Hyperthermia-Triggered Gemcitabine Release from Polymer-Coated Magnetite Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Functionalization with –COOH Terminal Groups
2.2.2. Adsorption of Gemcitabine to MagP–COOH
2.2.3. Morphology and Size Distribution
2.2.4. Electrophoretic Mobility and Zeta Potential
2.2.5. ATR-FTIR Characterization
2.2.6. Thermogravimetric Analysis
2.2.7. Magnetic Properties
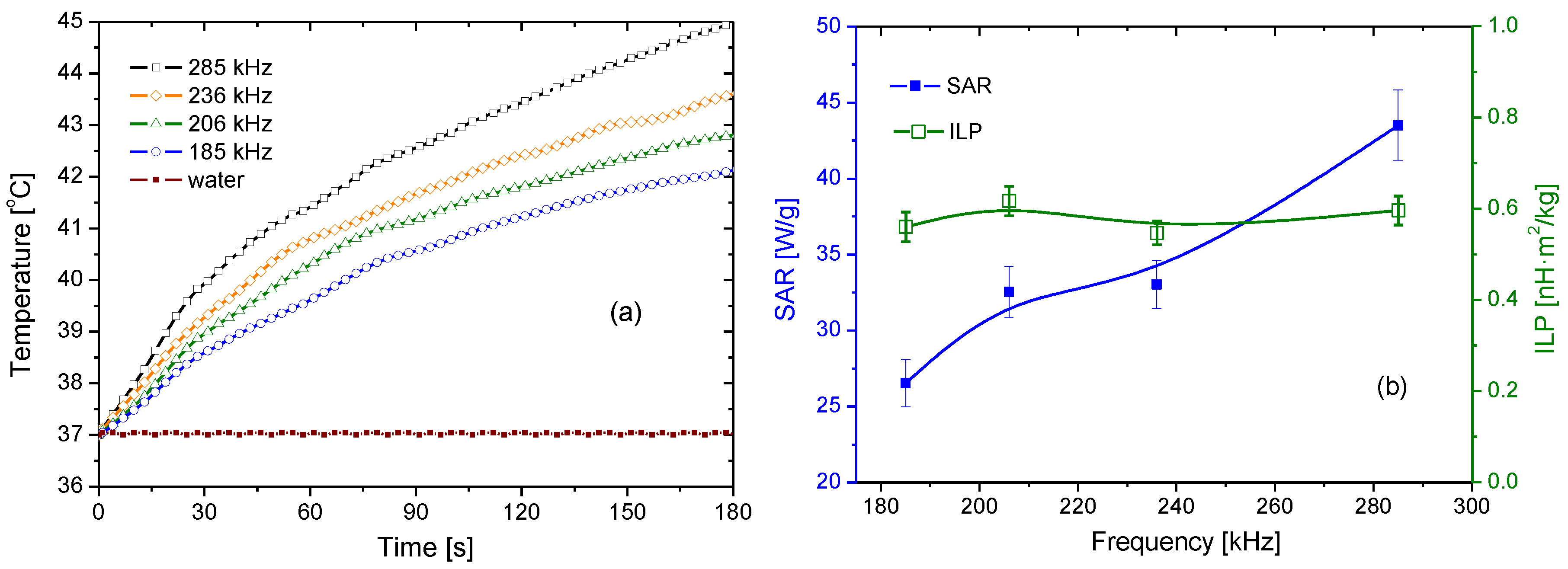
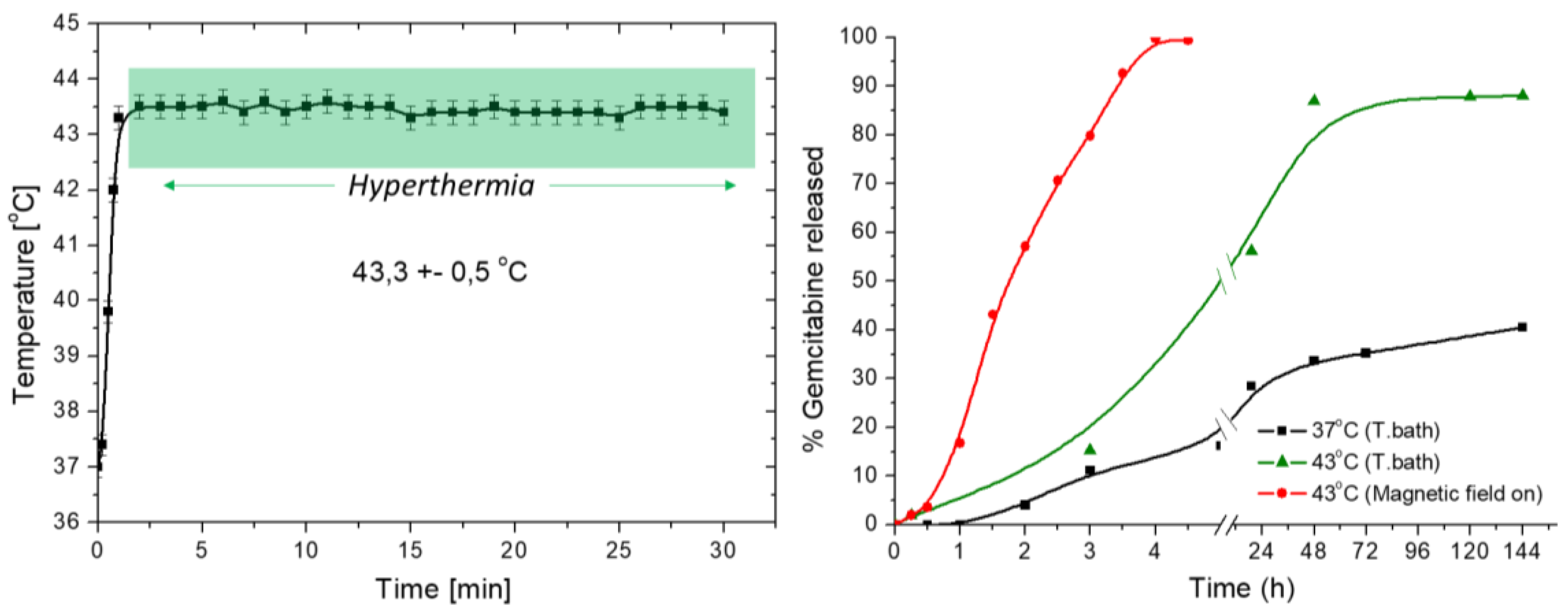
2.2.8. Magnetic Hyperthermia and Specific Absorption Rate Determination
2.2.9. Drug Loading and Release
3. Results and Discussion
3.1. Morphology and Particle Size Distribution
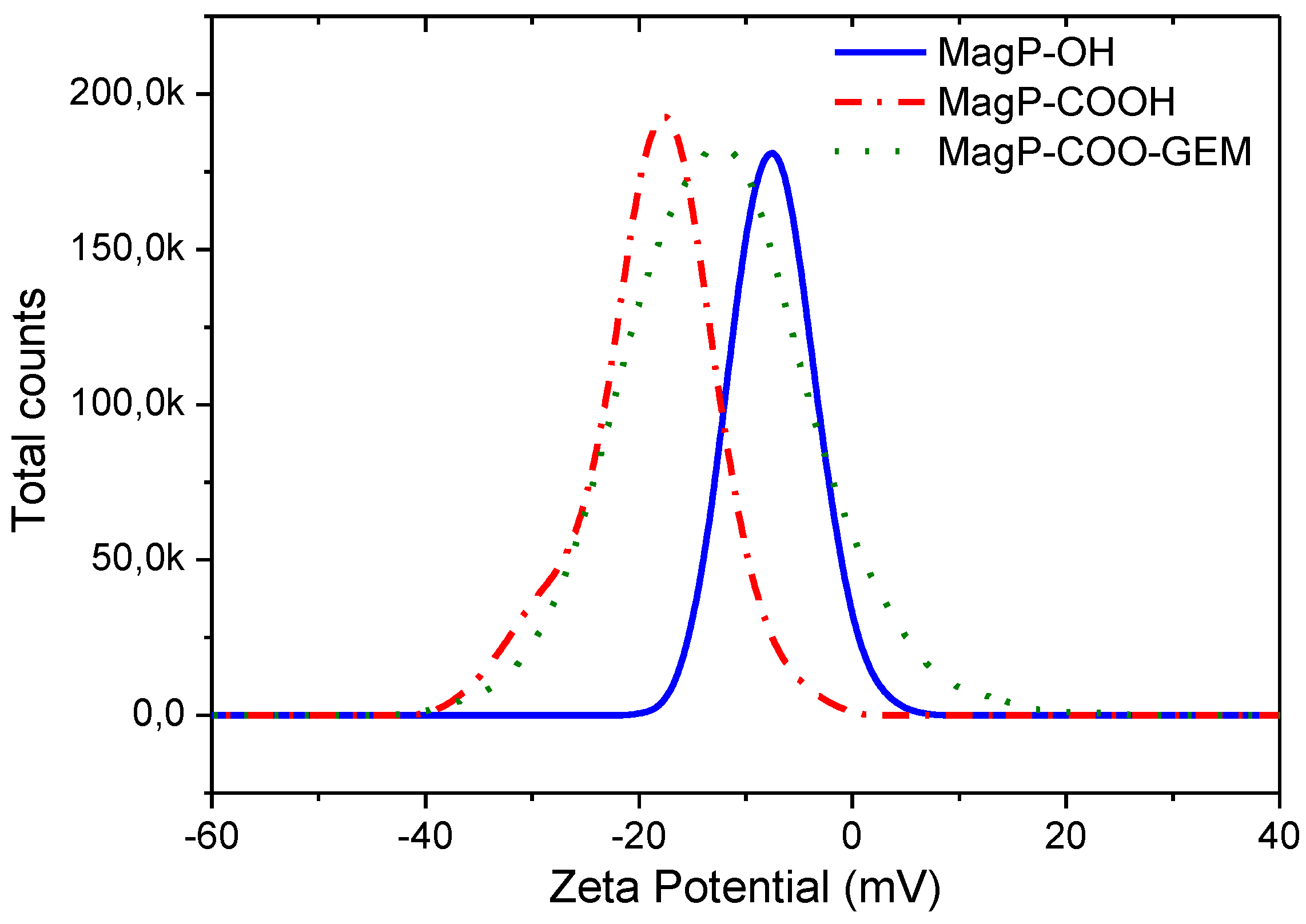
3.2. Zeta Potential
3.3. Drug Loading
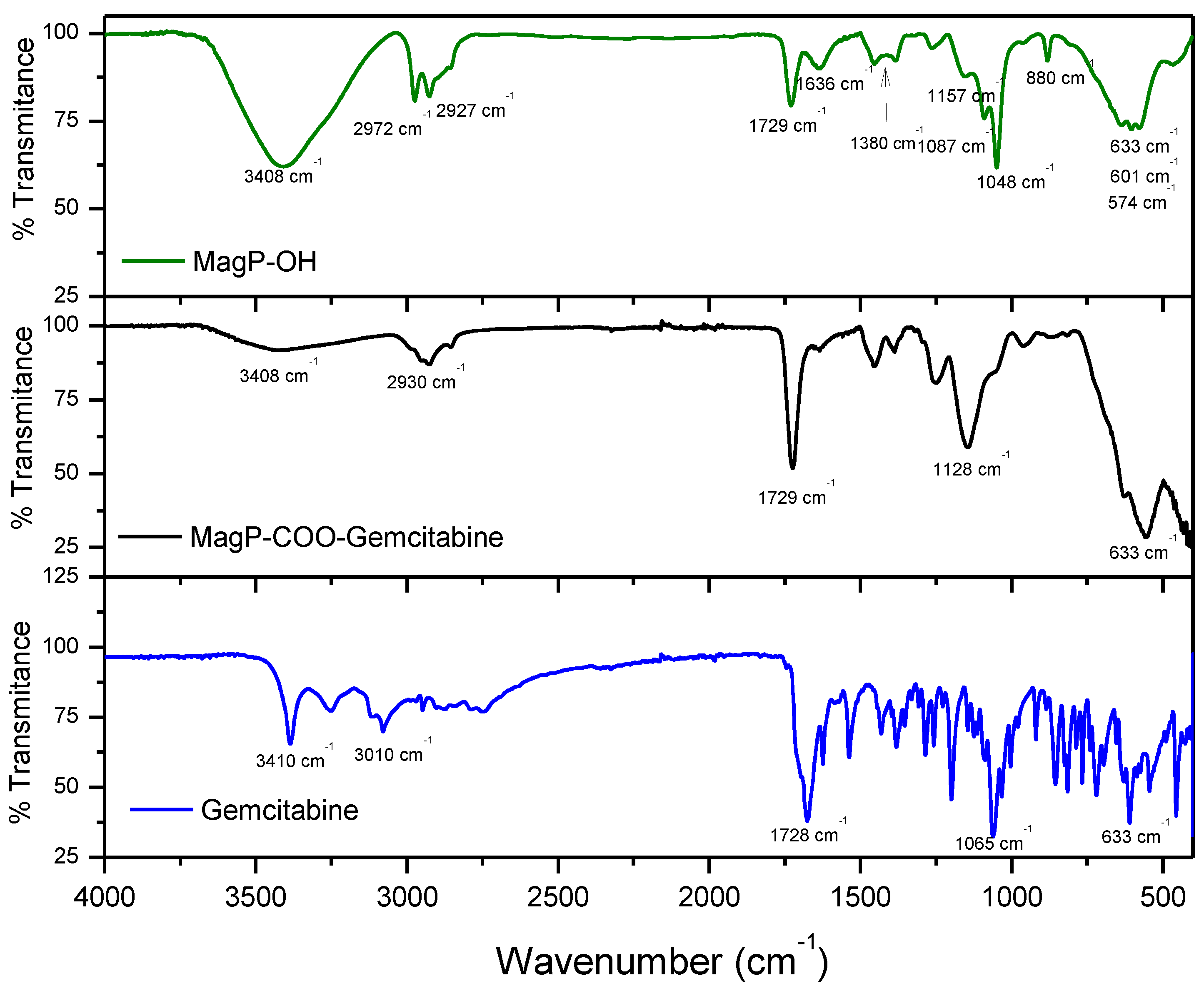
3.4. ATR-FTIR Characterization
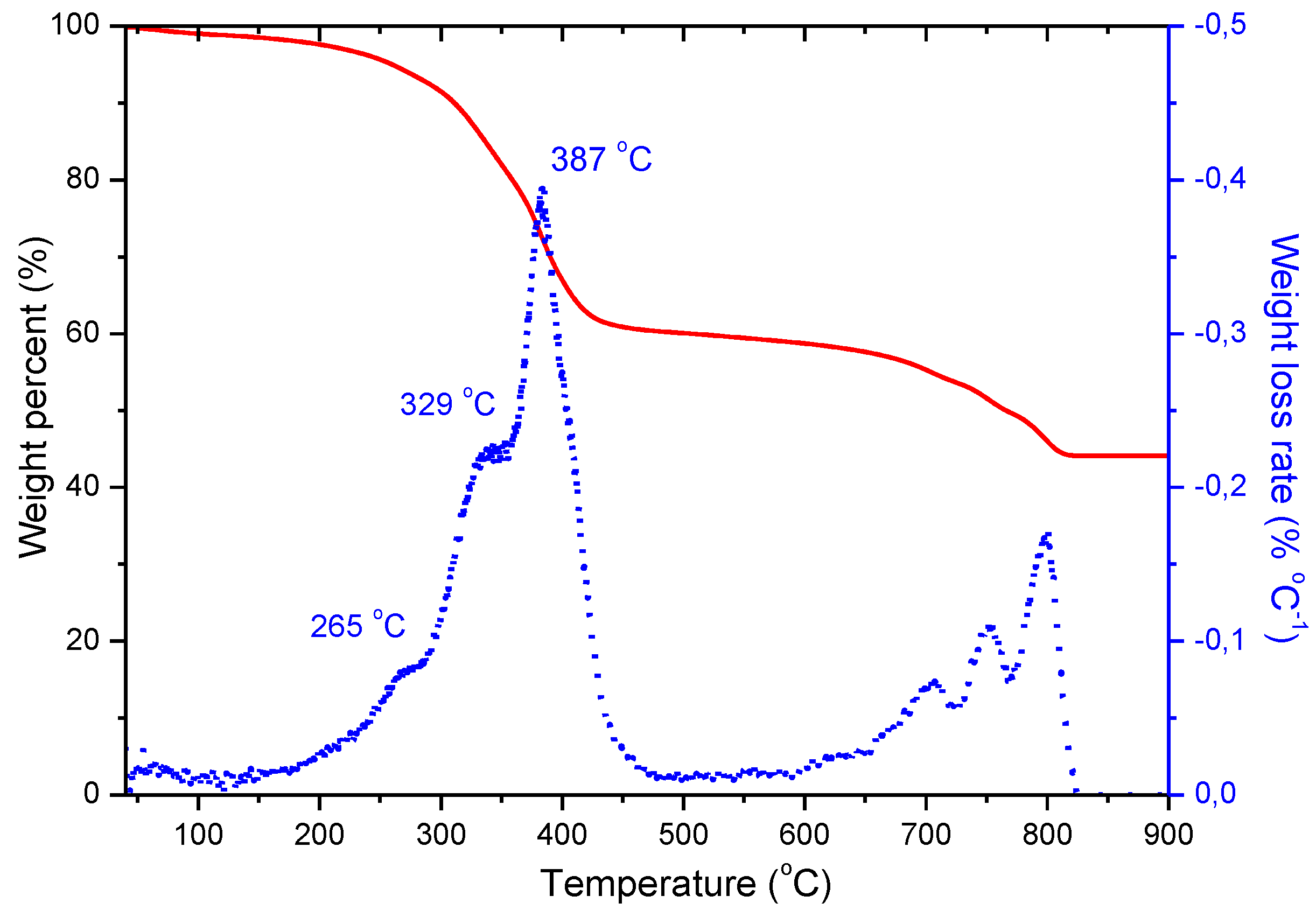
3.5. Thermogravimetric Analysis
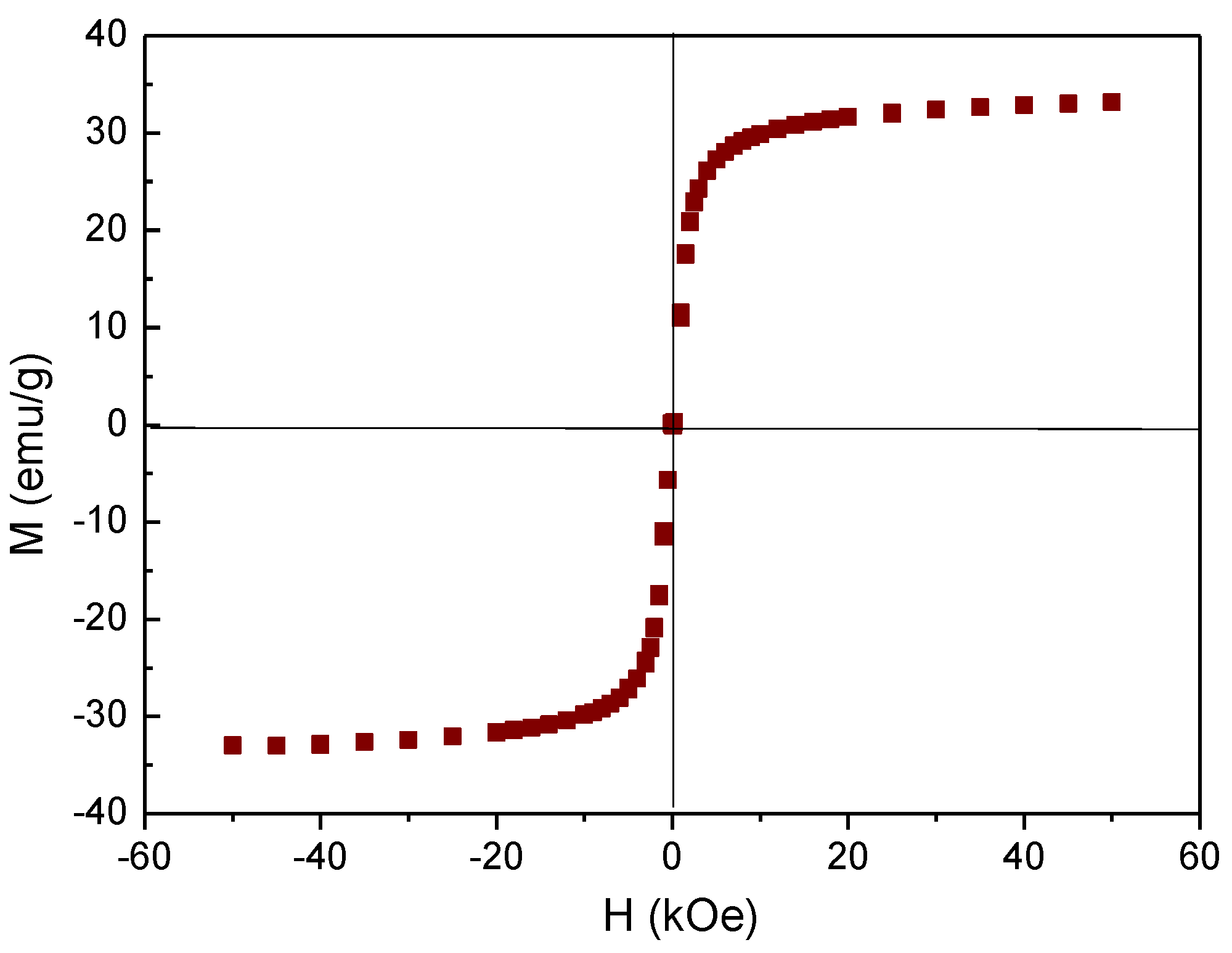
3.6. Magnetization and Hyperthermia
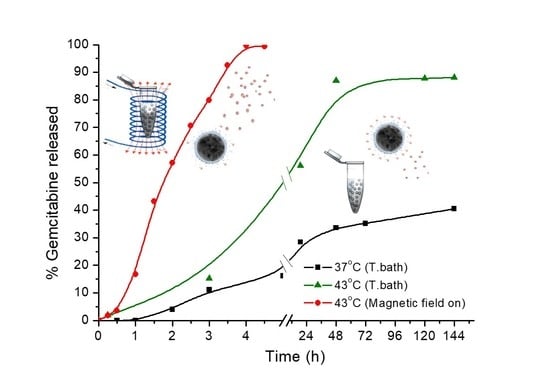
3.7. Drug Release
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mitragotri, S.; Lahann, J. Physical approaches to biomaterial design. Nat. Mater. 2009, 8, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S. Nanomedicine—Elastic clues in cancer detection. Nat. Nanotechnol. 2007, 2, 748–749. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. The ‘right’ size in nanobiotechnology. Nat. Biotechnol. 2003, 21, 1161–1165. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Ribeiro, A.M. Biomimetic nanoparticles: Preparation, characterization and biomedical applications. Int. J. Nanomed. 2010, 5, 249–259. [Google Scholar] [CrossRef]
- Thanh, N.T.K.; Green, L.A.W. Functionalisation of nanoparticles for biomedical applications. Nano Today 2010, 5, 213–230. [Google Scholar] [CrossRef]
- Kim, E.M.; Jeong, H.J. Current Status and Future Direction of Nanomedicine: Focus on Advanced Biological and Medical Applications. Nucl. Med. Mol. Imaging 2017, 51, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.R.; Wang, X.J.; Tang, X.; Hong, R.Y.; Wang, Y.Q.; Feng, W.G. Preparation and characterization of carbonyl iron/strontium hexaferrite magnetorheological fluids. Particuology 2015, 22, 134–144. [Google Scholar] [CrossRef]
