3D Printing and Electrospinning of Composite Hydrogels for Cartilage and Bone Tissue Engineering
Abstract
1. Tissue Engineering
2. Challenges in Tissue Engineering of Soft and Hard Tissues
2.1. Cartilage
2.2. Bone
2.3. Osteochondral Tissue
3. Hydrogels as Tissue Engineering Scaffolds
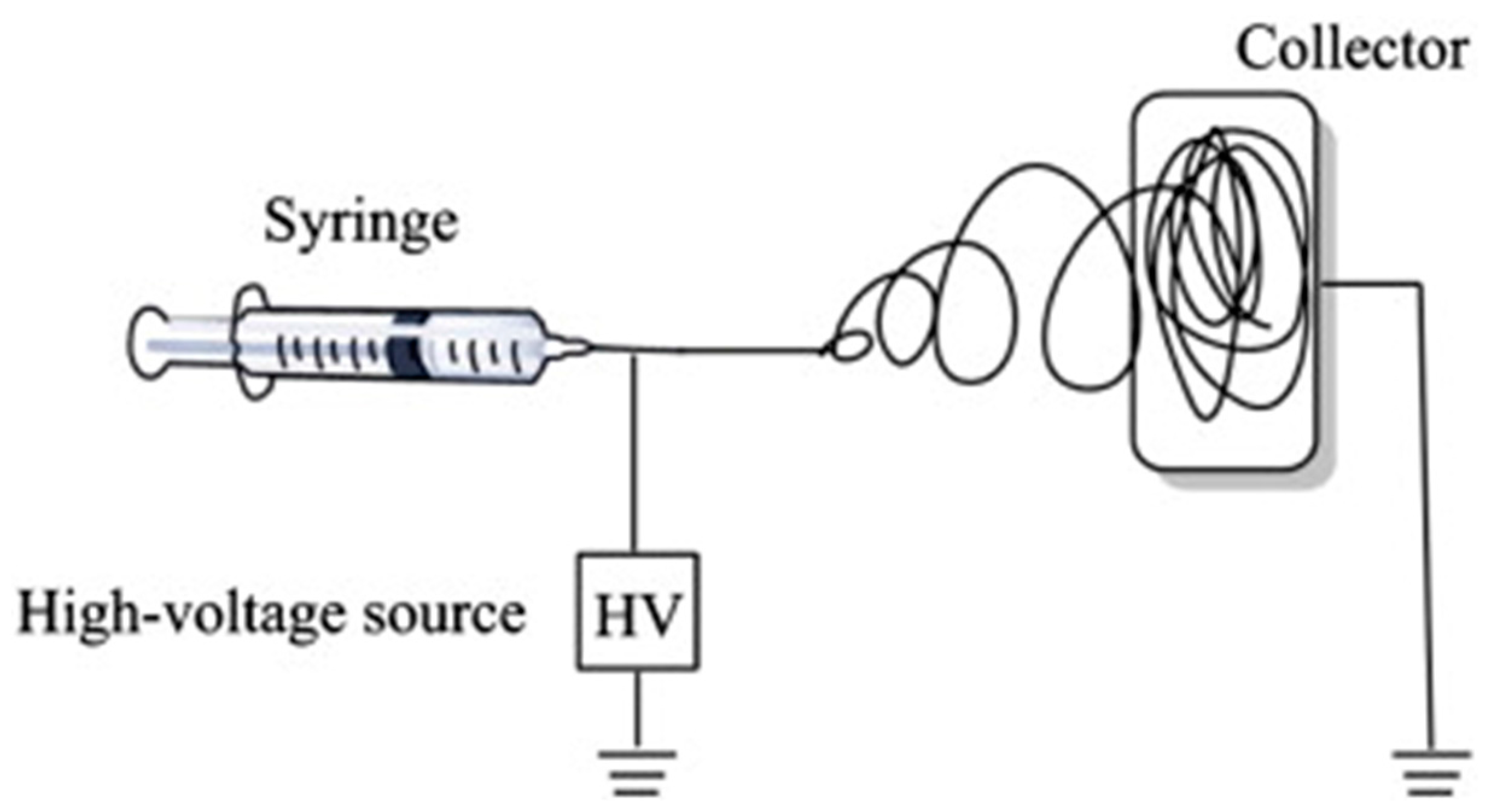
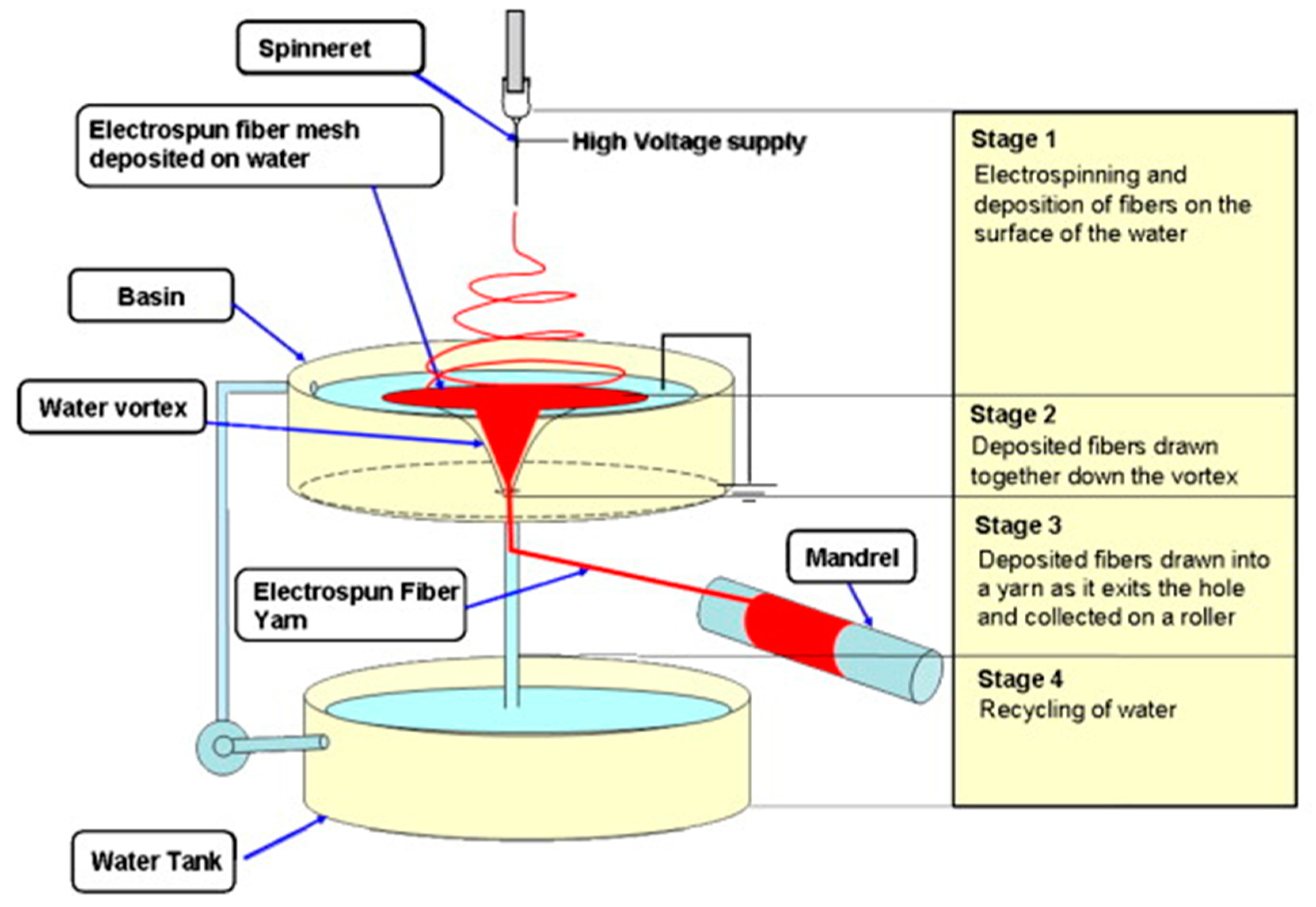
4. Electrospinning
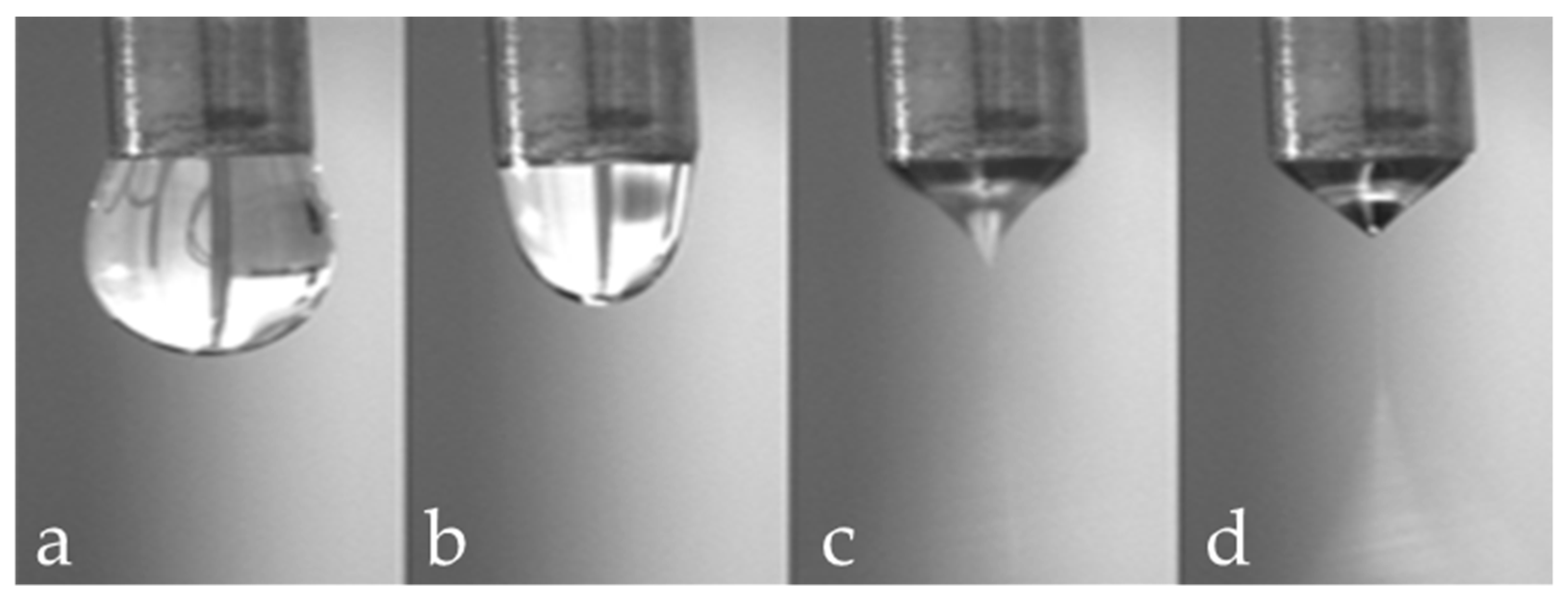
- Solution characteristics (viscosity, surface tension and conductivity): the electrospinning technique relies on the uniaxial stretching of a charged jet, which, in turn, is significantly affected by changing the concentration of the polymeric solution. Generally, by reducing the polymer concentration, the fibre diameter is decreased. However, when the concentration of the polymeric solution is lowered to a critical value, known as entanglement concentration (Ce), beaded fibres are produced. If the concentration is too high no fibres are produced due to the excessive viscosity [63]. Solution conductivity is fundamental to optimize both fibre diameter and stability of the Taylor cone. If the solution has a low conductivity the fluid surface cannot be charged, and no Taylor cone can be formed. By increasing the charge on the surface of the droplet a Taylor cone is formed and the fibre diameter is decreased [64]. The conductivity of a polymeric solution can be controlled by the addition of salts. Moreover, the solvent has a crucial role in determining the characteristics of the solution; an ideal solvent must to be able to solubilize the polymer at the required concentrations and be sufficiently volatile to evaporate in the space between the needle and the collector. However, if the boiling point is too low, the solvent will evaporate too quickly causing the drying of the jet at the needle tip [65].
