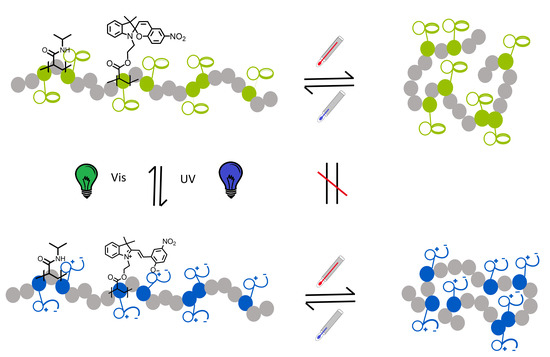
Dual Stimuli-Responsive P(NIPAAm-co-SPA) Copolymers: Synthesis and Response in Solution and in Films
Abstract
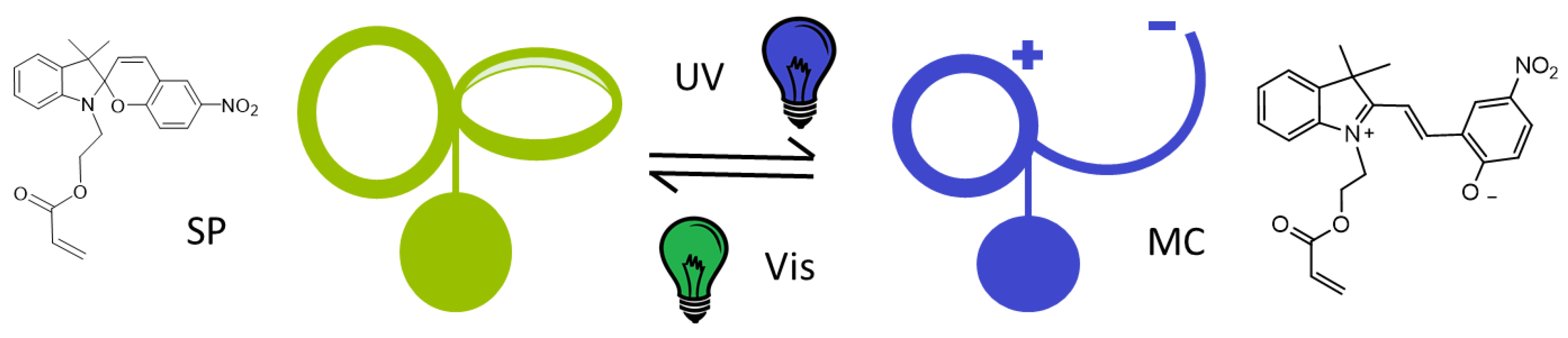
1. Introduction
2. Materials and Methods
2.1. General Information
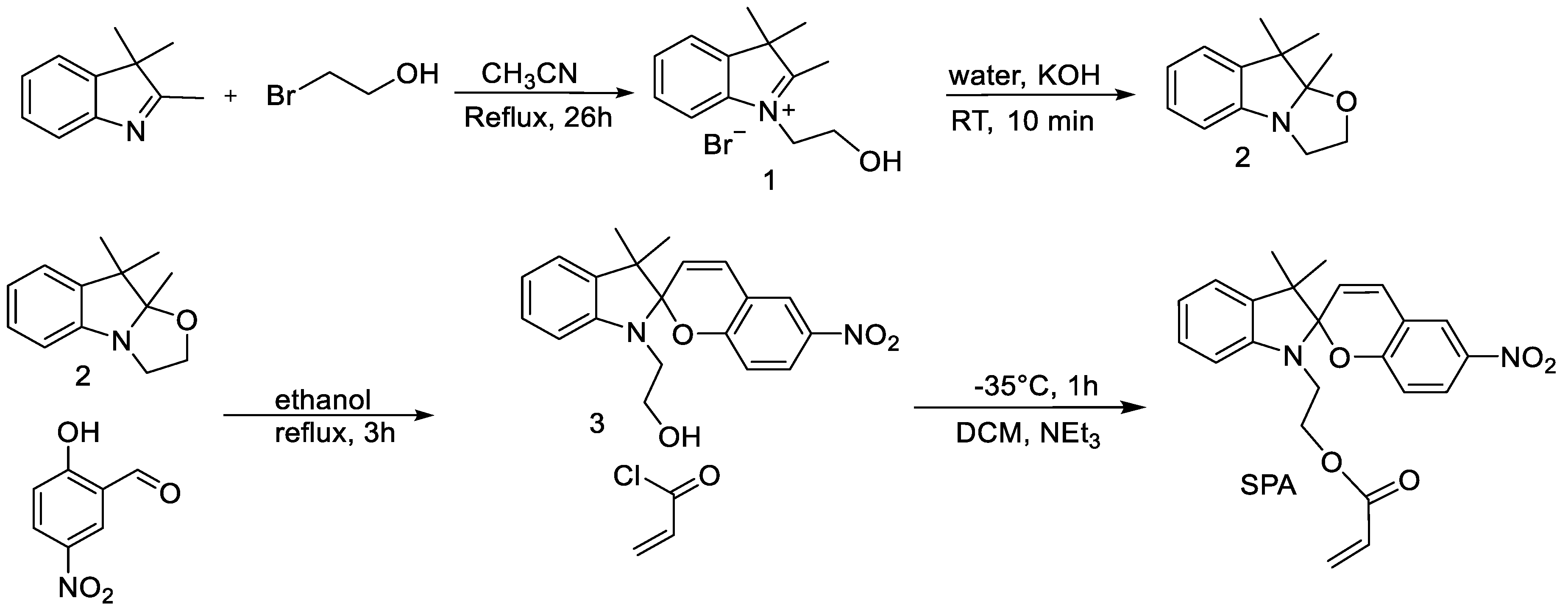
2.2. Synthesis of 1-(2-Hydroxyethyl)-2,3,3-trimethyl-3H-indolium Bromide
2.3. Synthesis of 9,9,9a-Trimethyl-2,3,9,9a-tetrahydro-oxazolo[2,3-a]indole
2.4. Synthesis of 2-(3′,3′-Dimethyl-6-nitro-3′H-spiro[chromene-2,2′-indol]-1′-yl)-ethanol
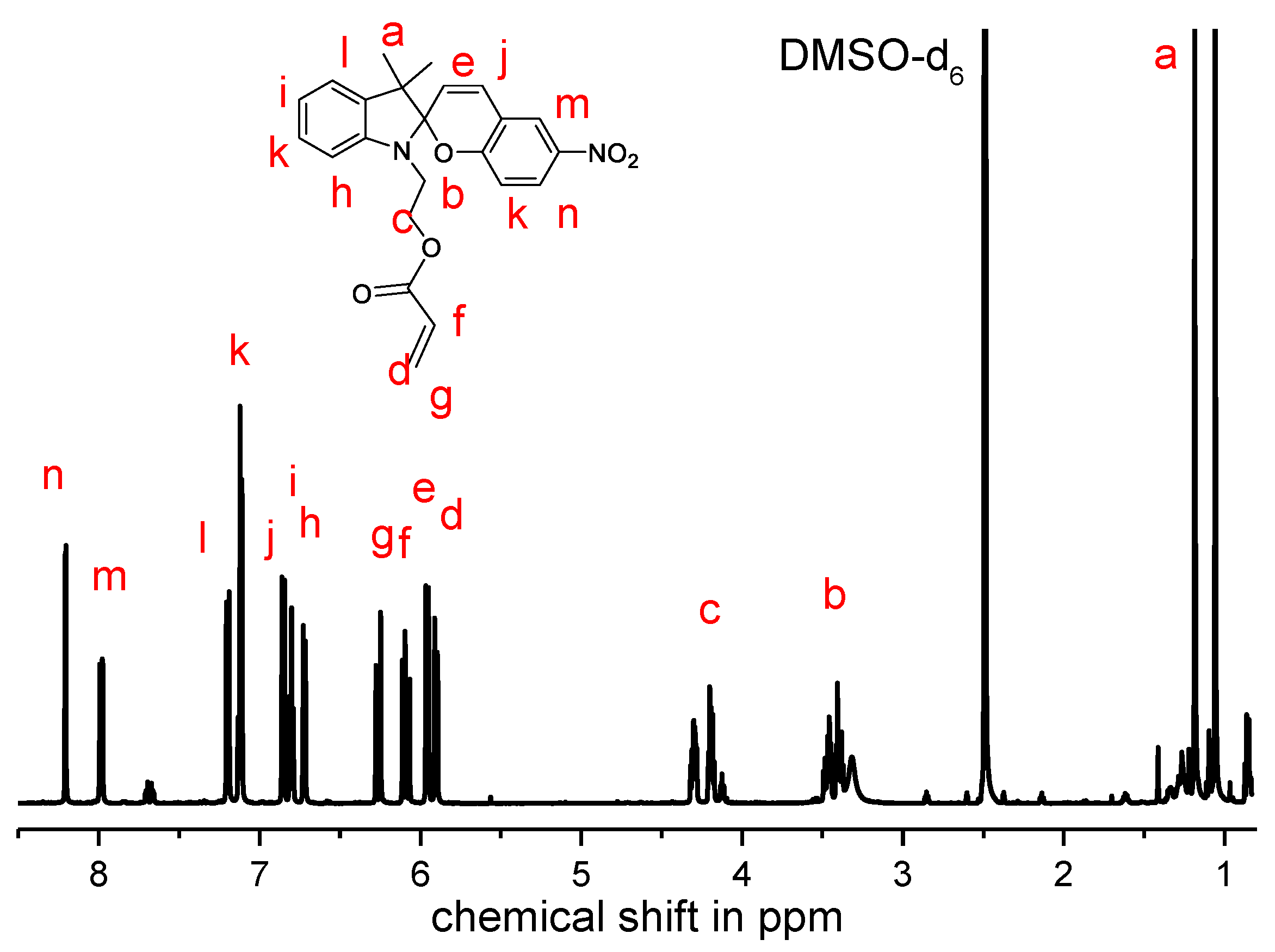
2.5. Synthesis of 2-(3′,3′-Dimethyl-6-nitrospiro[chromene-2,2′-indolin]-1′-yl)ethyl Acrylate (SPA)
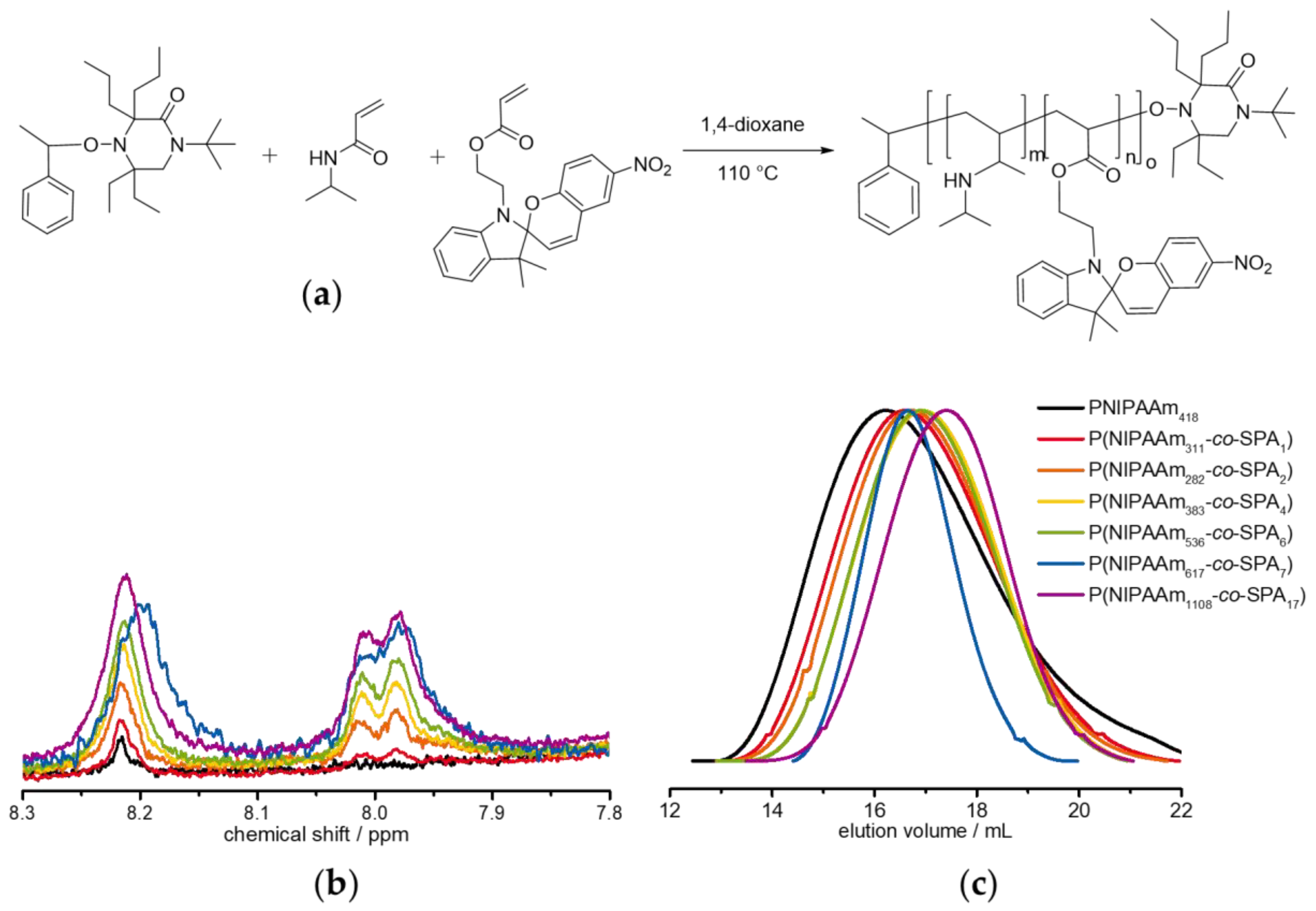
2.6. Synthesis of P(NIPAAm-co-SPA)
3. Results and Discussion
3.1. Synthesis of the Spiropyran Acrylate (SPA) Monomer
3.2. Synthesis of P(NIPAAm-co-SPA) Copolymers
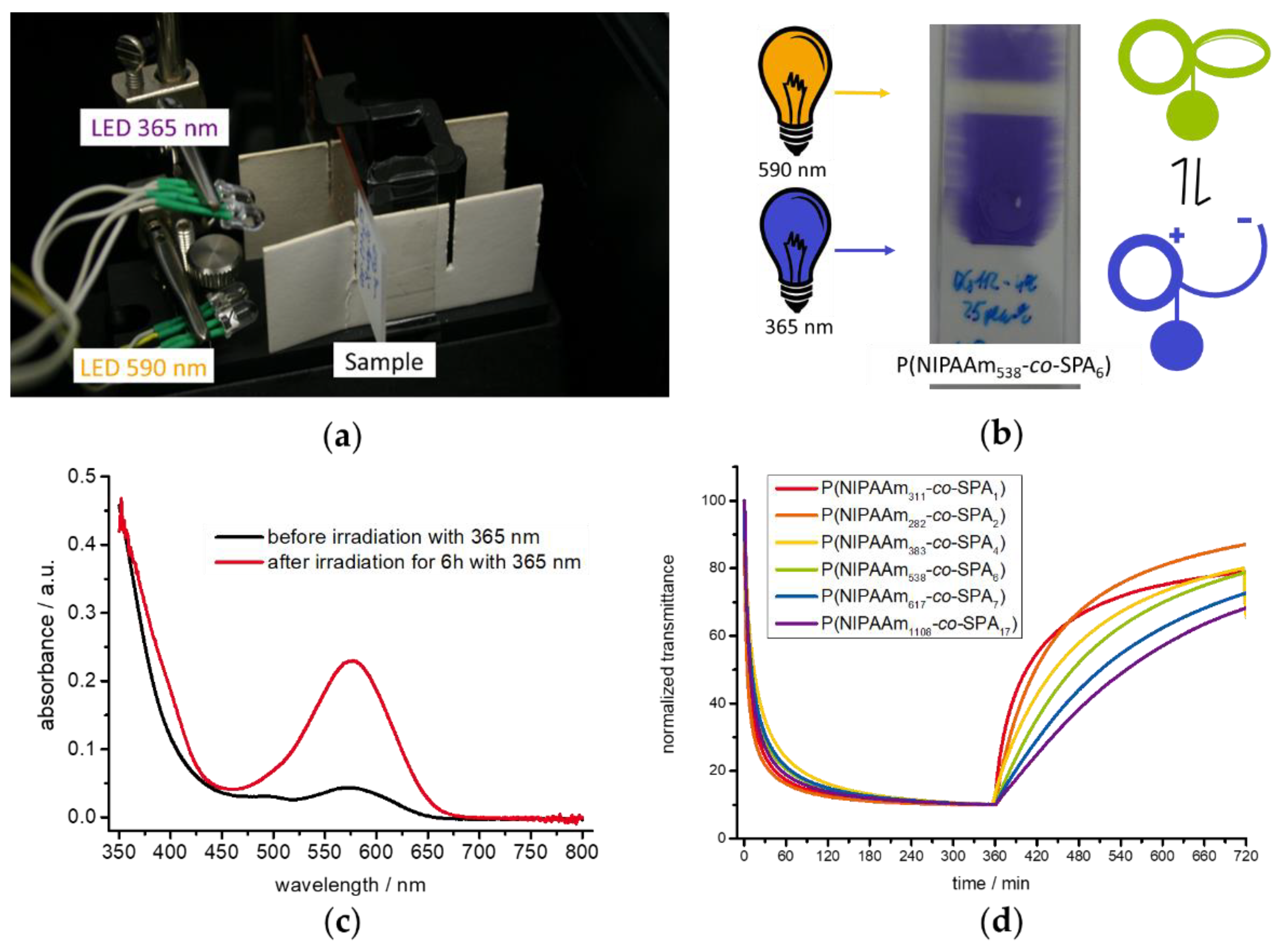
3.3. Photo-Response in the Solid State
3.4. Photo-Response in Aqueous Solution
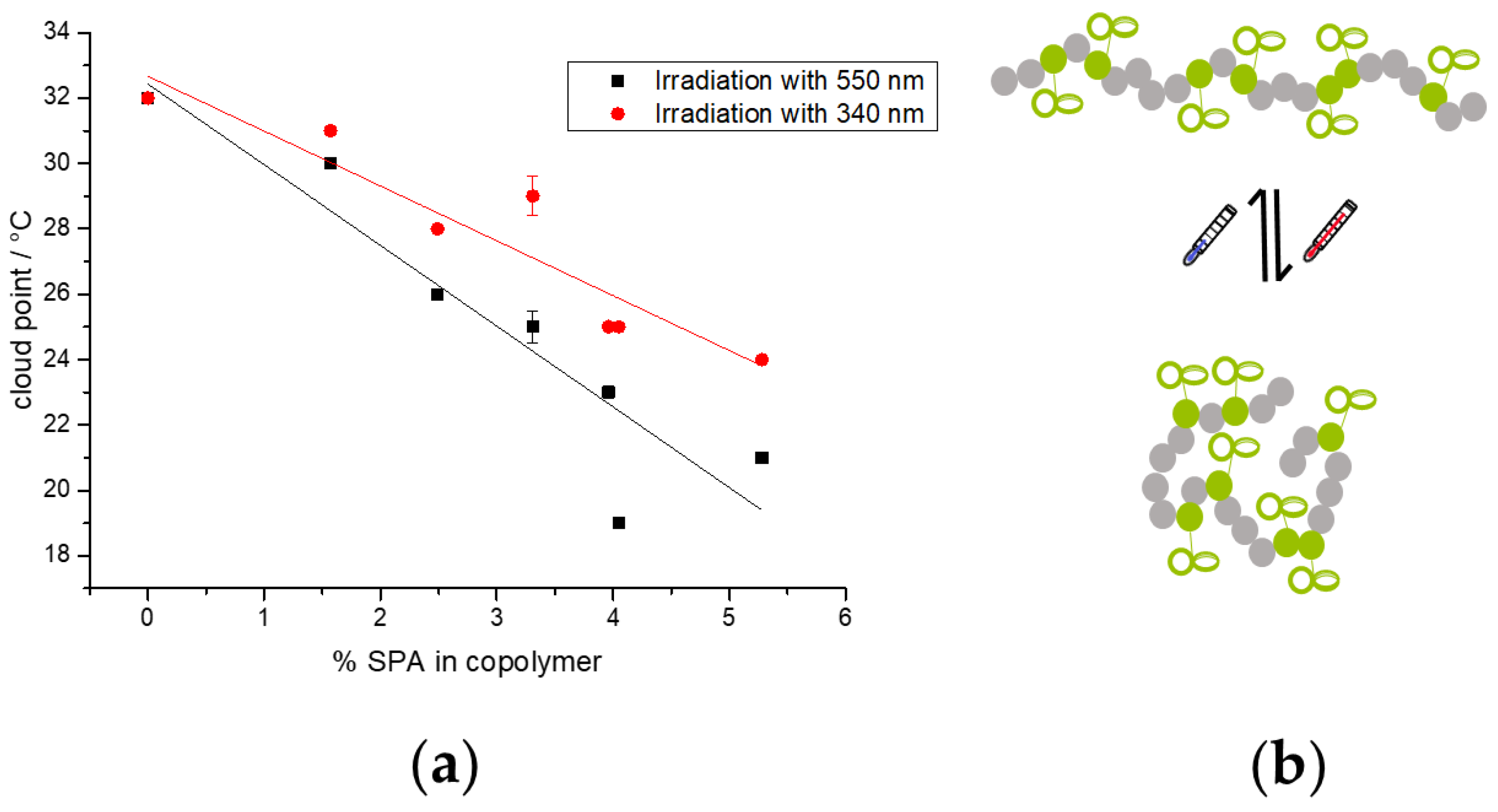
3.5. Temperature Response in Aqueous Solution
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Jochum, F.D.; Theato, P. Temperature- and light-responsive smart polymer materials. Chem. Soc. Rev. 2013, 42, 7468–7483. [Google Scholar] [CrossRef] [PubMed]
- Gohy, J.F.; Zhao, Y. Photo-responsive block copolymer micelles: Design and behavior. Chem. Soc. Rev. 2013, 42, 7117–7129. [Google Scholar] [CrossRef] [PubMed]
- Stumpel, J.E.; Liu, D.; Broer, D.J.; Schenning, A.P. Photoswitchable hydrogel surface topographies by polymerisation-induced diffusion. Chemistry 2013, 19, 10922–10927. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, I.; Trzebicka, B.; Müller, A.H.E.; Dworak, A.; Tsvetanov, C.B. Thermosensitive water-soluble copolymers with doubly responsive reversibly interacting entities. Prog. Polym. Sci. 2007, 32, 1275–1343. [Google Scholar] [CrossRef]
- Fan, X.; Chung, J.Y.; Lim, Y.X.; Li, Z.; Loh, X.J. Review of Adaptive Programmable Materials and Their Bioapplications. ACS Appl. Mater. Interfaces 2016, 8, 33351–33370. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Wang, X.; Cao, M.; Wang, C.; Hu, Z.; Wu, Y.-L.; Li, Z.; Loh, X.J. “Y”-shape armed amphiphilic star-like copolymers: Design, synthesis and dual-responsive unimolecular micelle formation for controlled drug delivery. Polym. Chem. 2017, 8, 5611–5620. [Google Scholar] [CrossRef]
