Preparation of Water-soluble Polyion Complex (PIC) Micelles Covered with Amphoteric Random Copolymer Shells with Pendant Sulfonate and Quaternary Amino Groups
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of P(SA)91
2.3. Preparation of P(SA)91S67
2.4. Preparation of P(SA)91A88
2.5. Preparation of PIC micelles
2.6. Measurements
3. Results and Discussion
3.1. Preparation of P(SA)91S67 and P(SA)91A88
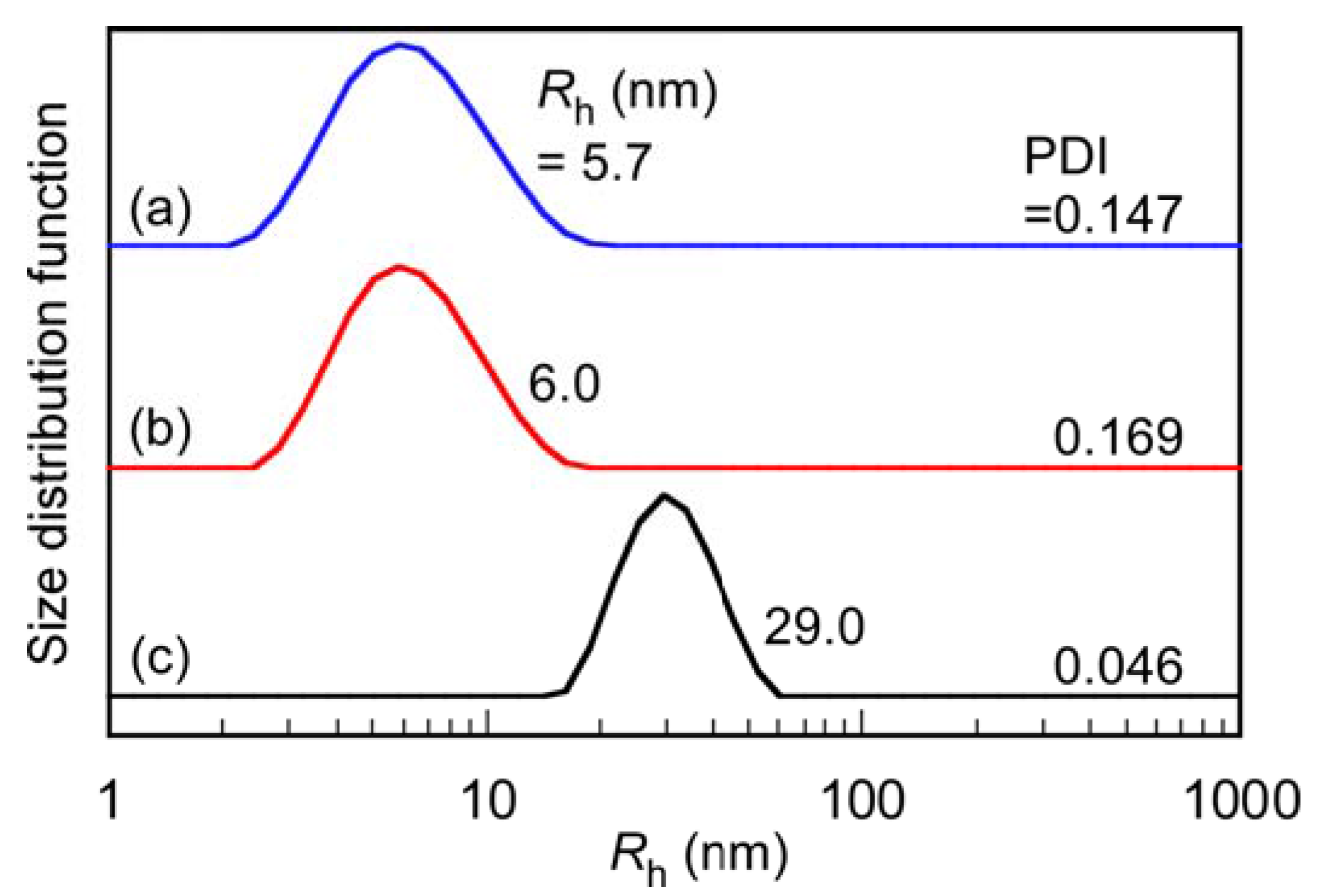
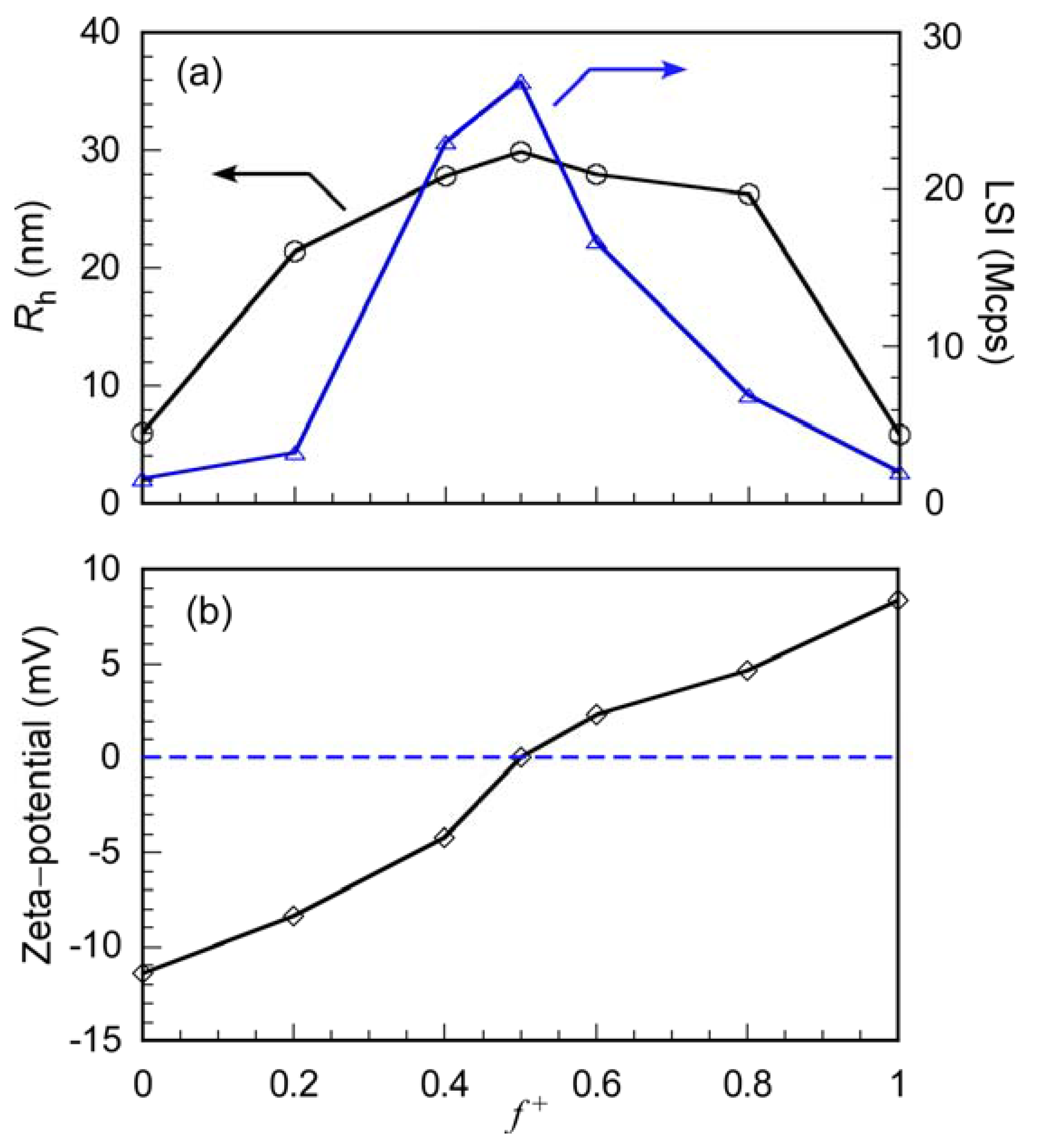
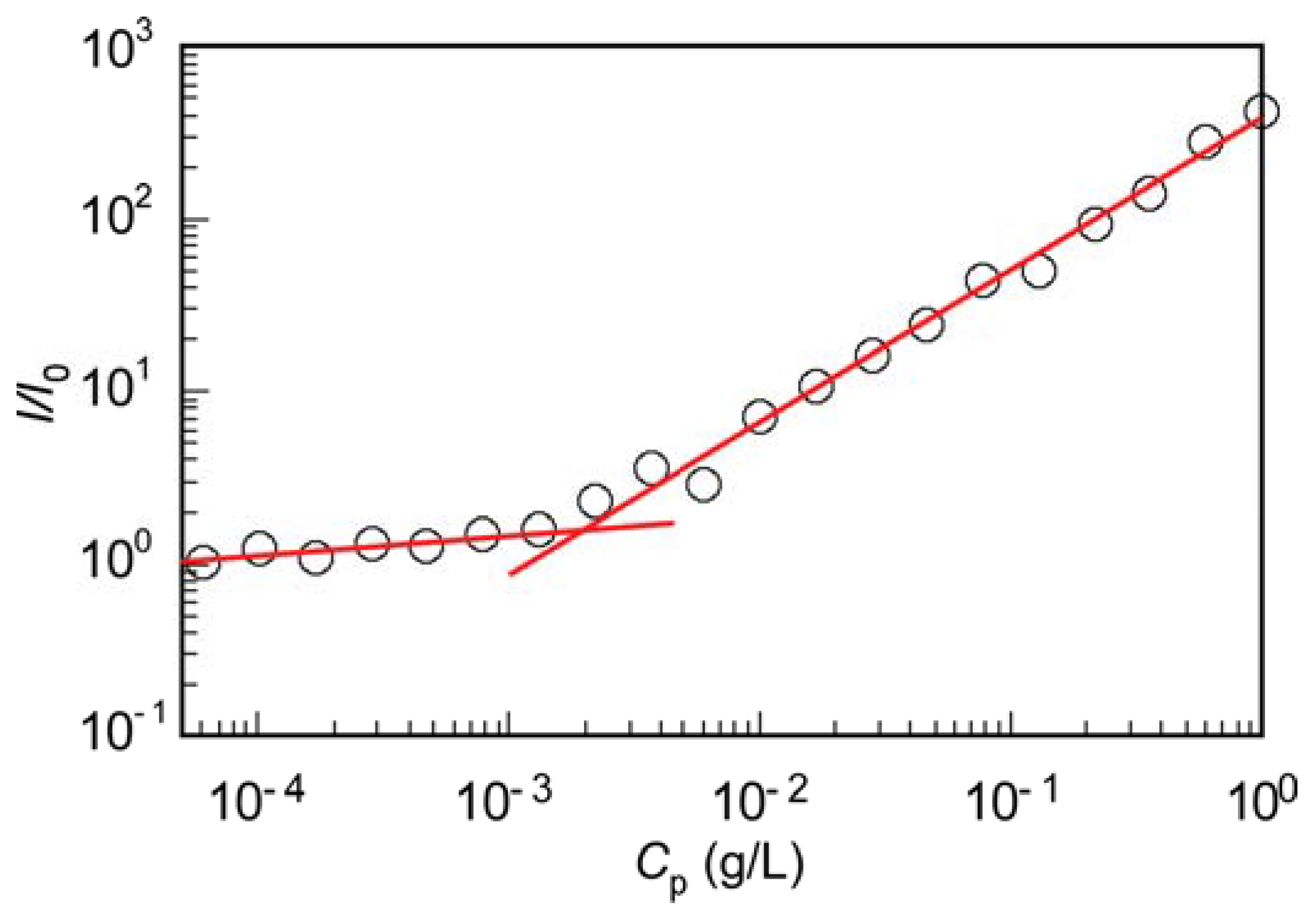
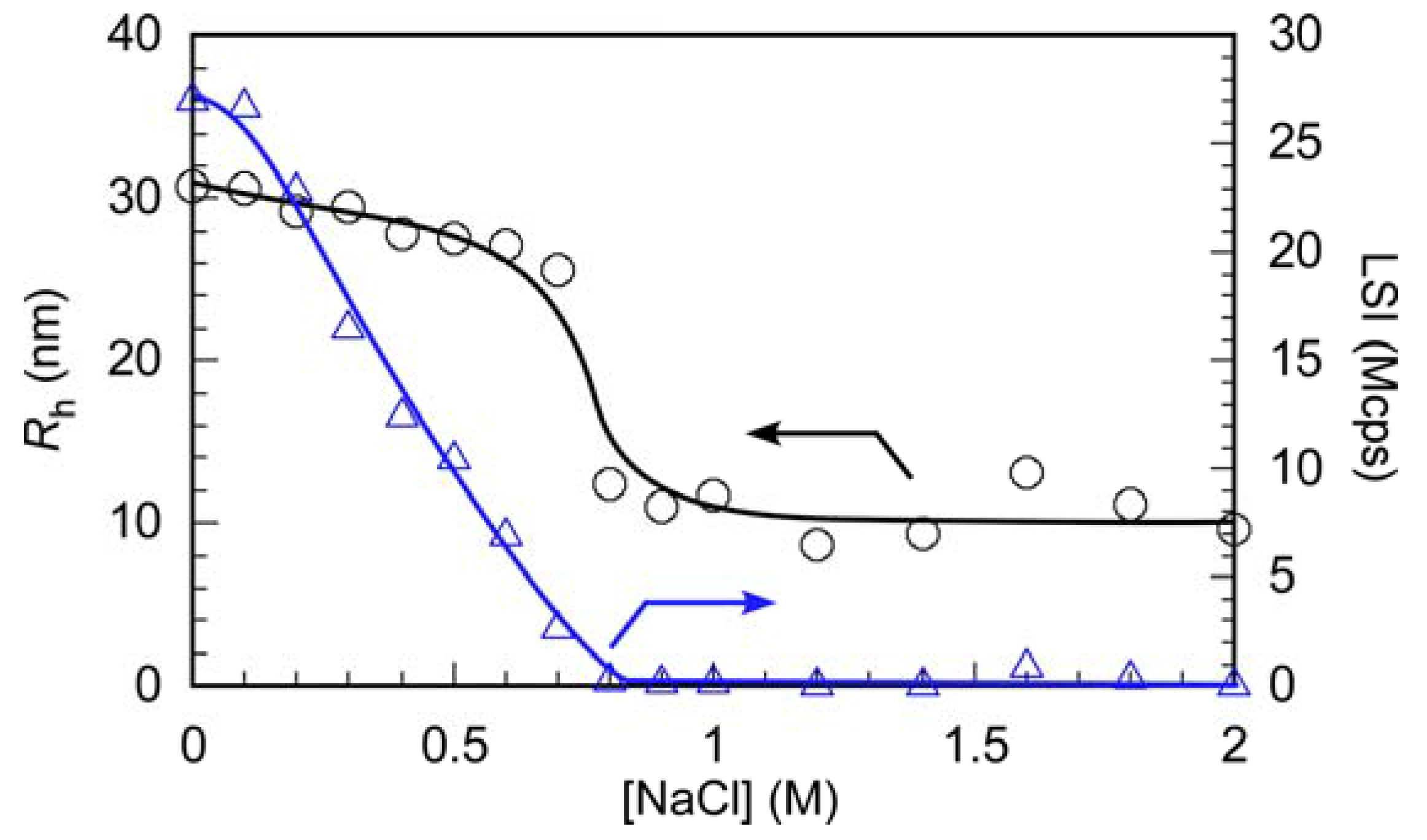
3.2. Preparation and Characterization of PIC Micelles
3.3. Interactions between PIC Micelles and Proteins
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Michaels, A.S.; Miekka, R.G. Polycation-polyanion complexes: preparation and properties of poly(vinylbenzyltrimethylammonium) poly(styrensulfonate). J. Phys. Chem. 1961, 65, 1765–1773. [Google Scholar] [CrossRef]
- Wibowo, A.; Osada, K.; Matsuda, H.; Anraku, Y.; Hirose, H.; Kishimura, A.; Kataoka, K. Morphology control in water of polyion complex nanoarchitectures of double-hydrophilic charged block copolymers through composition tuning and thermal treatment. Macromolecules 2014, 47, 3086–3092. [Google Scholar] [CrossRef]