- Singh, A.; Sahoo, S.K. Magnetic nanoparticles: A novel platform for cancer theranostics. Drug Discov. Today 2014, 19, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Tonga, G.Y.; Solfiell, D.; Rotello, V.M. Inorganic nanosystems for therapeutic delivery: Status and prospects. Adv. Drug Deliv. Rev. 2013, 65, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Bridot, J.L.; Elst, L.V.; Muller, R.N. Magnetic iron oxide nanoparticles for biomedical applications. Futur. Med. Chem. 2010, 2, 427–449. [Google Scholar] [CrossRef] [PubMed]
- Duran, J.D.G.; Arias, J.L.; Gallardo, V.; Delgado, A.V. Magnetic colloids as drug vehicles. J. Pharm. Sci. 2008, 97, 2948–2983. [Google Scholar] [CrossRef] [PubMed]
- McBain, S.C.; Yiu, H.H.P.; Dobson, J. Magnetic nanoparticles for gene and drug delivery. Int. J. Nanomed. 2008, 3, 169–180. [Google Scholar]
- Laurent, D.F.S.; Port, M.; Roch, A.; Robic, C.; Elst, L.V.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Naregalkar, R.R.; Vaidya, V.D.; Gupta, M. Recent advances on surface engineering of magnetic iron oxide nanoparticles and their biomedical applications. Nanomedicine 2007, 2, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Dobson, J. Magnetic nanoparticles for drug delivery. Drug Dev. Res. 2006, 67, 55–60. [Google Scholar] [CrossRef]
- Tartaj, P.; Morales, M.P.; Gonzalez-Carreno, T.; Veintemillas-Verdaguer, S.; Serna, C.J. Advances in magnetic nanoparticles for biotechnology applications. J. Magn. Magn. Mater. 2005, 290, 28–34. [Google Scholar] [CrossRef]
- Berry, C.C.; Curtis, A.S.G. Functionalisation of magnetic nanoparticles for applications in biomedicine. J. Phys. D-Appl. Phys. 2003, 36, R198–R206. [Google Scholar] [CrossRef]
- Angelakeris, M. Magnetic nanoparticles: A multifunctional vehicle for modern theranostics. Biochim. Biophys. Acta-Gen. Subj. 2017, 1861, 1642–1651. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, G.R.; Ruiz-Moron, L.F.; Duran, J.D.G.; Delgado, A.V. Dynamic and wear study of an extremely bidisperse magnetorheological fluid. Smart Mater. Struct. 2015, 24, 127001. [Google Scholar] [CrossRef]
- Estelrich, J.; Escribano, E.; Queralt, J.; Busquets, M.A. Iron Oxide Nanoparticles for Magnetically-Guided and Magnetically-Responsive Drug Delivery. Int. J. Mol. Sci. 2015, 16, 8070–8101. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.V.; López-Viota, J.; Ramos-Tejada, M.M.; Arias, J.L. Particle geometry, charge, and wettability: The fate of nanoparticle-based drug vehicles. In Colloid and Interface Science in Pharmaceutical Research and Development; Oshima, H., Makino, K., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2014; Volume 1, pp. 443–467. [Google Scholar]