- Process parameters (applied voltage, flow rate and tip to collector distance): these parameters influence the diameter and morphology of the fibres. As the feed rate increases, the charge density will decrease. Thus, by increasing the flow rate the diameter of fibres is increased and beaded morphology can be observed [64]. Fibres are formed just when the applied voltage is higher than the threshold voltage (the value depends on the solution). Generally, by increasing the voltage there is an increase of the electrostatic force on the solution and thus, a reduction of the fibre diameter. A critical distance between tip and collector is needed for the solvent evaporation and for the preparation of smooth and uniform electrospun fibres. Generally, the longer the distance, the thinner the fibres will be.
- Environment conditions (humidity and temperature) [66,67]. Ideal environmental conditions must be found for better and improved fibre production. Ambient humidity and temperature can affect both the morphology and diameter of the fibres. Depending on the chemistry of the polymer [68], the fibre diameter can increase or decrease and no definitive comparisons with experimental data can be currently made.
4.1. Fibrous Hydrogels for Cartilage Tissue Engineering via Electrospinning
4.2. Fibrous Hydrogels for Bone Tissue Engineering via Electrospinning
4.3. Fibrous Hydrogels for Osteochondral Engineering via Electrospinning
5. 3D Printing
5.1. 3D Printing of Hydrogels for Cartilage Tissue Engineering
5.2. 3D Printing of Hydrogels for Bone Tissue Engineering
5.3. 3D Printing of Hydrogels for Osteochondral Tissue Engineering
6. Future Trends
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pisani, P.; Renna, M.D.; Conversano, F.; Casciaro, E.; Di Paola, M.; Quarta, E.; Muratore, M.; Casciaro, S. Major osteoporotic fragility fractures: Risk factor updates and societal impact. World J. Orthop. 2016, 7, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Murphy, L.; Helmick, C.G. The Impact of Osteoarthritis in the United States. Orthop. Nurs. 2012, 31, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Tozzi, G.; De Mori, A.; Oliveira, A.; Roldo, M. Composite hydrogels for bone regeneration. Materials (Basel) 2016, 9, 267. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.G.; Shin, H.; Lim, D.W. Biomimetic Scaffolds for Tissue Engineering. Adv. Funct. Mater. 2012, 22, 2446–2468. [Google Scholar] [CrossRef]
- Khan, W.S.; Malik, A. Stem Cell Therapy and Tissue Engineering Applications for Cartilage Regeneration. Curr. Stem Cell Res. Ther. 2012, 7, 241–242. [Google Scholar] [CrossRef] [PubMed]
- Grottkau, B.E.; Lin, Y. Osteogenesis of Adipose-Derived Stem Cells. Bone Res. 2013, 1, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Bush, J.R.; Liang, H.; Dickinson, M.; Botchwey, E.A. Xylan hemicellulose improves chitosan hydrogel for bone tissue regeneration. Polym. Adv. Technol. 2016, 27, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Trickey, W.R.; Lee, G.M.; Guilak, F. Viscoelastic properties of chondrocytes from normal and osteoarthritic human cartilage. J. Orthop. Res. 2000, 18, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Pal, S. Design of Artificial Human Joints & Organs; Springer: New York, NY, USA, 2014; pp. 1–419. [Google Scholar]
- Mobasheri, A.; Csaki, C.; Clutterbuck, A.L.; Rahmanzadeh, M.; Shakibaei, M. Mesenchymal stem cells in connective tissue engineering and regenerative medicine: Applications in cartilage repair and osteoarthritis therapy. Histol. Histopathol. 2009, 24, 347–366. [Google Scholar] [CrossRef] [PubMed]
- Izadifar, Z.; Chen, X.; Kulyk, W. Strategic Design and Fabrication of Engineered Scaffolds for Articular Cartilage Repair. J. Funct. Biomater. 2012, 3, 799–838. [Google Scholar] [CrossRef] [PubMed]
- Reddi, A.H.; Becerra, J.; Andrades, J.A. Nanomaterials and hydrogel scaffolds for articular cartilage regeneration. Tissue Eng. B 2011, 17, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.K. Bones and Cartilage, 2nd ed.; Academic Press: San Diego, CA, USA, 2015; ISBN 978-0-12-416678-3. [Google Scholar]
- Benders, K.E.M.; van Weeren, P.R.; Badylak, S.F.; Saris, D.B.F.; Dhert, W.J.A.; Malda, J. Extracellular matrix scaffolds for cartilage and bone regeneration. Trends Biotechnol. 2013, 31, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Hadjidakis, D.J.; Androulakis, I.I. Bone Remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Kini, U.; Nandeesh, B.N. Physiology of Bone Formation, Remodeling, and Metabolism. In Radionuclide and Hybrid Bone Imaging; Fogelman, I., Gnanasegaran, G., van der Wall, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 29–57. ISBN 978-3-642-02400-9. [Google Scholar]
- Athanasiou, K.A.; Zhu, C.; Lanctot, D.R.; Agrawal, C.M.; Wang, X. Fundamentals of biomechanics in tissue engineering of bone. Tissue Eng. 2000, 6, 361–381. [Google Scholar] [CrossRef] [PubMed]
- Florencio-Silva, R.; Rodrigues da Silva Sasso, G.; Sasso-Cerri, E.; Simoes, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors that Influence Bone Cells. BioMed Res. Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Adler, C.-P. Bones and Bone Tissue. In Bone Diseases: Macroscopic, Histological, and Radiological Diagnosis of Structural Changes in the Skeleton; Adler, C.-P., Ed.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 1–11. ISBN 978-3-662-04088-1. [Google Scholar]
- Rho, J.Y.; Ashman, R.B.; Turner, C.H. Young’s modulus of trabecular and cortical bone material: Ultrasonic and microtensile measurements. J. Biomech. 2018, 26, 111–119. [Google Scholar] [CrossRef]
- Wu, S.; Liu, X.; Yeung, K.W.K.; Liu, C.; Yang, X. Biomimetic porous scaffolds for bone tissue engineering. Mater. Sci. Eng. R Rep. 2014, 80, 1–36. [Google Scholar] [CrossRef]
- Fratzl, P.; Weinkamer, R. Nature’s hierarchical materials. Prog. Mater. Sci. 2007, 52, 1263–1334. [Google Scholar] [CrossRef]
