholar] [CrossRef] [PubMed]
- Barnabé, S.; Beauchesne, I.; Cooper, D.G.; Nicell, J.A. Plasticizers and their degradation products in the process streams of a large urban physicochemical sewage treatment plant. Water Res. 2008, 42, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Beauchesne, I.; Barnabé, S.; Cooper, D.G.; Nicell, J.A. Plasticizers and related toxic degradation products in wastewater sludges. Water Sci. Technol. 2008, 57, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Erythropel, H.C.; Shipley, S.; Bormann, A.; Nicell, J.A.; Maric, M.; Leask, R.L. Designing green plasticizers: Influence of molecule geometry and alkyl chain length on the plasticizing effectiveness of diester plasticizers in PVC blends. Polymer 2016, 89, 18–27. [Google Scholar] [CrossRef]
- Firlotte, N.; Cooper, D.G.; Marić, M.; Nicell, J.A. Characterization of 1,5-pentanediol dibenzoate as a potential “green” plasticizer for poly(vinyl chloride). J. Vinyl Addit. Technol. 2009, 15, 99–107. [Google Scholar] [CrossRef]
- Wowkonowicz, P.; Kijeńska, M. Phthalate release in leachate from municipal landfills of central Poland. PLoS ONE 2017, 12, e0174986. [Google Scholar] [CrossRef] [PubMed]
- Schettler, T. Human exposure to phthalates via consumer products. Int. J. Androl. 2006, 29, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Erythropel, H.C. Evaluation of Maleate, Fumarate, and Succinate Diesters as Potential Green Plasticizers. Ph.D. Thesis, McGill University, Montreal, QC, Canada, December 2015. [Google Scholar]
- Lindström, A.; Hakkarainen, M. Migration resistant polymeric plasticizer for poly(vinyl chloride). J. Appl. Polym. Sci. 2007, 104, 2458–2467. [Google Scholar] [CrossRef]
- ASTM D.-1239. Astm D 1239: Standard Test Method for Resistance of Plastic Films to Extraction by Chemicals; American Society for Testing and Materials: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Navarro, R.; Pérez Perrino, M.; Gómez Tardajos, M.; Reinecke, H. Phthalate plasticizers covalently bound to PVC: Plasticization with suppressed migration. Macromolecules 2010, 43, 2377–2381. [Google Scholar] [CrossRef]
- Yuan, J.; Cheng, B. A strategy for nonmigrating highly plasticized PVC. Sci. Rep. 2017, 7, 9277. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Hu, L.; Shang, Q.; Wang, R.; Zhang, M.; Zhou, Y. Self-plasticization of pvc materials via chemical modification of mannich base of cardanol butyl ether. ACS Sustain. Chem. Eng. 2017, 5, 6665–6673. [Google Scholar] [CrossRef]
- Messori, M.; Toselli, M.; Pilati, F.; Fabbri, E.; Fabbri, P.; Pasquali, L.; Nannarone, S. Prevention of plasticizer leaching from PVC medical devices by using organic-inorganic hybrid coatings. Polymer 2004, 45, 805–813. [Google Scholar] [CrossRef]
- Barreto, M.C.; Borris, J.; Thomas, M.; Hänsel, R.; Stoll, M.; Klages, C.P. Reduction of plasticizer leaching from PVC by barrier coatings deposited using DBD processes at atmospheric pressure. Plasma Process. Polym. 2012, 9, 1208–1214. [Google Scholar] [CrossRef]
- Zhang, X.X.; Li, Y.X.; Hankett, J.M.; Chen, Z. The molecular interfacial structure and plasticizer migration behavior of “green” plasticized poly(vinyl chloride). Phys. Chem. Chem. Phys. 2015, 17, 4472–4482. [Google Scholar] [CrossRef] [PubMed]
- Chiellini, F.; Ferri, M.; Morelli, A.; Dipaola, L.; Latini, G. Perspectives on alternatives to phthalate plasticized poly(vinyl chloride) in medical devices applications. Prog. Polym. Sci. 2013, 38, 1067–1088. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass: Volume i-Results of Screening for Potential Candidates from Sugars Ad Synthesis Gas; US DOE; Office of Biomass Program: Washington, DC, USA, 2004. [Google Scholar]
- Debabov, V.G. Prospects for biosuccinic acid production. Appl. Biochem. Microbiol. 2015, 51, 787–791. [Google Scholar] [CrossRef]
- Mazière, A.; Prinsen, P.; García, A.; Luque, R.; Len, C. A review of progress in (bio)catalytic routes from/to renewable succinic acid. Biofuel Bioprod. Bioredin. 2017, 11, 908–931. [Google Scholar] [CrossRef]
- Stuart, A.; McCallum, M.M.; Fan, D.; LeCaptain, D.J.; Lee, C.Y.; Mohanty, D.K. Poly(vinyl chloride) plasticized with succinate esters: Synthesis and characterization. Polym. Bull. 2010, 65, 589–598. [Google Scholar] [CrossRef]
- Van den Bergh, J.C.J.M.; Bruinsma, F.R. Managing the Transition to Renewable Energy: Theory and Practice from Local, Regional and Macro Perspectives; Edward Elgar Publishing Limited: Cheltenham, UK, 2008; p. 235. [Google Scholar]
- De Corato, U.; De Bari, I.; Viola, E.; Pugliese, M. Assessing the main opportunities of integrated biorefining from agro-bioenergy co/by-products and agroindustrial residues into high-value added products associated to some emerging markets: A review. Renew. Sustain. Energy Rev. 2018, 88, 326–346. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamarani, R.; Erythropel, H.C.; Nicell, J.A.; Leask, R.L.; Marić, M. How Green is Your Plasticizer? Polymers 2018, 10, 834. https://doi.org/10.3390/polym10080834
Jamarani R, Erythropel HC, Nicell JA, Leask RL, Marić M. How Green is Your Plasticizer? Polymers. 2018; 10(8):834. https://doi.org/10.3390/polym10080834
Chicago/Turabian StyleJamarani, Roya, Hanno C. Erythropel, James A. Nicell, Richard L. Leask, and Milan Marić. 2018. "How Green is Your Plasticizer?" Polymers 10, no. 8: 834. https://doi.org/10.3390/polym10080834
APA StyleJamarani, R., Erythropel, H. C., Nicell, J. A., Leask, R. L., & Marić, M. (2018). How Green is Your Plasticizer? Polymers, 10(8), 834. https://doi.org/10.3390/polym10080834