- Klostergaard, J.; Seeney, C.E. Magnetic nanovectors for drug delivery. Nanomed.-Nanotechnol. Biol. Med. 2012, 8, S37–S50. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Xu, S.J. Detection of magnetic nanomaterials in molecular imaging and diagnosis applications. Nanotechnol. Rev. 2014, 3, 247–268. [Google Scholar] [CrossRef]
- Arami, H.; Stephen, Z.; Veiseh, O.; Zhang, M. Chitosan-Coated Iron Oxide Nanoparticles for Molecular Imaging and Drug Delivery. In Chitosan for Biomaterials I; Jayakumar, R., Prabaharan, M., Muzzarelli, R.A.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 163–184. [Google Scholar]
- Wang, Y.X. Superparamagnetic iron oxide based MRI contrast agents: Current status of clinical application. Quant. Med. Imaging Surg. 2011, 1, 6. [Google Scholar]
- Hong, R.Y.; Feng, B.; Chen, L.L.; Liu, G.H.; Li, H.Z.; Zheng, Y.; Wei, D.G. Synthesis, characterization and MRI application of dextran-coated Fe3O4 magnetic nanoparticles. Biochem. Eng. J. 2008, 42, 290–300. [Google Scholar] [CrossRef]
- Deatsch, A.E.; Evans, B.A. Heating efficiency in magnetic nanoparticle hyperthermia. J. Magn. Magn. Mater. 2014, 354 (Suppl. C), 163–172. [Google Scholar] [CrossRef]
- Wildeboer, R.R.; Southern, P.; Pankhurst, Q.A. On the reliable measurement of specific absorption rates and intrinsic loss parameters in magnetic hyperthermia materials. J. Phys. D-Appl. Phys. 2014, 47, 495003. [Google Scholar] [CrossRef]
- Ortega, D.; Pankhurst, Q.A. Magnetic hyperthermia. In Nanoscience Volume 1: Nanostructure through Chemistry; O’Brien, P., Ed.; Royal Society of Chemistry: Cambridge, UK, 2013; Volume 1, pp. 60–88. [Google Scholar]
- Kobayashi, T. Cancer hyperthermia using magnetic nanoparticles. Biotechnol. J. 2011, 6, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Dutz, S.; Hafeli, U.O.; Mahmoudi, M. Magnetic fluid hyperthermia: Focus on superparamagnetic iron oxide nanoparticles. Adv. Colloid Interface Sci. 2011, 166, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, S.; Choi, B.H.; Park, M.T.; Lee, J.; Jeong, S.Y.; Choi, E.K.; Lim, B.U.; Kim, C.; Park, H.J. Hyperthermia improves therapeutic efficacy of doxorubicin carried by mesoporous silica nanocontainers in human lung cancer cells. Int. J. Hyperth. 2011, 27, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.X.; Kawashita, M.; Araki, N.; Mitsumori, M.; Hiraoka, M.; Doi, M. Magnetite nanoparticles with high heating efficiencies for application in the hyperthermia of cancer. Mater. Sci. Eng. C Mater. Biol. Appl. 2010, 30, 990–996. [Google Scholar] [CrossRef]
