Epoxy-Thiol Systems Filled with Boron Nitride for High Thermal Conductivity Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Differential Scanning Calorimetry (DSC)
2.2.2. Density Measurements
2.2.3. Thermal Conductivity Measurements
3. Results and Discussion
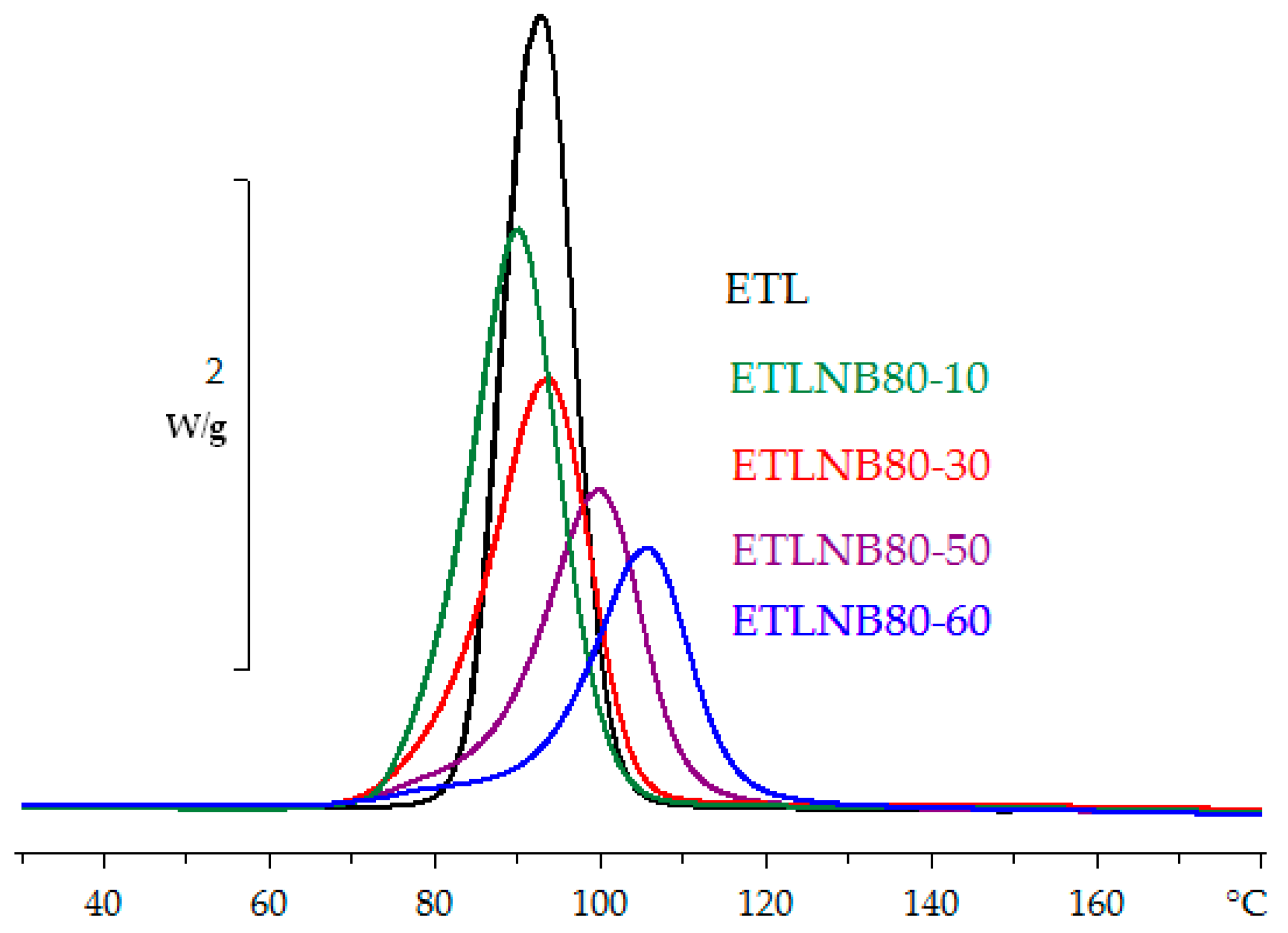
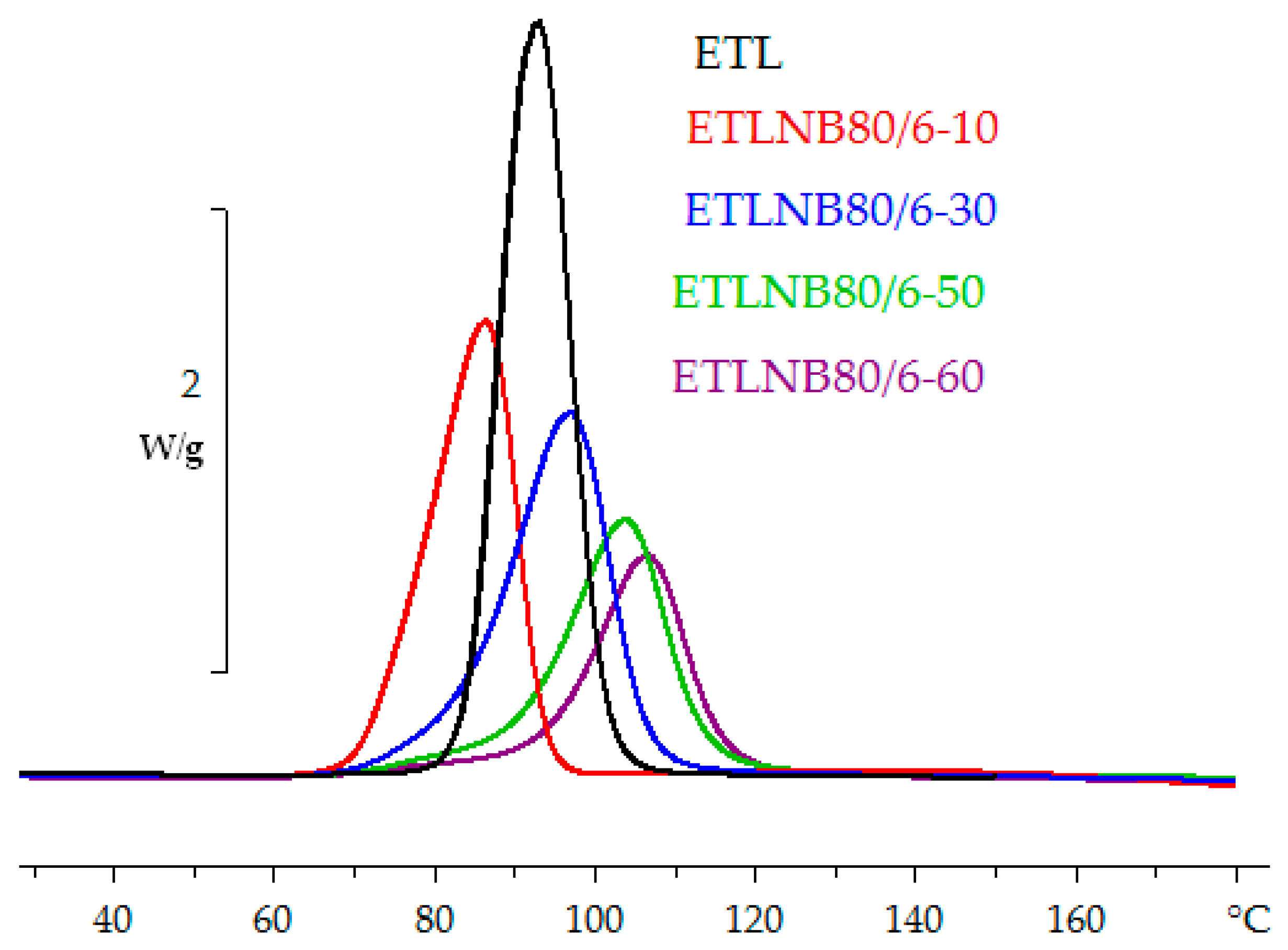
3.1. Differential Scanning Calorimetry (DSC)
3.2. Density Measurements
3.3. Thermal Conductivity Measurements
4. Conclusions
- The heat of reaction in both non-isothermal and isothermal cure is independent of the BN content, as is also the glass transition temperature of the fully cured