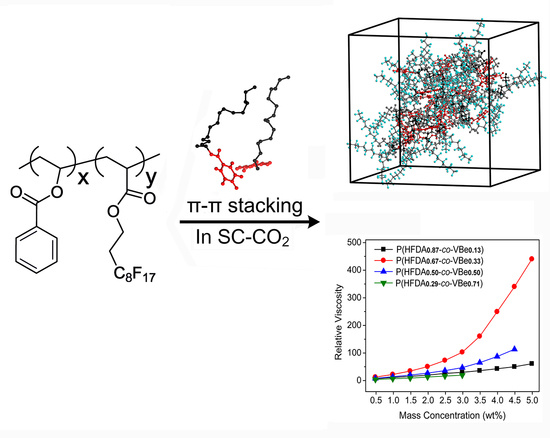
Thickening Supercritical CO2 with π-Stacked Co-Polymers: Molecular Insights into the Role of Intermolecular Interaction
Abstract
1. Introduction
2. Methodology
2.1. Synthesis and Characterization
2.1.1. Materials
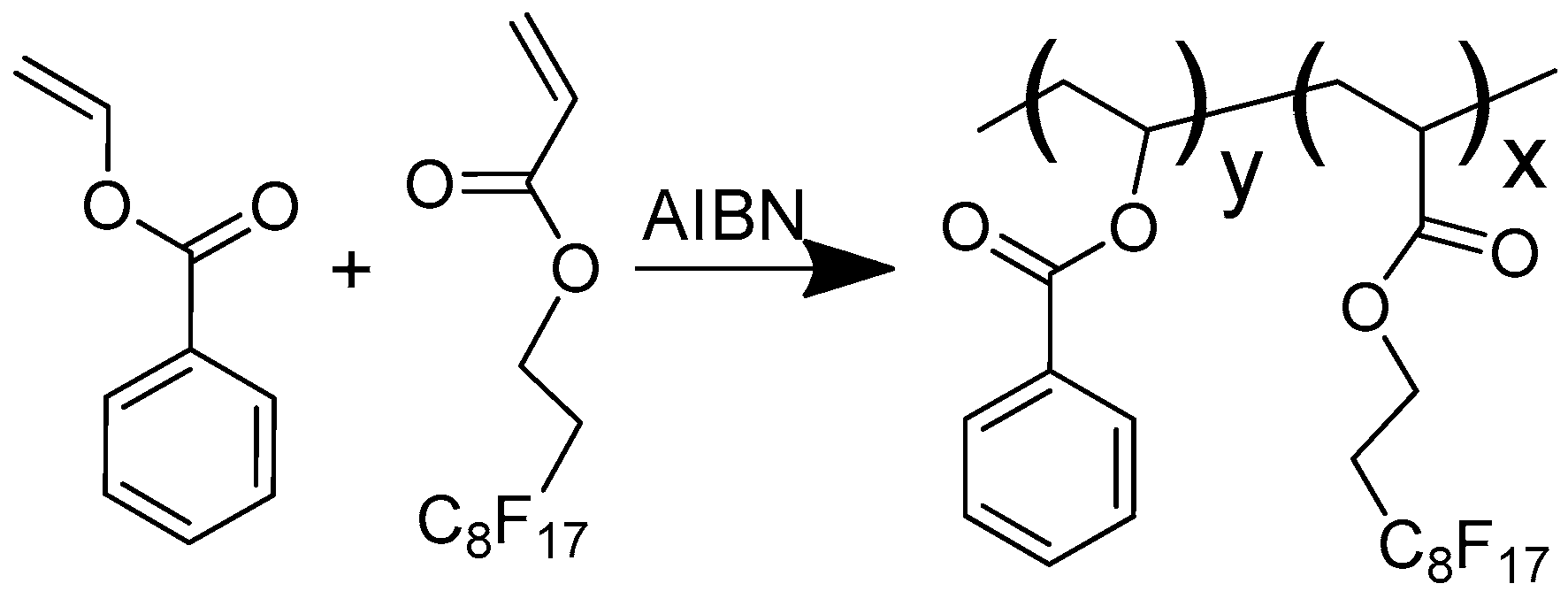
2.1.2. Preparation of Co-Polymer
2.1.3. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.1.4. Differential Scanning Calorimetry
2.1.5. Molar Mass Analysis
2.1.6. Surface Tension Measurements
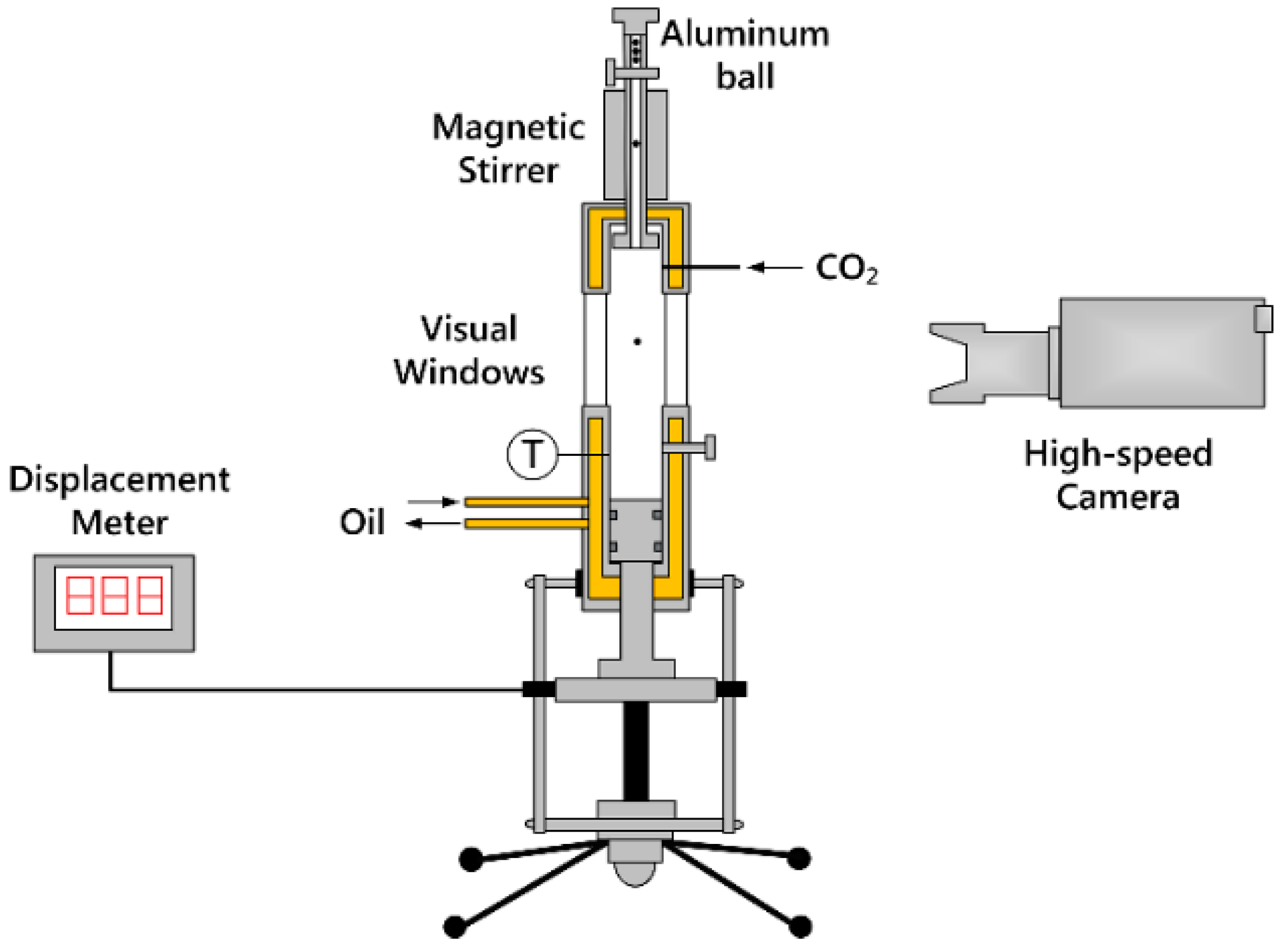
2.2. Cloud Point and Viscosity Measurements
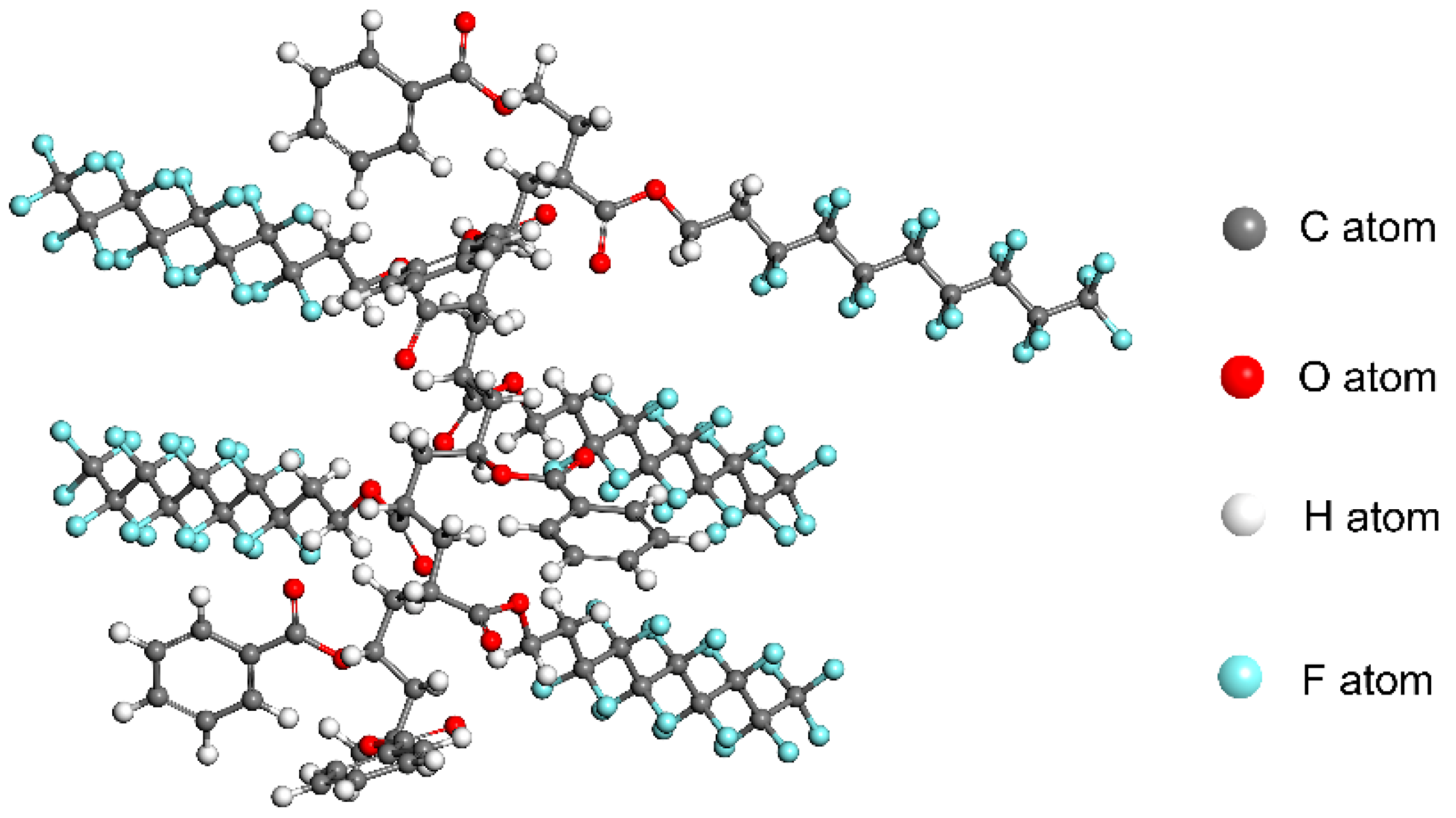
2.3. Molecular Dynamics Simulation
3. Results and Discussion
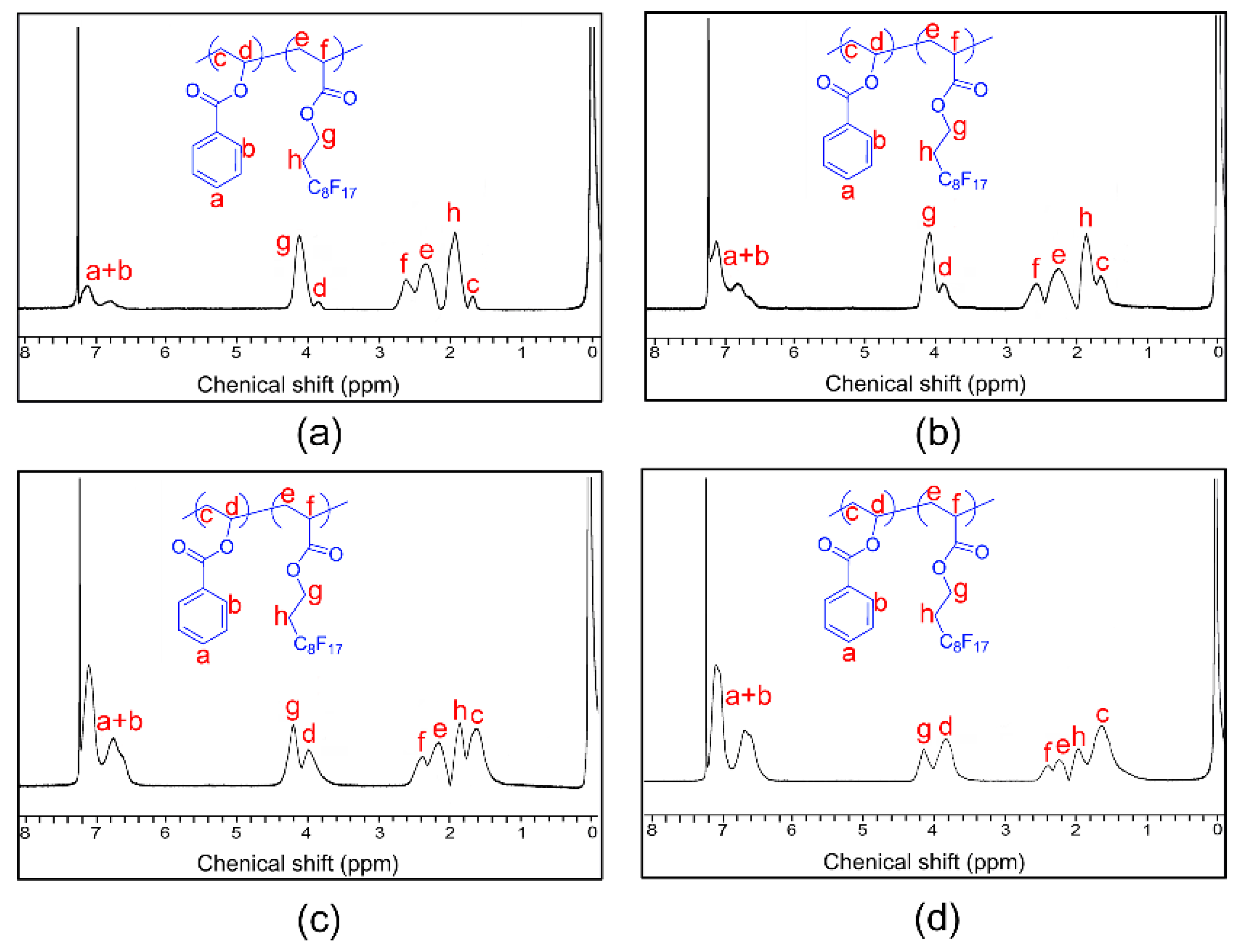
3.1. Synthesis and Characterization
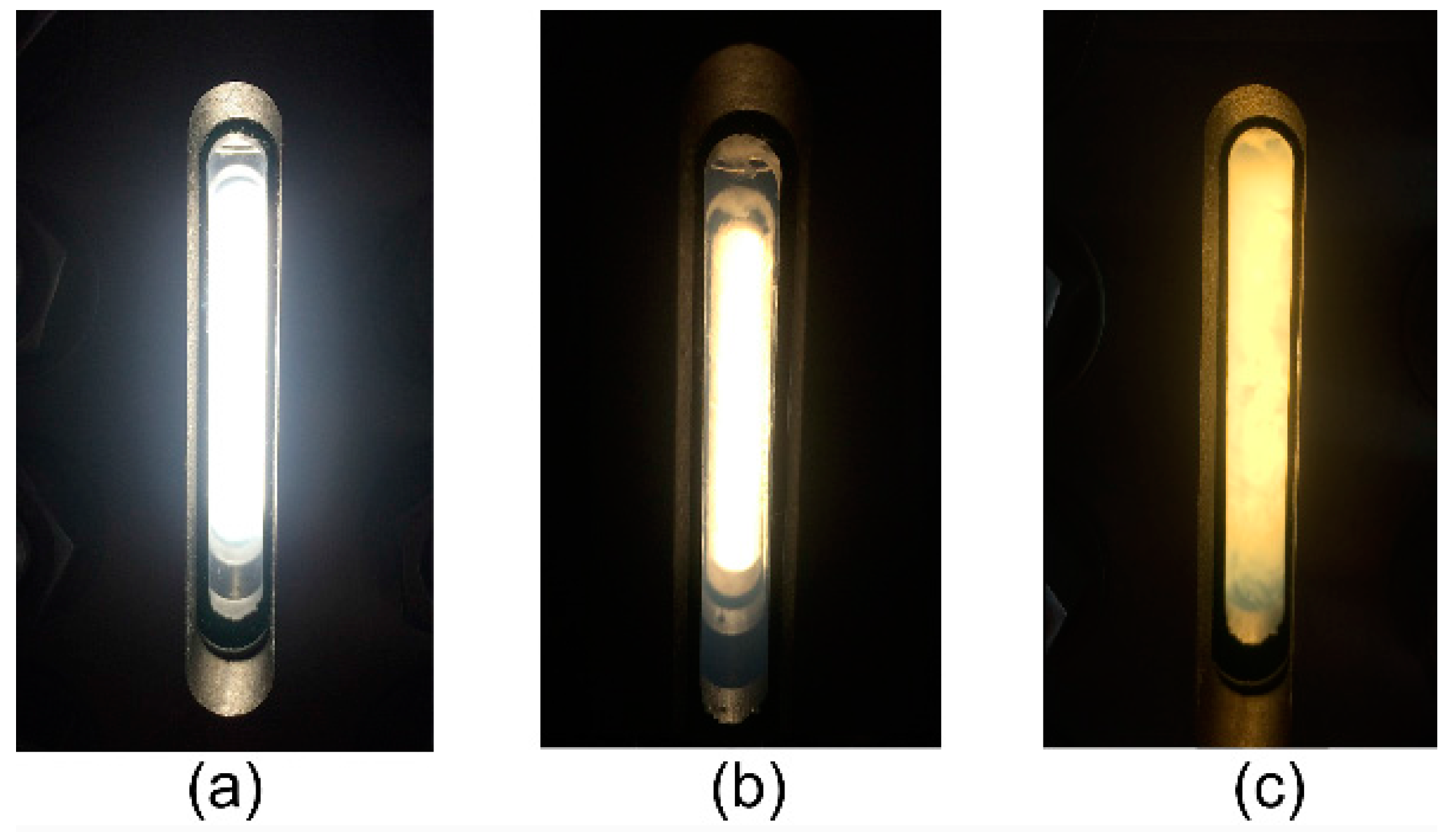
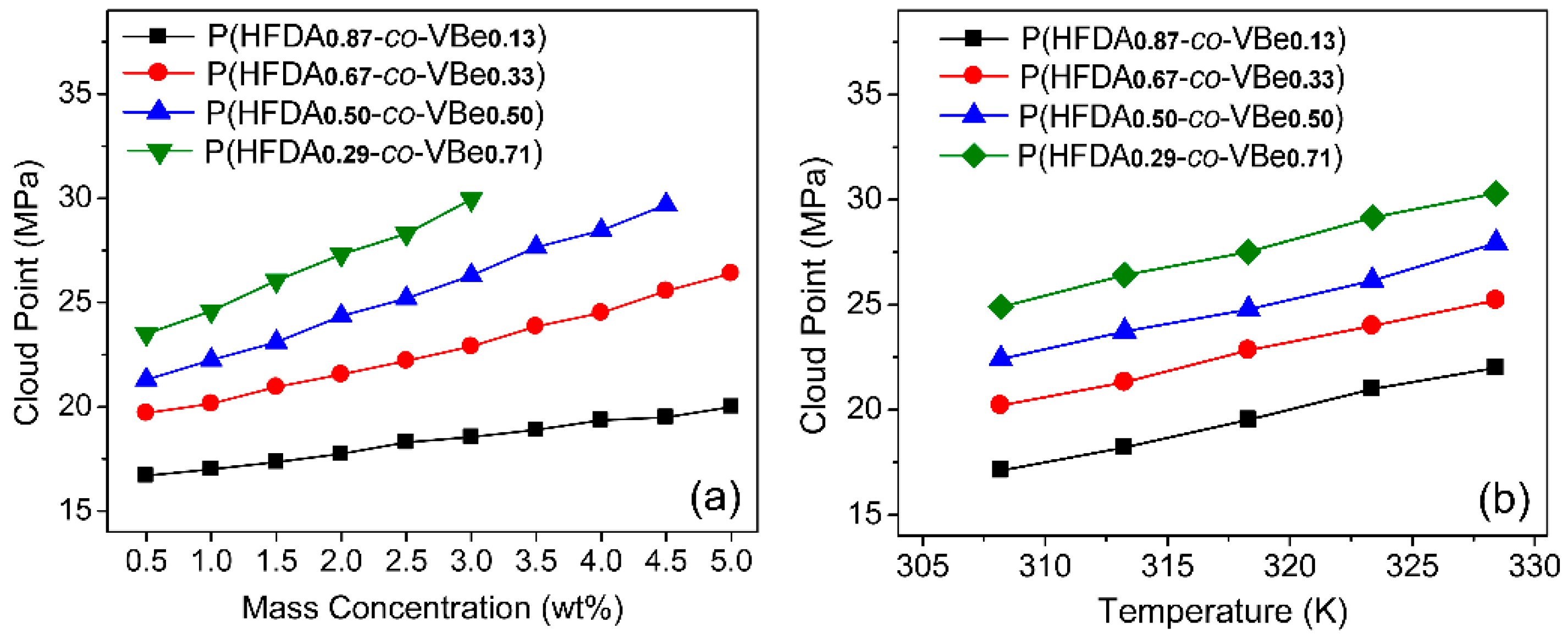
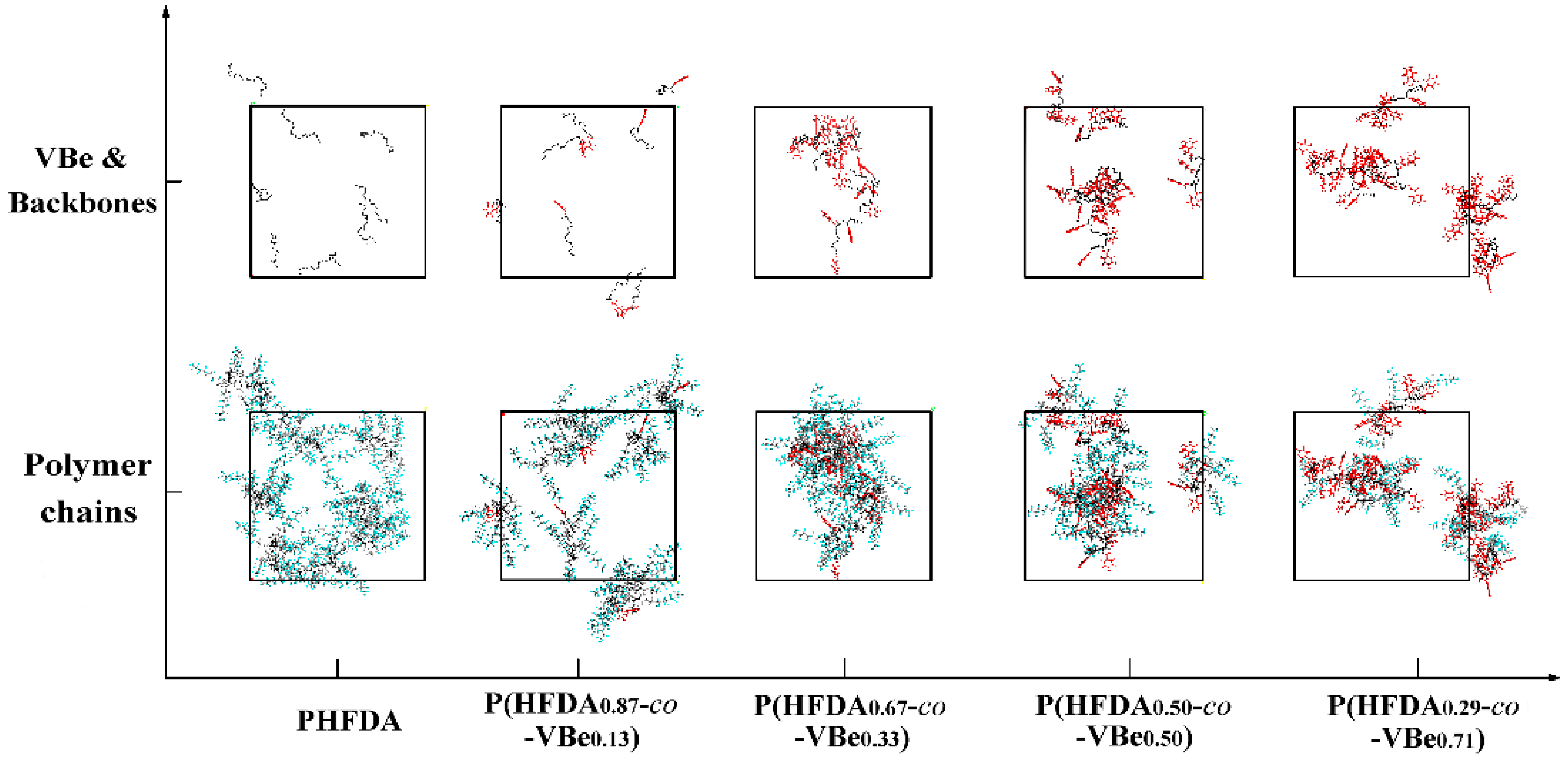
3.2. Solubility of Co-Polymers in SC-CO2
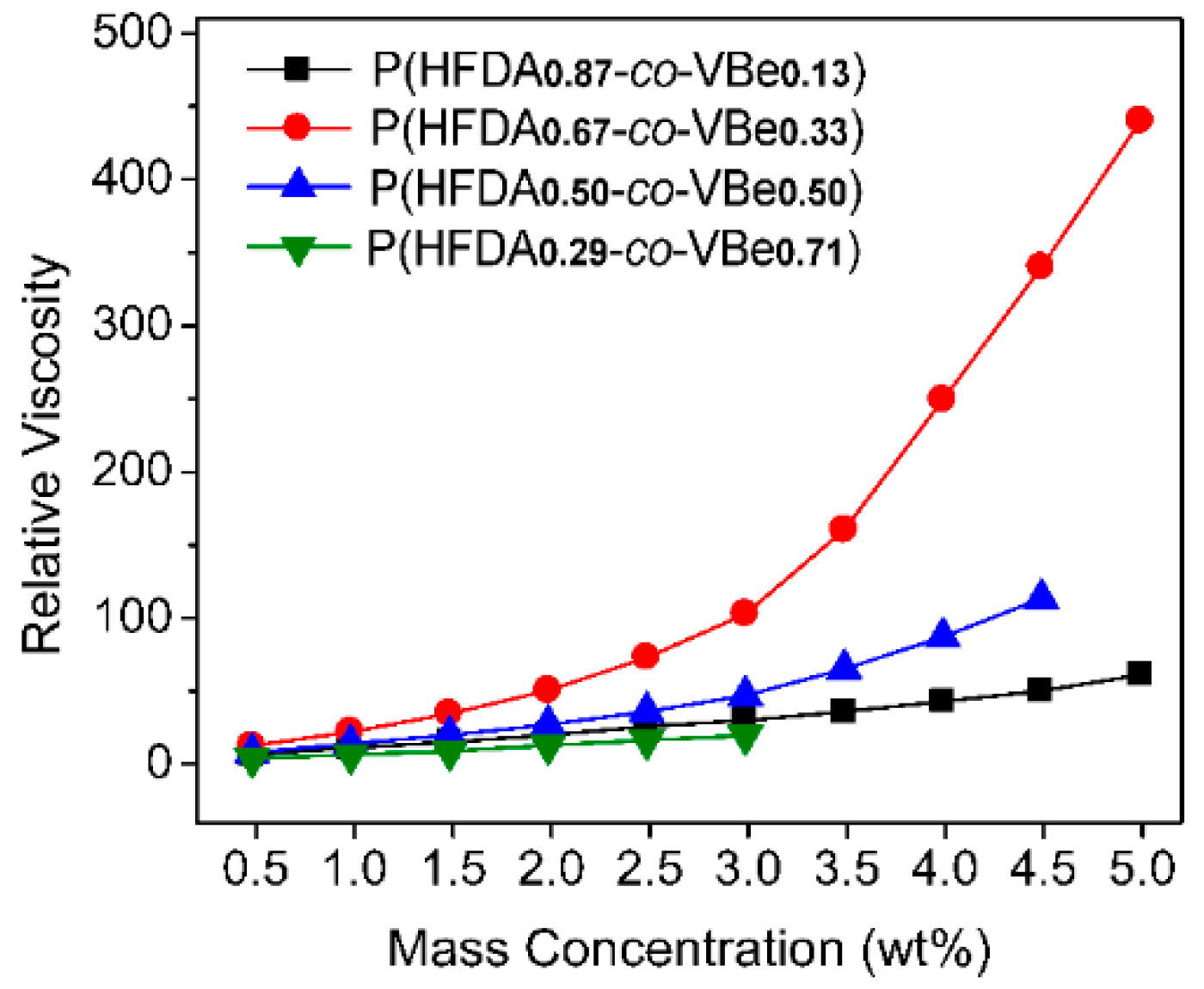
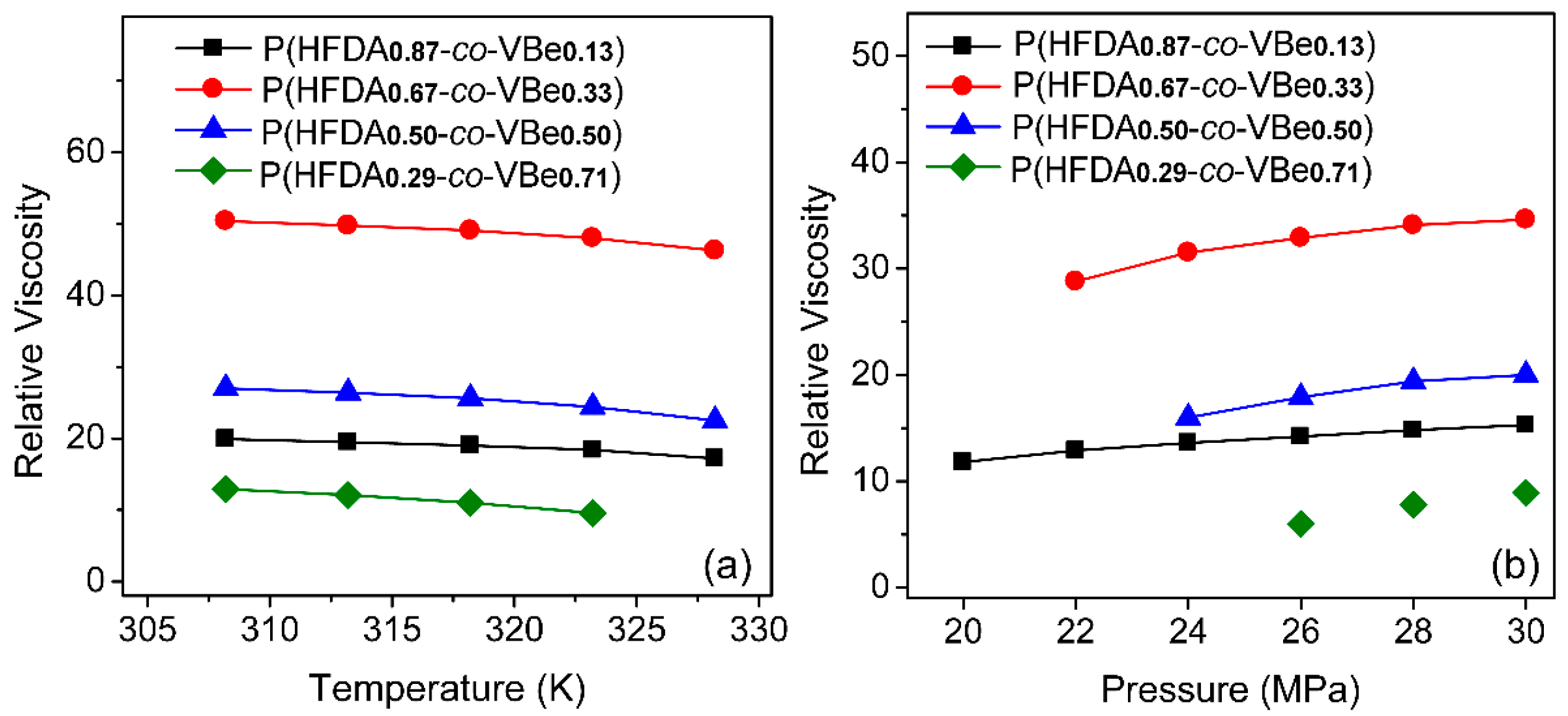
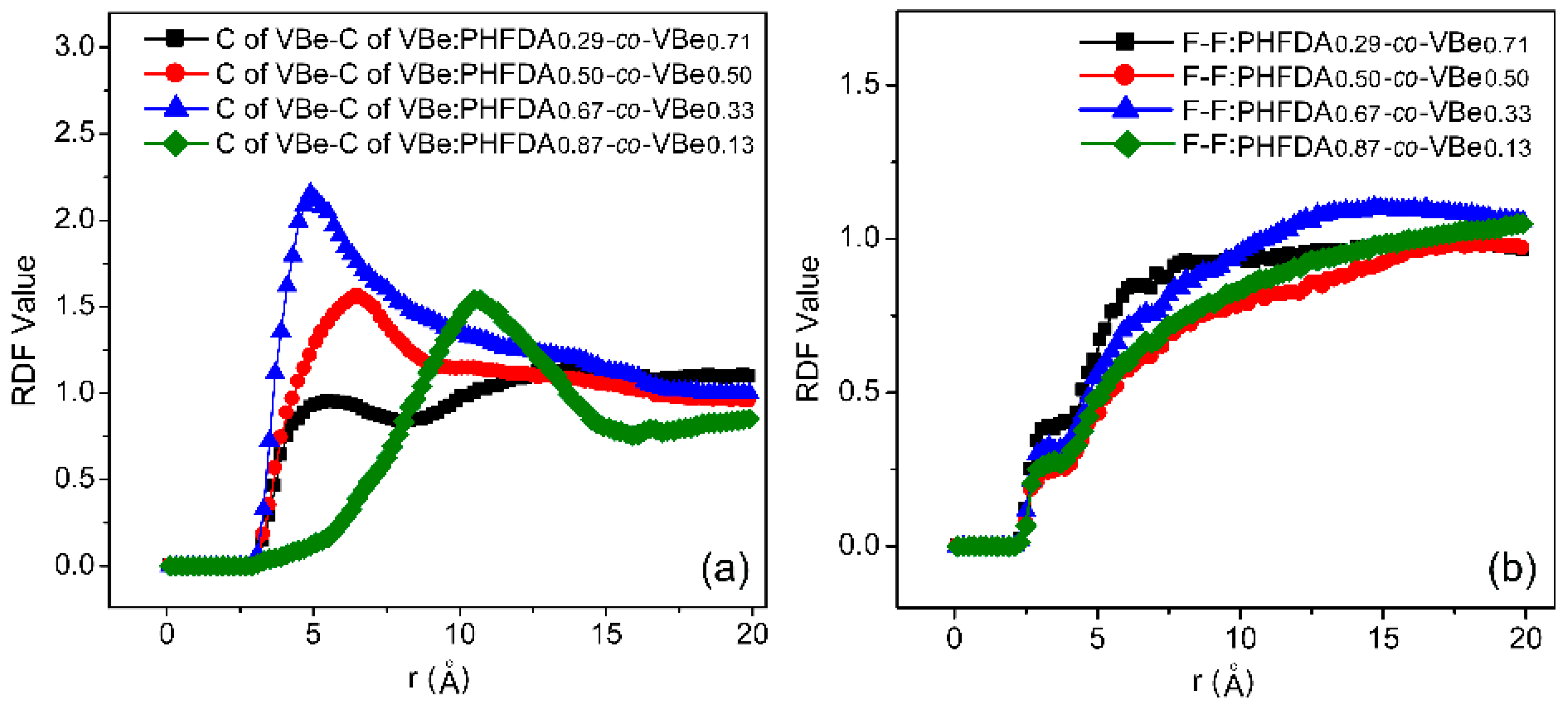
3.3. Thickening Capability of Co-Polymers in SC-CO2
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sampath, K.H.S.M.; Perera, M.S.A.; Ranjith, P.G.; Matthai, S.K.; Rathnaweer, T.; Zhang, G.; Tao, X. CH4-CO2 gas exchange and supercritical CO2 based hydraulic fracturing as CBM production-accelerating techniques: A review. J. CO2 Util. 2017, 22, 212–230. [Google Scholar] [CrossRef]
- Hou, L.; Sun, B.; Geng, X.; Jiang, T.; Wang, Z. Study of the slippage of particle/supercritical CO2 two-phase flow. J. Supercrit. Fluids 2017, 120, 173–180. [Google Scholar] [CrossRef]
- Middleton, R.S.; Carey, J.W.; Currier, R.P.; Hyman, D.; Kang, Q.; Karra, S.; Jiménez-Martínez, J.M.; Porter, L.; Viswanathan, H.S. Shale gas and non-aqueous fracturing fluids: Opportunities and challenges for supercritical CO2. Appl. Energy 2015, 147, 500–509. [Google Scholar] [CrossRef]
- Xu, J. CO2 Thickening Agents for Reduced CO2 Mobility; University of Pittsburgh: Pittsburgh, PA, USA, 2003. [Google Scholar]
- Hou, L.; Sun, B.; Wang, Z.; Li, Q. Experimental study of particle settling in supercritical carbon dioxide. J. Supercrit. Fluids 2015, 100, 121–128. [Google Scholar] [CrossRef]
- Xu, J.; Wlaschin, A.; Enick, R.M. Thickening carbon dioxide with the fluoroacrylate-styrene copolymer. SPE J. 2003, 8, 85–91. [Google Scholar] [CrossRef]