- Aqil, A.; Vasseur, S.; Duguet, E.; Passirani, C.; Benoit, J.P.; Jerome, R.; Jerome, C. Magnetic nanoparticles coated by temperature responsive copolymers for hyperthermia. J. Mater. Chem. 2008, 18, 3352–3360. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S. Magnetic particle hyperthermia-biophysical limitations of a visionary tumour therapy. J. Magn. Magn. Mater. 2007, 311, 187–192. [Google Scholar] [CrossRef]
- Rosensweig, R.E. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater. 2002, 252, 370–374. [Google Scholar] [CrossRef]
- Falk, M.H.; Issels, R.D. Hyperthermia in oncology. Int. J. Hyperth. 2001, 17, 1–18. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Thanh, N.T.K.; Jones, S.K.; Dobson, J. Progress in applications of magnetic nanoparticles in biomedicine. J. Phys. D-Appl. Phys. 2009, 42, 220301. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D-Appl. Phys. 2003, 36, R167–R181. [Google Scholar] [CrossRef]
- Iglesias, G.; Delgado, A.V.; Kujda, M.; Ramos-Tejada, M.M. Magnetic hyperthermia with magnetite nanoparticles: Electrostatic and polymeric stabilization. Colloid Polym. Sci. 2016, 294, 1541–1550. [Google Scholar] [CrossRef]
- Bonvin, D.; Bastiaansen, J.A.M.; Stuber, M.; Hofmann, H.; Ebersold, M.M. Folic acid on iron oxide nanoparticles: Platform with high potential for simultaneous targeting, MRI detection and hyperthermia treatment of lymph node metastases of prostate cancer. Dalton Trans. 2017, 46, 12692–12704. [Google Scholar] [CrossRef] [PubMed]
- Lima-Tenorio, M.K.; Pineda, E.A.G.; Ahmad, N.M.; Fessi, H.; Elaissari, A. Magnetic nanoparticles: In vivo cancer diagnosis and therapy. Int. J. Pharm. 2015, 493, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Lima, E., Jr.; Torres, T.E.; Rossi, L.M.; Rechenberg, H.R.; Berquo, T.S.; Ibarra, A.; Marquina, C.; Ibarra, M.R.; Goya, G.F. Size dependence of the magnetic relaxation and specific power absorption in iron oxide nanoparticles. J. Nanopart. Res. 2013, 15, 1654. [Google Scholar] [CrossRef]
- Long, N.V.; Yang, Y.; Teranishi, T.; Thi, C.M.; Cao, Y.; Nogami, M. Biomedical Applications of Advanced Multifunctional Magnetic Nanoparticles. J. Nanosci. Nanotechnol. 2015, 15, 10091–10107. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.; Mohammad, F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 789–808. [Google Scholar] [CrossRef] [PubMed]
