3.1. Electrochemical Polymerizations
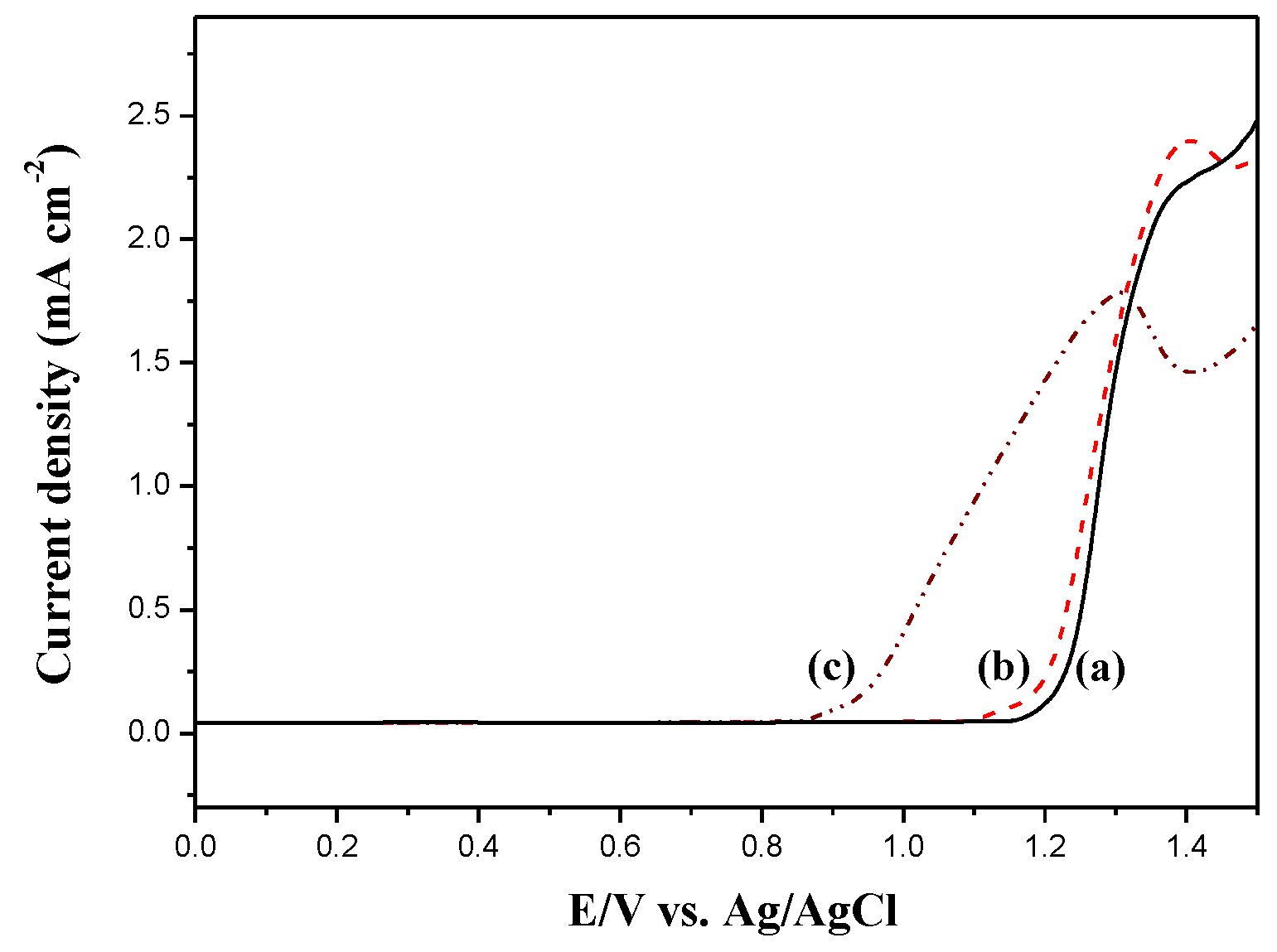
The electrooxidation of DiCP, CPDTK, and CPDT in 0.2 M LiClO
4 ACN/DCM solution were shown in
Figure 2. The onset potential of oxidation for DiCP, CPDT, and CPDTK were 1.15, 0.95, and 1.10 V, respectively. The onset potential of CPDT is smaller than that of CPDTK, this can be attributed to the withdrawing keto groups of CPDTK increasing the onset potential significantly. Moreover, the differences between onset potential of oxidation for DiCP vs. CPDTK and DiCP vs. CPDT are less than 0.2 V, indicating the copolymerizations of DiCP with CPDTK (or CPDT) are practicable.