- Hou, L.; Jiang, T.; Liu, H.; Geng, X.; Sun, B.; Li, G.; Meng, S. An evaluation method of supercritical CO2 thickening result for particle transporting. J. CO2 Util. 2017, 21, 247–252. [Google Scholar] [CrossRef]
- O’Neill, M.L.; Cao, Q.; Fang, A.M.; Johnston, K.P.; Wilkinson, S.P.; Smith, C.D.; Kerschner, J.L.; Jureller, S.H. Solubility of Homopolymers and Copolymers in Carbon Dioxide. Ind. Eng. Chem. Res. 1998, 37, 3067–3079. [Google Scholar] [CrossRef]
- Sarbu, T.; Styranec, T.J.; Beckman, E.J. Design and Synthesis of Low Cost, Sustainable CO2-philes. Ind. Eng. Chem. Res. 2000, 39, 4678–4683. [Google Scholar] [CrossRef]
- Guan, Z.; Combes, J.R.; Menceloglu, Y.Z.; Desimone, J.M. Homogeneous free radical polymerizations in supercritical carbon dioxide. Macromolecules 1993, 26, 2663–2669. [Google Scholar] [CrossRef]
- McHugh, M.A.; Park, I.H.; Reisinger, J.J.; Ren, Y.; Lodge, T.P.; Hillmyer, M.A. Solubility of CF2-modified polybutadiene and polyisoprene in supercritical carbon dioxide. Macromolecules 2002, 35, 4653–4657. [Google Scholar] [CrossRef]
- Kazarian, S.G.; Vincent, M.F.; Bright, F.V.; Lioota, C.L.; Eckert, C.A. Specific intermolecular interaction of carbon dioxide with polymers. J. Am. Chem. Soc. 1996, 118, 1729–1736. [Google Scholar] [CrossRef]
- Wang, Y.; Hong, L.; Tapriyal, D.; Kim, I.C.; Paik, I.-H.; Crosthwaite, J.M.; Hamilton, A.D.; Thies, M.C.; Beckman, E.J.; Enick, R.M.; et al. Design and evaluation of nonfluorous CO2-soluble oligomers and polymers. J. Phys. Chem. B 2009, 113, 14971–14980. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, P.; Wallen, S.L. Cooperative C–H···O hydrogen bonding in CO2 lewis base complexes: Implications for solvation in supercritical CO2. J. Am. Chem. Soc. 2002, 124, 12590–12599. [Google Scholar] [CrossRef] [PubMed]
- Blatchford, M.A.; Raveendran, P.; Wallen, S.L. Spectroscopic studies of model carbonyl compounds in CO2: Evidence for cooperative C–H···O interactions. J. Phys. Chem. A 2003, 107, 10311–10323. [Google Scholar] [CrossRef]
- Blatchford, M.A.; Raveendran, P.; Wallen, S.L. Raman spectroscopic evidence for cooperative C–H···O interactions in the acetaldehyde-CO2 complex. J. Am. Chem. Soc. 2002, 124, 14818–14819. [Google Scholar] [CrossRef] [PubMed]