- Kaviti, A.K.; Yadav, A.; Shukla, A. Inclined solar still designs: A review. Renew. Sustain. Energy Rev. 2016, 54, 429–451. [Google Scholar] [CrossRef]
- Soares, P.I.P.; Sousa, A.I.; Silva, J.C.; Ferreira, I.M.M.; Novo, C.M.M.; Borges, J.P. Chitosan-based nanoparticles as drug delivery systems for doxorubicin: Optimization and modelling. Carbohydr. Polym. 2016, 147, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.I.P.; Sousa, A.I.; Ferreira, I.M.M.; Novo, C.M.M.; Borges, J.P. Towards the development of multifunctional chitosan-based iron oxide nanoparticles: Optimization and modelling of doxorubicin release. Carbohydr. Polym. 2016, 153, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Quinto, C.A.; Mohindra, P.; Tong, S.; Bao, G. Multifunctional superparamagnetic iron oxide nanoparticles for combined chemotherapy and hyperthermia cancer treatment. Nanoscale 2015, 7, 12728–12736. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, G.R.; Delgado, A.V.; Gonzalez-Caballero, E.; Ramos-Tejada, M.M. Simultaneous hyperthermiaanddoxorubicindelivery frompolymer-coatedmagnetitenanoparticles. J. Magn. Magn. Mater. 2017, 431, 294–296. [Google Scholar] [CrossRef]
- Eckel, F.; Schmid, R.M. Chemotherapy in advanced biliary tract carcinoma: A pooled analysis of clinical trials. Br. J. Cancer 2007, 96, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Kamat, A.M.; Hahn, N.M.; Efstathiou, J.A.; Lerner, S.P.; Malmstrom, P.U.; Choi, W.; Guo, C.C.; Lotan, Y.; Kassouf, W. Bladder cancer. Lancet 2016, 388, 2796–2810. [Google Scholar] [CrossRef]
- Kaufman, D.S.; Shipley, W.U.; Feldman, A.S. Bladder cancer. Lancet 2009, 374, 239–249. [Google Scholar] [CrossRef]
- Conroy, T.; Bachet, J.B.; Ayav, A.; Huguet, F.; Lambert, A.; Caramella, C.; Marechal, R.; van Laethem, J.L.; Ducreux, M. Current standards and new innovative approaches for treatment of pancreatic cancer. Eur. J. Cancer 2016, 57, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, D.S.; Akerley, W.; Bepler, G.; Blum, M.G.; Chang, A.; Cheney, R.T.; Chirieac, L.R.; D’Amico, T.A.; Demmy, T.L.; Ganti, A.K.P.; et al. Non Small Cell Lung Cancer. J. Nat. Compr. Cancer Netw. 2010, 8, 740–801. [Google Scholar] [CrossRef]