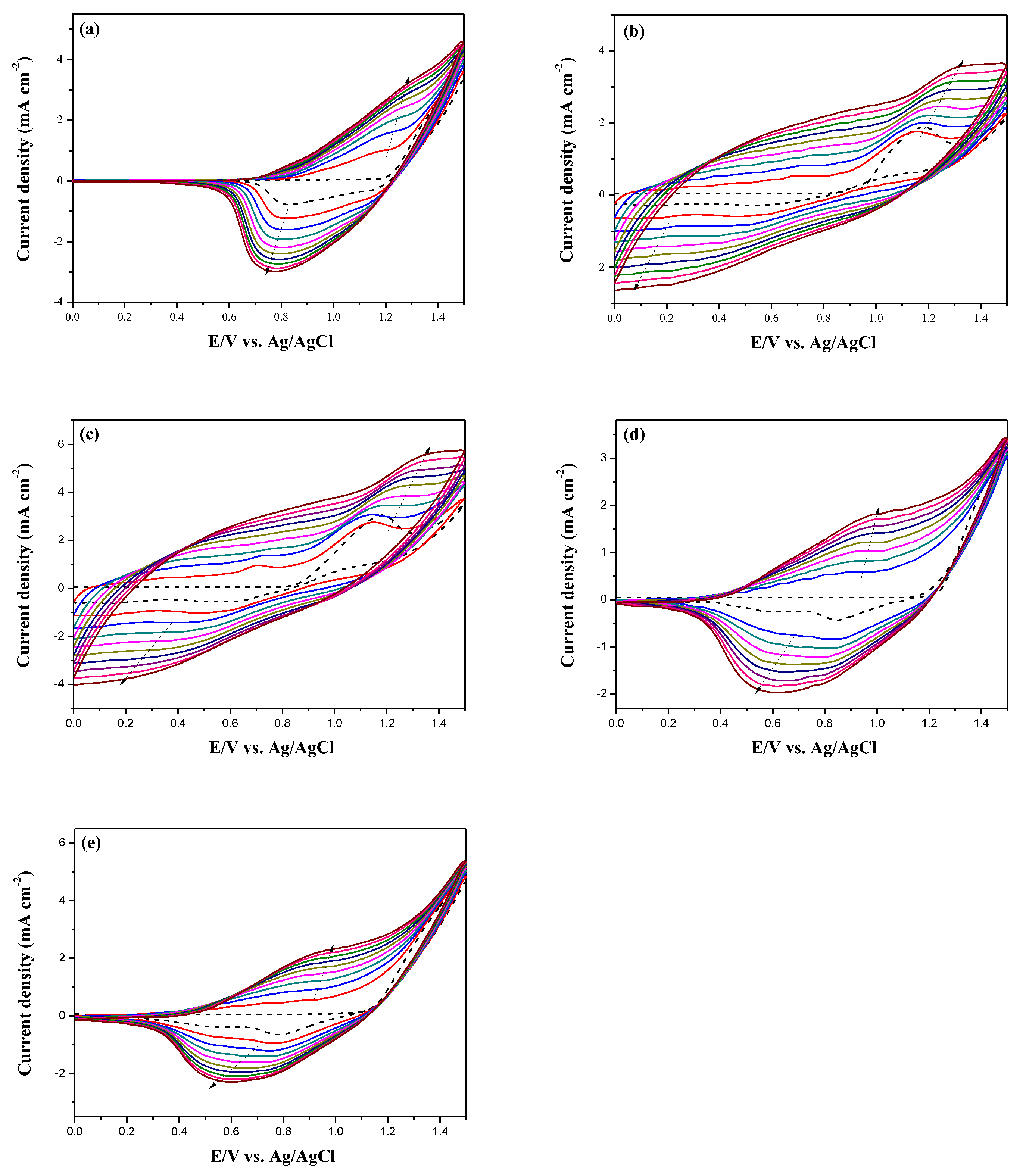
Figure 3 shows the electrochemical synthesis of PDiCP, P(DiCP-
co-CPDT), P(DiCP-
co-CPDT2), P(DiCP-
co-CPDTK), and P(DiCP-
co-CPDTK2) in 0.2 M LiClO
4/ACN/DCM solution (ACN:DCM = 1:1, by volume) at 100 mV s
−1 on ITO working electrode.
The current densities of the redox peaks increase with increasing scanning CV cycles, implying both deposition of the polymer films and that the deposited polymers are conducting [
32]. The oxidation peaks of PDiCP, P(DiCP-
co-CPDT), P(DiCP-
co-CPDT2), P(DiCP-
co-CPDTK), and P(DiCP-
co-CPDTK2) located at 1.15, 1.10, 1.10, 0.9 and 0.9 V, respectively, whereas the reduction peaks of PDiCP, P(DiCP-
co-CPDT), P(DiCP-
co-CPDT2), P(DiCP-
co-CPDTK), and P(DiCP-
co-CPDTK2) located at 0.75, 0.45, 0.5, 0.6, and 0.6 V, respectively. The redox peaks and CV curves of P(DiCP-
co-CPDT), P(DiCP-
co-CPDT2), P(DiCP-
co-CPDTK), and P(DiCP-
co-CPDTK2) are different from the oxidation and reduction peaks and CV curves of PDiCP, indicating the formation of P(DiCP-
co-CPDT), P(DiCP-
co-CPDT2), P(DiCP-
co-CPDTK), and P(DiCP-
co-CPDTK2) films on ITO electrodes. Specific capacitances of PDiCP, P(DiCP-
co-CPDT), P(DiCP-
co-CPDT2), P(DiCP-
co-CPDTK), and P(DiCP-
co-CPDTK2) electrodes are 76, 92, 94, 85, and 88 F/g, respectively.