- Kane, R.; Ma, P.X. Mimicking the nanostructure of bone matrix to regenerate bone. Mater. Today 2013, 16, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.Y.; Kuhn-Spearing, L.; Zioupos, P. Mechanical properties and the hierarchical structure of bone. Med. Eng. Phys. 1998, 20, 92–102. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, Y.; Li, J.; Guo, Q.; Peng, J.; Liu, S.; Yang, J.; Wang, Y. Cell-Derived Extracellular Matrix: Basic Characteristics and Current Applications in Orthopedic Tissue Engineering. Tissue Eng. B 2015, 22, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.C.; Sun, L.; Kohanski, R.A. Balanced Regulation of Proliferation, Growth, Differentiation, and Degradation in Skeletal Cells. Ann. N. Y. Acad. Sci. 2007, 1116, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Sims, N.A.; Gooi, J.H. Bone remodeling: Multiple cellular interactions required for coupling of bone formation and resorption. Semin. Cell Dev. Biol. 2008, 19, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Monazzah, S.; Bigham-Sadegh, A. Bone injury and fracture healing biology. Biomed. Environ. Sci. 2015, 28, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Schubert, T.; Lafont, S.; Beaurin, G.; Grisay, G.; Behets, C.; Gianello, P.; Dufrane, D. Critical size bone defect reconstruction by an autologous 3D osteogenic-like tissue derived from differentiated adipose MSCs. Biomaterials 2013, 34, 4428–4438. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Einhorn, T.A.; Marsh, D. Fracture healing: The diamond concept. Injury 2018, 38, S3–S6. [Google Scholar] [CrossRef]
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Stevens, M.M. Biomaterials for bone tissue engineering. Mater. Today 2008, 11, 18–25. [Google Scholar] [CrossRef]
- Nauth, A.; Ristevski, B.; Li, R.; Schemitsch, E.H. Growth factors and bone regeneration: How much bone can we expect? Injury 2011, 42, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Garcia, A.J. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug Deliv. Rev. 2015, 94, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Younger, E.M.; Chapman, M.W. Morbidity at bone graft donor sites. J. Orthop. Trauma 1989, 3, 192–195. [Google Scholar] [CrossRef] [PubMed]
- García-Gareta, E.; Coathup, M.J.; Blunn, G.W. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone 2015, 81, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Yunus Basha, R.; Sampath Kumar, T.S.; Doble, M. Design of biocomposite materials for bone tissue regeneration. Mater. Sci. Eng. C 2015, 57, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, B.; Swieszkowski, W.; Godlinski, D.; Kurzydlowski, K.J. Highly porous titanium scaffolds for orthopaedic applications. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 95, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.K.; Lee, B.T. Hard tissue regeneration using bone substitutes: An update on innovations in materials. Korean J. Intern. Med. 2015, 30, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Atala, A.; Kasper, F.K.; Mikos, A.G. Engineering complex tissues. Sci. Transl. Med. 2012, 4, 160rv12. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.M.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions—A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef] [PubMed]
- Khorshidi, S.; Karkhaneh, A. A review on gradient hydrogel/fiber scaffolds for osteochondral regeneration. J. Tissue Eng. Regen. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Nukavarapu, S.P.; Dorcemus, D.L. Osteochondral tissue engineering: Current strategies and challenges. Biotechnol. Adv. 2013, 31, 706–721. [Google Scholar] [CrossRef] [PubMed]
- Nooeaid, P.; Salih, V.; Beier, J.P.; Boccaccini, A.R. Osteochondral tissue engineering: Scaffolds, stem cells and applications. J. Cell. Mol. Med. 2012, 16, 2247–2270. [Google Scholar] [CrossRef] [PubMed]
- Gomoll, A.H.; Madry, H.; Knutsen, G.; van Dijk, N.; Seil, R.; Brittberg, M.; Kon, E. The subchondral bone in articular cartilage repair: Current problems in the surgical management. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Seliktar, D. Designing cell-compatible hydrogels for biomedical applications. Science 2012, 336, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xia, K.; Lu, Z.; Li, G.; Chen, J.; Deng, Y.; Li, S.; Zhou, F.; He, N. Efficient and Facile Synthesis of Gold Nanorods with Finely Tunable Plasmonic Peaks from Visible to Near-IR Range. Chem. Mater. 2014, 26, 1794–1798. [Google Scholar] [CrossRef]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Vega, S.; Kwon, M.; Burdick, J. Recent advances in hydrogels for cartilage tissue engineering. Eur. Cells Mater. 2017, 33, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.; Melchels, F.P.W.; Jeon, J.E.; van Bussel, E.M.; Kimpton, L.S.; Byrne, H.M.; Dhert, W.J.A.; Dalton, P.D.; Hutmacher, D.W.; Malda, J. Reinforcement of hydrogels using three-dimensionally printed microfibres. Nat. Commun. 2015, 6, 6933. [Google Scholar] [CrossRef] [PubMed]
- Vedadghavami, A.; Minooei, F.; Mohammadi, M.H.; Khetani, S.; Rezaei Kolahchi, A.; Mashayekhan, S.; Sanati-Nezhad, A. Manufacturing of hydrogel biomaterials with controlled mechanical properties for tissue engineering applications. Acta Biomater. 2017, 62, 42–63. [Google Scholar] [CrossRef] [PubMed]
- Snyder, T.N.; Madhavan, K.; Intrator, M.; Dregalla, R.C.; Park, D. A fibrin/hyaluronic acid hydrogel for the delivery of mesenchymal stem cells and potential for articular cartilage repair. J. Biol. Eng. 2014, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yuan, T.; Xiao, Z.; Tang, P.; Xiao, Y.; Fan, Y.; Zhang, X. Hydrogels of collagen/chondroitin sulfate/hyaluronan interpenetrating polymer network for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2012, 23, 2267–2279. [Google Scholar] [CrossRef] [PubMed]
- Yanagawa, F.; Sugiura, S.; Kanamori, T. Hydrogel microfabrication technology toward three dimensional tissue engineering. Regen. Ther. 2016, 3, 45–57. [Google Scholar] [CrossRef]