samples, indicating that the network structure of the epoxy-thiol matrix is not influenced by the presence of BN particles.
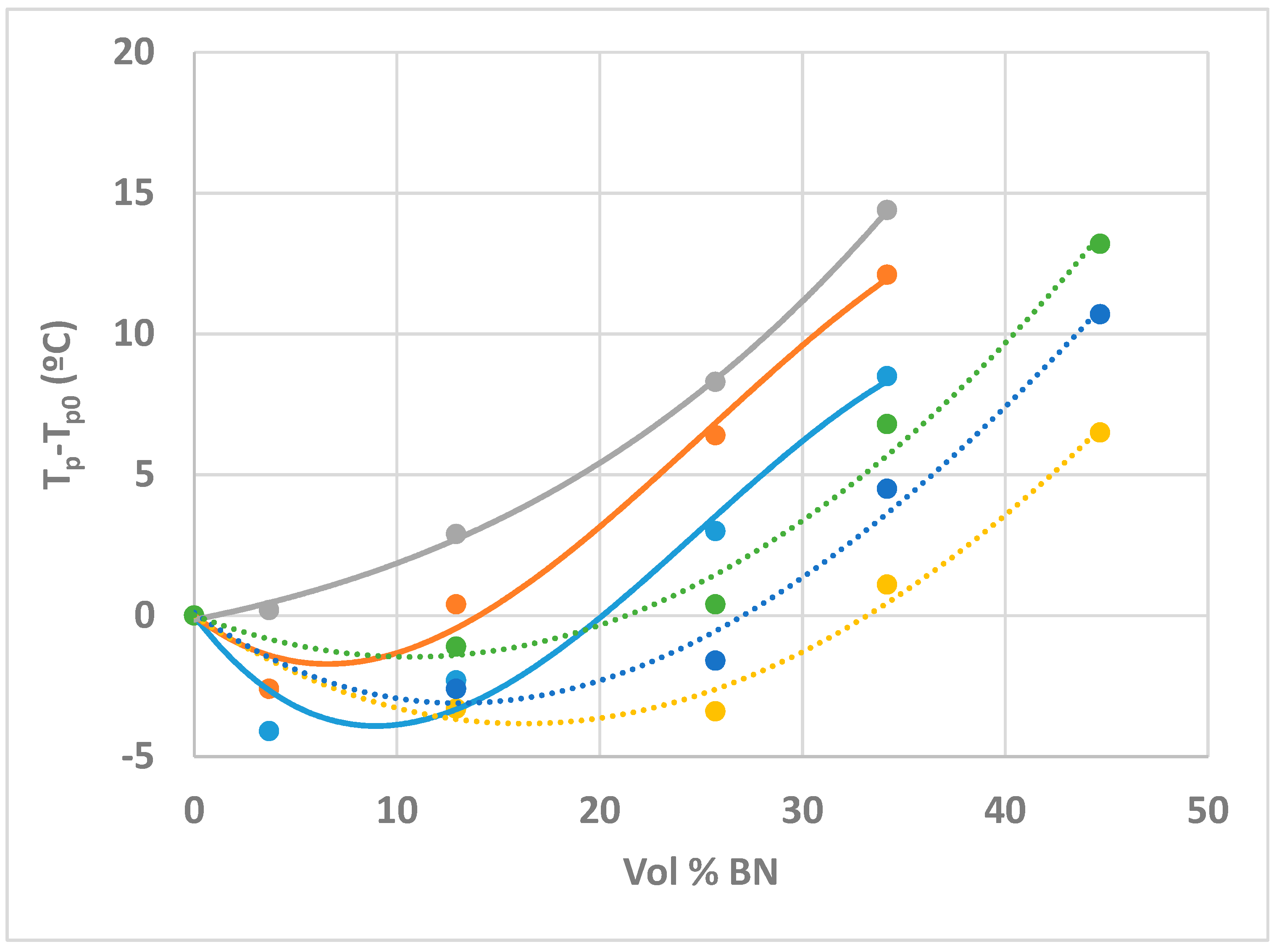
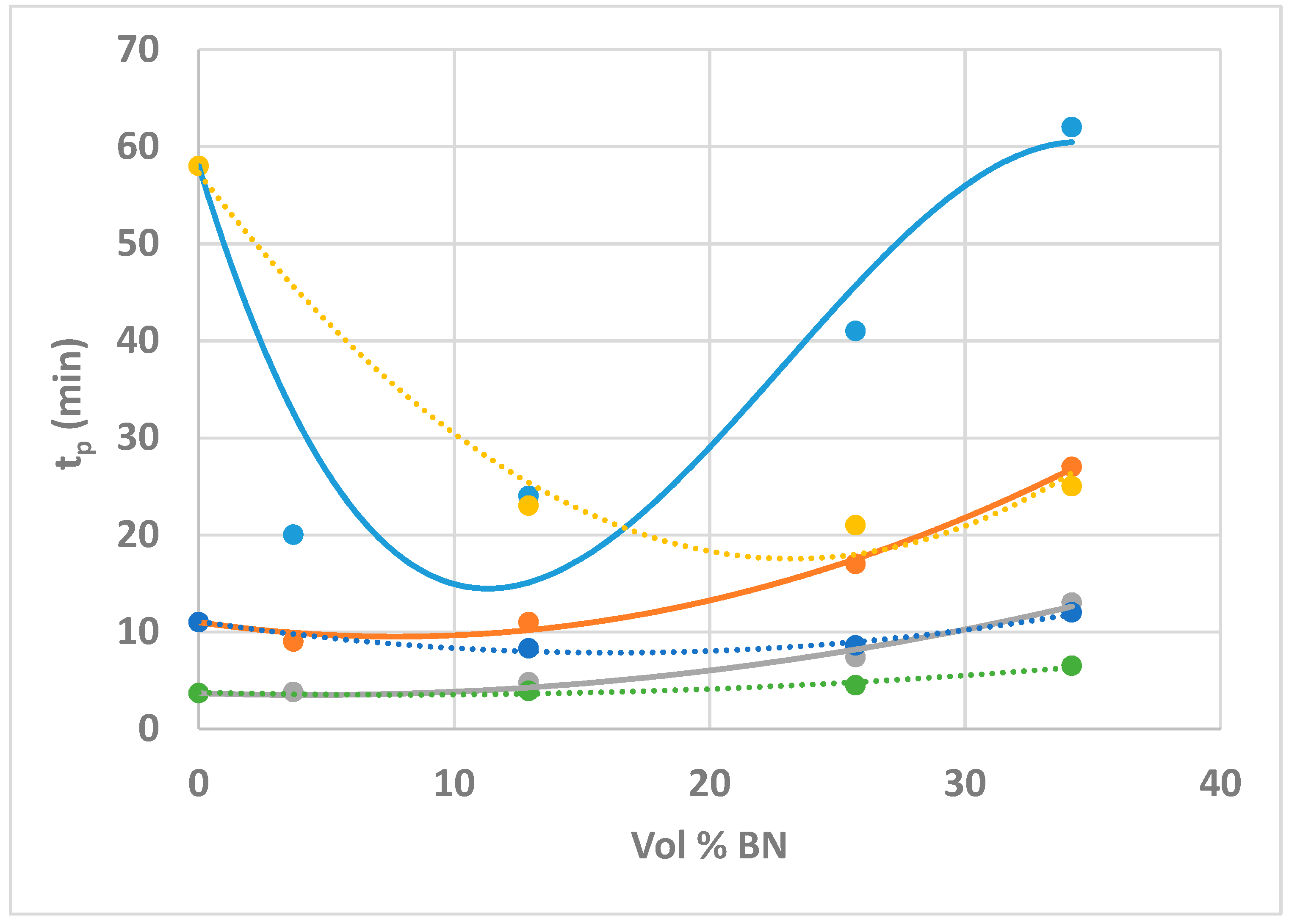
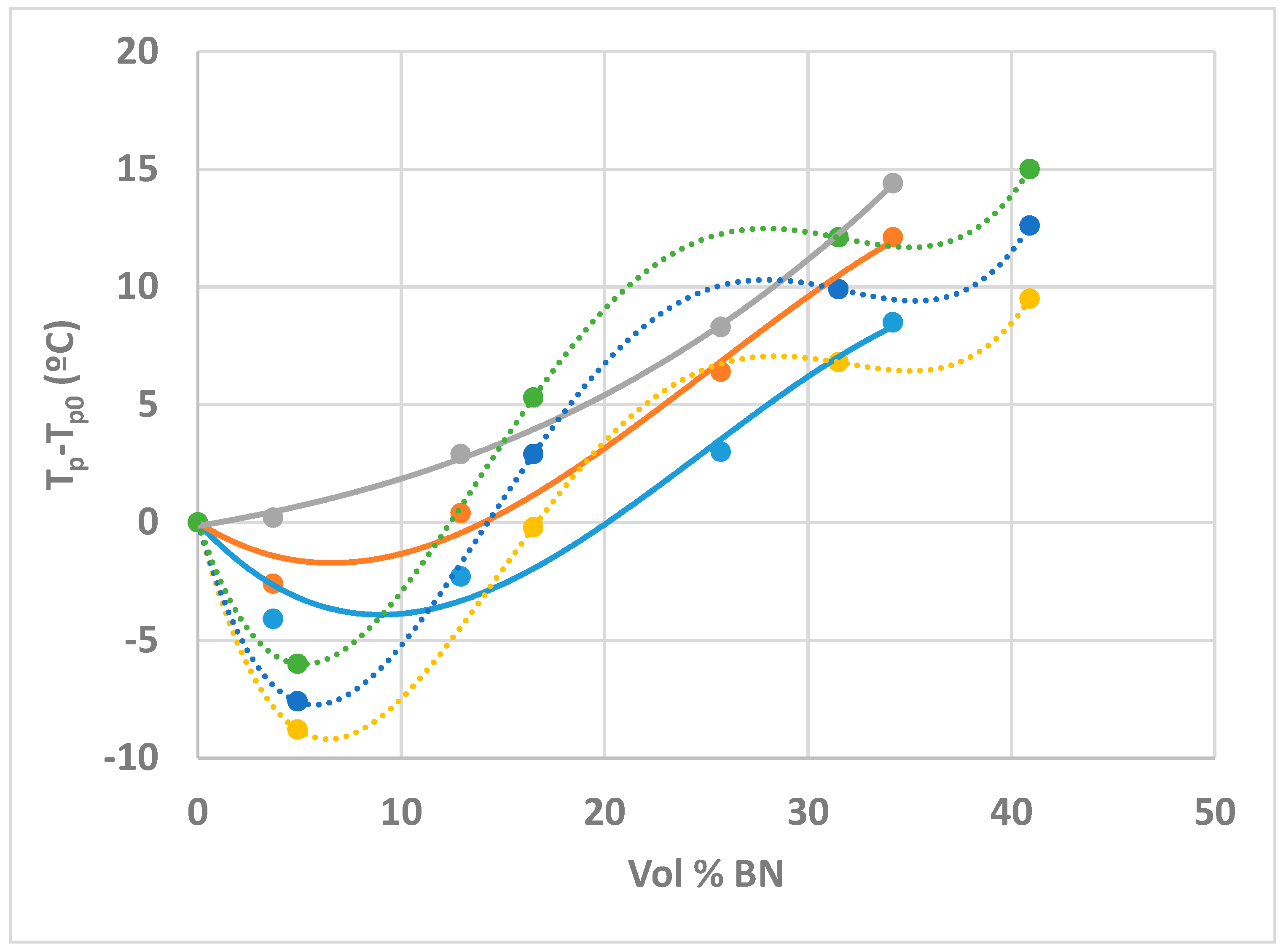
- On the other hand, the exothermic curing reaction has a maximum heat flow that occurs at a temperature (for non-isothermal cure) or a time (for isothermal cure) which depends systematically on the BN content, first decreasing at low BN contents, and then increasing as the BN content increases.