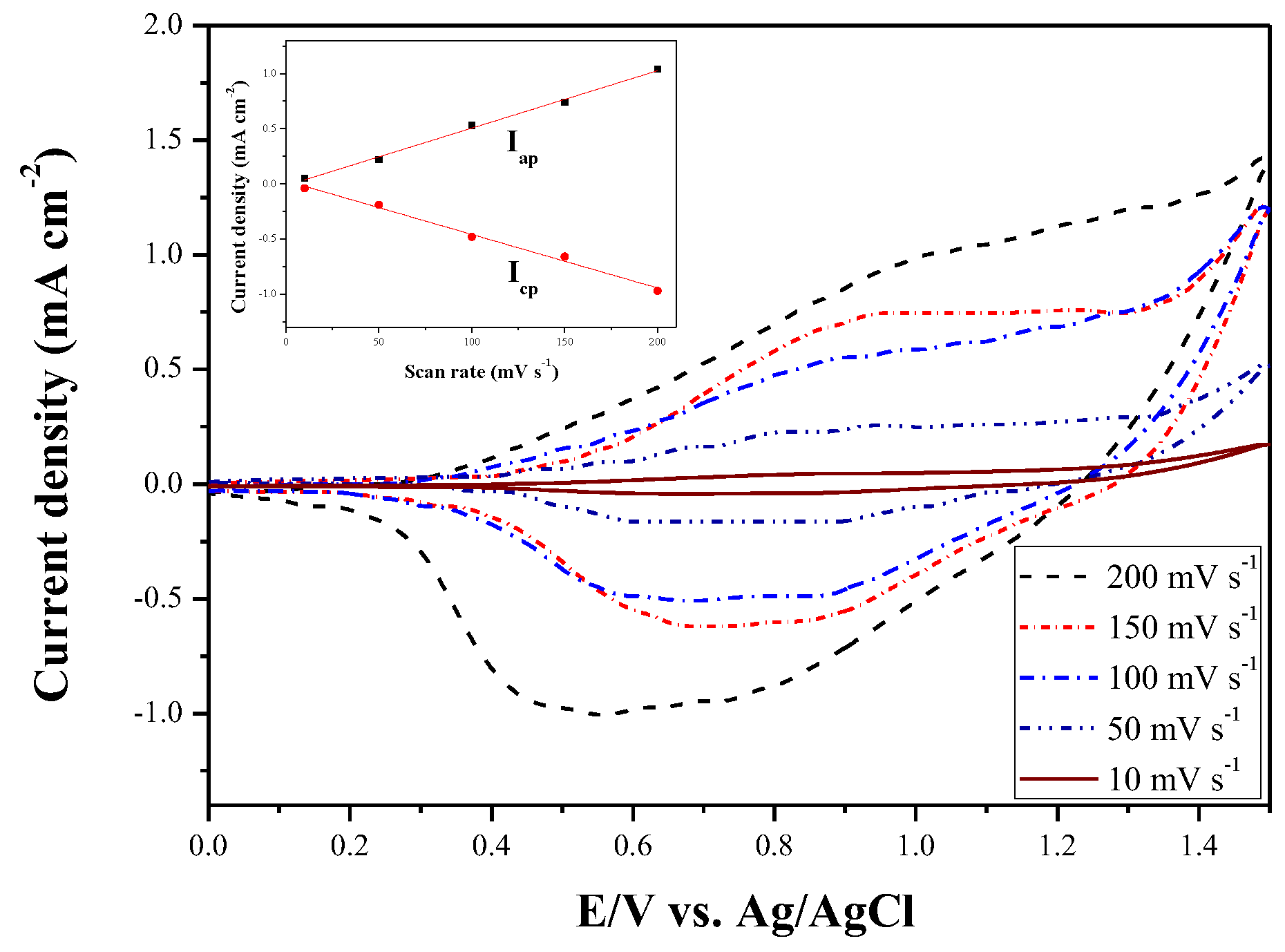
Figure 4 shows the CV curves of deposited P(DiCP-
co-CPDTK) film at 10, 50, 100, 150, and 200 mV s
−1 in 0.2 M LiClO
4/ACN/DCM solution, the P(DiCP-
co-CPDTK) film shows well-defined redox peaks at various scan rates and the anodic and cathodic peak current densities are proportional to the scan rates (inset of
Figure 4), inferring the P(DiCP-
co-CPDTK) film was stuck onto ITO glass and the redox process of the copolymer film was not diffusional control [
33].
3.2. Spectroelectrochemical Characterizations of Homopolymer and Copolymer Films
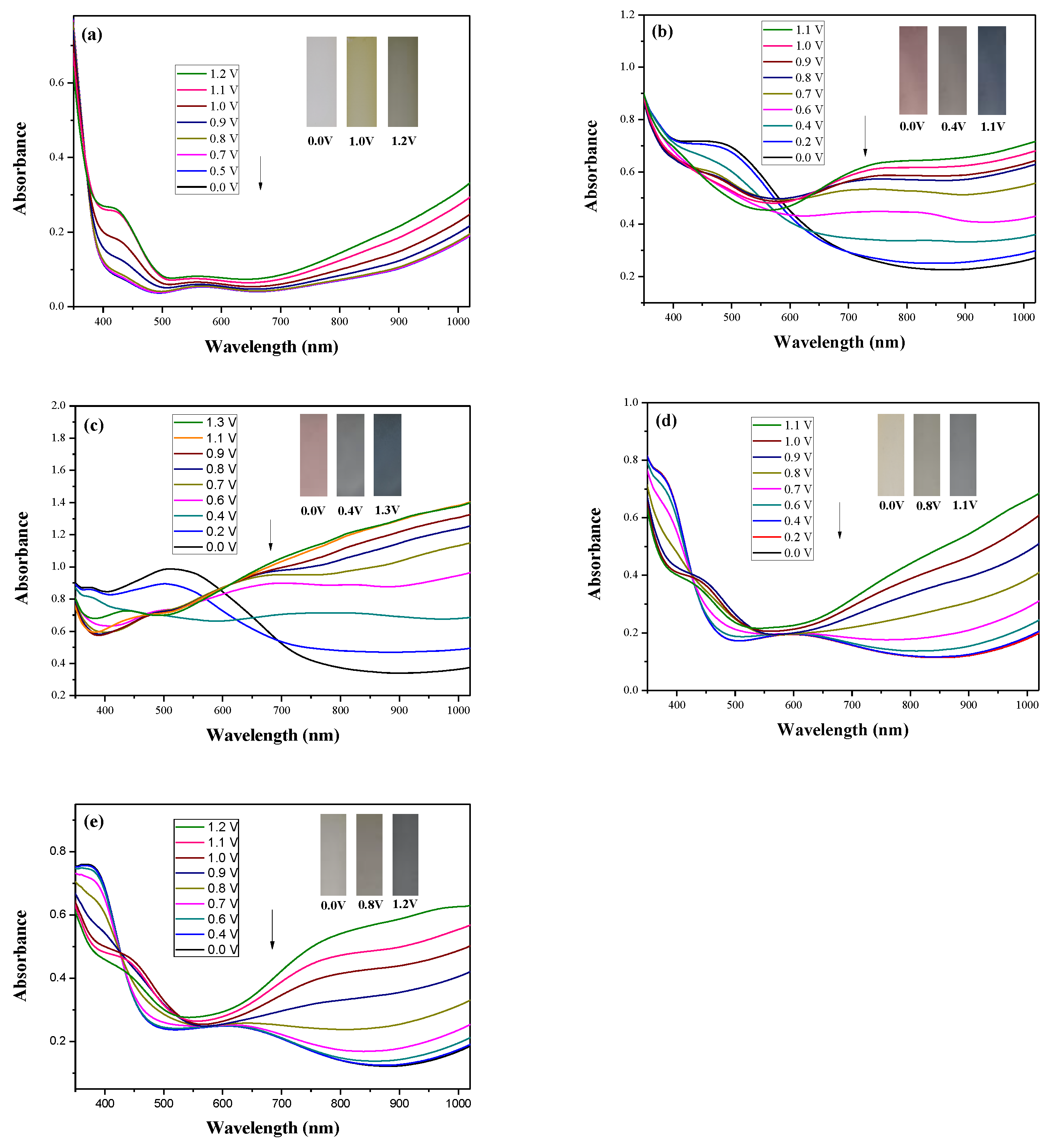
Figure 5 shows the spectroelectrochemical spectra of PDiCP, P(DiCP-
co-CPDT), P(DiCP-
co-CPDT2), P(DiCP-
co-CPDTK), and P(DiCP-
co-CPDTK2) films at various potentials in 0.2 M LiClO
4/ACN/DCM solution, and that there is no prominent absorption peak of PDiCP film at 0.0 V.
Upon increasing the potential progressively, new bands appeared at 425 and 1000 nm, which could be assigned to the formation of charge carriers for PDiCP film [
34]. However, P(DiCP-
co-CPDT), P(DiCP-
co-CPDT2), P(DiCP-
co-CPDTK), and P(DiCP-
co-CPDTK2) films showed π-π* (or n-π*) transition peaks at 485, 500, 392, and 395 nm, respectively, which were different to the absorption spectra of PDiCP film in the neutral state. The UV–VIS spectra of P(DiCP-
co-CPDT) film recorded for various potentials exhibit isosbestic points, suggesting the presence of neutral and oxidized P(DiCP-
co-CPDT) films which are active towards different irradiations at different potentials. The charge carrier bands of P(DiCP-