- Yusa, S.; Yokoyama, Y.; Morishima, Y. Synthesis of oppositely charged block copolymers of poly(ethylene glycol) via reversible addition–fragmentation chain transfer (RAFT) radical polymerization and characterization of their polyion complex (PIC) micelles in water. Macromolecules 2009, 42, 376–383. [Google Scholar] [CrossRef]
- Oparin, A.I. Origin and evolution of metabolism. Comp. Biochem. Physiol. 1962, 4, 371–377. [Google Scholar] [CrossRef]
- Oishi, M.; Nagasaki, Y.; Itaka, K.; Nishiyama, N.; Kataoka, K. Lactosylated poly(ethylene glycol)-siRNA conjugate through acid-labile β-thiopropionate linkage to construct pH-sensitive polyion complex micelles achieving enhanced gene silencing in hepatoma cells. J. Am. Chem. Soc. 2005, 127, 1624–1625. [Google Scholar] [CrossRef] [PubMed]
- Van der Gucht, J.; Spruijt, E.; Lemmers, M.; Cohen Stuart, M.A. Polyelectrolyte complexes: Bulk phases and colloidal systems. J. Colloid Interface Sci. 2011, 361, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Müller, M. Sizing, shaping and pharmaceutical applications of polyelectrolyte complex nanoparticles. Adv. Polym. Sci. 2014, 256, 197–260. [Google Scholar]
- Pergushov, D.V.; Mueller, A.H.; Schacher, F.H. Micellar interpolyelectrolyte complexes. Chem. Soc. Rev. 2012, 41, 6888–6901. [Google Scholar] [CrossRef] [PubMed]
- Voets, I.K.; de Keizer, A.; Stuart, M.A.C. Complex coacervate core micelles. Adv. Colloid Interface Sci. 2009, 147, 300–318. [Google Scholar] [CrossRef] [PubMed]