- Girard, E.; Tassaing, T.; Marty, J.-D.; Destarac, M. Influence of macromolecular characteristics of RAFT/MADIX poly(vinyl acetate)-based (co)polymers on their solubility in supercritical carbon dioxide. Polym. Chem. 2011, 2, 2222–2230. [Google Scholar] [CrossRef]
- Gregorowicz, J.; Wawrzyńska, E.P.; Parzuchowski, P.G.; Fraś, Z.; Rokicki, G.; Wojciechowski, K.; Wieczorek, S.A.; Wiśniewska, A.; Plichta, A.; Dąbrowski, K.; et al. Synthesis, characterization, and solubility in supercritical carbon dioxide of hyperbranched copolyesters. Macromolecules 2013, 46, 7180–7195. [Google Scholar] [CrossRef]
- Clancy, T.C.; Mattice, W.L. Miscibility of poly(vinyl chloride) melts composed of mixtures of chains with differing stereochemical composition and stereochemical sequence. Macromolecules 2001, 34, 6482–6486. [Google Scholar] [CrossRef]
- Baradie, B.; Shoichet, M.S.; Shen, Z.; McHugh, M.; Hong, L.; Wang, Y.; Johnson, J.K.; Beckman, E.J.; Enick, R.M. Synthesis and solubility of linear poly(tetrafluoroethylene-co-vinyl acetate) in dense CO2: Experimental and molecular modeling results. Macromolecules 2004, 37, 7799–7807. [Google Scholar] [CrossRef]
- Huang, Z.; Shi, C.; Xu, J.; Kilic, S.; Enick, R.M.; Beckman, E.J. Enhancement of the viscosity of carbon dioxide using styrene/fluoroacrylate copolymers. Macromolecules 2000, 33, 5437–5442. [Google Scholar] [CrossRef]
- Zhang, S.; Luo, Y.; Yang, H.; Yang, H.-J.; Tan, B. Functional oligo(vinyl acetate) bearing bipyridine moieties by RAFT polymerization and extraction of metal ions in supercritical carbon dioxide. Polym. Chem. 2013, 4, 3507–3513. [Google Scholar] [CrossRef]
- Foltran, S.; Maisonneuve, L.; Cloutet, E.; Gadenne, B.; Alfos, C.; Tassaing, T.; Cramail, H. Solubility in CO2 and swelling studies by in situ IR spectroscopy of vegetable-based epoxidized oils as polyurethane precursors. Polym. Chem. 2012, 3, 525–532. [Google Scholar] [CrossRef]
- Jennings, J.; Bassett, S.P.; Hermida-Merino, D.; Portale, G.; Bras, W.; Knight, L.; Titman, J.; Higuchi, T.; Jinnaic, H.; Howdle, S.M. How does dense phase CO2 influence the phase behavior of block copolymers synthesised by dispersion polymerisation? Polym. Chem. 2016, 7, 905–916. [Google Scholar] [CrossRef]