- This behaviour is attributed to the effects of heat transfer both into (before the exothermic reaction) and out of (after the exothermic reaction) the sample, the effect being more marked for the epoxy-BN composites with 80 µm particles than it is for those with 6 µm particles.
- The dependence of the cure reaction kinetics on BN content is not observed for epoxy-BN composites in which the epoxy is cured with a diamine, from which it is concluded that the Lewis acid-base interaction between the thiol and the BN particles results in an improved matrix-filler interface.
- The fully cured epoxy-BN composites filled with 80 µm particles, for which the systematic effect of BN on the cure kinetics was more pronounced, have a higher thermal conductivity than do the composites filled with 6 µm particles, which correlates with the DSC observations.
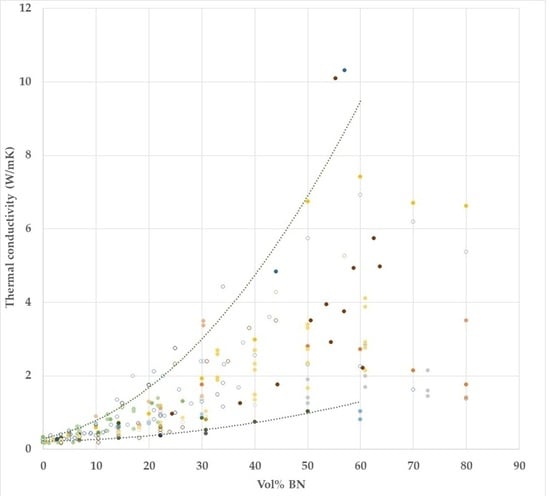
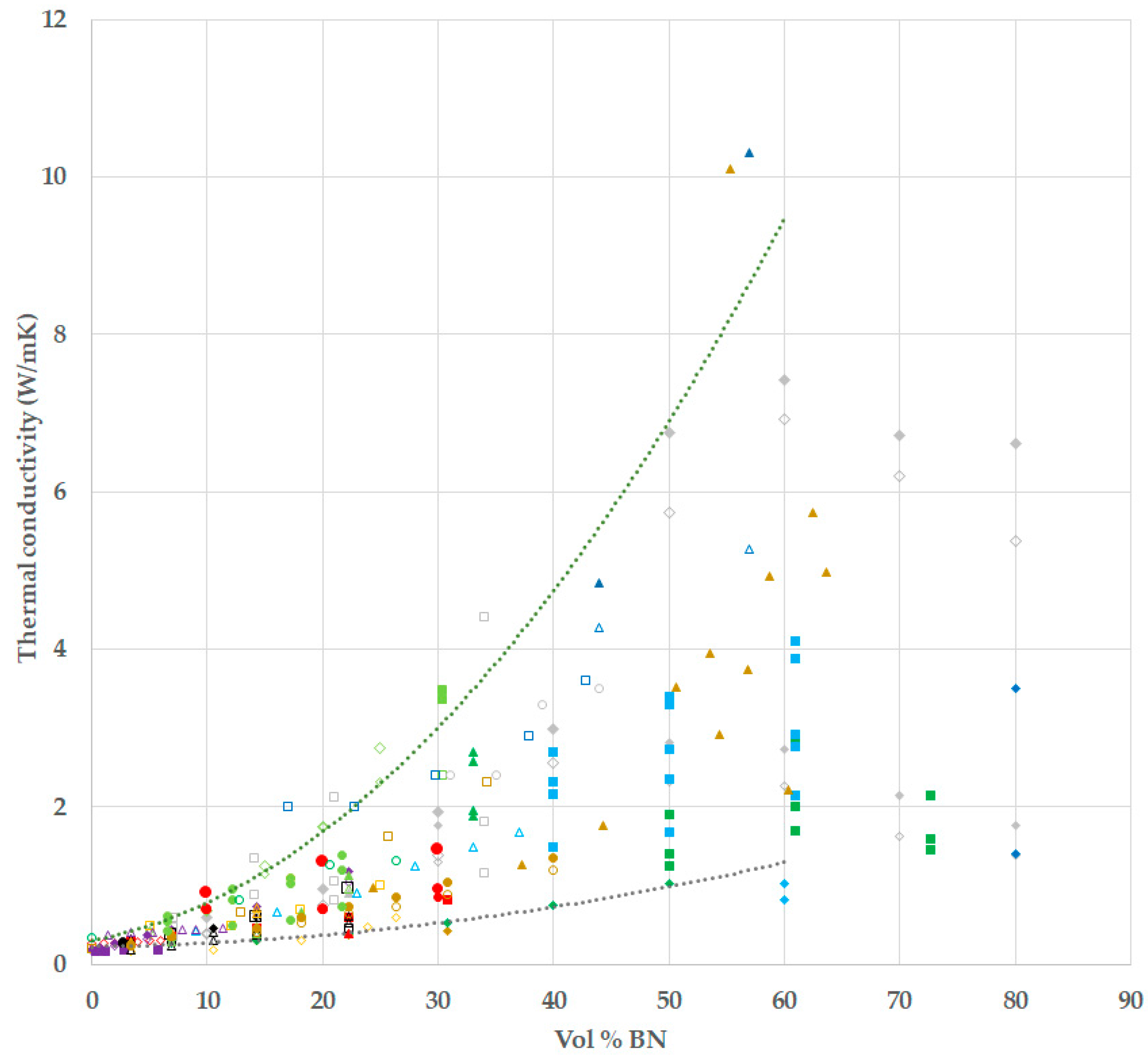
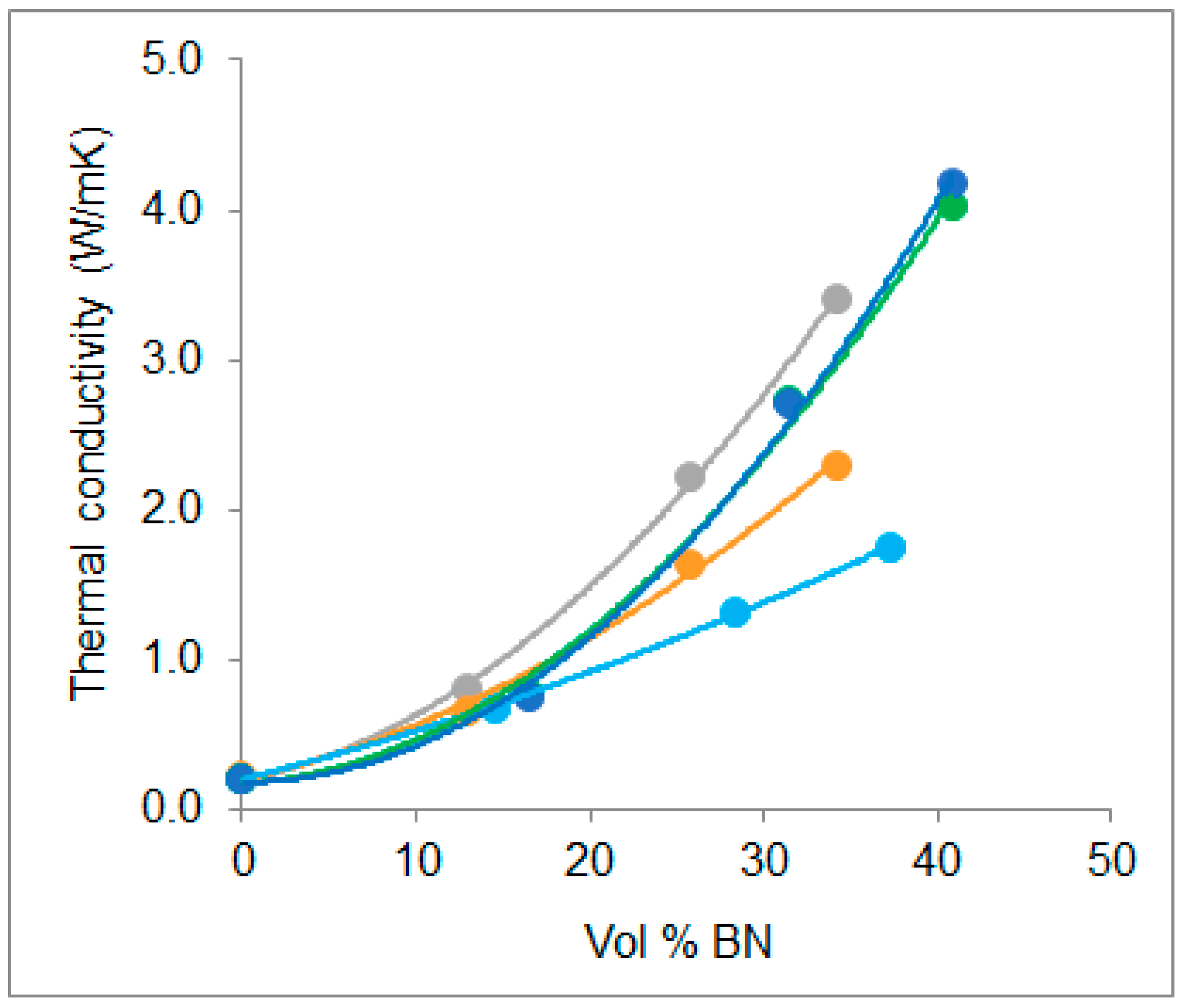
- The thermal conductivity for all the composites increases with BN content, and for a given BN vol %, the values obtained in this work are higher than most others in the literature.
- The use of hybrid samples, in which BN particles of two different sizes, 80 and 6 µm, are combined, allows a greater vol % BN to be achieved, and results in a thermal conductivity of over 4.0 W/mK for a BN content of 42 vol %.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Insulated Metal Substrate Laminates for Metal Core Printed Circuit Boards (MCPCB). Available online: https://www.aitechnology.com/products/insulated-metal-substrates/thermclads/ (accessed on 20 October 2017).
- Technology by MOS, Printed Circuit Board Technology for the Future, 10th ed.; July 2016; p. 46. Available online: http://www.mos-electronic.com/en/downloads/english/Technology_en_07_2016.pdf (accessed on 20 October 2017).
- Metal Backed PCB Materials. Available online: http://www.pwcircuits.co.uk/metalbacked.html (accessed on 20 October 2017).
