1. Introduction
With the consumer’s enhanced awareness of eco-safety, widespread interest has emerged in the application of sustainable and eco-friendly materials [
1,
2,
3]. In the textile industry, a constantly increasing interest in biomass pigments has been aroused in recent years [
4,
5,
6], which has been regarded as an ecological, as well as sustainable dyeing technology to address environmental contamination issues caused by the application of synthetic dyestuffs [
6,
7,
8,
9,
10].
With the ever-increasing demand of biomass colorants [
11], several methods have been made to prepare biomass dyestuffs biologically, and to further enhance the content of pigments over the past few years [
12,
13,
14,
15]. The most promising is the application of enzyme generated by microorganism to synthesize biopigment [
16,
17,
18]. Biosynthesis is green and secure compared to chemical synthesis [
19,
20,
21,
22], which could give rise to effective preparation for target product via biotransformation [
12,
23].
Considering safety, energy, and water conservation, as well as environmental responsibility, enzymes are gaining an increasing role in textile wet processing [
24], and the textile industry has become one of the main fields for industrial application of enzymes [
25,
26]. Moreover, new enzymes are being introduced to the field of textile processing [
27,
28].
Laccase is regarded as an ideal biocatalyst to take the place of chemical catalysts, by virtue of numerous strengths [
29,
30], such as high catalytic efficiency, mild reaction conditions, as well as renewability, etc. [
31,
32]. Accordingly, laccase has been considerably highlighted by many researchers in recent years. Laccase has strategic significance to address severe environmental pollution issues [
33], and meet the tendency concerning green manufacturing, as well as sustainable development [
34]. In addition, laccase has been employed in biosynthesis of biomass pigments and decolorization of synthetic dyestuffs in dyeing industry [
27,
34,
35].
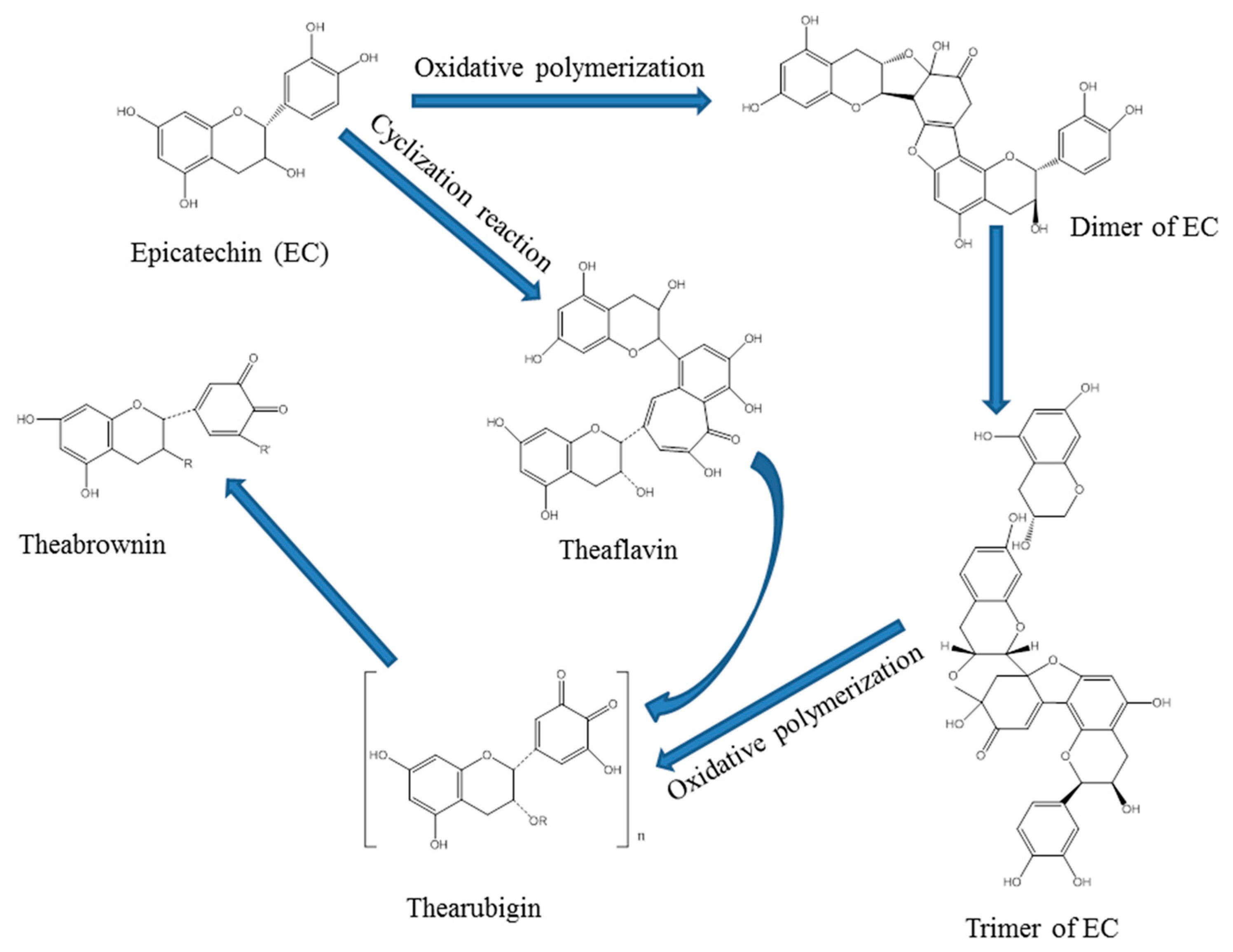
Tea polyphenols are the main component in green tea [
36], which also is one of typical substrates for laccase-catalyzed oxidative polymerization [
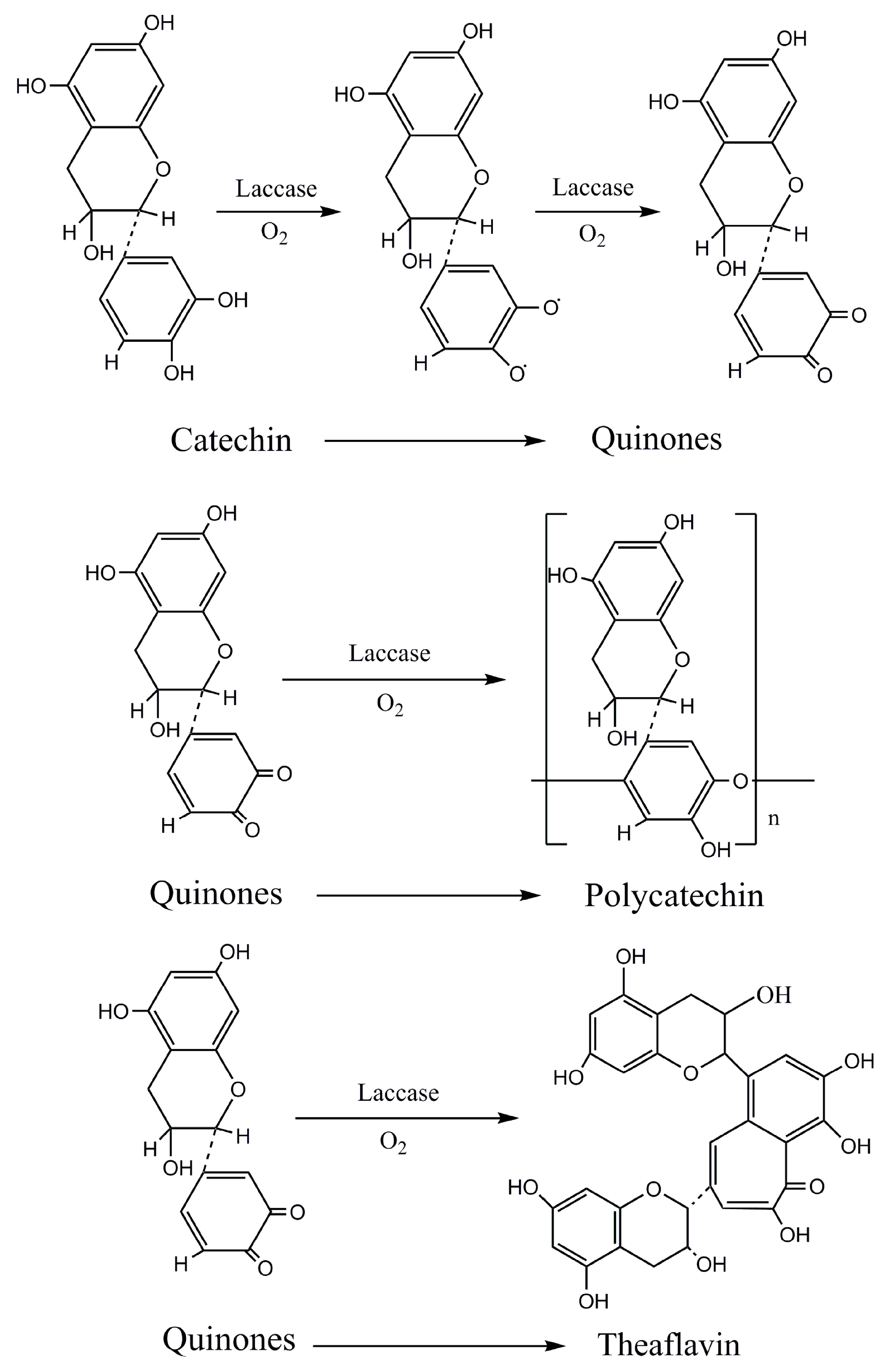
37]. Tea polyphenols would be firstly catalyzed into quinones [