- Schild, H.G.; Tirrell, D.A. Microcalorimetric detection of lower critical solution temperatures in aqueous polymer solutions. J. Phys. Chem. B 1990, 94, 4352–4356. [Google Scholar] [CrossRef]
- Halperin, A.; Kröger, M.; Winnik, F.M. Poly(N-isopropylacrylamide) Phase Diagrams: Fifty Years of Research. Angew. Chem. Int. Ed. 2015, 54, 15342–15367. [Google Scholar] [CrossRef] [PubMed]
- Glassner, M.; Vergaelen, M.; Hoogenboom, R. Poly(2-oxazoline)s: A comprehensive overview of polymer structures and their physical properties. Polym. Int. 2018, 67, 32–45. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Schlaad, H. Thermoresponsive poly(2-oxazoline)s, polypeptoids, and polypeptides. Polym. Chem. 2017, 8, 24–40. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Thijs, H.M.; Jochems, M.J.; van Lankvelt, B.M.; Fijten, M.W.; Schubert, U.S. Tuning the LCST of poly(2-oxazoline)s by varying composition and molecular weight: Alternatives to poly(N-isopropylacrylamide)? Chem. Commun. 2008. [Google Scholar] [CrossRef] [PubMed]
- Diehl, C.; Schlaad, H. Thermo-responsive polyoxazolines with widely tuneable LCST. Macromol. Biosci. 2009, 9, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Z.; Liu, K.L.; Ni, X.; Li, J. Biodegradable hyperbranched amphiphilic polyurethane multiblock copolymers consisting of poly(propylene glycol), poly(ethylene glycol), and polycaprolactone as in situ thermogels. Biomacromolecules 2012, 13, 3977–3989. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tan, B.H.; Jin, G.; Li, K.; He, C. Design of polyhedral oligomeric silsesquioxane (POSS) based thermo-responsive amphiphilic hybrid copolymers for thermally denatured protein protection applications. Polym. Chem. 2014, 5, 6740–6753. [Google Scholar] [CrossRef]
- Su, X.; Tan, M.J.; Li, Z.; Wong, M.; Rajamani, L.; Lingam, G.; Loh, X.J. Recent Progress in Using Biomaterials as Vitreous Substitutes. Biomacromolecules 2015, 16, 3093–3102. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.-F.; Hoth, A. Preparation of Ideal PEG Analogues with a Tunable Thermosensitivity by Controlled Radical Copolymerization of 2-(2-Methoxyethoxy)ethyl Methacrylate and Oligo(ethylene glycol) Methacrylate. Macromolecules 2006, 39, 893–896. [Google Scholar] [CrossRef]