- Thermal Substrates. Available online: http://www.bergquistcompany.com/thermal_substrates/index.htm (accessed on 20 October 2017).
- Chung, S.-L.; Lin, J.-S. Thermal conductivity of epoxy resin composites filled with combustion synthesized h-BN particles. Molecules 2016, 21, 670. [Google Scholar] [CrossRef] [PubMed]
- Donnay, M.; Tzvalas, S.; Logakis, E. Boron nitride filled epoxy with improved thermal conductivity and dielectric breakdown strength. Compos. Sci. Technol. 2015, 110, 152–158. [Google Scholar] [CrossRef]
- Firdaus, S.M.; Mariatti, M. Nano-sized boron nitride epoxy composites for underfill application: Effect of diluent and filler loading. J. Mater. Sci. Mater. Electron. 2015, 26, 774–783. [Google Scholar] [CrossRef]
- Fu, Y.; He, Z.; Mo, D.; Lu, S. Thermal conductivity enhancement with different fillers for epoxy resin adhesives. Appl. Therm. Eng. 2014, 66, 493–498. [Google Scholar] [CrossRef]
- Fu, J.; Shi, L.; Zhang, D.; Zhong, Q.; Chen, Y. Effect of nanoparticles on the performance of thermally conductive epoxy adhesives. Polym. Eng. Sci. 2010, 50, 1809–1819. [Google Scholar] [CrossRef]
- Gaska, K.; Rybak, A.; Kapusta, C.; Sekula, R.; Siwek, A. Enhanced thermal conductivity of epoxy-matrix composites with hybrid fillers. Polym. Adv. Technol. 2015, 26, 26–31. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, Q.; Dang, J.; Xie, C. Thermal conductivity epoxy resin composites filled with boron nitride. Polym. Adv. Technol. 2012, 23, 1025–1028. [Google Scholar] [CrossRef]
- Hong, J.; Yoon, S.; Hwang, T.; Lee, Y.; Won, S.; Nam, J. Interphase control of boron nitride/epoxy composites for high thermal conductivity. Korea-Aust. Rheol. J. 2010, 22, 259–264. [Google Scholar]
- Hong, J.; Yoon, S.; Hwang, T.; Oh, J.; Hong, S.; Lee, Y.; Nam, J. High thermal conductivity epoxy composites with bimodal distribution of aluminum nitride and boron nitride fillers. Thermochim. Acta 2012, 537, 70–75. [Google Scholar] [CrossRef]
- Hou, J.; Li, G.; Yang, N.; Qin, L.; Grami, M.E.; Zhang, Q.; Wang, N.; Qu, X. Preparation and characterization of surface modified boron nitride epoxy composites with enhanced thermal conductivity. RSC Adv. 2014, 4, 44282–44290. [Google Scholar] [CrossRef]
- Hu, J.; Huang, Y.; Yao, Y.; Pan, G.; Sun, J.; Zeng, X.; Sun, R.; Xu, J.; Song, B.; Wong, C. Polymer composite with improved thermal conductivity by constructing a hierarchically ordered three-dimensional interconnected network of BN. ACS Appl. Mater. Interfaces 2017, 9, 13544–13553. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhu, P.; Li, G.; Zhou, F.; Lu, D.; Sun, R.; Wong, C. Spherical and flake-like BN filled epoxy composites: Morphological effect on the thermal conductivity, thermo-mechanical and dielectric properties. J. Mater. Sci. Mater. Electron. 2015, 26, 3564–3572. [Google Scholar] [CrossRef]
- Huang, T.; Zeng, X.; Yao, Y.; Sun, R.; Meng, F.; Xu, J.; Wong, C. Boron nitride & graphene oxide hybrids for epoxy composites with enhanced thermal conductivity. RSC Adv. 2016, 6, 35847–35854. [Google Scholar] [CrossRef]
- Huang, T.; Zeng, X.; Yao, Y.; Sun, R.; Meng, F.; Xu, J.; Wong, C. A novel h-BN-RGO hybrids for epoxy resin composites achieving enhanced high thermal conductivity and energy density. RSC Adv. 2017, 7, 23355–23362. [Google Scholar] [CrossRef]
- Hutchinson, J.M.; Román, F.; Cortés, P.; Calventus, Y. Epoxy composites filled with boron nitride and aluminium nitride for improves thermal conductivity. Polimery 2017, 62, 560–566. [Google Scholar] [CrossRef]
- Jang, I.; Shin, H.; Yang, I.; Kim, H.; Kim, J.; Kim, W.; Jeon, S.; Kim, J. Enhancement of thermal conductivity of BN/epoxy composite through surface modification with silane coupling agents. Colloids Surf. A Physicochem. Eng. Aspects 2017, 518, 64–72. [Google Scholar] [CrossRef]
- Kim, K.; Kim, J. Fabrication of thermally conductive composite with surface modified boron nitride by epoxy wetting method. Ceram. Int. 2014, 40, 5181–5189. [Google Scholar] [CrossRef]
- Kim, K.; Kim, M.; Hwang, Y.; Kim, J. Chemically modified boron nitride-epoxy terminated dimethylsiloxane composite for improving the thermal conductivity. Ceram. Int. 2014, 40, 2047–2056. [Google Scholar] [CrossRef]
- Kim, K.; Kim, M.; Kim, J. Thermal and mechanical properties of epoxy composites with a binary particle filler system consisting of aggregated and whisker type boron nitride particles. Compos. Sci. Technol. 2014, 103, 72–77. [Google Scholar] [CrossRef]