co-CPDT), P(DiCP-
co-CPDT2), P(DiCP-
co-CPDTK), and P(DiCP-
co-CPDTK2) films in 0.2 M LiClO
4/ACN/DCM solution showed significant variations at 1000 nm from neutral to oxidation states.
The PDiCP film showed three kinds of colour variations from neutral to oxidation state, PDiCP film was light gray at 0.0 V, dark khaki at 1.0 V, and grey black at 1.2 V. For the copolymer films, P(DiCP-
co-CPDT) and P(DiCP-
co-CPDT2) films were light brown at 0.0 V, light cadet blue at 0.4 V, and navy blue at 1.1 (or 1.3 V), whereas P(DiCP-
co-CPDTK) and P(DiCP-
co-CPDTK2) films were light yellow at 0.0 V, grey at 0.8 V, and rock grey at 1.1 V. The colorimetric values (
L*,
a*, and
b*) and CIE chromaticity diagrams of PDiCP, P(DiCP-
co-CPDT2), and P(DiCP-
co-CPDTK) films at various potentials are displayed in
Table 2.
The optical band gap (
Eg) of PDiCP homopolymer film calculated using Planck equation was 2.58 eV:
where the onset UV absorption wavelength (
λonset) of the π-π* transition band of PDiCP was 481 nm [
35,
36]. The onset oxidation potential vs. Ag/AgCl (3 M KCl) was 0.82 V, the
EFOC of ferrocene/ferrocenium vs. Ag/AgCl (3 M KCl) determined using cyclic voltammetry was 0.80 V, and the onset oxidation potential vs.
EFOC was found to be 0.02 V. The HOMO and LUMO energy levels of PDiCP determined from the onset oxidation potential with respect to the energy level of ferrocene/ferrocenium couple (−4.8 eV less than vacuum energy level) [
37,
38] and
Eg were taken as −4.82 and −2.24 eV, respectively.