- Hedir, G.G.; Arno, M.C.; Langlais, M.; Husband, J.T.; O’Reilly, R.K.; Dove, A.P. Poly(oligo(ethylene glycol) vinyl acetate)s: A Versatile Class of Thermoresponsive and Biocompatible Polymers. Angew. Chem. Int. Ed. 2017, 129, 9306–9310. [Google Scholar] [CrossRef]
- Langer, M.; Brandt, J.; Lederer, A.; Goldmann, A.S.; Schacher, F.H.; Barner-Kowollik, C. Amphiphilic block copolymers featuring a reversible hetero Diels-Alder linkage. Polym. Chem. 2014, 5, 5330–5338. [Google Scholar] [CrossRef]
- Heskins, M.; Guillet, J.E. Solution Properties of Poly(N-isopropylacrylamide). J. Macromol. Sci. A 1968, 2, 1441–1455. [Google Scholar] [CrossRef]
- Schild, H.G. Poly(N-isopropylacrylamide): Experiment, theory and application. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Hofmann, C.; Schönhoff, M. Do additives shift the LCST of poly(N-isopropylacrylamide) by solvent quality changes or by direct interactions? Colloid Polym. Sci. 2009, 287, 1369–1376. [Google Scholar] [CrossRef]
- Hou, L.; Wu, P. Comparison of LCST-transitions of homopolymer mixture, diblock and statistical copolymers of NIPAM and VCL in water. Soft Matter 2015, 11, 2771–2781. [Google Scholar] [CrossRef] [PubMed]
- Grimm, O.; Wendler, F.; Schacher, F. Micellization of Photo-Responsive Block Copolymers. Polymers 2017, 9, 396. [Google Scholar] [CrossRef]
- Fihey, A.; Perrier, A.; Browne, W.R.; Jacquemin, D. Multiphotochromic molecular systems. Chem. Soc. Rev. 2015, 44, 3719–3759. [Google Scholar] [CrossRef] [PubMed]
- Bléger, D. Orchestrating Molecular Motion with Light—From Single (macro)Molecules to Materials. Macromol. Chem. Phys. 2016, 217, 189–198. [Google Scholar] [CrossRef]
- Hartley, G.S. The Cis-form of Azobenzene. Nat. Chem. 1937, 140, 281. [Google Scholar] [CrossRef]
- Kumar, G.S.; Neckers, D.C. Photochemistry of azobenzene-containing polymers. Chem. Rev. 1989, 89, 1915–1925. [Google Scholar] [CrossRef]
- Darcy, P.J.; Hart, R.J.; Heller, H.G. Overcrowded molecules. Part 14. Photochromic systems involving (Z)-1-methylpropylidene(diphenylmethylene)succinic and (E)-3,5-dimethoxybenzylidene(alkyl-substituted methylene)succinic anhydrides. J. Chem. Soc. Perkin Trans. 1978. [Google Scholar] [CrossRef]