- Da Costa, F.F.P.; Araujo, E.S.; Nascimineto, M.L.F.; de Oliveira, H.P. Electrospun Fibers of Enteric Polymer for Controlled Drug Delivery. Int. J. Polym. Sci. 2015. [Google Scholar] [CrossRef]
- Scholten, E.; Bromberg, L.; Rutledge, G.C.; Hatton, T.A. Electrospun Polyurethane Fibers for Absorption of Volatile Organic Compounds from Air. ACS Appl. Mater. Interfaces 2011, 3, 3902–3909. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, T.; Li, M.; Fu, N.; Fu, Y.; Ba, K.; Deng, S.; Jiang, Y.; Hu, J.; Peng, Q.; et al. Electrospun fibers for dental and craniofacial applications. Curr. Stem Cell Res. Ther. 2014, 9, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhou, W.; Ma, D.; Ma, Q.; Bridges, D.; Ma, Y.; Hu, A. Electrospinning of Nanofibers and Their Applications for Energy Devices. J. Nanomater. 2015, 2015, 140716. [Google Scholar] [CrossRef]
- Zhu, P.; Lin, A.; Tang, X.; Lu, X.; Zheng, J.; Zheng, G.; Lei, T. Fabrication of three-dimensional nanofibrous macrostructures by electrospinning. AIP Adv. 2016, 6, 55304. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.-K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2015. [Google Scholar] [CrossRef]
- Ray, S.S.; Chen, S.-S.; Li, C.-W.; Nguyen, N.C.; Nguyen, H.T. A comprehensive review: Electrospinning technique for fabrication and surface modification of membranes for water treatment application. RSC Adv. 2016, 6, 85495–85514. [Google Scholar] [CrossRef]
- Erdem-Kuruca, S.; Kayaman-Apohan, N.; Oktay, B. Fabrication of nanofiber mats from electrospinning of functionalized polymers. IOP Conf. Ser. Mater. Sci. Eng. 2014, 64, 12011. [Google Scholar]
- Thompson, C.J.; Chase, G.G.; Yarin, A.L.; Reneker, D.H. Effects of parameters on nanofiber diameter determined from electrospinning model. Polymer (Guildford) 2007, 48, 6913–6922. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.-T.; Jamshidi, A. A new strategy for fabrication of bone scaffolds using electrospun nano-HAp/PHB fibers and protein hydrogels. Chem. Eng. J. 2016, 289, 38–47. [Google Scholar] [CrossRef]
- Bosworth, L.A.; Turner, L.-A.; Cartmell, S.H. State of the art composites comprising electrospun fibres coupled with hydrogels: A review. Nanomedicine 2013, 9, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Coburn, J.; Gibson, M.; Bandalini, P.A.; Laird, C.; Mao, H.-Q.; Moroni, L.; Seliktar, D.; Elisseeff, J. Biomimetics of the Extracellular Matrix: An Integrated Three-Dimensional Fiber-Hydrogel Composite for Cartilage Tissue Engineering. Smart Struct. Syst. 2011, 7, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Mirahmadi, F.; Tafazzoli-Shadpour, M.; Shokrgozar, M.A.; Bonakdar, S. Enhanced mechanical properties of thermosensitive chitosan hydrogel by silk fibers for cartilage tissue engineering. Mater. Sci. Eng. C 2013, 33, 4786–4794. [Google Scholar] [CrossRef] [PubMed]
- Mohabatpour, F.; Karkhaneh, A.; Sharifi, A.M. A hydrogel/fiber composite scaffold for chondrocyte encapsulation in cartilage tissue regeneration. RSC Adv. 2016, 6, 83135–83145. [Google Scholar] [CrossRef]
- Coburn, J.M.; Gibson, M.; Monagle, S.; Patterson, Z.; Elisseeff, J.H. Bioinspired nanofibers support chondrogenesis for articular cartilage repair. Proc. Natl. Acad. Sci. USA 2012, 109, 10012–10017. [Google Scholar] [CrossRef] [PubMed]
- Bas, O.; De-Juan-Pardo, E.M.; Meinert, C.; D’Angella, D.; Baldwin, J.G.; Bray, L.J.; Wellard, R.M.; Kollmannsberger, S.; Rank, E.; Werner, C.; et al. Biofabricated soft network composites for cartilage tissue engineering. Biofabrication 2017, 9, 25014. [Google Scholar] [CrossRef] [PubMed]
- Tonsomboon, K.; Butcher, A.L.; Oyen, M.L. Strong and tough nanofibrous hydrogel composites based on biomimetic principles. Mater. Sci. Eng. C 2017, 72, 220–227. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Wang, Z.; Yan, Y.; Zheng, J.; Cai, S. Polymer nanofiber reinforced double network gel composite: Strong, tough and transparent. Extrem. Mech. Lett. 2016, 9, 165–170. [Google Scholar] [CrossRef]
- Wright, L.D.; McKeon-Fischer, K.D.; Cui, Z.; Nair, L.S.; Freeman, J.W. PDLA/PLLA and PDLA/PCL nanofibers with a chitosan-based hydrogel in composite scaffolds for tissue engineered cartilage. J. Tissue Eng. Regen. Med. 2014, 8, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Bas, O.; De-Juan-Pardo, E.M.; Chhaya, M.P.; Wunner, F.M.; Jeon, J.E.; Klein, T.J.; Hutmacher, D.W. Enhancing structural integrity of hydrogels by using highly organised melt electrospun fibre constructs. Eur. Polym. J. 2015, 72, 451–463. [Google Scholar] [CrossRef]
- Formica, F.A.; Öztürk, E.; Hess, S.C.; Stark, W.J.; Maniura-Weber, K.; Rottmar, M.; Zenobi-Wong, M. A Bioinspired Ultraporous Nanofiber-Hydrogel Mimic of the Cartilage Extracellular Matrix. Adv. Healthc. Mater. 2016, 5, 3129–3138. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhan, J.; Su, Y.; Wu, T.; Ramakrishna, S.; Liao, S.; Mo, X. Injectable hydrogel incorporating with nanoyarn for bone regeneration. J. Biomater. Sci. Polym. Ed. 2014, 25, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.-E.; Gopal, R.; Ramaseshan, R.; Fujihara, K.; Ramakrishna, S. A dynamic liquid support system for continuous electrospun yarn fabrication. Polymer (Guildford) 2007, 48, 3400–3405. [Google Scholar] [CrossRef]
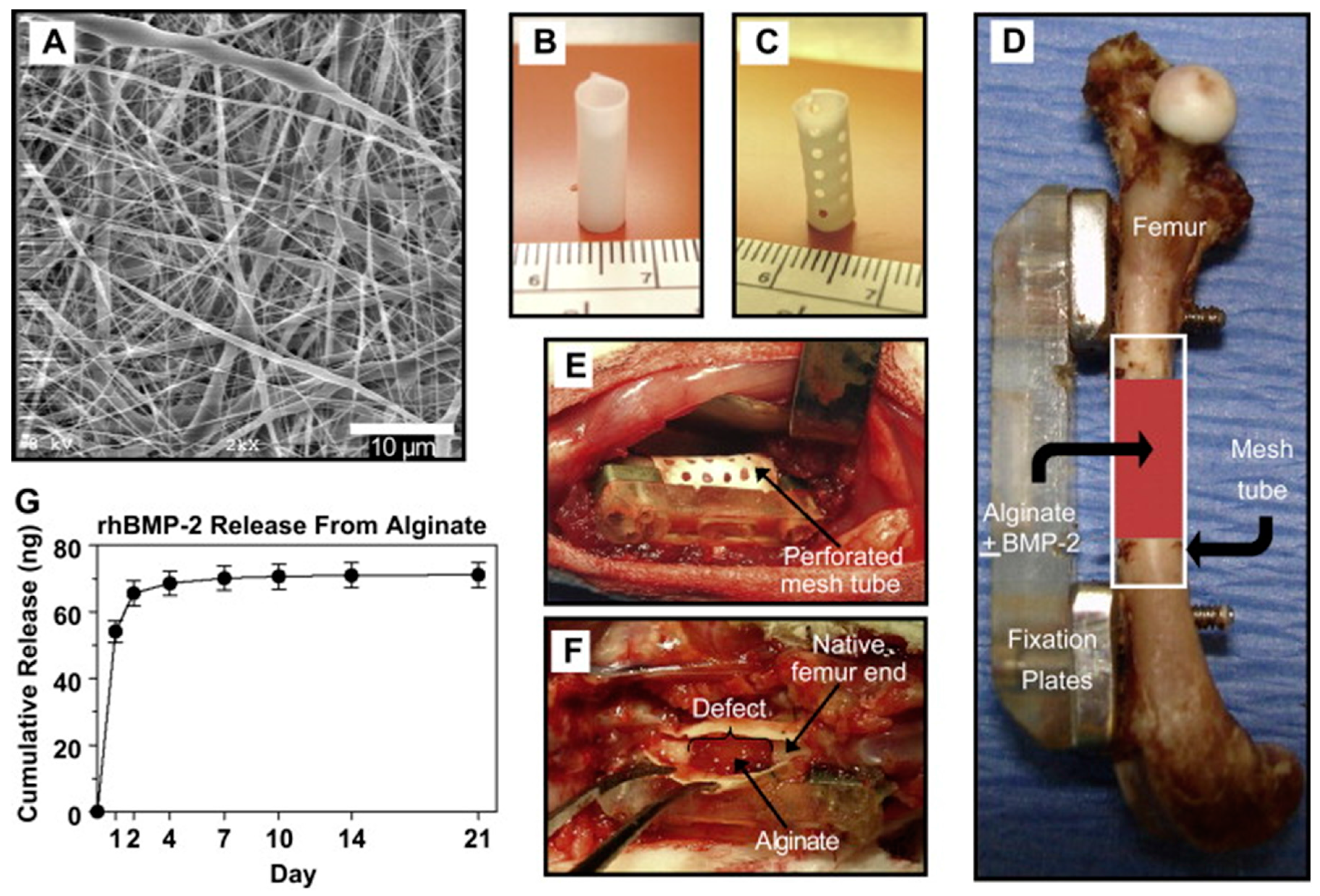
- Kolambkar, Y.M.; Dupont, K.M.; Boerckel, J.D.; Huebsch, N.; Mooney, D.J.; Hutmacher, D.W.; Guldberg, R.E. An alginate-based hybrid system for growth factor delivery in the functional repair of large bone defects. Biomaterials 2011, 32, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Filova, E.; Rampichova, M.; Litvinec, A.; Drzik, M.; Mickova, A.; Buzgo, M.; Kostakova, E.; Martinova, L.; Usvald, D.; Prosecka, E.; et al. A cell-free nanofiber composite scaffold regenerated osteochondral defects in miniature pigs. Int. J. Pharm. 2013, 447, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.K.; Leong, K.F.; Lim, C.S. Rapid Prototyping. Principles and Applications, 3rd ed.; World Scientific: Singapore, 2010; ISBN 978-981-277-897-0. [Google Scholar]