- Steinschulte, A.A.; Gelissen, A.P.H.; Jung, A.; Brugnoni, M.; Caumanns, T.; Lotze, G.; Mayer, J.; Pergushov, D.V.; Plamper, F.A. Facile screening of various micellar morphologies by blending miktoarm stars and diblock copolymers. ACS Macro Lett. 2017, 6, 711–715. [Google Scholar] [CrossRef]
- Dähling, C.; Lotze, G.; Mori, H.; Pergushov, D.V.; Plamper, F.A. Thermoresponsive segments retard the formation of equilibrium micellar interpolyelectrolyte complexes by detouring to various intermediate structures. J. Phys. Chem. B 2017, 121, 6739–6748. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Nishiuchi, M.; Inoue, M.; Ishihara, K.; Sanada, Y.; Sakurai, K.; Yusa, S. Preparation and characterization of polyion complex micelles with phoshobetaine shells. Langmuir 2013, 29, 9651–9661. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Ishihara, K.; Yusa, S. Preparation of giant polyion complex vesicles (G-PICsomes) with polyphosphobetaine shells composed of oppositely charged diblock copolymers. Chem. Lett. 2017, 46, 824–827. [Google Scholar] [CrossRef]
- Nakai, K.; Ishihara, K.; Kappl, M.; Fujii, S.; Nakamura, Y.; Yusa, S. Polyion complex vesicles with solvated phosphobetaine shells formed from oppositely charged diblock copolymers. Polymers 2017, 9, 49. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Ishihara, K. Cell membrane-inspired phospholipid polymers for developing medical devices with excellent biointerfaces. Sci. Technol. Adv. Mater. 