- Irie, M.; Mohri, M. Thermally irreversible photochromic systems. Reversible photocyclization of diarylethene derivatives. J. Org. Chem. 1988, 53, 803–808. [Google Scholar] [CrossRef]
- Irie, M. Diarylethenes for Memories and Switches. Chem. Rev. 2000, 100, 1685–1716. [Google Scholar] [CrossRef] [PubMed]
- Kobatake, S.; Takami, S.; Muto, H.; Ishikawa, T.; Irie, M. Rapid and reversible shape changes of molecular crystals on photoirradiation. Nature 2007, 446, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Löwenbein, A.; Katz, W. Über substituiertespiro-Dibenzopyrane. Ber. Dtsch. Chem. Ges. 1926, 59, 1377–1383. [Google Scholar] [CrossRef]
- Smets, G. Photochromic behaviour of polymeric systems and related phenomena. Pure Appl. Chem. 1972, 30, 1–24. [Google Scholar] [CrossRef]
- Balmond, E.I.; Tautges, B.K.; Faulkner, A.L.; Or, V.W.; Hodur, B.M.; Shaw, J.T.; Louie, A.Y. Comparative Evaluation of Substituent Effect on the Photochromic Properties of Spiropyrans and Spirooxazines. J. Org. Chem. 2016, 81, 8744–8758. [Google Scholar] [CrossRef] [PubMed]
- Berkovic, G.; Krongauz, V.; Weiss, V. Spiropyrans and Spirooxazines for Memories and Switches. Chem. Rev. 2000, 100, 1741–1754. [Google Scholar] [CrossRef] [PubMed]
- Hirshberg, Y.; Fischer, E. Photochromism and reversible multiple internal transitions in some spiropyrans at low temperatures. Part I. J. Chem. Soc. 1954. [Google Scholar] [CrossRef]
- Hirshberg, Y.; Fischer, E. Photochromism and reversible multiple internal transitions in some spiroPyrans at low temperatures. Part II. J. Chem. Soc. 1954. [Google Scholar] [CrossRef]
- Bergmann, E.D.; Weizmann, A.; Fischer, E. Structure and Polarity of Some Polycyclic Spirans. J. Am. Chem. Soc. 1950, 72, 5009–5012. [Google Scholar] [CrossRef]
- Vlassiouk, I.; Park, C.D.; Vail, S.A.; Gust, D.; Smirnov, S. Control of nanopore wetting by a photochromic spiropyran: A light-controlled valve and electrical switch. Nano Lett. 2006, 6, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Liu, G.; Ji, J. Micelles and reverse micelles with a photo and thermo double-responsive block copolymer. J. Polym. Sci. Part A 2010, 48, 2855–2861. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Sumiya, S.; Manabe, K.; Hirai, T. Thermoresponsive copolymer containing a coumarin-spiropyran conjugate: Reusable fluorescent sensor for cyanide anion detection in water. ACS Appl. Mater. Interfaces 2011, 3, 4649–4656. [Google Scholar] [CrossRef] [PubMed]