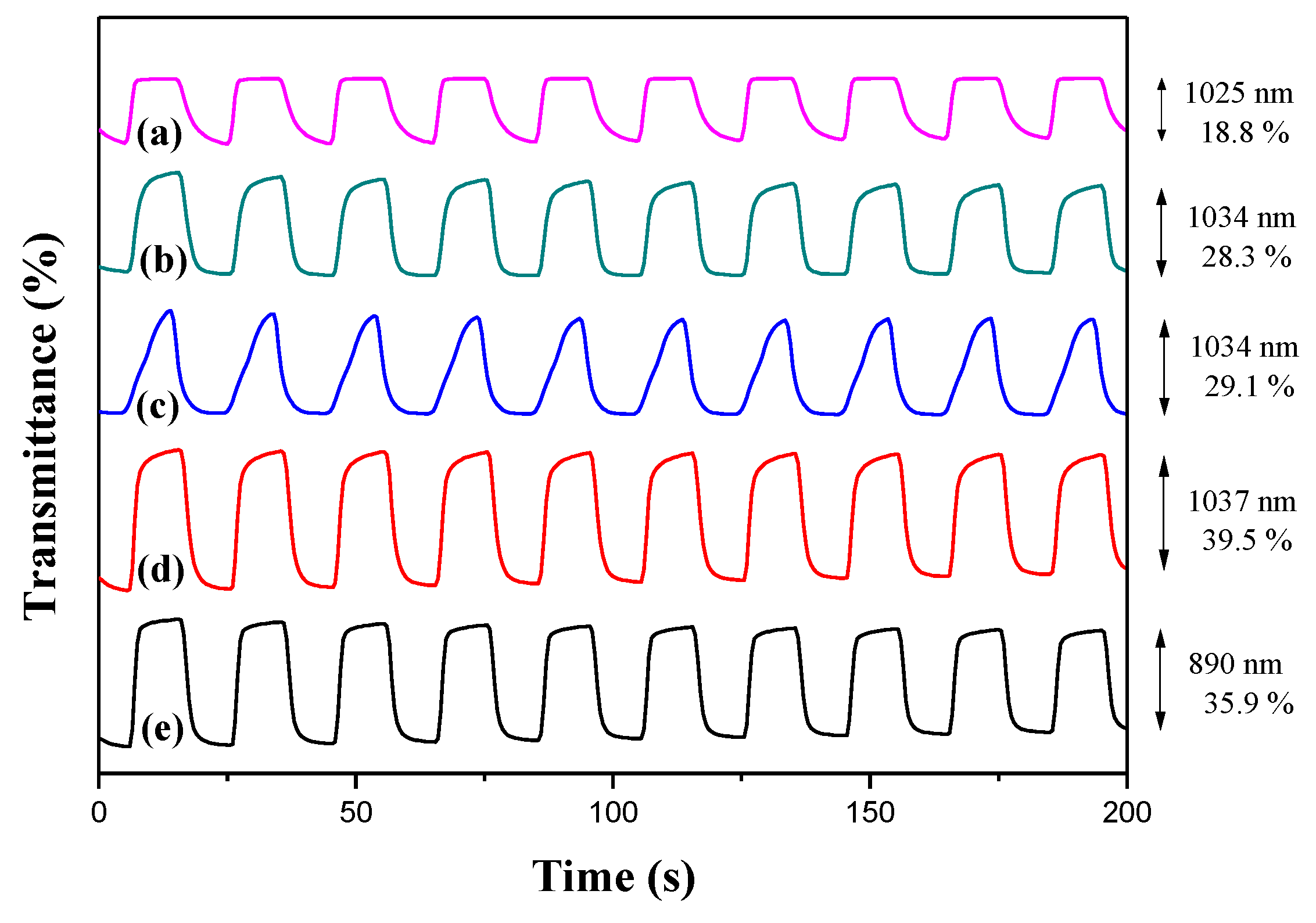
Figure 6 showed the electrochromic switching profiles of PDiCP, P(DiCP-
co-CPDT), P(DiCP-
co-CPDT2), P(DiCP-
co-CPDTK), and P(DiCP-
co-CPDTK2) films in 0.2 M LiClO
4/ACN/DCM solution, which were monitored using double potential-step chronoamperometry by repeating potentials between 0.0 and 1.2 V with a time interval of 10 s. The Δ
T of PDiCP at 1025 nm, P(DiCP-
co-CPDT) at 1034 nm, P(DiCP-
co-CPDT2) at 1034 nm, P(DiCP-
co-CPDTK) at 1037 nm, and P(DiCP-
co-CPDTK2) at 890 nm from the bleaching state to the colouration state in 0.2 M LiClO
4/ACN/DCM solution were calculated to be 18.8, 28.3, 29.1, 39.5, and 35.9%, respectively. Among these polymer films, P(DiCP-
co-CPDTK) film presents the highest Δ
T, and copolymers (P(DiCP-
co-CPDT), P(DiCP-
co-CPDT2), P(DiCP-
co-CPDTK), and P(DiCP-
co-CPDTK2)) present higher Δ
T than that of homopolymer (PDiCP) in 0.2 M LiClO
4/ACN/DCM solution, implying the copolymerization of DiCP with CPDT (or CPDTK) monomer results in the increase of Δ
T in 0.2 M LiClO
4/ACN/DCM solution. The colouration switching time (
τc) and bleaching switching time (
τb) of polymer films in 0.2 M LiClO
4/ACN/DCM solution are listed in
Table 3, the
τc and
τb are determined at 90% of the whole transmittance change. The
τc and
τb of PDiCP, P(DiCP-
co-CPDT), P(DiCP-
co-CPDT2), P(DiCP-
co-CPDTK), and P(DiCP-
co-CPDTK2) films in 0.2 M LiClO
4/ACN/DCM solution are in the range of 1.9–6.7 s.
ΔOD is the change of optical density, which can be calculated using the equation [
39]:
where
Tox and
Tred refer to the transmittance (%) of oxidized and neutral states, respectively. The ΔOD of PDiCP, P(DiCP-
co-CPDT), P(DiCP-
co-CPDT2), P(DiCP-
co-CPDTK), and P(DiCP-
co-CPDTK2) films at specific wavelengths in 0.2 M LiClO
4/ACN/DCM solution are listed in
Table 3. P(DiCP-
co-CPDT2) film shows the largest ΔOD among these polymer films in 0.2 M LiClO
4/ACN/DCM solution.
Colouration efficiency (
η) is an important parameter for electrochromic materials and devices, and can be calculated using the formula [
40]:
where
Qd is the injected/ejected charge of the polymer films (or devices) per active area.
Table 3 lists the
η of homopolymer and copolymer films in 0.2 M LiClO
4/ACN/DCM solution, the
η of PDiCP at 1025 nm, P(DiCP-
co-CPDT) at 1034 nm, P(DiCP-
co-CPDT2) at 1034 nm, P(DiCP-
co-CPDTK) at 1037 nm, and P(DiCP-
co-CPDTK2) at 890 nm are 123.8, 149.5, 80.9, 184.1, and 111.5 cm
2 C
−1, respectively.