- Kochetov, R.; Andritsch, T.; Lafont, U.; Morshuis, P.H.F.; Picken, S.J.; Smit, J.J. Thermal behaviour of epoxy resin filled with high thermal conductivity nanopowders. In Proceedings of the IEEE Electrical Insulation Conference, Montreal, QC, Canada, 31 May–3 June 2009. [Google Scholar] [CrossRef]
- Lee, W.S.; Yu, J. Comparative study of thermally conductive fillers in underfill for the electronic components. Diam. Relat. Mater. 2005, 14, 1647–1653. [Google Scholar] [CrossRef]
- Lin, Z.; McNamara, A.; Liu, Y.; Moon, K.; Wong, C. Exfoliated hexagonal boron nitride-based polymer nanocomposite with enhanced thermal conductivity for electronic encapsulation. Compos. Sci. Technol. 2014, 90, 123–128. [Google Scholar] [CrossRef]
- Permal, A.; Devarajan, M.; Hung, H.L.; Zahner, T.; Lacey, D.; Ibrahim, K. Thermal and mechanical properties of epoxy composite filled with binary particle system of polygonal aluminum oxide and boron nitride platelets. J. Mater. Sci. 2016, 51, 7415–7426. [Google Scholar] [CrossRef]
- Qu, T.; Yang, N.; Hou, J.; Li, G.; Yao, Y.; Zhang, Q.; He, L.; Wua, D.; Qu, X. Flame retarding epoxy composites with poly(phosphazene-co-bisphenol A)-coated boron nitride to improve thermal conductivity and thermal stability. RSC Adv. 2017, 7, 6140–6151. [Google Scholar] [CrossRef]
- Tanaka, T.; Wang, Z.; Iizuka, T.; Kozako, M.; Ohki, Y. High thermal conductivity epoxy/BN composites with sufficient dielectric breakdown strength. In Proceedings of the IEEE 2011 Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Cancun, Mexico, 16–19 October 2011; Volumes 1 and 2, pp. 691–694, ISBN 978-1-4577-0986-9. [Google Scholar]
- Teng, C.; Ma, C.M.; Chiou, K.; Lee, T.; Shih, Y. Synergetic effect of hybrid boron nitride and multi-walled carbon nanotubes on the thermal conductivity of epoxy composites. Mater. Chem. Phys. 2011, 126, 722–728. [Google Scholar] [CrossRef]
- Wattanakul, K.; Manuspiya, H.; Yanumet, N. Effective surface treatments for enhancing the thermal conductivity of BN-filled epoxy composite. J. Appl. Polym. Sci. 2011, 119, 3234–3243. [Google Scholar] [CrossRef]
- Wattanakul, K.; Manuspiya, H.; Yanumet, N. Thermal conductivity and mechanical properties of BN-filled epoxy composite: Effects of filler content, mixing conditions, and BN agglomerate size. J. Compos. Mater. 2011, 45, 1967–1980. [Google Scholar] [CrossRef]
- Xu, Y.; Chung, D.D.L. Increasing the thermal conductivity of boron nitride and aluminum nitride particle epoxy-matrix composites by particle surface treatments. Compos. Interfaces 2000, 7, 243–256. [Google Scholar] [CrossRef]
- Yadav, A.K. Thermal Characteristics of Boron Nitride Filled Epoxy Composites. Master’s Thesis, National Institute of Technology, Rourkela, India, June 2013. [Google Scholar]
- Yu, C.; Zhang, J.; Li, Z.; Tian, W.; Wang, L.; Luo, J.; Li, Q.; Fan, X.; Yao, Y. Enhanced through-plane thermal conductivity of boron nitride/epoxy composites. Compos. Part A 2017, 98, 25–31. [Google Scholar] [CrossRef]
- Yu, J.; Huang, X.; Wu, C.; Wu, X.; Wang, G.; Jiang, P. Interfacial modification of boron nitride nanoplatelets for epoxy composites with improved thermal properties. Polymer 2012, 53, 471–480. [Google Scholar] [CrossRef]
- Yung, K.C.; Wang, J.; Yue, T.M. Thermal management for boron nitride filled metal core printed circuit board. J. Compos. Mater. 2008, 42, 2615–2627. [Google Scholar] [CrossRef]
- Yung, K.C.; Zhu, B.L.; Yue, T.M.; Xie, C.S. Development of epoxy-matrix composite with both high-thermal conductivity and low-dielectric constant via hybrid filler systems. J. Appl. Polym. Sci. 2010, 116, 518–527. [Google Scholar] [CrossRef]
- Zhou, W.; Zuo, J.; Zhang, X.; Zhou, A. Thermal, electrical, and mechanical properties of hexagonal boron nitride-reinforced epoxy composites. J. Compos. Mater. 2014, 48, 2517–2526. [Google Scholar] [CrossRef]
- Zhu, B.L.; Ma, J.; Wu, J.; Yung, K.C.; Xie, C.S. Study on the properties of the epoxy-matrix composites filled with thermally conductive AlN and BN ceramic particles. J. Appl. Polym. Sci. 2010, 118, 2754–2764. [Google Scholar] [CrossRef]
- Zivkovic, I.; Murk, A. Boron nitride loading for thermal conductivity improvement of composite microwave absorbers. Electron. Lett. 2012, 48, 1130–1131. [Google Scholar] [CrossRef]