2012, 13, 064101. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Ijuin, M.; Mikami, A.; Nakabayashi, N.; Ishihara, K. Behavior of blood cells in contact with water-soluble phospholipid polymer. J. Biomed. Mater. Res. 1999, 6, 360–367. [Google Scholar] [CrossRef]
- Ishihara, K.; Ueda, T.; Nakabayasi, N. Preparation of phospholipid polymers and their properties as hydrogel sheet. Polym. J. 1990, 22, 355–360. [Google Scholar] [CrossRef]
- Ostuni, E.; Chapman, R.G.; Holmlin, R.E.; Takayama, S.; Whitesides, G.M. A survey of structure-Property relationships of surfaces that resist the adsorption of protein. Langmuir 2001, 17, 5605–5620. [Google Scholar] [CrossRef]
- Holmlin, R.E.; Chen, X.; Chapman, R.G.; Takayama, S.; Whitesides, G.M. Zwitterionic SAMs that resist nonspecific adsorption of protein from aqueous buffer. Langmuir 2001, 17, 2841–2850. [Google Scholar] [CrossRef]
- Zhao, T.; Chen, K.; Gu, H. Investigations on the interactions of proteins with polyampholyte-coated magnetite nanoparticles. J. Phys. Chem. B 2013, 117, 14129–14135. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Chen, S.; Zhang, Z.; Jiang, S. Highly protein-resistant coatings from well-defined diblock copolymers containing sulfobetaines. Langmuir 2006, 22, 2222–2226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, S.; Jiang, S. Dual-functional biomimetic materials: Nonfouling poly(carboxybetaine) with active functional groups for protein immobilization. Biomacromolecules 2006, 7, 3311–3315. [Google Scholar] [CrossRef] [PubMed]