- Sarbu, T.; Styranec, T.; Beckman, E.J. Non-fluorous polymers with very high solubility in supercritical CO2 down to low pressures. Nature 2000, 405, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Kilic, S.; Michalik, S.; Wang, Y.; Johnson, J.K.; Enick, R.M.; Beckman, E.J. Effect of grafted lewis base groups on the phase behavior of model Poly(dimethyl siloxanes) in CO2. Ind. Eng. Chem. Res. 2003, 42, 6415–6424. [Google Scholar] [CrossRef]
- Maisonneuve, L.; Lebarbe, T.; Grau, E.; Cramail, H. Structure-properties relationship of fatty acid-based thermoplastics as synthetic polymer mimics. Polym. Chem. 2013, 4, 5472–5517. [Google Scholar] [CrossRef]
- Girard, E.; Liu, X.; Marty, J.-D.; Destarac, M. RAFT/MADIX (co)polymerization of vinyl trifl uoroacetate: A means to many ends. Polym. Chem. 2014, 5, 1013–1022. [Google Scholar] [CrossRef]
- Kilic, S.; Wang, Y.; Johnson, J.K.; Beckman, E.J.; Enick, R.M. Influence of tert-amine groups on the solubility of polymers in CO2. Polymer 2009, 50, 2436–2444. [Google Scholar] [CrossRef][Green Version]
- Hu, D.; Sun, S.; Yuan, P.; Zhao, L.; Liu, T. Evaluation of CO2-philicity of poly(vinyl acetate) and poly(vinyl acetate-alt-maleate) copolymers through molecular modeling and dissolution behavior measurement. J. Phys. Chem. B 2015, 119, 3194–3204. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Sun, S.; Yuan, P.; Zhao, L.; Liu, T. Effect of molecular weight on CO2-philicity of poly(vinyl acetate) with different molecular chain structure. J. Supercrit. Fluids 2016, 118, 96–106. [Google Scholar] [CrossRef]
- Pandiyan, S.; Brown, D.; Neyertz, S.; van der Vegt, N.F. Carbon dioxide solubility in three fluorinated polyimides studied by molecular dynamics simulations. Macromolecules 2010, 43, 2605–2621. [Google Scholar] [CrossRef]
- Hu, D.; Sun, S.; Yuan, P.; Zhao, L.; Liu, T. Exploration of CO2-philicity of poly(vinyl acetate-co-alkyl vinyl ether) through molecular modeling and dissolution behavior measurement. J. Phys. Chem. B 2015, 119, 12490–12501. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Sun, B.; Li, Y.; Fan, H.; Gao, Y.; Sun, H.; Li, G. Microcosmic understanding on thickening capability of copolymers in supercritical carbon dioxide: The key role of π-π stacking. RSC Adv. 2017, 7, 34567–34573. [Google Scholar] [CrossRef]