- Hammerschmidt, U.; Meier, V. New Transient Hot-Bridge sensor to measure thermal conductivity, thermal diffusivity, and volumetric specific heat. Int. J. Thermophys. 2006, 27, 840–865. [Google Scholar] [CrossRef]
- Li, C.; Potter, K.; Wisnom, M.R.; Stringer, G. In-situ measurement of chemical shrinkage of MY750 epoxy resin by a novel gravimetric method. Compos. Sci. Technol. 2004, 64, 55–64. [Google Scholar] [CrossRef]
- Yu, H.; Mhaisalkar, S.G.; Wong, E.H. Cure shrinkage measurement of nonconductive adhesives by means of a thermomechanical analyser. J. Electron. Mater. 2005, 34, 1177–1182. [Google Scholar] [CrossRef]


| Samples | Parts by mass | wt % BN | vol % BN calculated | ||||
|---|---|---|---|---|---|---|---|
| Simple mixtures | Epoxy | BN filler, 80 µm | Thiol | LC-80 | |||
| ETL | 100.0 | 0 | 66.7 | 2.0 | 0 | 0 | |
| ETLBN80-10 | 90.0 | 10.0 | 60.0 | 1.8 | 6.2 | 3.7 | |
| ETLBN80-30 | 70.0 | 30.0 | 46.7 | 1.4 | 20.3 | 12.9 | |
| ETLBN80-50 | 50.0 | 50.0 | 33.3 | 1.0 | 37.2 | 25.7 | |
| ETLBN80-60 | 40.0 | 60.0 | 26.7 | 0.8 | 47.1 | 34.2 | |
| 80/6 hybrids | 80 µm | 6 µm | |||||
| ETLBN80/6-10 | 90.0 | 10.0 | 3.3 | 60.0 | 1.8 | 8.1 | 4.9 |
| ETLBN80/6-30 | 70.0 | 30.0 | 10.0 | 46.7 | 1.4 | 25.3 | 16.5 |
| ETLBN80/6-50 | 50.0 | 50.0 | 16.7 | 33.3 | 1.0 | 44.2 | 31.6 |
| ETLBN80/6-60 | 40.0 | 60.0 | 20.0 | 26.7 | 0.8 | 54.2 | 40.9 |
| 80/2 hybrids | 80 µm | 2 µm | |||||
| ETLBN80/2-10 | 90.0 | 10.0 | 3.3 | 60.0 | 1.8 | 8.1 | 4.9 |
| ETLBN80/2-30 | 70.0 | 30.0 | 10.0 | 46.7 | 1.4 | 25.3 | 16.5 |
| ETLBN80/2-50 | 50.0 | 50.0 | 16.7 | 33.3 | 1.0 | 44.2 | 31.6 |
| ETLBN80/2-60 | 40.0 | 60.0 | 20.0 | 26.7 | 0.8 | 54.2 | 40.9 |
| Sample | β (K/min) | Tg0 (°C) | ∆H (J/g) | ∆H (kJ/ee) | Tg∞ (°C) | Tp (°C) |
|---|---|---|---|---|---|---|
| ETL | 2 | −37.3 | 425 | 131 | 53.8 | 83.8 |
| 5 | −37.7 | 420 | 129 | 53.0 | 93.9 | |
| 10 | −37.2 | 416 | 128 | 52.5 | 102.7 | |
| ETLBN80-10 | 2 | −37.9 | 403 | 132 | 55.8 | 79.7 |
| 5 | −36.9 | 404 | 133 | 55.0 | 91.3 | |
| 10 | −35.4 | 397 | 131 | 54.7 | 102.9 | |
| ETLBN80-30 | 2 | −38.1 | 334 | 128 | 54.1 | 81.5 |
| 5 | −36.9 | 333 | 128 | 52.9 | 94.3 | |
| 10 | −35.6 | 334 | 129 | 52.8 | 105.6 | |
| ETLBN80-50 | 2 | −38.4 | 267 | 131 | 54.7 | 86.7 |
| 5 | −37.4 | 264 | 130 | 54.8 | 100.3 | |
| 10 | −36.7 | 260 | 128 | 53.9 | 111.0 | |
| ETLBN80-60 | 2 | −38.8 | 228 | 132 | 54.8 | 92.3 |
| 5 | −37.7 | 220 | 128 | 54.8 | 105.9 | |
| 10 | −36.2 | 220 | 128 | 54.5 | 117.1 |
| Sample | Tc (°C) | ∆H (J/g) | ∆H (kJ/ee) | Tg∞ (°C) | tp (min) |
|---|---|---|---|---|---|
| ETL | 60 | 431 | 132 | 53.6 | 58 |
| 70 | 399 | 122 | 54.7 | 11 | |
| 80 | 430 | 132 | 53.3 | 3.7 | |
| ETLBN80-10 | 60 | 401 | 131 | 52.8 | 20 |
| 70 | 396 | 130 | 53.4 | 9.0 | |
| 80 | 398 | 131 | 52.7 | 3.8 | |
| ETLBN80-30 | 60 | 329 | 126 | 52.0 | 24 |
| 70 | 344 | 133 | 53.7 | 11 | |
| 80 | 329 | 127 | 52.6 | 4.8 | |
| ETLBN80-50 | 60 | 257 | 126 | 52.6 | 41 |
| 70 | 256 | 126 | 53.6 | 17 | |
| 80 | 260 | 128 | 51.6 | 7.5 | |
| ETLBN80-60 | 60 | 212 | 123 | 52.3 | 62 |
| 70 | 221 | 129 | 53.0 | 27 | |
| 80 | 206 | 120 | 51.7 | 13 |
| Sample | Density (g/cm3) | vol % BN measured | vol % BN calculated | λ (W/mK) |
|---|---|---|---|---|
| ETL | 0.0 | 0.0 | 0.20 | |
| ETLBN80-30 | 1.39 | 13.4 | 12.9 | 0.80 |
| ETLBN80-50 | 1.51 | 26.7 | 25.7 | 2.21 |
| ETLBN80-60 | 1.56 | 35.0 | 34.2 | 3.40 |
| ETLBN80/2-30 | 1.42 | 17.1 | 16.5 | 0.77 |
| ETLBN80/2-50 | 1.54 | 32.4 | 31.5 | 2.72 |
| ETLBN80/2-60 | 1.58 | 40.8 | 40.9 | 4.02 |
| ETLBN80/6-30 | 1.42 | 17.1 | 16.5 | 0.75 |
| ETLBN80/6-50 | 1.57 | 33.0 | 31.5 | 2.71 |
| ETLBN80/6-60 | 1.63 | 42.1 | 40.9 | 4.17 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hutchinson, J.M.; Román, F.; Folch, A. Epoxy-Thiol Systems Filled with Boron Nitride for High Thermal Conductivity Applications. Polymers 2018, 10, 340. https://doi.org/10.3390/polym10030340
Hutchinson JM, Román F, Folch A. Epoxy-Thiol Systems Filled with Boron Nitride for High Thermal Conductivity Applications. Polymers. 2018; 10(3):340. https://doi.org/10.3390/polym10030340
Chicago/Turabian StyleHutchinson, John M., Frida Román, and Adrià Folch. 2018. "Epoxy-Thiol Systems Filled with Boron Nitride for High Thermal Conductivity Applications" Polymers 10, no. 3: 340. https://doi.org/10.3390/polym10030340
APA StyleHutchinson, J. M., Román, F., & Folch, A. (2018). Epoxy-Thiol Systems Filled with Boron Nitride for High Thermal Conductivity Applications. Polymers, 10(3), 340. https://doi.org/10.3390/polym10030340