- Muro, E.; Pons, T.; Lequeux, N.; Fragola, A.; Sanson, N.; Lenkei, Z.; Dubertret, B. Small and stable sulfobetaine zwitterionic quantum dots for functional live-cell imaging. J. Am. Chem. Soc. 2010, 132, 4556–4557. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.; Chang, Y.; Quemener, D.; Yang, H.; Jhong, J.; Ho, F.; Higuchi, A.; Chang, Y. Hemocompatibility of polyampholyte copolymers with well-defined charge bias in human blood. Langmuir 2014, 30, 6489–6496. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, R.; Yusa, S. Solution properties of amphoteric random copolymers bearing pendant sulfonate and quaternary ammonium groups with controlled structures. Langmuir 2018. [Google Scholar] [CrossRef] [PubMed]
- Mitsukami, Y.; Donovan, S.M.; Lowe, B.A.; McCormick, L.C. Water-soluble polymers. 81. Direct synthesis of hydrophilic styrenic-based homopolymers and block copolymers in aqueous solution via RAFT. Macromolecules 2001, 34, 2248–2256. [Google Scholar] [CrossRef]
- Koppel, E.D. Analysis of macromolecular polydispersity in intensity correlation spectroscopy: The method of cumulants. J. Chem. Phys. 1972, 57, 4814–4820. [Google Scholar] [CrossRef]