- Birkin, N.A.; Wildig, O.J.; Howdle, S.M. Effects of poly(vinyl pivalate)-based stabilizer architecture on CO2-solubility and stabilising ability in dispersion polymerisation of N-vinyl pyrrolidone. Polym. Chem. 2013, 4, 3791–3799. [Google Scholar] [CrossRef]
- Girard, E.; Tassaing, T.; Ladavière, C.; Marty, J.-D.; Destarac, M. Distinctive features of solubility of RAFT/MADIX-derived partially trifluoromethylated poly(vinyl acetate) in supercritical CO2. Macromolecules 2012, 45, 9674–9681. [Google Scholar] [CrossRef]
- Girard, E.; Tassaing, T.; Camy, S.; Condoret, J.S.; Marty, J.D.; Destarac, M. Enhancement of poly(vinyl ester) solubility in supercritical CO2 by partial fluorination: The key role of polymer–polymer interactions. J. Am. Chem. Soc. 2012, 134, 11920–11923. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Jones, N.; Lyle, S.; Venkataramani, S.; Clark, J.; Kranbuehl, D. Laser light-scattering molecular weight analysis of a poly(fluoroacrylate). J. Appl. Polym. Sci. 2010, 91, 3447–3454. [Google Scholar] [CrossRef]
- Evain, K.; Illien, B.; Chabanel, M.; Beignon, M. Comparison of proutière and Carr-Zimm theories for static light-scattering molecular weight determination: Application to surfactants in solution. J. Phys. Chem. B 2007, 111, 1597–1603. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Monsuur, F.; Denoulet, B.; Dobrak, A.; Vandezande, P.; Vankelecom, I.J. Evaporative light scattering detector: Toward a general molecular weight cutoff characterization of nanofiltration membranes. Anal. Chem. 2009, 81, 1801–1809. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mya, K.Y.; Ni, X.; He, C.; Leong, K.W.; Li, J. Dynamic and static light scattering studies on self-aggregation behavior of biodegradable amphiphilic poly(ethylene oxide)-poly[(R)-3-hydroxybutyrate]−poly(ethylene oxide) triblock copolymers in aqueous solution. J. Phys. Chem. B 2006, 110, 5920–5926. [Google Scholar] [CrossRef] [PubMed]
- Pencer, J.; Hallett, F.R. Effects of vesicle size and shape on static and dynamic light scattering measurements. Langmuir 2003, 19, 7488–7497. [Google Scholar] [CrossRef]
- Schatz, C.; Pichot, C.; Delair, T.; Viton, A.C.; Domard, A. Static light scattering studies on chitosan solutions: From macromolecular chains to colloidal dispersions. Langmuir 2003, 19, 9896–9903. [Google Scholar] [CrossRef]