- Sumaru, K.; Kameda, M.; Kanamori, T.; Shinbo, T. Characteristic Phase Transition of Aqueous Solution of Poly(N-isopropylacrylamide) Functionalized with Spirobenzopyran. Macromolecules 2004, 37, 4949–4955. [Google Scholar] [CrossRef]
- Ueki, T.; Nakamura, Y.; Lodge, T.P.; Watanabe, M. Light-Controlled Reversible Micellization of a Diblock Copolymer in an Ionic Liquid. Macromolecules 2012, 45, 7566–7573. [Google Scholar] [CrossRef]
- Fissi, A.; Pieroni, O.; Angelini, N.; Lenci, F. Photoresponsive Polypeptides. Photochromic and Conformational Behavior of Spiropyran-Containing Poly(l-glutamate)s under Acid Conditions. Macromolecules 1999, 32, 7116–7121. [Google Scholar] [CrossRef]
- Szilágyi, A.; Sumaru, K.; Sugiura, S.; Takagi, T.; Shinbo, T.; Zrínyi, M.; Kanamori, T. Rewritable Microrelief Formation on Photoresponsive Hydrogel Layers. Chem. Mater. 2007, 19, 2730–2732. [Google Scholar] [CrossRef]
- Stumpel, J.E.; Ziolkowski, B.; Florea, L.; Diamond, D.; Broer, D.J.; Schenning, A.P. Photoswitchable ratchet surface topographies based on self-protonating spiropyran-NIPAAM hydrogels. ACS Appl. Mater. Interfaces 2014, 6, 7268–7274. [Google Scholar] [CrossRef] [PubMed]
- Sumaru, K.; Kameda, M.; Kanamori, T.; Shinbo, T. Reversible and Efficient Proton Dissociation of Spirobenzopyran-Functionalized Poly(N-isopropylacrylamide) in Aqueous Solution Triggered by Light Irradiation and Temporary Temperature Rise. Macromolecules 2004, 37, 7854–7856. [Google Scholar] [CrossRef]
- Kameda, M.; Sumaru, K.; Kanamori, T.; Shinbo, T. Probing the dielectric environment surrounding poly(N-isopropylacrylamide) in aqueous solution with covalently attached spirobenzopyran. Langmuir ACS J. Surfaces Colloids 2004, 20, 9315–9319. [Google Scholar] [CrossRef] [PubMed]
- Sumaru, K.; Takagi, T.; Satoh, T.; Kanamori, T. Photo- and Thermoresponsive Dehydration of Spiropyran-Functionalized Polymer Regulated by Molecular Recognition. Macromol. Rapid Commun. 2018, 39, 1700234. [Google Scholar] [CrossRef] [PubMed]
- Sumaru, K.; Ohi, K.; Takagi, T.; Kanamori, T.; Shinbo, T. Photoresponsive properties of poly(N-isopropylacrylamide) hydrogel partly modified with spirobenzopyran. Langmuir ACS J. Surfaces Coll. 2006, 22, 4353–4356. [Google Scholar] [CrossRef] [PubMed]