- Huber, K.; Bantle, S.; Lutz, P.; Burchard, W. Hydrodynamic and thermodynamic behavior of short-chain polystyrene in toluene and cyclohexane at 34.5 °C. Macromolecules 1985, 18, 1461–1467. [Google Scholar] [CrossRef]
- Konishi, T.; Yoshizaki, T.; Yamakawa, H. On the “Universal Constants” ρ and Φ. of flexible polymers. Macromolecules 1991, 24, 5614–5622. [Google Scholar] [CrossRef]
- Zimm, H.B. Apparatus and methods for measurement and interpretation of the angular variation of light scattering; Preliminary results on polystyrene solutions. J. Chem. Phys. 1948, 16, 1099–1116. [Google Scholar] [CrossRef]
- Ali, I.S.; Heuts, A.P.J.; van Herk, A.M. Controlled synthesis of polymeric nanocapsules by RAFT-based vesicle templating. Langmuir 2010, 26, 7848–7858. [Google Scholar] [CrossRef] [PubMed]
- Akcasu, A.Z.; Han, C.C. Molecular weight and temperature dependence of polymer dimensions in solution. Macromolecules 1979, 12, 276–280. [Google Scholar] [CrossRef]
- Matsuda, Y.; Kobayashi, M.; Annaka, M.; Ishihara, K.; Takahara, A. Dimensions of a free linear polymer immobilized on silica nanoparticles of a zwitterionic polymer in aqueous solutions with various ionic strength. Langmuir 2008, 28, 8772–8778. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Hou, G.; Miao, J.; Chen, D.; Jiang, M.; Zhu, L. Efficient synthesis of unimolecular polymeric janus nanoparticles and their unique self-assembly behavior in a common solvent. Macromolecules 2008, 41, 8159–8166. [Google Scholar] [CrossRef]