- Peltola, S.M.; Melchels, F.P.W.; Grijpma, D.W.; Kellomaki, M. A review of rapid prototyping techniques for tissue engineering purposes. Ann. Med. 2008, 40, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Sachlos, E.; Czernuszka, J.T. Making tissue engineering scaffolds work. Review: The application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Eur. Cell Mater. 2003, 5, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Do, A.-V.; Khorsand, B.; Geary, S.M.; Salem, A.K. 3D Printing of Scaffolds for Tissue Regeneration Applications. Adv. Healthc. Mater. 2015, 26, 1742–1762. [Google Scholar] [CrossRef] [PubMed]
- Billiet, T.; Vandenhaute, M.; Schelfhout, J.; Van Vlierberghe, S.; Dubruel, P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials 2012, 33, 6020–6041. [Google Scholar] [CrossRef] [PubMed]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef] [PubMed]
- Liska, R.; Schuster, M.; Inführ, R.; Turecek, C.; Fritscher, C.; Seidl, B.; Schmidt, V.; Kuna, L.; Haase, A.; Varga, F.; et al. Photopolymers for rapid prototyping. J. Coat. Technol. Res. 2007, 4, 505–510. [Google Scholar] [CrossRef]
- Dhariwala, B.; Hunt, E.; Boland, T. Rapid prototyping of tissue-engineering constructs, using photopolymerizable hydrogels and stereolithography. Tissue Eng. 2004, 10, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.; Nam, J.; Sun, W. Multi-nozzle deposition for construction of 3D biopolymer tissue scaffolds. Rapid Prototyp. J. 2005, 11, 9–17. [Google Scholar] [CrossRef]
- Liu, L.; Xiong, Z.; Yan, Y.; Zhang, R.; Wang, X.; Jin, L. Multinozzle low-temperature deposition system for construction of gradient tissue engineering scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 88, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Vozzi, G.; Ahluwalia, A. Microfabrication for tissue engineering: Rethinking the cells-on-a scaffold approach. J. Mater. Chem. 2007, 17, 1248. [Google Scholar] [CrossRef]
- Sachs, E.; Cima, M.; Cornie, J. Three-Dimensional Printing: Rapid Tooling and Prototypes Directly from a CAD Model. CIRP Ann. 1990, 39, 201–204. [Google Scholar] [CrossRef]
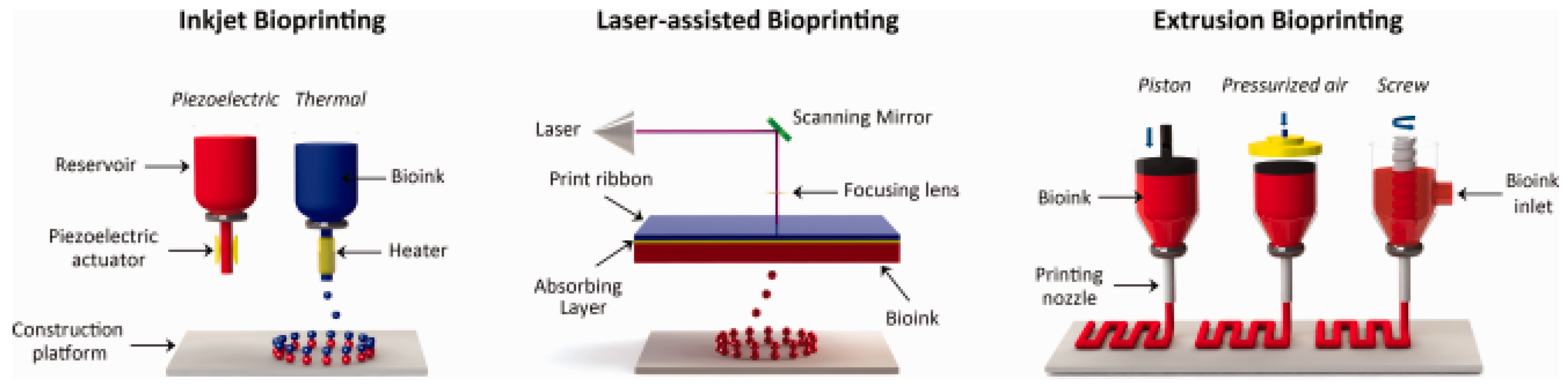
- Nakamura, M.; Kobayashi, A.; Takagi, F.; Watanabe, A.; Hiruma, Y.; Ohuchi, K.; Iwasaki, Y.; Horie, M.; Morita, I.; Takatani, S. Biocompatible inkjet printing technique for designed seeding of individual living cells. Tissue Eng. 2005, 11, 1658–1666. [Google Scholar] [CrossRef] [PubMed]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.; Bartolo, P.J. 3D bioprinting of photocrosslinkable hydrogel constructs. J. Appl. Polym. Sci. 2015. [Google Scholar] [CrossRef]
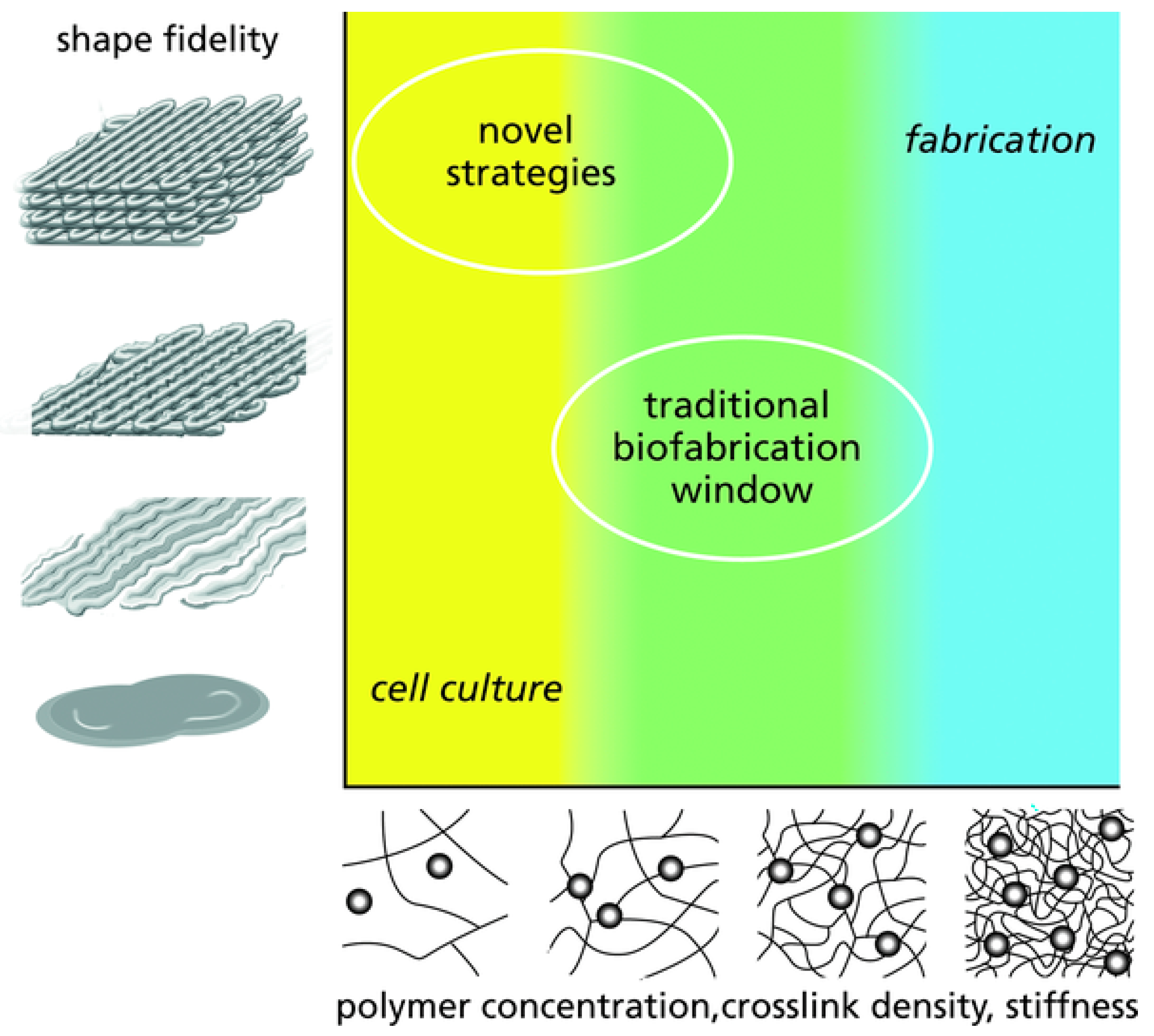
- Guvendiren, M.; Lu, H.D.; Burdick, J.A. Shear-thinning hydrogels for biomedical applications. Soft Matter 2012, 8, 260–272. [Google Scholar] [CrossRef]
- Stanton, M.M.; Samitier, J.; Sanchez, S. Bioprinting of 3D hydrogels. Lab Chip 2015, 15, 3111–3115. [Google Scholar] [CrossRef] [PubMed]
- Holzl, K.; Lin, S.; Tytgat, L.; Van Vlierberghe, S.; Gu, L.; Ovsianikov, A. Bioink properties before, during and after 3D bioprinting. Biofabrication 2016, 8, 32002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y. Tissue Engineering Applications of Three-Dimensional Bioprinting. Cell Biochem. Biophys. 2015, 72, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Malda, J.; Visser, J.; Melchels, F.P.; Jüngst, T.; Hennink, W.E.; Dhert, W.J.A.; Groll, J.; Hutmacher, D.W. 25th Anniversary Article: Engineering Hydrogels for Biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.; Sun, W. Bioprinting endothelial cells with alginate for 3D tissue constructs. J. Biomech. Eng. 2009, 131, 111002. [Google Scholar] [CrossRef] [PubMed]
- Tirella, A.; Orsini, A.; Vozzi, G.; Ahluwalia, A. A phase diagram for microfabrication of geometrically controlled hydrogel scaffolds. Biofabrication 2009, 1, 45002. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhai, D.; Huan, Z.; Zhu, H.; Xia, L.; Chang, J.; Wu, C. Three-Dimensional Printing of Hollow-Struts-Packed Bioceramic Scaffolds for Bone Regeneration. ACS Appl. Mater. Interfaces 2015, 7, 24377–24383. [Google Scholar] [CrossRef] [PubMed]
- Osterbur, L.W. 3D Printing of Hyaluronic Acid Scaffolds for Tissue Engineering Applications; University of Illinois at Urbana-Champaign: Champaign, IL, USA, 2013. [Google Scholar]
- Zhao, S.; Zhang, J.; Zhu, M.; Zhang, Y.; Liu, Z.; Tao, C.; Zhu, Y.; Zhang, C. Three-dimensional printed strontium-containing mesoporous bioactive glass scaffolds for repairing rat critical-sized calvarial defects. Acta Biomater. 2015, 12, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Jakus, A.E.; Taylor, S.L.; Geisendorfer, N.R.; Dunand, D.C.; Shah, R.N. Metallic Architectures from 3D-Printed Powder-Based Liquid Inks. Adv. Funct. Mater. 2015, 25, 6985–6995. [Google Scholar] [CrossRef]
- You, F.; Eames, B.F.; Chen, X. Application of Extrusion-Based Hydrogel Bioprinting for Cartilage Tissue Engineering. Int. J. Mol. Sci. 2017, 18, 1597. [Google Scholar] [CrossRef] [PubMed]
- You, F.; Wu, X.; Zhu, N.; Lei, M.; Eames, B.F.; Chen, X. 3D Printing of Porous Cell-Laden Hydrogel Constructs for Potential Applications in Cartilage Tissue Engineering. ACS Biomater. Sci. Eng. 2016, 2, 1200–1210. [Google Scholar] [CrossRef]
- Klein, T.J.; Rizzi, S.C.; Reichert, J.C.; Georgi, N.; Malda, J.; Schuurman, W.; Crawford, R.W.; Hutmacher, D.W. Strategies for Zonal Cartilage Repair using Hydrogels. Macromol. Biosci. 2009, 9, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hägg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose–Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Möller, T.; Amoroso, M.; Hägg, D.; Brantsing, C.; Rotter, N.; Apelgren, P.; Lindahl, A.; Kölby, L.; Gatenholm, P. In Vivo Chondrogenesis in 3D Bioprinted Human Cell-laden Hydrogel Constructs. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1227. [Google Scholar] [CrossRef] [PubMed]