- Khare, V.; Singh, A.; Mahajan, G.; Alam, N.; Kour, S.; Gupta, M.; Kumar, A.; Singh, G.; Singh, S.K.; Saxena, A.K.; et al. Long-circulatory nanoparticles for gemcitabine delivery: Development and investigation of pharmacokinetics and in vivo anticancer efficacy. Eur. J. Pharm. Sci. 2016, 92, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Celia, C.; Cosco, D.; Paolino, D.; Fresta, M. Gemcitabine-loaded innovative nanocarriers vs. GEMZAR: Biodistribution, pharmacokinetic features and in vivo antitumor activity. Expert Opin. Drug Deliv. 2011, 8, 1609–1629. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, M.; di Cicilia, R.; Macchini, M.; Nobili, E.; Vecchirelli, S.; Brandi, G.; Biasco, G. Metastatic pancreatic cancer: Is gemcitabine still the best standard treatment? (Review). Oncol. Rep. 2010, 23, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Manga, G.; Lluch, A.; Alba, E.; Moreno-Nogueira, J.A.; Palomero, M.; García-Conde, J.; Khayat, D.; Rivelles, N. Gemcitabine in Combination with Doxorubicin in Advanced Breast Cancer: Final Results of a Phase II Pharmacokinetic Trial. J. Clin. Oncol. 2000, 18, 2545–2552. [Google Scholar] [CrossRef] [PubMed]
- Storm, G.; Belliot, S.O.; Daemen, T.; Lasic, D.D. Surface modification of nanoparticles to oppose uptake by the mononuclear phagocyte system. Target. Drugs Deliv. Syst. 1995, 17, 31–48. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, S.; Hou, P.; Yang, Y.; Weng, J.; Li, X.; Li, M. Synthesis and characterization of biocompatible Fe3O4 nanoparticles. J. Biomed. Mater. Res. A 2007, 80, 333–341. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, R.W.; White, L.R. Electrophoretic mobility of a spherical colloidal particle. J. Chem. Soc. Faraday Trans. II 1978, 74, 1607–1626. [Google Scholar] [CrossRef]
- Obaidat, I.M.; Issa, B.; Haik, Y. Magnetic Properties of Magnetic Nanoparticles for Efficient Hyperthermia. Nanomaterials 2015, 5, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Raikher, Y.L.; Stepanov, V.I. Physical aspects of magnetic hyperthermia: Low-frequency ac field absorption in a magnetic colloid. J. Magn. Magn. Mater. 2014, 368, 421–427. [Google Scholar] [CrossRef]
- Schlorf, T.; Meincke, M.; Kossel, E.; Glueer, C.C.; Jansen, O.; Mentlein, R. Biological Properties of Iron Oxide Nanoparticles for Cellular and Molecular Magnetic Resonance Imaging. Int. J. Mol. Sci. 2011, 12, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Shakya, A.K.; Naik, R.; Shalan, N. Stability-Indicating HPLC Determination of Gemcitabine in Pharmaceutical Formulations. Int. J. Anal. Chem. 2015, 2015, 862592. [Google Scholar] [CrossRef] [PubMed]