- Achilleos, D.S.; Vamvakaki, M. Multiresponsive Spiropyran-Based Copolymers Synthesized by Atom Transfer Radical Polymerization. Macromolecules 2010, 43, 7073–7081. [Google Scholar] [CrossRef]
- Wu, T.; Zou, G.; Hu, J.; Liu, S. Fabrication of Photoswitchable and Thermotunable Multicolor Fluorescent Hybrid Silica Nanoparticles Coated with Dye-Labeled Poly(N-isopropylacrylamide) Brushes. Chem. Mater. 2009, 21, 3788–3798. [Google Scholar] [CrossRef]
- Guragain, S.; Bastakoti, B.P.; Ito, M.; Yusa, S.-I.; Nakashima, K. Aqueous polymeric micelles of poly[N-isopropylacrylamide-b-sodium 2-(acrylamido)-2-methylpropanesulfonate] with a spiropyran dimer pendant: Quadruple stimuli-responsiveness. Soft Matter 2012, 8, 9628. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Pang, M.; Zhang, W. Synthesis and micellization of multi-stimuli responsive block copolymer based on spiropyran. Polym. Chem. 2016. [Google Scholar] [CrossRef]
- Chen, S.; Gao, Y.; Cao, Z.; Wu, B.; Wang, L.; Wang, H.; Dang, Z.; Wang, G. Nanocomposites of Spiropyran-Functionalized Polymers and Upconversion Nanoparticles for Controlled Release Stimulated by Near-Infrared Light and pH. Macromolecules 2016, 49, 7490–7496. [Google Scholar] [CrossRef]
- Raymo, F.M.; Giordani, S. Signal Processing at the Molecular Level. J. Am. Chem. Soc. 2001, 123, 4651–4652. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.I.; Wu, W.; Oh, J.K.; Mueller, L.; Sherwood, G.; Peteanu, L.; Kowalewski, T.; Matyjaszewski, K. Light-induced reversible formation of polymeric micelles. Angew. Chem. Int. Ed. 2007, 46, 2453–2457. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, A.; Mahdavian, A.R.; Salehi-Mobarakeh, H. Preparation of Stimuli-Responsive Functionalized Latex Nanoparticles: The Effect of Spiropyran Concentration on Size and Photochromic Properties. Langmuir ACS J. Surfaces Colloids 2015. [Google Scholar] [CrossRef] [PubMed]
- Miele, S.; Nesvadba, P.; Studer, A. 1-tert-Butyl-3,3,5,5-tetraalkyl-2-piperazinon-4-oxyls: Highly Efficient Nitroxides for Controlled Radical Polymerization. Macromolecules 2009, 42, 2419–2427. [Google Scholar] [CrossRef]