- Apelgren, P.; Amoroso, M.; Lindahl, A.; Brantsing, C.; Rotter, N.; Gatenholm, P.; Kölby, L. Chondrocytes and stem cells in 3D-bioprinted structures create human cartilage in vivo. PLoS ONE 2017, 12, e0189428. [Google Scholar] [CrossRef] [PubMed]
- Kesti, M.; Eberhardt, C.; Pagliccia, G.; Kenkel, D.; Grande, D.; Boss, A.; Zenobi-Wong, M. Bioprinting Complex Cartilaginous Structures with Clinically Compliant Biomaterials. Adv. Funct. Mater. 2015, 25, 7406–7417. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic Acid Hydrogels for Biomedical Applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Becher, J.; Schnabelrauch, M.; Zenobi-Wong, M. Nanostructured Pluronic hydrogels as bioinks for 3D bioprinting. Biofabrication 2015, 7, 35006. [Google Scholar] [CrossRef] [PubMed]
- Pescosolido, L.; Schuurman, W.; Malda, J.; Matricardi, P.; Alhaique, F.; Coviello, T.; van Weeren, P.R.; Dhert, W.J.A.; Hennink, W.E.; Vermonden, T. Hyaluronic Acid and Dextran-Based Semi-IPN Hydrogels as Biomaterials for Bioprinting. Biomacromolecules 2011, 12, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tuomi, J.; Mäkitie, A.A.; Paloheimo, K.-S.; Partanen, J.; Yliperttula, M. The Integrations of Biomaterials and Rapid Prototyping Techniques for Intelligent Manufacturing of Complex Organs; Pignatello, R., Ed.; InTech: Rijeka, Croatia, 2013; p. 17. [Google Scholar]
- Ifkovits, J.L.; Burdick, J.A. Review: Photopolymerizable and degradable biomaterials for tissue engineering applications. Tissue Eng. 2007, 13, 2369–2385. [Google Scholar] [CrossRef] [PubMed]
- Billiet, T.; Gevaert, E.; De Schryver, T.; Cornelissen, M.; Dubruel, P. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials 2014, 35, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Levato, R.; Webb, W.R.; Otto, I.A.; Mensinga, A.; Zhang, Y.; van Rijen, M.; van Weeren, R.; Khan, I.M.; Malda, J. The bio in the ink: Cartilage regeneration with bioprintable hydrogels and articular cartilage-derived progenitor cells. Acta Biomater. 2017, 61, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Costantini, M.; Idaszek, J.; Szöke, K.; Jaroszewicz, J.; Dentini, M.; Barbetta, A.; Brinchmann, J.E.; Święszkowski, W. 3D bioprinting of BM-MSCs-loaded ECM biomimetic hydrogels for in vitro neocartilage formation. Biofabrication 2016, 8, 35002. [Google Scholar] [CrossRef] [PubMed]
- Schuurman, W.; Levett, P.A.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.A.; Hutmacher, D.W.; Melchels, F.P.W.; Klein, T.J.; Malda, J. Gelatin-Methacrylamide Hydrogels as Potential Biomaterials for Fabrication of Tissue-Engineered Cartilage Constructs. Macromol. Biosci. 2013, 13, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Breitenkamp, K.; Finn, M.G.; Lotz, M.; D’Lima, D.D. Direct Human Cartilage Repair Using Three-Dimensional Bioprinting Technology. Tissue Eng. Part A 2012, 18, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Schilling, A.F.; Hubbell, K.; Yonezawa, T.; Truong, D.; Hong, Y.; Dai, G.; Cui, X. Improved properties of bone and cartilage tissue from 3D inkjet-bioprinted human mesenchymal stem cells by simultaneous deposition and photocrosslinking in PEG-GelMA. Biotechnol. Lett. 2015, 37, 2349–2355. [Google Scholar] [CrossRef] [PubMed]
- Schuurman, W.; Khristov, V.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.; Malda, J. Bioprinting of hybrid tissue constructs with tailorable mechanical properties. Biofabrication 2011, 3, 21001. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.; Shim, J.-H.; Jang, J.; Kim, S.-W.; Cho, D.-W. An additive manufacturing-based PCL-alginate-chondrocyte bioprinted scaffold for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2015, 9, 1286–1297. [Google Scholar] [CrossRef] [PubMed]
- Izadifar, Z.; Chang, T.; Kulyk, W.; Chen, X.; Eames, B.F. Analyzing Biological Performance of 3D-Printed, Cell-Impregnated Hybrid Constructs for Cartilage Tissue Engineering. Tissue Eng. Part C Methods 2016, 22, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Skardal, A.; Atala, A. Evaluation of hydrogels for bio-printing applications. J. Biomed. Mater. Res. A 2013, 101, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef] [PubMed]
- Maas, M.; Hess, U.; Rezwan, K. The contribution of rheology for designing hydroxyapatite biomaterials. Curr. Opin. Colloid Interface Sci. 2014, 19, 585–593. [Google Scholar] [CrossRef]
- Hayrapetyan, A.; Bongio, M.; Leeuwenburgh, S.C.G.; Jansen, J.A.; van den Beucken, J.J.J.P. Effect of Nano-HA/Collagen Composite Hydrogels on Osteogenic Behavior of Mesenchymal Stromal Cells. Stem Cell Rev. Rep. 2016, 12, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Wust, S.; Godla, M.E.; Muller, R.; Hofmann, S. Tunable hydrogel composite with two-step processing in combination with innovative hardware upgrade for cell-based three-dimensional bioprinting. Acta Biomater. 2014, 10, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Sadat-Shojai, M.; Khorasani, M.-T.; Jamshidi, A. 3-Dimensional cell-laden nano-hydroxyapatite/protein hydrogels for bone regeneration applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 49, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Thakur, T.; Xavier, J.R.; Cross, L.; Jaiswal, M.K.; Mondragon, E.; Kaunas, R.; Gaharwar, A.K. Photocrosslinkable and elastomeric hydrogels for bone regeneration. J. Biomed. Mater. Res. A 2016, 104, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-F.; Lu, P.-J.; Song, Y.; Sun, Y.-C.; Wang, Y.-G.; Wang, Y. Nano hydroxyapatite particles promote osteogenesis in a three-dimensional bio-printing construct consisting of alginate/gelatin/hASCs. RSC Adv. 2016, 6, 6832–6842. [Google Scholar] [CrossRef]
- Poldervaart, M.T.; Goversen, B.; de Ruijter, M.; Abbadessa, A.; Melchels, F.P.W.; Öner, F.C.; Dhert, W.J.A.; Vermonden, T.; Alblas, J. 3D bioprinting of methacrylated hyaluronic acid (MeHA) hydrogel with intrinsic osteogenicity. PLoS ONE 2017, 12, e0177628. [Google Scholar] [CrossRef] [PubMed]