- Viota, J.L.; Carazo, A.; Munoz-Gamez, J.A.; Rudzka, K.; Gomez-Sotomayor, R.; Ruiz-Extremera, A.; Salmeron, J.; Delgado, A.V. Functionalized magnetic nanoparticles as vehicles for the delivery of the antitumor drug gemcitabine to tumor cells. Physicochemical in vitro evaluation. Mater. Sci. Eng. C-Mater. Biol. Appl. 2013, 33, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Betsiou, M.; Bantsis, G.; Zoi, I.; Sikalidis, C. Adsorption and release of gemcitabine hydrochloride and oxaliplatin by hydroxyapatite. Ceram. Int. 2012, 38, 2719–2724. [Google Scholar] [CrossRef]
- Khare, V.; Al Sakarchi, W.; Gupta, P.N.; Curtis, A.D.M.; Hoskins, C. Synthesis and characterization of TPGS-gemcitabine prodrug micelles for pancreatic cancer therapy. RSC Adv. 2016, 6, 60126–60137. [Google Scholar] [CrossRef]
- Ayyappan, S.; Gnanaprakash, G.; Panneerselvam, G.; Antony, M.P.; Philip, J. Effect of Surfactant Monolayer on Reduction of Fe3O4 Nanoparticles under Vacuum. J. Phys. Chem. C 2008, 112, 18376–18383. [Google Scholar] [CrossRef]
- Gałka, P.; Kowalonek, J.; Kaczmarek, H. Thermogravimetric analysis of thermal stability of poly(methyl methacrylate) films modified with photoinitiators. J. Therm. Anal. Calorim. 2014, 115, 1387–1394. [Google Scholar] [CrossRef]
- Demirelli, K.; Coskun, M.; Kaya, E. A detailed study of thermal degradation of poly(2-hydroxyethyl methacrylate). Polym. Degrad. Stab. 2001, 72, 75–80. [Google Scholar] [CrossRef]
- Han, S.; Hagiwara, M.; Ishizone, T. Synthesis of Thermally Sensitive Water-Soluble Polymethacrylates by Living Anionic Polymerizations of Oligo(ethylene glycol) Methyl Ether Methacrylates. Macromolecules 2003, 36, 8312–8319. [Google Scholar] [CrossRef]
- Das, R.; Alonso, J.; Porshokouh, Z.N.; Kalappattil, V.; Torres, D.; Phan, M.H.; Garaio, E.; Garcia, J.A.; Llamazares, J.L.S.; Srikanth, H. Tunable High Aspect Ratio Iron Oxide Nanorods for Enhanced Hyperthermia. J. Phys. Chem. C 2016, 120, 10086–10093. [Google Scholar] [CrossRef]
- Lu, H.M.; Zheng, W.T.; Jiang, Q. Saturation magnetization of ferromagnetic and ferrimagnetic nanocrystals at room temperature. J. Phys. D-Appl. Phys. 2007, 40, 320–325. [Google Scholar] [CrossRef]
- Johannsen, M.; Gneveckow, U.; Eckelt, L.; Feussner, A.; Waldofner, N.; Scholz, R.; Deger, S.; Wust, P.; Loening, S.A.; Jordan, A. Clinical hyperthermia of prostate cancer using magnetic nanoparticles: Presentation of a new interstitial technique. Int. J. Hyperth. 2005, 21, 637–647. [Google Scholar] [CrossRef]