- And, GL.; Yan, X.; Duncan, S. Polystyrene-block-polyisoprene nanofiber fractions. 1. Preparation and static light-scattering study. Macromolecules 2002, 35, 9788–9793. [Google Scholar]
- Lavi, B.; Marmur, A.; Bachmann, J. Porous media characterization by the Two-Liquid method: Effect of dynamic contact angle and inertia. Langmuir 2008, 24, 1918–1923. [Google Scholar] [CrossRef] [PubMed]
- Tamai, Y.; Matsunaga, T.; Horiuchi, K. Surface energy analysis of several organic polymers: Comparison of the two-liquid-contact-angle method with the one-liquid-contact-angle method. J. Colloid Interface Sci. 1977, 60, 112–116. [Google Scholar] [CrossRef]
- Katoh, K.; Yu, T.; Yamamoto, M.; Azuma, T.; Fujita, H. A new method for measuring contact angle and liquid surface tension applying detachment of Two-Dimensional meniscus. J. Colloid Interface Sci. 1998, 202, 54–62. [Google Scholar] [CrossRef]
- Tan, B.; Bray, C.L.; Cooper, A.I. Fractionation of poly(vinyl acetate) and the phase behavior of end-group modified oligo(vinyl acetate)s in CO2. Macromolecules 2009, 42, 7945–7952. [Google Scholar] [CrossRef]
- Shi, C.; Huang, Z.; Beckman, E.J.; Enick, R.M.; Kim, S.-Y.; Curran, D.P. Semi-Fluorinated trialkyltin fluorides and fluorinated telechelic ionomers as viscosity-enhancing agents for carbon dioxide. Ind. Eng. Chem. Res. 2001, 40, 908–913. [Google Scholar] [CrossRef]
- Matsuda, H.; Yamamoto, H.; Kurihara, K.; Tochigi, K. Computer-aided reverse design for ionic liquids by QSPR using descriptors of group contribution type for ionic conductivities and viscosities. Fluid Phase Equilibria 2007, 261, 434–443. [Google Scholar] [CrossRef]
- Fang, Z.; Qiao, Y.; Di, Z.; Huo, Y.; Ma, P.; Xia, S. Viscosities of p-Xylene, Acetic Acid, and p-Xylene + Acetic Acid at (313.15 to 473.15)K and (0.10 to 3.20)MPa: Determined by the Rolling-Ball Method. J. Chem. Eng. Data 2008, 53, 2787–2792. [Google Scholar] [CrossRef]
- Costa, G.M.N.; Rocha, P.S.D.M.V.; Ribeiro, A.L.C.; de Menezes, P.R.F.; de Lima, R.C.A.; Costa, P.U.O.; de Almeida Rodrigues, E. An improved method for calculating CO2 minimum miscibility pressure based on solubility parameter. J. Petrol. Sci. Eng. 2012, 98–99, 144–155. [Google Scholar] [CrossRef]