The comparisons of transmittance changes and colouration efficiencies with reported polymer films were displayed in
Table 4, P(DiCP-
co-CPDTK) film showed higher transmittance change than those reported for PETCB film at 1100 nm [
41], PMCzP film at 460 nm [
42], and PBCz film at 1050 nm [
43]. However, P(DiCP-
co-CPDTK) film showed a lower transmittance change than those reported for P(BCz1-
co-Inc1) film at 787 nm [
44] and P(NO
2-3Cz) film at 710 nm [
45]. On the other hand, P(DiCP-
co-CPDTK) film revealed higher
η than those reported for P(BCz1-
co-Inc1) [
44], P(NO
2-3Cz) [
45], and PBCz films [
43].
3.3. Spectroelectrochemical Characterizations of Electrochromic Devices
Five dual type ECDs based on PDiCP, P(DiCP-
co-CPDT), P(DiCP-
co-CPDT2), P(DiCP-
co-CPDTK), and P(DiCP-
co-CPDTK2) films as anodically-colouring polymers and PEDOT-PSS film as cathodically-colouring polymer were fabricated, which were denominated as PDiCP/PEDOT-PSS, P(DiCP-
co-CPDT)/PEDOT-PSS, P(DiCP-
co-CPDT2)/PEDOT-PSS, P(DiCP-
co-CPDTK)/PEDOT-PSS, and P(DiCP-
co-CPDTK2)/PEDOT-PSS ECDs, respectively.
Figure 7a–e displayed the UV–VIS spectra of PDiCP/PEDOT-PSS, P(DiCP-
co-CPDT)/PEDOT-PSS, P(DiCP-
co-CPDT2)/PEDOT-PSS, P(DiCP-
co-CPDTK)/PEDOT-PSS, and P(DiCP-
co-CPDTK2)/PEDOT-PSS ECDs, respectively.
At 0.0 V, PDiCP/PEDOT-PSS ECD did not show distinct absorption peaks in the ultraviolet and visible zones. At this moment, anodic PDiCP film was in its reduced state, displaying a light grey colour. The cathodic PEDOT-PSS film was in the oxidized state, displaying a limpid colour. Accordingly, the PDiCP/PEDOT-PSS ECD displayed light grey colour at 0.0 V. Upon increasing the voltages gradually, PDiCP film started to oxidize and PEDOT-PSS film began to reduce. Accordingly, new peaks at 420 and 637 nm emerged gradually and the PDiCP/PEDOT-PSS ECD presented as dark blue at 1.9 V. The main electrochromic response of PDiCP/PEDOT-PSS ECD comes from the PEDOT-PSS layer.
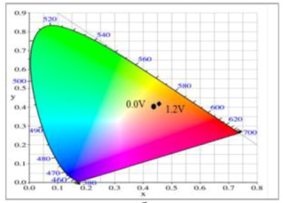
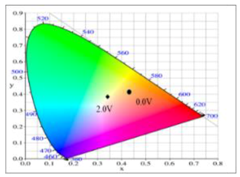
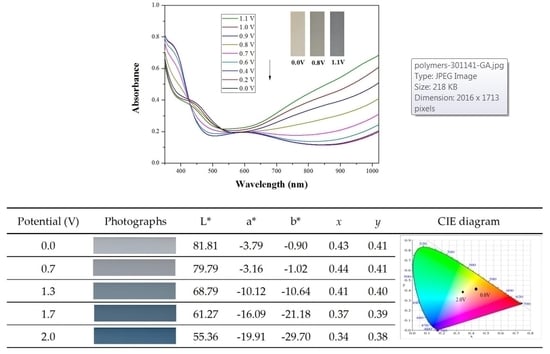
Table 5 summarized the electrochromic photographs, colorimetric values (
L*,
a*, and
b*) and CIE chromaticity diagrams of the P(DiCP-
co-CPDTK)/PEDOT-PSS ECD at various potentials. P(DiCP-
co-CPDTK)/PEDOT-PSS ECD showed light silverish-yellow at 0.0 V, light grey at 0.7 V, grey at 1.3 V, light greyish blue at 1.7 V, and greyish blue at 2.0 V.