- Savoji, M.T.; Strandman, S.; Zhu, X.X. Switchable vesicles formed by diblock random copolymers with tunable pH-and thermo-responsiveness. Langmuir 2013, 29, 6823–6832. [Google Scholar] [CrossRef] [PubMed]
- Anraku, Y.; Kishimura, A.; Oba, M.; Yamasaki, Y.; Kataoka, K. Spontaneous formation of nanosized unilamellar polyion complex vesicles with tunable size and properties. J. Am. Chem. Soc. 2010, 132, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Santis, D.S.; Ladogana, D.R.; Diociaiuti, M.; Masci, G. Pegylated and thermosensitive polyion comples micelles by self-assembly of two oppositely and permanently charged diblock copolymers. Macromolecules 2010, 43, 1992–2001. [Google Scholar] [CrossRef]
- Maggi, F.; Ciccarelli, S.; Diociaiuti, M.; Casciardi, S.; Masci, G. Chitosan nanogels by template chemical cross-linking in polyion complex micelle nanpreactors. Biomacromolecules 2011, 12, 3499–3507. [Google Scholar] [CrossRef] [PubMed]



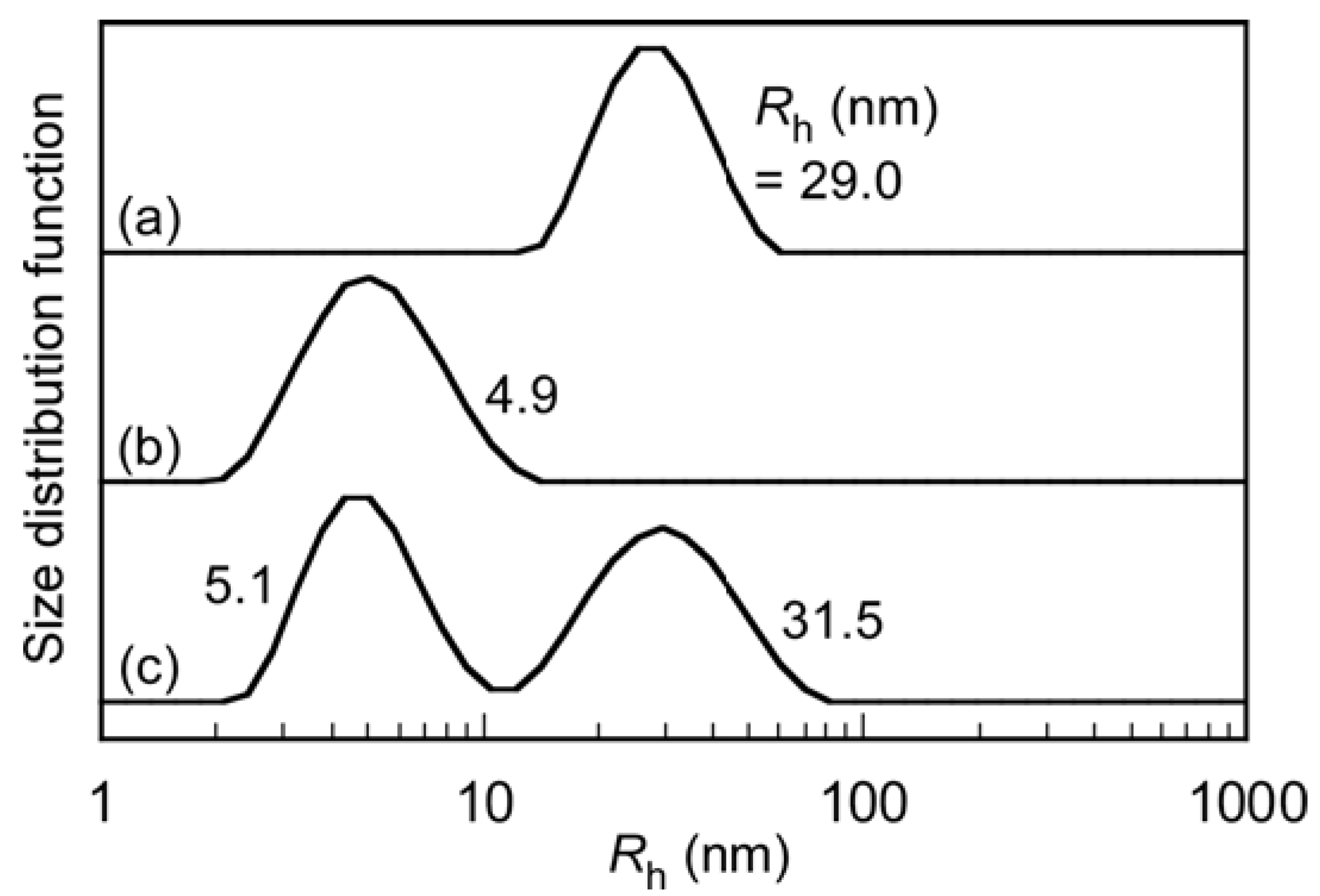
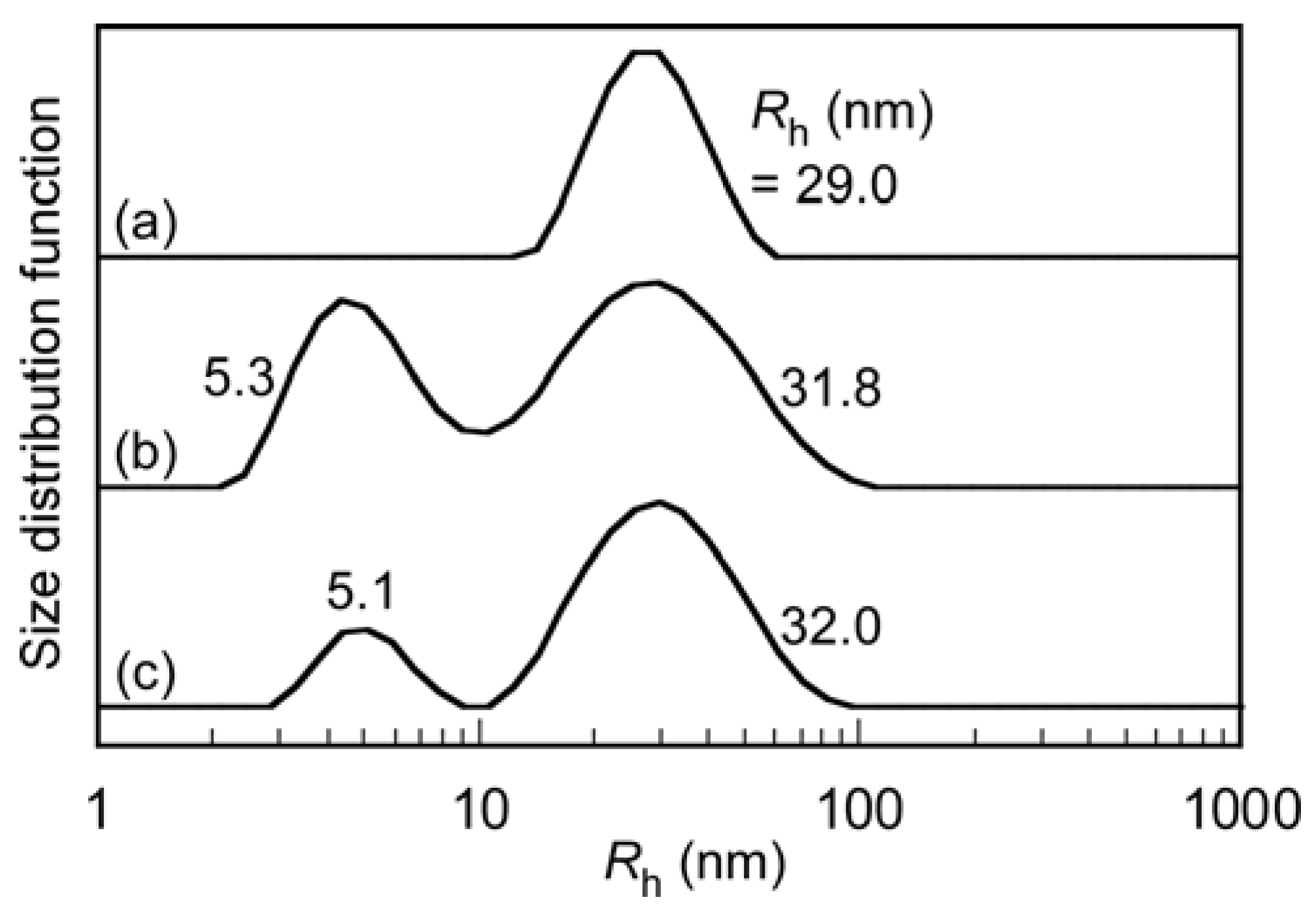
| Sample | DP (theory) a | Mn(theory) b × 104 (g/mol) | DP (NMR) c | Mn(NMR) × 104 (g/mol) | Mn(GPC) × 104 (g/mol) | Mw/Mn |
|---|---|---|---|---|---|---|
| P(SA)91 | 91 | 1.91 | - d | - d | 1.54 | 1.27 |
| P(SA)91S67 | 61 | 3.30 | 67 | 3.27 | 2.38 | 1.04 |
| P(SA)91A88 | 90 | 3.73 | 88 | 3.70 | 1.87 | 1.15 |
| Samples | Mw × 104 (g/mol) | Nagg | Rg (nm) | Rh (nm) | Rg/Rh | A2 × 10−4 (cm3⋅mol/g2) | d a (g/cm3) | dn/dCp (mL/g) |