- Zhou, G.; Chen, J.; Wang, M.; Zhang, M.; Guo, J.; Shen, S.; Liu, Z.; Liu, Z.; Jiang, J.; Lu, J. Insight into the role of intermolecular interactions on the enhanced solubility of fluorinated epoxide oligomers in supercritical CO2. Green Chem. 2015, 17, 4489–4498. [Google Scholar] [CrossRef]
- Chremos, A.; Glynos, E.; Green, P.F. Structure and dynamical intra-molecular heterogeneity of star polymer melts above glass transition temperature. J. Chem. Phys. 2015, 142, 572–946. [Google Scholar] [CrossRef] [PubMed]
- Kolev, V.; Freger, V. Hydration, porosity and water dynamics in the polyamide layer ofreverse osmosis membranes: A molecular dynamics study. Polymer 2014, 55, 1420–1426. [Google Scholar]
- Hou, M.; Zhang, X.; Han, B.; Song, J.; Liu, G.; Zhang, Z.; Zhang, J. New topic of supercritical fluids: Local activity coefficients of supercritical solvent and cosolvent around solute. J. Chem. Phys. 2008, 10, 104510. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, X.; Liu, Z.; Hu, D.; Zhang, C.; Xue, D.; Xiao, J.; Liu, Z. Intermolecular-interaction-dominated solvation behaviors of liquid monomers and polymers in gaseous and supercritical carbon dioxide. Macromolecules 2012, 45, 4907–4919. [Google Scholar] [CrossRef]
- Lal, M.; Plummer, M.; Smith, W. Solvent density effects on the solvation behavior and configurational structure of bare and passivated 38-Atom gold nanoparticle in supercritical ethane. J. Phys. Chem. B 2006, 110, 20879–20888. [Google Scholar] [CrossRef] [PubMed]
- Gestoso, P.; Brisson, J. Towards the simulation of poly(vinyl phenol)/poly(vinyl methyl ether) blends by atomistic molecular modelling. Polymer 2003, 44, 2321–2329. [Google Scholar] [CrossRef]
- Fu, Y.; Liao, L.; Yang, L.; Lan, Y.; Mei, L.; Liu, Y.; Hu, S. Molecular dynamics and dissipative particle dynamics simulations for prediction of miscibility in polyethylene terephthalate/polylactide blends. Mol. Simul. 2013, 39, 415–422. [Google Scholar] [CrossRef]
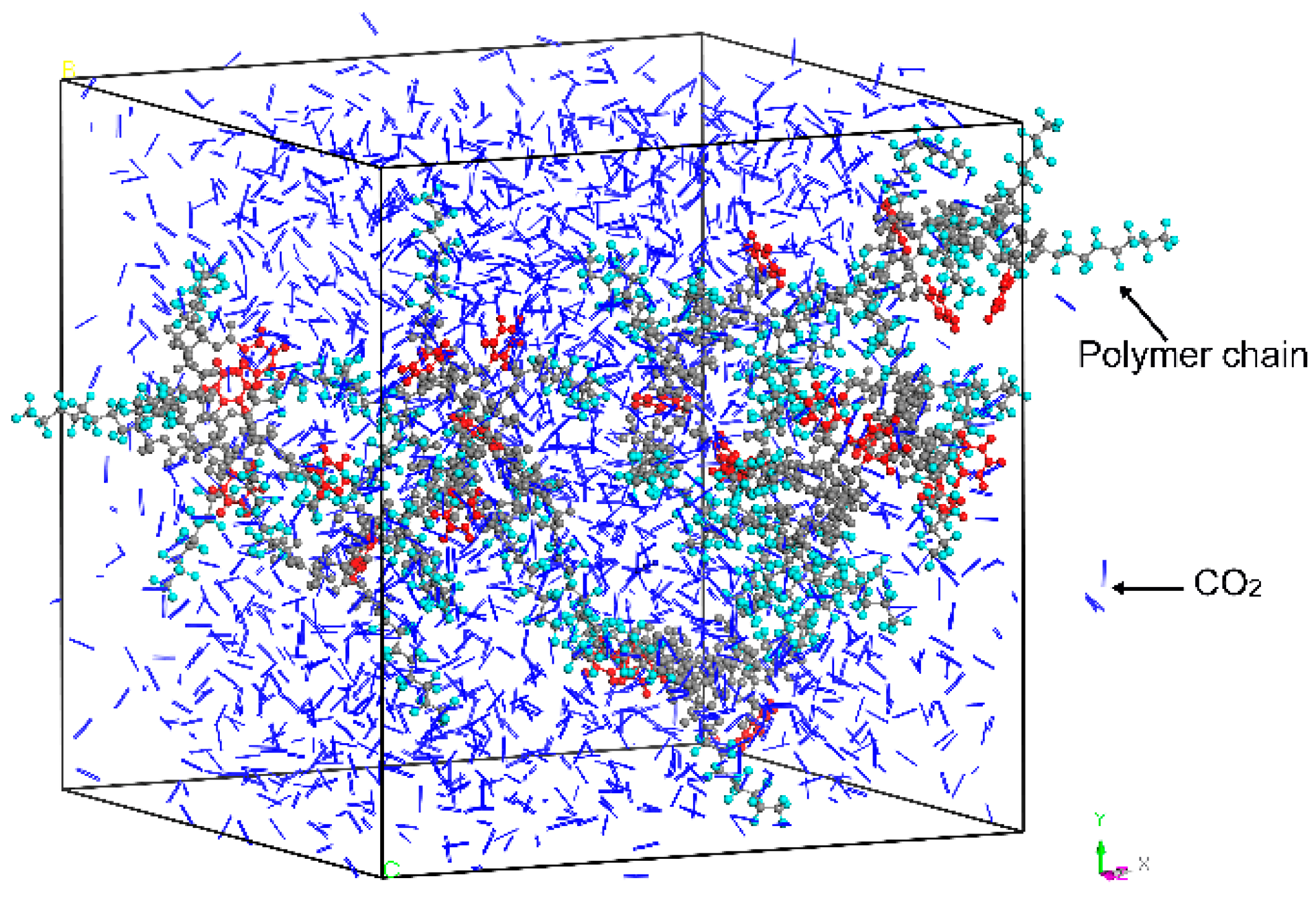
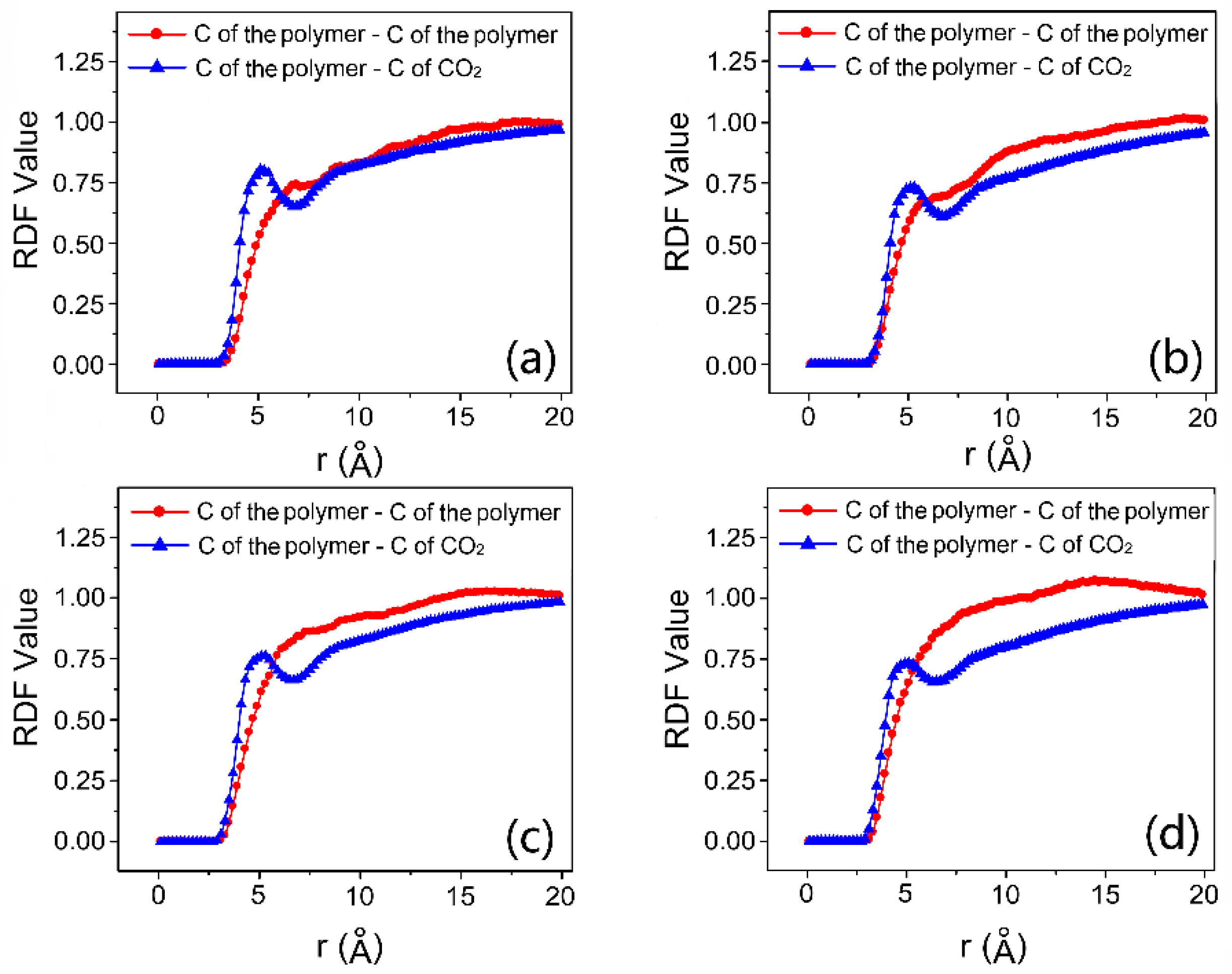
| Run | System | Mn of One Chain | Number of Chains | Number of HFDA Units in One Chain | Number of VBe Units in One Chain | Number of CO2 |
|---|---|---|---|---|---|---|
| 1 | CO2+PHFDA | 3630 | 8 | 7 | 0 | 2000 |
| 2 | CO2+P(HFDA0.87-co-VBe0.13) | 3777 | 8 | 7 | 1 | 2000 |
| 3 | CO2+P(HFDA0.67-co-VBe0.33) | 3555 | 8 | 6 | 3 | 2000 |
| 4 | CO2+P(HFDA0.50-co-VBe0.50) | 3333 | 8 | 5 | 5 | 2000 |