- Johannsen, M.; Gneveckow, U.; Taymoorian, K.; Thiesen, B.; Waldoefner, N.; Scholz, R.; Jung, K.; Jordan, A.; Wust, P.; Loening, S.A. Morbidity and quality of life during thermotherapy using magnetic nanoparticles in locally recurrent prostate cancer: Results of a prospective phase I trial. J. Magn. Magn. Mater. 2007, 23, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm.-Drug Res. 2010, 67, 217–223. [Google Scholar]
- Jaidev, L.R.; Krishnan, U.M.; Sethuraman, S. Gemcitabine loaded biodegradable PLGA nanospheres for in vitro pancreatic cancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 47, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Haley, B.; Frenkel, E. Nanoparticles for drug delivery in cancer treatment. Urol. Oncol. Sem. Orig. Investig. 2008, 26, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Karna, S.; Chaturvedi, S.; Agrawal, V.; Alim, M. Formulation approaches for sustained release dosage forms: A review. Asian J. Pharm. Clin. Res. 2015, 8, 46–53. [Google Scholar]
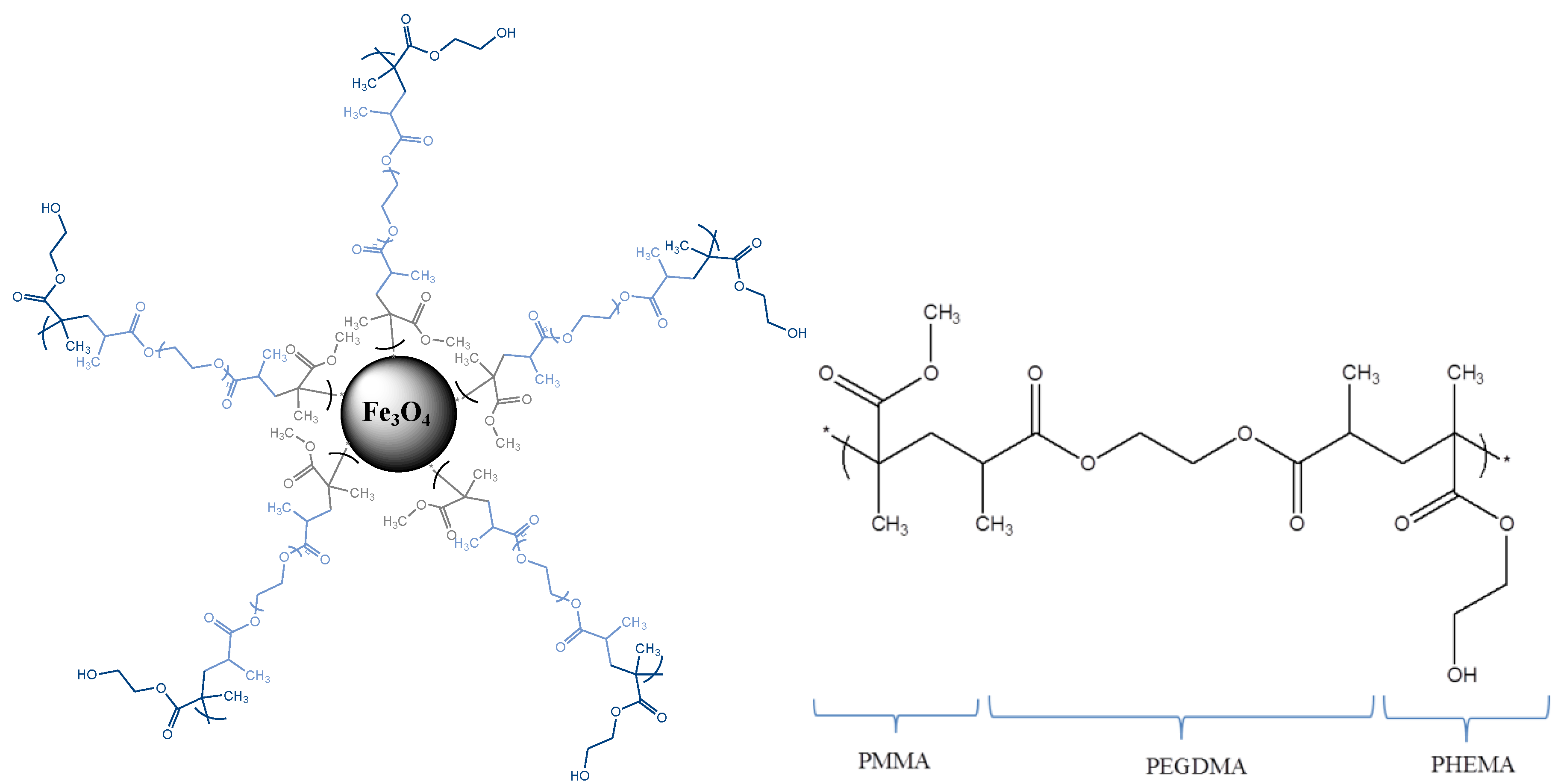
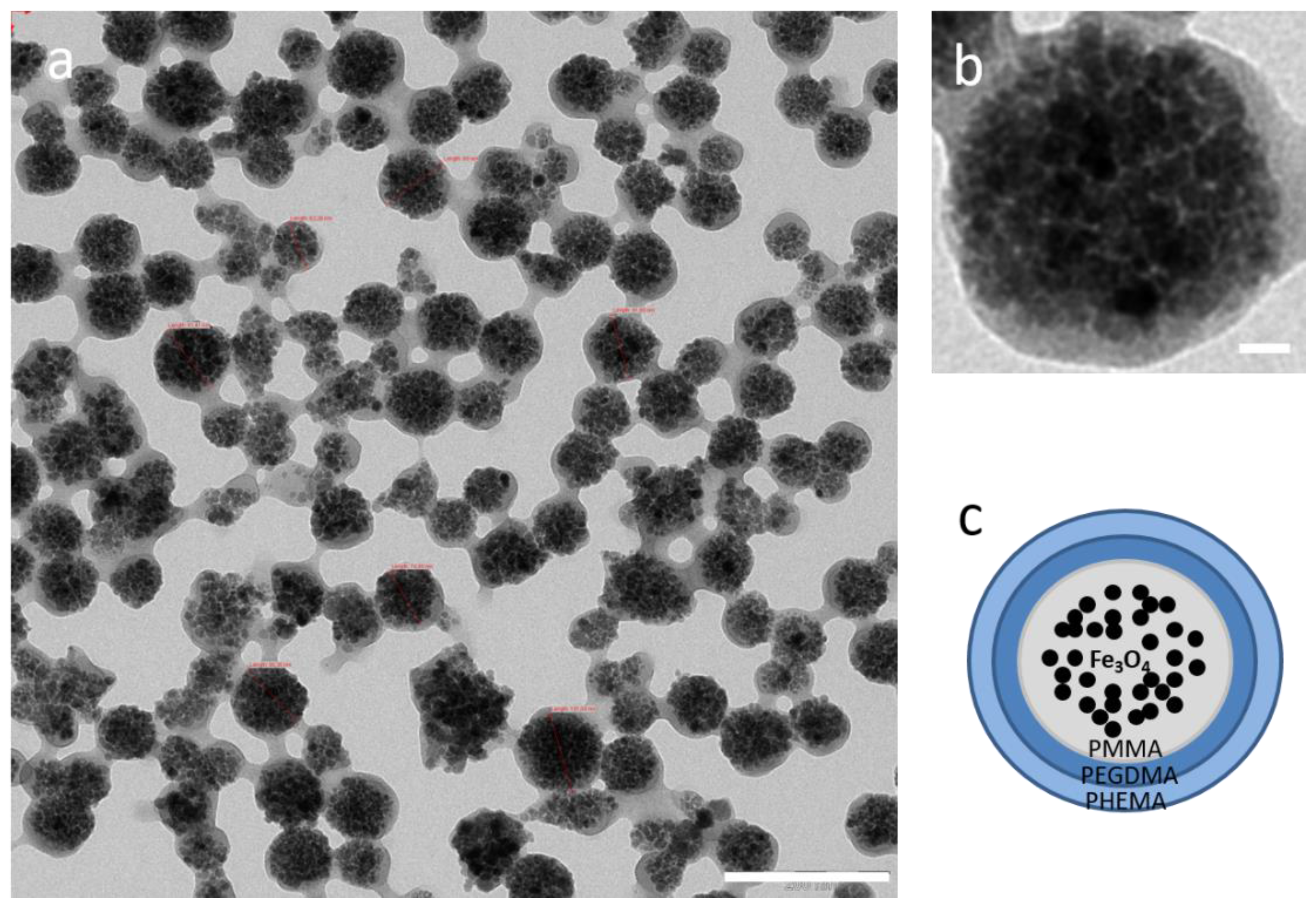
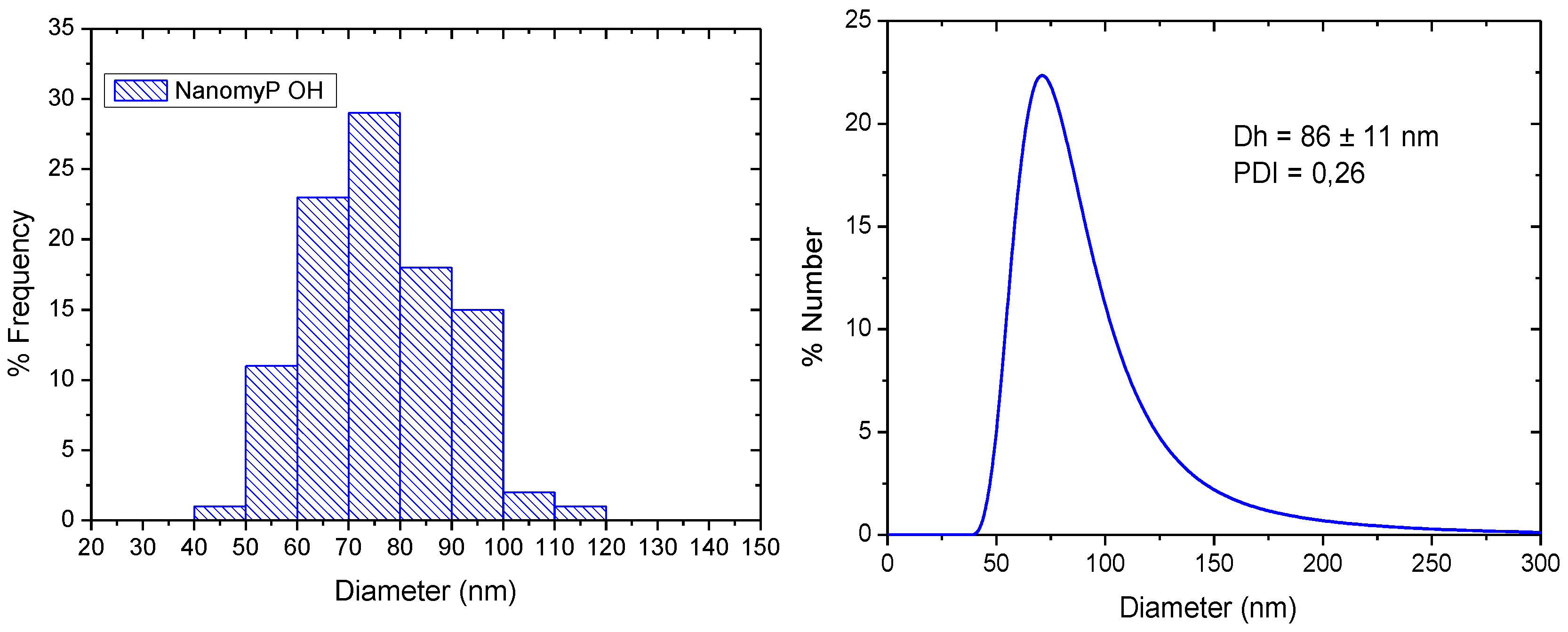

| Initial Drug/MNPs Ratio (mg/g) | Adsorbed GEM (mg/g MNPs) | Fraction of Adsorbed Drug (%) |
|---|---|---|
| 33.3 | 4.3 | 13.9 |
| 66.7 | 4.0 | 6.0 |
| 100 | 3.0 | 3.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iglesias, G.R.; Reyes-Ortega, F.; Checa Fernandez, B.L.; Delgado, Á.V. Hyperthermia-Triggered Gemcitabine Release from Polymer-Coated Magnetite Nanoparticles. Polymers 2018, 10, 269. https://doi.org/10.3390/polym10030269
Iglesias GR, Reyes-Ortega F, Checa Fernandez BL, Delgado ÁV. Hyperthermia-Triggered Gemcitabine Release from Polymer-Coated Magnetite Nanoparticles. Polymers. 2018; 10(3):269. https://doi.org/10.3390/polym10030269
Chicago/Turabian StyleIglesias, G. R., Felisa Reyes-Ortega, B. L. Checa Fernandez, and Ángel V. Delgado. 2018. "Hyperthermia-Triggered Gemcitabine Release from Polymer-Coated Magnetite Nanoparticles" Polymers 10, no. 3: 269. https://doi.org/10.3390/polym10030269
APA StyleIglesias, G. R., Reyes-Ortega, F., Checa Fernandez, B. L., & Delgado, Á. V. (2018). Hyperthermia-Triggered Gemcitabine Release from Polymer-Coated Magnetite Nanoparticles. Polymers, 10(3), 269. https://doi.org/10.3390/polym10030269