- Lai, J.T. Hindered Amines; III1. Highly Regioselective Syntheses of 1,3,3,5,5-Pentasubstituted 2-Piperazinones and their Nitroxyl Radicals. Synthesis 1981, 1981, 40–42. [Google Scholar] [CrossRef]
- Stitzel, S.; Byrne, R.; Diamond, D. LED switching of spiropyran-doped polymer films. J. Mater. Sci. 2006, 41, 5841–5844. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Hurley, E.P.; Desper, J.; Natali, M.; Douglawi, A.; Giordani, S. The balance between closed and open forms of spiropyrans in the solid state. CrystEngComm 2010, 12, 1027–1033. [Google Scholar] [CrossRef]
- Kohl-Landgraf, J.; Braun, M.; Ozcoban, C.; Goncalves, D.P.; Heckel, A.; Wachtveitl, J. Ultrafast dynamics of a spiropyran in water. J. Am. Chem. Soc. 2012, 134, 14070–14077. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Zhang, Z.; Xu, K. Spectrally Resolved Super-Resolution Microscopy Unveils Multipath Reaction Pathways of Single Spiropyran Molecules. J. Am. Chem. Soc. 2017. [Google Scholar] [CrossRef] [PubMed]
- Paramonov, S.V.; Lokshin, V.; Fedorova, O.A. Spiropyran, chromene or spirooxazine ligands: Insights into mutual relations between complexing and photochromic properties. J. Photochem. Photobiol. C 2011, 12, 209–236. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Q.; Zhou, Y.-N.; Zhu, S. Let spiropyran help polymers feel force! Prog. Polym. Sci. 2017. [Google Scholar] [CrossRef]
- Dilthey, W.; Berres, C.; Hölterhoff, E.; Wübken, H. Beitrag Zur Kenntnis der Spiro-di-benzopyrane (Heteropolare Kohlenstoffverbindungen. IV). J. Prakt. Chem. 1926, 114, 179–198. [Google Scholar] [CrossRef]
- Hammarson, M.; Nilsson, J.R.; Li, S.; Beke-Somfai, T.; Andreasson, J. Characterization of the thermal and photoinduced reactions of photochromic spiropyrans in aqueous solution. J. Phys. Chem. B 2013, 117, 13561–13571. [Google Scholar] [CrossRef] [PubMed]
- Stafforst, T.; Hilvert, D. Kinetic characterization of spiropyrans in aqueous media. Chem. Commun. 2009. [Google Scholar] [CrossRef] [PubMed]
- Baillet, G.; Giusti, G.; Guglielmetti, R. Comparative photodegradation study between spiro[indoline—oxazine] and spiro[indoline—pyran] derivatives in solution. J. Photochem. Photobiol. A 1993, 70, 157–161. [Google Scholar] [CrossRef]
- Baillet, G.; Campredon, M.; Guglielmetti, R.; Giusti, G.; Aubert, C. Dealkylation of N-substituted indolinospironaphthoxazine photochromic compounds under UV irradiation. J. Photochem. Photobiol. A 1994, 83, 147–151. [Google Scholar] [CrossRef]