- Duarte Campos, D.F.; Blaeser, A.; Buellesbach, K.; Sen, K.S.; Xun, W.; Tillmann, W.; Fischer, H. Bioprinting Organotypic Hydrogels with Improved Mesenchymal Stem Cell Remodeling and Mineralization Properties for Bone Tissue Engineering. Adv. Healthc. Mater. 2016, 5, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-F.; Song, Y.; Liu, Y.-S.; Sun, Y.; Wang, Y.; Wang, Y.; Lyu, P.-J. Osteogenic Differentiation of Three-Dimensional Bioprinted Constructs Consisting of Human Adipose-Derived Stem Cells In Vitro and In Vivo. PLoS ONE 2016, 11, e0157214. [Google Scholar] [CrossRef] [PubMed]
- Ang, T.H.; Sultana, F.S.A.; Hutmacher, D.W.; Wong, Y.S.; Fuh, J.Y.H.; Mo, X.M.; Loh, H.T.; Burdet, E.; Teoh, S.H. Fabrication of 3D chitosan-hydroxyapatite scaffolds using a robotic dispensing system. Mater. Sci. Eng. C 2002, 20, 35–42. [Google Scholar] [CrossRef]
- Demirtaş, T.T.; Irmak, G.; Gümüşderelioğlu, M. A bioprintable form of chitosan hydrogel for bone tissue engineering. Biofabrication 2017, 9, 35003. [Google Scholar] [CrossRef] [PubMed]
- Wenz, A.; Borchers, K.; Tovar, G.E.M.; Kluger, P.J. Bone matrix production in hydroxyapatite-modified hydrogels suitable for bone bioprinting. Biofabrication 2017, 9, 44103. [Google Scholar] [CrossRef] [PubMed]
- Bendtsen, S.T.; Quinnell, S.P.; Wei, M. Development of a novel alginate-polyvinyl alcohol-hydroxyapatite hydrogel for 3D bioprinting bone tissue engineered scaffolds. J. Biomed. Mater. Res. Part A 2017, 105, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Ciardelli, G.; Chiono, V.; Vozzi, G.; Pracella, M.; Ahluwalia, A.; Barbani, N.; Cristallini, C.; Giusti, P. Blends of poly-(epsilon-caprolactone) and polysaccharides in tissue engineering applications. Biomacromolecules 2005, 6, 1961–1976. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Wang, S.-J.; Zhao, X.-R.; Zhu, Y.-F.; Yu, J.-K. 3D-Printed Poly(ε-caprolactone) Scaffold Integrated with Cell-laden Chitosan Hydrogels for Bone Tissue Engineering. Sci. Rep. 2017, 7, 13412. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Kuss, M.A.; Harms, R.; Wu, S.; Wang, Y.; Untrauer, J.B.; Carlson, M.A.; Duan, B. Short-term hypoxic preconditioning promotes prevascularization in 3D bioprinted bone constructs with stromal vascular fraction derived cells. Rsc Adv. 2017, 7, 29312–29320. [Google Scholar] [CrossRef] [PubMed]
- Bracaglia, L.G.; Messina, M.J.; Winston, S.; Kuo, C.-Y.; Lerman, M.; Fisher, J.P. 3D Printed Pericardium Hydrogels to Promote Wound Healing in Vascular Applications. Biomacromolecules 2017. [Google Scholar] [CrossRef] [PubMed]
- Fedorovich, N.E.; Schuurman, W.; Wijnberg, H.M.; Prins, H.-J.; van Weeren, P.R.; Malda, J.; Alblas, J.; Dhert, W.J.A. Biofabrication of Osteochondral Tissue Equivalents by Printing Topologically Defined, Cell-Laden Hydrogel Scaffolds. Tissue Eng. C 2012, 18, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Levato, R.; Visser, J.; Planell, J.A.; Engel, E.; Malda, J.; Mateos-Timoneda, M.A. Biofabrication of tissue constructs by 3D bioprinting of cell-laden microcarriers. Biofabrication 2014, 6, 35020. [Google Scholar] [CrossRef] [PubMed]
- Castro, N.J.; O’Brien, J.; Zhang, L.G. Integrating Biologically Inspired Nanomaterials and Table-top Stereolithography for 3D Printed Biomimetic Osteochondral Scaffolds. Nanoscale 2015, 7, 14010–14022. [Google Scholar] [CrossRef] [PubMed]
- Gillette, B.M.; Jensen, J.A.; Tang, B.; Yang, G.J.; Bazargan-Lari, A.; Zhong, M.; Sia, S.K. In situ collagen assembly for integrating microfabricated three-dimensional cell-seeded matrices. Nat. Mater. 2008, 7, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Chung, C.; Khetan, S.; Burdick, J.A. Hydrolytically Degradable Hyaluronic Acid Hydrogels with Controlled Temporal Structures. Biomacromolecules 2008, 9, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Choi, J.-C.; Shim, J.-H.; Lee, J.-S.; Park, H.; Kim, S.W.; Doh, J.; Cho, D.-W. A comparative study on collagen type I and hyaluronic acid dependent cell behavior for osteochondral tissue bioprinting. Biofabrication 2014, 6, 35004. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.-H.; Jang, K.-M.; Hahn, S.K.; Park, J.Y.; Jung, H.; Oh, K.; Park, K.M.; Yeom, J.; Park, S.H.; Kim, S.W.; et al. Three-dimensional bioprinting of multilayered constructs containing human mesenchymal stromal cells for osteochondral tissue regeneration in the rabbit knee joint. Biofabrication 2016, 8, 14102. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Heo, D.N.; Park, J.S.; Kwon, S.K.; Lee, J.H.; Lee, J.H.; Kim, W.D.; Kwon, I.K.; Park, S.A. Characterization and preparation of bio-tubular scaffolds for fabricating artificial vascular grafts by combining electrospinning and a 3D printing system. Phys. Chem. Chem. Phys. 2015, 17, 2996–2999. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Hua, S.; Yang, M.; Fu, Z.; Teng, S.; Niu, K.; Zhao, Q.; Yi, C. Fabrication and characterization of electrospinning/3D printing bone tissue engineering scaffold. RSC Adv. 2016, 6, 110557–110565. [Google Scholar] [CrossRef]
- Naghieh, S.; Foroozmehr, E.; Badrossamay, M.; Kharaziha, M. Combinational processing of 3D printing and electrospinning of hierarchical poly(lactic acid)/gelatin-forsterite scaffolds as a biocomposite: Mechanical and biological assessment. Mater. Des. 2017, 133, 128–135. [Google Scholar] [CrossRef]
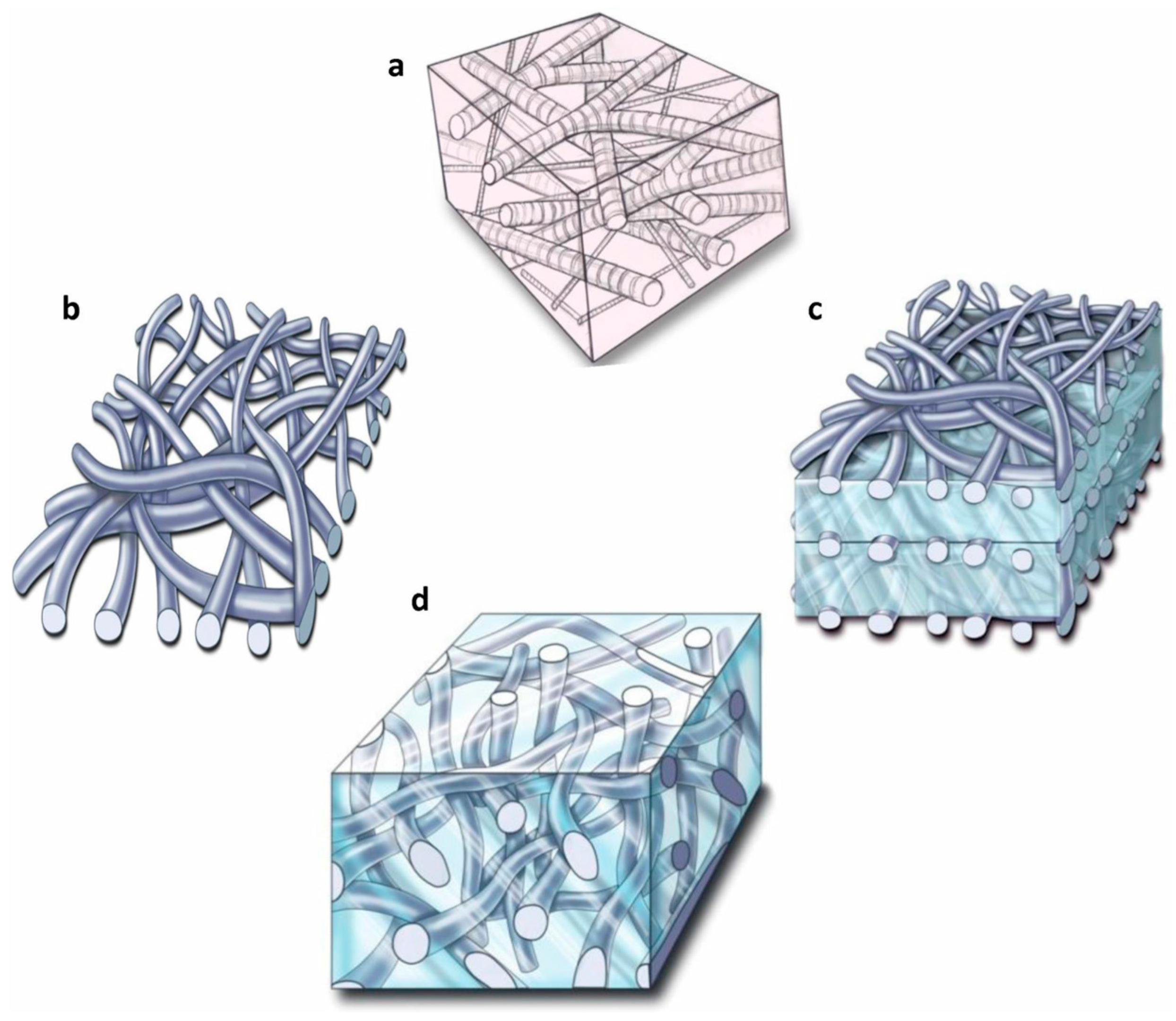


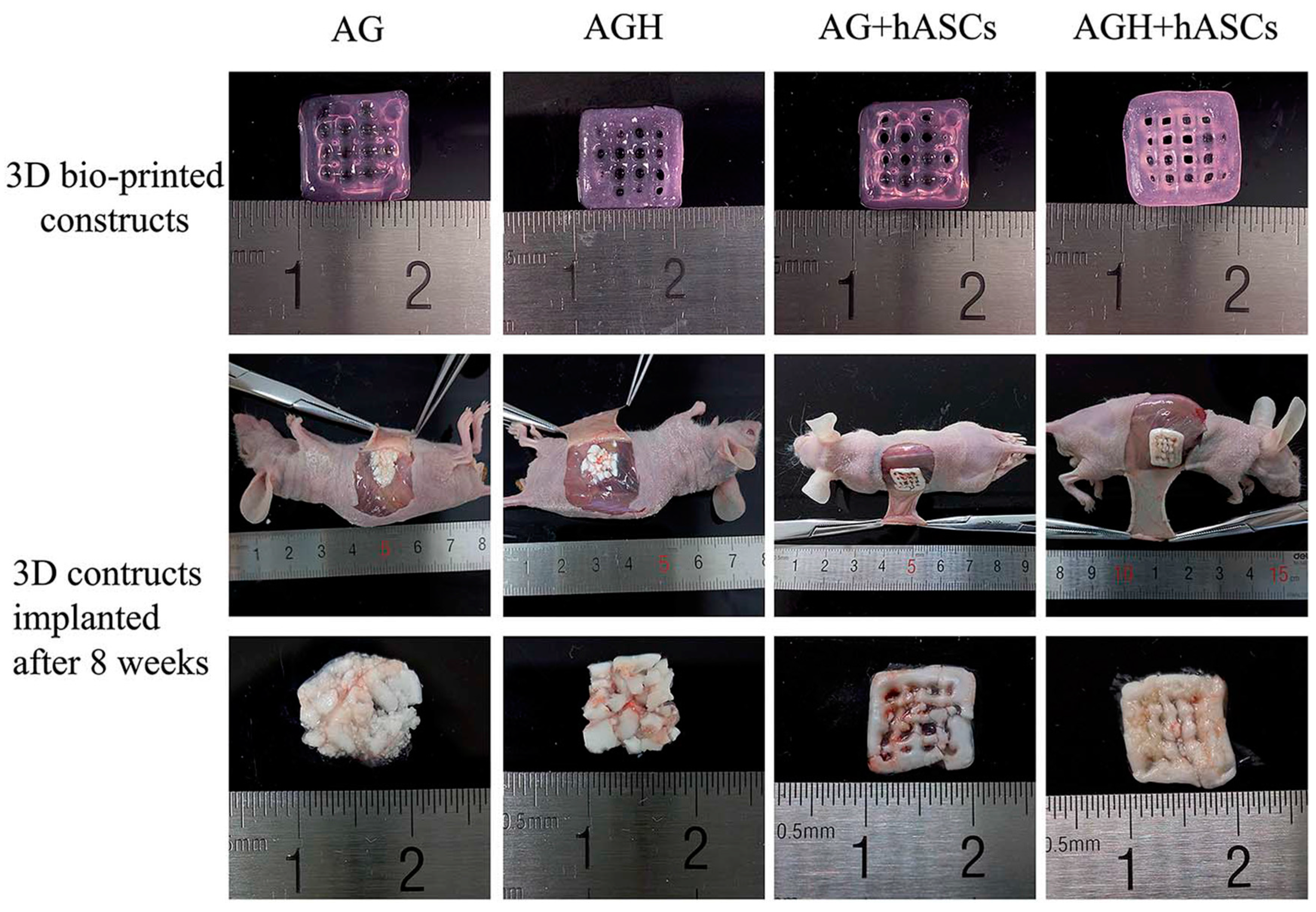
| Fibre(s) | Hydrogel | Fabrication method | Mechanical properties | Cytocompatibility | Reference |
|---|---|---|---|---|---|
| PCL or CSMA/PVAMA | PEG-diacrylate | Fibres mixed with the hydrogel | Not reported | Chondrogenic differentiation | [71] |
| Polyacrylonitrile | Alginate-polyacrylamide | Sandwich-like structure | Young modulus: 3.4 MPa | Not reported | [77] |
| PDLA/PLLA or PDLA/PCL | Chitosan | Fibre infiltrated with hydrogel | Compressive modulus: 2–12 MPa | Cartilage ECM deposition | [78] |
| PCL | GelMA and GelMA/HAMA | Fibres infiltrated with hydrogels | Compressive modulus: 20–1500 kPa | Not reported | [79] |