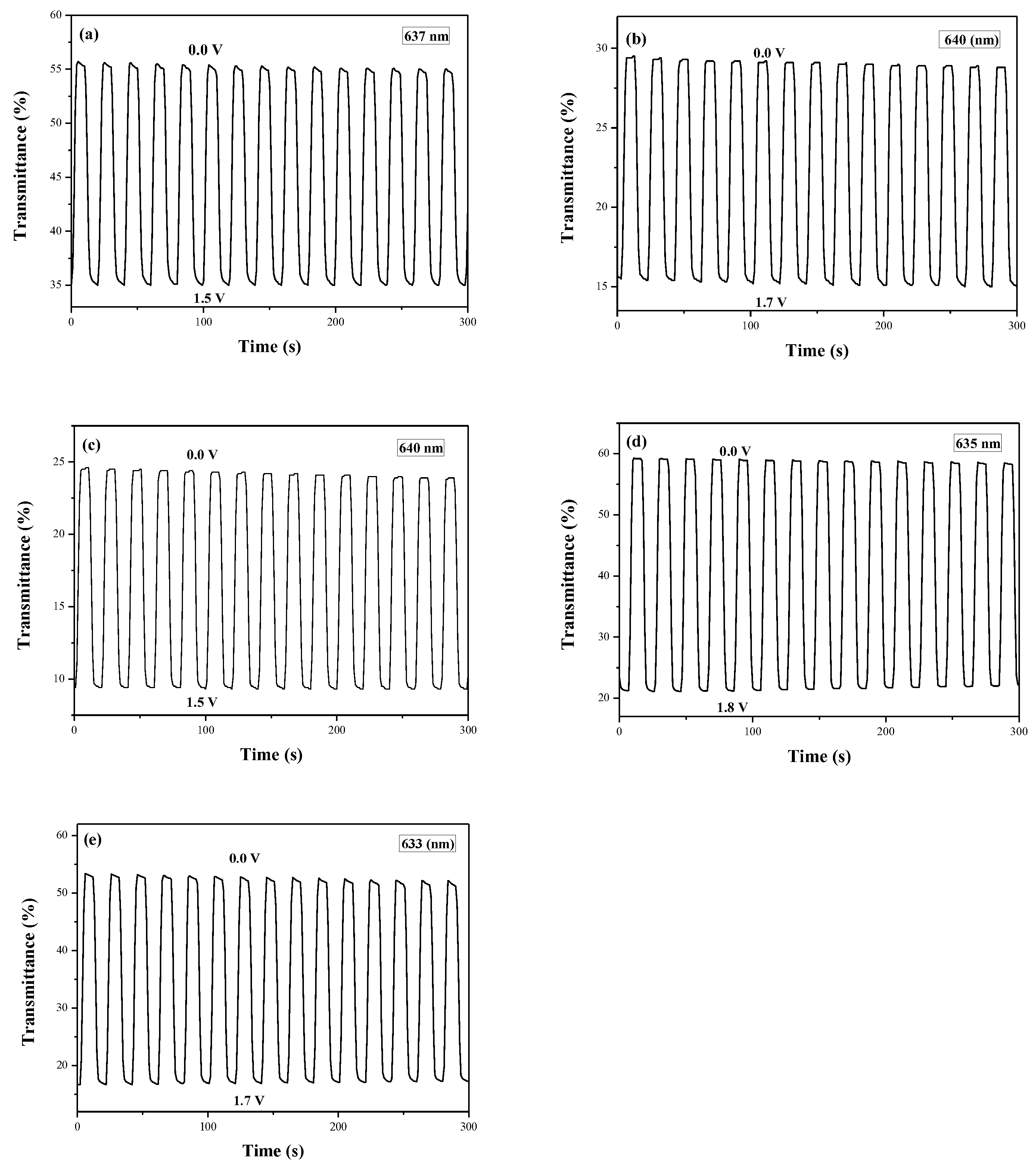
Figure 8 showed the electrochromic switching profiles of PDiCP/PEDOT-PSS, P(DiCP-
co-CPDT)/PEDOT-PSS, P(DiCP-
co-CPDT2)/PEDOT-PSS, P(DiCP-
co-CPDTK)/PEDOT-PSS, and P(DiCP-
co-CPDTK2)/PEDOT-PSS ECDs, the transmittance variations of these ECDs were carried out between 0.0 V and 1.8 V with a time interval of 10 s. The Δ
T, ΔOD,
Qd,
η,
τc, and
τb of PDiCP/PEDOT-PSS, P(DiCP-
co-CPDT)/PEDOT-PSS, P(DiCP-
co-CPDT2)/PEDOT-PSS, P(DiCP-
co-CPDTK)/PEDOT-PSS, and P(DiCP-
co-CPDTK2)/PEDOT-PSS ECDs estimated at the second cycle are listed in
Table 6.
P(DiCP-co-CPDTK)/PEDOT-PSS and P(DiCP-co-CPDTK2)/PEDOT-PSS ECDs revealed larger ΔT than that of PDiCP/PEDOT-PSS ECD, whereas P(DiCP-co-CPDT)/PEDOT-PSS and P(DiCP-co-CPDT2)/PEDOT-PSS ECDs revealed smaller ΔT than that of PDiCP/PEDOT-PSS ECD, implying the incorporation of P(DiCP-co-CPDTK) and P(DiCP-co-CPDTK2) as the anodically-colouring polymers led to a higher ΔT than those of PDiCP, P(DiCP-co-CPDT), and P(DiCP-co-CPDT2) electrodes. In other aspects, the colouration efficiencies of PDiCP/PEDOT-PSS, P(DiCP-co-CPDT)/PEDOT-PSS, P(DiCP-co-CPDT2)/PEDOT-PSS, P(DiCP-co-CPDTK)/PEDOT-PSS, and P(DiCP-co-CPDTK2)/PEDOT-PSS ECDs were 259.7 mC cm−2 at 637 nm, 358.0 mC cm−2 at 640 nm, 461.5 mC cm−2 at 640 nm, 633.8 mC cm−2 at 635 nm, and 510.4 mC cm−2 at 633 nm, respectively. The colouration efficiencies of P(DiCP-co-CPDT)/PEDOT-PSS, P(DiCP-co-CPDT2)/PEDOT-PSS, P(DiCP-co-CPDTK)/PEDOT-PSS, and P(DiCP-co-CPDTK2)/PEDOT-PSS ECDs were greater than that of PDiCP/PEDOT-PSS ECD.
The comparisons of transmittance changes and colouration efficiencies with reported ECDs were displayed in
Table 7, P(DiCP-
co-CPDTK)/PEDOT-PSS ECD showed higher transmittance change than those reported for PtCz/PProDOT-Me
2 [
46], poly(2,5-bis(9-methyl-9H-carbazol-3-yl)-1,3,4-oxadiazole)/PEDOT [
47], poly(4,4′-di(
N-carbazolyl)biphenyl)/PEDOT ECDs [
48], and was comparable to those reported for poly(4,4′-di(
N-carbazoyl)biphenyl-
co-4H-cyclopenta[2,1-b:3,4-b′]dithiophene)/PEDOT [
49] and P(tnCz1-
co-bTp2)/PProDOT-Me
2 ECDs [
50]. On the other side, P(DiCP-
co-CPDTK)/PEDOT-PSS ECD revealed higher
η at 635 nm than those reported for PtCz/PProDOT-Me
2 [
46], poly(4,4′-di(
N-carbazoyl)biphenyl-
co-4H-cyclopenta[2,1-b:3,4-b′]dithiophene)/PEDOT [
49], and P(tnCz1-
co-bTp2)/PProDOT-Me
2 ECDs [
50], which made P(DiCP-
co-CPDTK)/PEDOT-PSS ECD desirable for applications in rear-view mirrors of vehicles.