- Wolff, C.; Kind, J.; Schenderlein, H.; Bartling, H.; Feldmeier, C.; Gschwind, R.M.; Biesalski, M.; Thiele, C.M. Studies of a photochromic model system using NMR with ex-situ and in-situ irradiation devices. Magn. Reson. Chem. 2016, 54, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Gupta, R.B. Thermosensitive and ampholytic hydrogels for salt solution. J. Appl. Polym. Sci. 2003, 88, 2032–2037. [Google Scholar] [CrossRef]
- Bittrich, E.; Kuntzsch, M.; Eichhorn, K.-J.; Uhlmann, P. Complex pH- and temperature-sensitive swelling behavior of mixed polymer brushes. J. Polym. Sci. Part B 2010, 48, 1606–1615. [Google Scholar] [CrossRef]

| Composition b,d | xSPA Reaction mixture (%) | Mna | Ða | % SPA b | % SPA c | Mwd | Extinction coefficient c/mol·mL−1·cm−1 |
|---|---|---|---|---|---|---|---|
| PNIPAAm418 | 0 | 22,900 | 2.47 | 0 | - | 47,300 | 10,000 |
| P(NIPAAm311-co-SPA1) | 1 | 25,700 | 1.86 | 1.5 | 1.5 | 35,800 | 13,000 |
| P(NIPAAm282-co-SPA2) | 2 | 25,800 | 1.76 | 2.5 | 3.6 | 32,700 | 29,000 |
| P(NIPAAm383-co-SPA4) | 3 | 25,500 | 1.58 | 3.5 | 5.7 | 44,800 | 63,000 |
| P(NIPAAm538-co-SPA6) | 4 | 26,300 | 1.55 | 4 | 7.5 | 63,400 | 116,000 |
| P(NIPAAm617-co-SPA7) | 5 | 19,300 | 1.47 | 4 | 10.3 | 72,700 | 253,000 |
| P(NIPAAm1108-co-SPA17) | 6 | 22,600 | 1.41 | 5.5 | 10.8 | 132,300 | 350,000 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grimm, O.; Schacher, F.H. Dual Stimuli-Responsive P(NIPAAm-co-SPA) Copolymers: Synthesis and Response in Solution and in Films. Polymers 2018, 10, 645. https://doi.org/10.3390/polym10060645
Grimm O, Schacher FH. Dual Stimuli-Responsive P(NIPAAm-co-SPA) Copolymers: Synthesis and Response in Solution and in Films. Polymers. 2018; 10(6):645. https://doi.org/10.3390/polym10060645
Chicago/Turabian StyleGrimm, Oliver, and Felix H. Schacher. 2018. "Dual Stimuli-Responsive P(NIPAAm-co-SPA) Copolymers: Synthesis and Response in Solution and in Films" Polymers 10, no. 6: 645. https://doi.org/10.3390/polym10060645
APA StyleGrimm, O., & Schacher, F. H. (2018). Dual Stimuli-Responsive P(NIPAAm-co-SPA) Copolymers: Synthesis and Response in Solution and in Films. Polymers, 10(6), 645. https://doi.org/10.3390/polym10060645