| 5 | CO2+P(HFDA0.29-co-VBe0.71) | 3556 | 8 | 4 | 10 | 2000 |
| 6 | PHFDA | 3630 | 8 | 7 | 0 | 0 |
| 7 | P(HFDA0.87-co-VBe0.13) | 3777 | 8 | 7 | 1 | 0 |
| 8 | P(HFDA0.67-co-VBe0.33) | 3555 | 8 | 6 | 3 | 0 |
| 9 | P(HFDA0.50-co-VBe0.50) | 3333 | 8 | 5 | 5 | 0 |
| 10 | P(HFDA0.29-co-VBe0.71) | 3556 | 8 | 4 | 10 | 0 |
| Run | Sample | Mw (g/mol) a | Composition b | Feed Ratio | Tg (°C) c | γ (mN/m−1) d |
|---|---|---|---|---|---|---|
| HFDA:VBe | HFDA:VBe | |||||
| 1 | P(HFDA0.87-co-VBe0.13) | 152,100 | 87:33 | 85:15 | 52.6 | 27.4 |
| 2 | P(HFDA0.67-co-VBe0.33) | 145,600 | 67:33 | 70:30 | 55.4 | 29.7 |
| 3 | P(HFDA0.50-co-VBe0.50) | 142,900 | 50:50 | 50:50 | 58.1 | 32.5 |
| 4 | P(HFDA0.29-co-VBe0.71) | 150,400 | 29:71 | 30:70 | 62.8 | 35.8 |
| Run | System | Echain−CO2 | Echain | ECO2 | Einter |
|---|---|---|---|---|---|
| 1 | CO2+PHFDA | 4341.63 | 1357.22 | 1556.01 | 1428.40 |
| 2 | CO2+P(HFDA0.87-co-VBe0.13) | 3986.58 | 1089.34 | 1698.63 | 1198.61 |
| 3 | CO2+P(HFDA0.67-co-VBe0.33) | 3643.29 | 773.12 | 1805.42 | 1064.75 |
| 4 | CO2+P(HFDA0.50-co-VBe0.50) | 3327.36 | 680.63 | 1718.76 | 927.98 |
| 5 | CO2+P(HFDA0.29-co-VBe0.71) | 3438.42 | 769.19 | 1960.29 | 708.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, W.; Sun, B.; Li, Y.; Huang, X.; Fan, H.; Zhao, X.; Sun, H.; Sun, W. Thickening Supercritical CO2 with π-Stacked Co-Polymers: Molecular Insights into the Role of Intermolecular Interaction. Polymers 2018, 10, 268. https://doi.org/10.3390/polym10030268
Sun W, Sun B, Li Y, Huang X, Fan H, Zhao X, Sun H, Sun W. Thickening Supercritical CO2 with π-Stacked Co-Polymers: Molecular Insights into the Role of Intermolecular Interaction. Polymers. 2018; 10(3):268. https://doi.org/10.3390/polym10030268
Chicago/Turabian StyleSun, Wenchao, Baojiang Sun, Ying Li, Xiaonan Huang, Haiming Fan, Xinxin Zhao, Haoyang Sun, and Wenxia Sun. 2018. "Thickening Supercritical CO2 with π-Stacked Co-Polymers: Molecular Insights into the Role of Intermolecular Interaction" Polymers 10, no. 3: 268. https://doi.org/10.3390/polym10030268
APA StyleSun, W., Sun, B., Li, Y., Huang, X., Fan, H., Zhao, X., Sun, H., & Sun, W. (2018). Thickening Supercritical CO2 with π-Stacked Co-Polymers: Molecular Insights into the Role of Intermolecular Interaction. Polymers, 10(3), 268. https://doi.org/10.3390/polym10030268