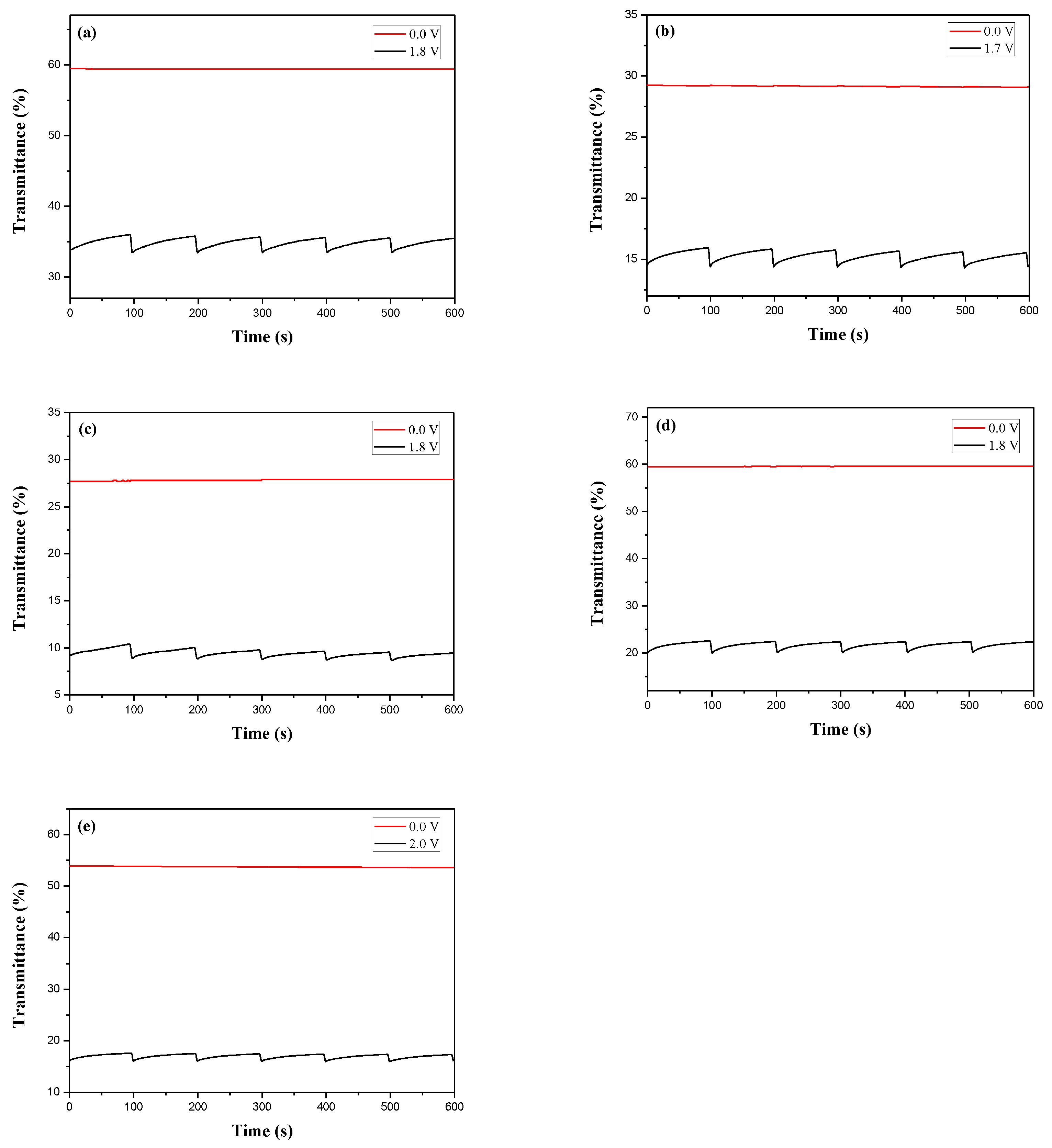
3.4. Optical Memory of ECDs
The ability to maintain a bleached (or coloured) state at open circuit of PDiCP/PEDOT-PSS, P(DiCP-
co-CPDT)/PEDOT-PSS, P(DiCP-
co-CPDT2)/PEDOT-PSS, P(DiCP-
co-CPDTK)/PEDOT-PSS, and P(DiCP-
co-CPDTK2)/PEDOT-PSS ECDs was measured in bleached and coloured states by applying a potential for 1 s in a time interval of each 100 s [
51]. As shown in
Figure 9, the PDiCP/PEDOT-PSS, P(DiCP-
co-CPDT)/PEDOT-PSS, P(DiCP-
co-CPDT2)/PEDOT-PSS, P(DiCP-
co-CPDTK)/PEDOT-PSS, and P(DiCP-
co-CPDTK2)/PEDOT-PSS ECDs showed almost no transmittance change in bleached state, displaying satisfactory optical memories in bleached state. However, ECDs were rather less stable than those in the bleached state, but the transmittance variations were less than 4% in the coloured state, indicating the PDiCP/PEDOT-PSS, P(DiCP-
co-CPDT)/PEDOT-PSS, P(DiCP-
co-CPDT2)/PEDOT-PSS, P(DiCP-
co-CPDTK)/PEDOT-PSS, and P(DiCP-
co-CPDTK2)/PEDOT-PSS ECDs had desirable optical memories.