|---|---|---|---|---|---|---|---|---|
| P(SA)91S67 | 5.41 | 1 | 7.3 | 5.7 | 1.28 | 3.73 | 0.116 | 0.118 |
| P(SA)91A88 | 4.98 | 1 | 6.7 | 6.0 | 1.12 | 3.77 | 0.0914 | 0.138 |
| PIC micelles | 752 | 218 | 26.5 | 29.0 | 0.91 | 0.0127 | 0.122 | 0.128 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakahata, R.; Yusa, S.-i. Preparation of Water-soluble Polyion Complex (PIC) Micelles Covered with Amphoteric Random Copolymer Shells with Pendant Sulfonate and Quaternary Amino Groups. Polymers 2018, 10, 205. https://doi.org/10.3390/polym10020205
Nakahata R, Yusa S-i. Preparation of Water-soluble Polyion Complex (PIC) Micelles Covered with Amphoteric Random Copolymer Shells with Pendant Sulfonate and Quaternary Amino Groups. Polymers. 2018; 10(2):205. https://doi.org/10.3390/polym10020205
Chicago/Turabian StyleNakahata, Rina, and Shin-ichi Yusa. 2018. "Preparation of Water-soluble Polyion Complex (PIC) Micelles Covered with Amphoteric Random Copolymer Shells with Pendant Sulfonate and Quaternary Amino Groups" Polymers 10, no. 2: 205. https://doi.org/10.3390/polym10020205
APA StyleNakahata, R., & Yusa, S.-i. (2018). Preparation of Water-soluble Polyion Complex (PIC) Micelles Covered with Amphoteric Random Copolymer Shells with Pendant Sulfonate and Quaternary Amino Groups. Polymers, 10(2), 205. https://doi.org/10.3390/polym10020205