| PCL | PEG-heparin | Fibres infiltrated with hydrogel | Compressive modulus: 10–1500 kPa | Cell viability > 80% Chondrogenic differentiation | [75] |
| PLA | Alginate-graft-hyaluronate | Hydrogel mixed fibres and gelled | Compressive modulus: 3–5.4 kPa | Cell viability > 85% Chondrogenic differentiation | [73] |
| SIlk | Chitosan | Sandwich-like structure | Compressive modulus: 0.5–0.6 kPa | Cell viability > 90% Chondrogenic differentiation | [72] |
| PCL | Alginate or alginate sulphate | Hydrogel pipetted onto the scaffolds | Shear modulus: 0.5–5 kPa | Cartilage ECM deposition | [80] |
| Material(s) | Cell source(s) | Printing method | Mechanical properties | Cytocompatibility | Reference |
|---|---|---|---|---|---|
| Sodium alginate | ATDC5 chondrogenic cell line | Inkjet bioprinting | Compressive modulus: 20–70 kPa | ~85% cell viability Cartilage ECM deposition | [113] |
| Alginate with cellulose nanofibers | Human nasoseptal chondrocytes | Inkjet bioprinting | Compressive modulus: 75–250 kPa | 73–86% cell viability | [115] |
| Alginate with cellulose nanofibers | Human nasoseptal chondrocytes | Extrusion bioprinting | Compressive stress: 15–88 kPa | Cartilage ECM deposition | [116] |
| Alginate with cellulose nanofibers | Human nasoseptal chondrocytes and MSCs | Extrusion bioprinting | Not reported | Chondrogenic differentiation Chondrocytes proliferation | [117] |
| Alginate with gellan | Bovine articular chondrocytes | Extrusion bioprinting | Tensile modulus: 116–230 kPa | 80–96% cell viability. Cartilage ECM deposition Chondrocytes proliferation | [118] |
| Methacrylated HA with diacrylated Pluronic | Bovine articular chondrocytes | Inkjet bioprinting | Compressive modulus: 1.5–6.5 kPa | 62–86% cell viability | [120] |
| HA with dextran derived | Equine articular chondrocytes | Extrusion bioprinting | Ultimate compressive stress: 100–160 kPa | >75% cell viability | [121] |
| GelMA with HA | Equine articular chondrocytes | Inkjet bioprinting | Compressive modulus: 5–180 kPa | >73% cell viability | [127] |
| GelMA with HA-methacrylate | Human bone marrow MSCs | Extrusion bioprinting | Compressive modulus: 48–100 kPa | 85–95% cell viability.Chondrogenic differentiation | [126] |
| GelMA | Equine ACPCs/Chondrocytes/MSCs | Inkjet bioprinting | Compressive modulus: 100–187 kPa | >75% cell viability | [125] |
| PEGDMA | Human articular chondrocytes | Inkjet bioprinting | Compressive modulus: ~400 kPa | 89% cell viability Cartilage ECM deposition | [128] |
| PEG-GelMA | Human MSCs | Inkjet bioprinting | Compressive modulus: ~1 MPa | ~80% cell viability Cartilage ECM deposition Chondrogenic differentiation | [129] |
| Alginate reinforced with PCL | Embryonic chick chondrocytes | Extrusion bioprinting | Not reported | 77–85% cell viability Cartilage ECM deposition | [132] |
| Alginate reinforced with PCL + TGFβ | Human nososeptal chondrocytes | Extrusion bioprinting | Not reported | 85% cell viability Cartilage ECM deposition | [131] |
| Alginate reinforced with PCL | C20A4 human chondrocyte cell line | Extrusion bioprinting | Compressive modulus: 6 MPa | ~70% cell viability | [130] |
| Material(s) | Cell source(s) | Printing method | Mechanical properties | Cytocompatibility | Reference |
|---|---|---|---|---|---|
| MeHA | Human BM MSCs | Inkjet bioprinting | Elastic modulus: ~11 kPa | ~65% cell viability Osteogenic differentiation | [141] |
| Agarose with collagen type I | Human BM MSCs | Inkjet bioprinting | Compressive modulus: 18–90 kPa | >98% cell viability Osteogenic differentiation | [142] |
| Alginate-gelatin | hASCs | Inkjet bioprinting | Not reported | Osteogenic differentiation Bone matrix formation | [143] |
| Chitosan-HAp | Human osteoblasts | Extrusion printing | Not reported | Good cell attachment and proliferation | [144] |
| Chitosan-HAp/Alginate-HAp | MC3T3-E1 | Inkjet bioprinting | Elastic modulus: 4.6–15 kPa/3.5–19 kPa | >90% cell viability Cell proliferation Osteogenic differentiation | [145] |
| MeHA with HAp or GelMA with HAp | hASCs | Extrusion bioprinting | Not reported | Osteogenic differentiation Bone matrix formation | [146] |
| Alginate-gelatin/alginate-gelatin with nano-HAp | hASCs | Extrusion bioprinting | Not reported | >88% cell viability Osteogenic differentiation | [140] |
| Alginate-polyvinyl alcohol with HAp | MC3T3-E1 cells | Extrusion bioprinting | Compressive modulus: 2.4–10.3 kPa | 77–95% cell viability | [147] |
| Chitosan reinforced with PCL | Rabbit BM MSCs | Extrusion bioprinting | Compressive strength: 6.7 MPa | Osteogenic differentiation Bone matrix formation | [149] |
| HA and Gelatin reinforced with PCL/TCP | Human amniotic fluid-SCs | Extrusion bioprinting | Not reported | 91% cell viability Osteogenic differentiation Bone matrix formation | [150] |
| MeHA and GelMA reinforced with PCL/HAp | Stromal vascular fraction derived cells | Extrusion bioprinting | Not reported | Osteogenic differentiation | [151] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Mori, A.; Peña Fernández, M.; Blunn, G.; Tozzi, G.; Roldo, M. 3D Printing and Electrospinning of Composite Hydrogels for Cartilage and Bone Tissue Engineering. Polymers 2018, 10, 285. https://doi.org/10.3390/polym10030285
De Mori A, Peña Fernández M, Blunn G, Tozzi G, Roldo M. 3D Printing and Electrospinning of Composite Hydrogels for Cartilage and Bone Tissue Engineering. Polymers. 2018; 10(3):285. https://doi.org/10.3390/polym10030285
Chicago/Turabian StyleDe Mori, Arianna, Marta Peña Fernández, Gordon Blunn, Gianluca Tozzi, and Marta Roldo. 2018. "3D Printing and Electrospinning of Composite Hydrogels for Cartilage and Bone Tissue Engineering" Polymers 10, no. 3: 285. https://doi.org/10.3390/polym10030285
APA StyleDe Mori, A., Peña Fernández, M., Blunn, G., Tozzi, G., & Roldo, M. (2018). 3D Printing and Electrospinning of Composite Hydrogels for Cartilage and Bone Tissue Engineering. Polymers, 10(3), 285. https://doi.org/10.3390/polym10030285