32], which could be transformed into theaflavin, since generated quinones were unstable. Theaflavin could be converted into theabrownin via non-enzymatic browning reaction through adding exogenous additive amino acids [
38], which could not only further enhance the content of tea pigments, but also could endow aromatic flavor by dyeing fabrics. Therefore, this technology is able to achieve the processes of both dyeing and functional finishing [
39].
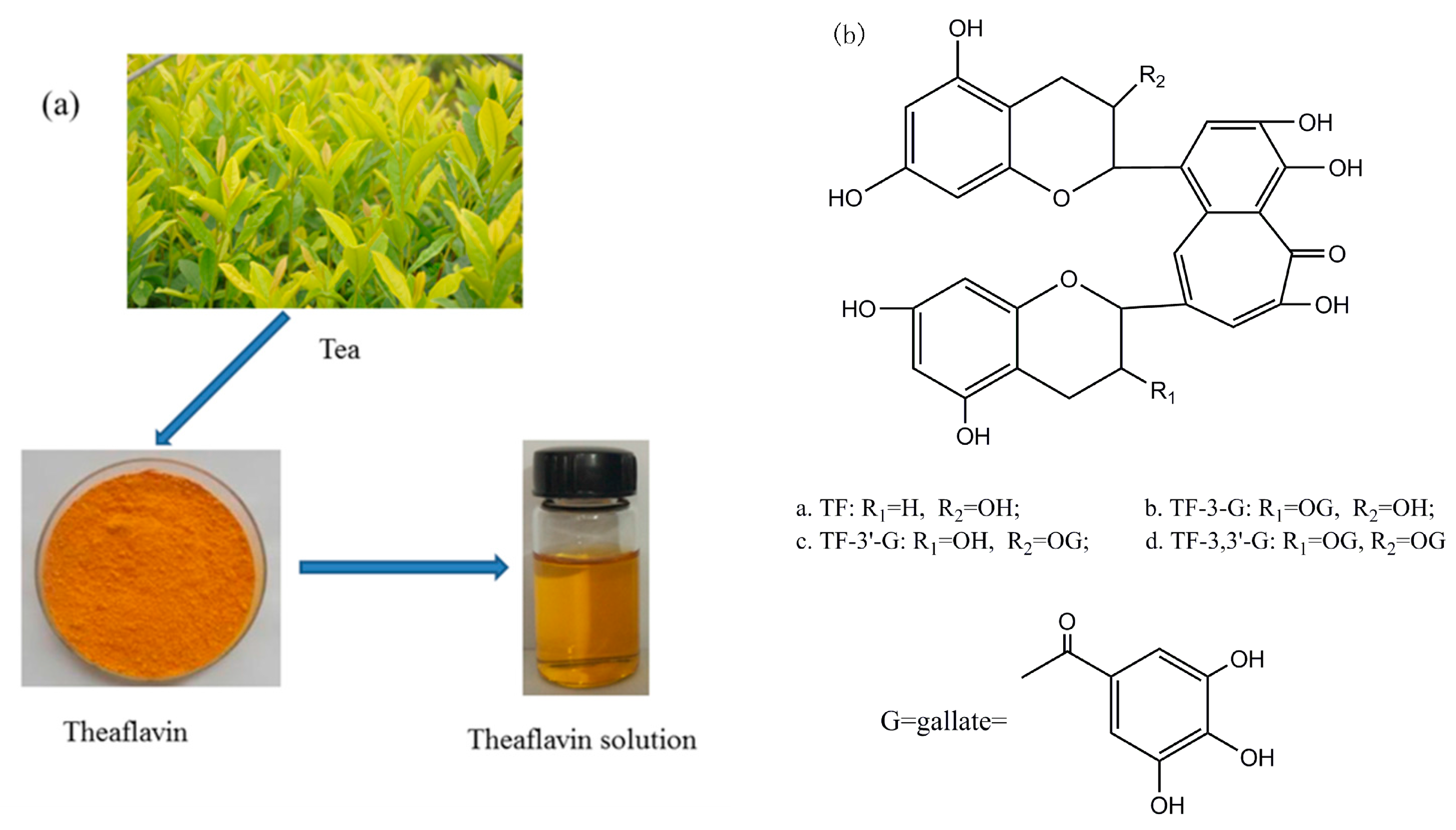
Theaflavin is the major component in black tea (
Figure 1a), which is the primary oxidation product during the process of tea fermentation [
40]. Laccase is able to catalyze precursor tea polyphenols transformed into theaflavin [
41], and theaflavin is the mixture. There are four principal substances in theaflavin (
Figure 1b), and the chemical structural formula depends on theaflavin (TF), theaflavin-3-gallate (TF-3-G), theaflavin-3′-gallate (TF-3′-G), as well as theaflavin-3,3′-gallate (TF-3,3′-G) [
42].
In this study, biocolorant prepared from phenolic compound was achieved, and dyeing of silk and wool fabrics with pigment derived from tea polyphenols was investigated under the influence of pH variation. Accordingly, a novel dyeing method based on pH-induced fixation was established for protein textiles.
2. Materials and Methods
2.1. Materials
Food-grade colorless tea polyphenols were purchased from Liyuan Food Additives Limited Company of Guangzhou in Guangdong Province of China, which were treated by decolorization processing, and the content of effective substance was 99%.
The wool fabric (warp density 86 yarns per inch, weft density 51 yarns per inch; weight 132.0 g/m2) was purchased from Jiangsu Huaxi Spinning Limited Company (Suzhou, China). The silk fabric (warp density 325 yarns per inch, weft density 34 yarns per inch; weight 75.0 g/m2) was bought from FING SILK Limited Company (Hangzhou, China).
Both citric acid and disodium hydrogen phosphate were analytical reagents, and purchased from Tianjin Comio Chemical Reagent Co., Ltd. (Tianjin, China). Laccase (EC1.10.3.2) Denilite II S was bought from Novozymes Corporation (Beijing, China), which was prepared from Aspergillus through utilizing submerged fermentation, and the standard enzyme activity was 120 LAMU/g (LAMU= Laccase Units of Modified Aspergillus).
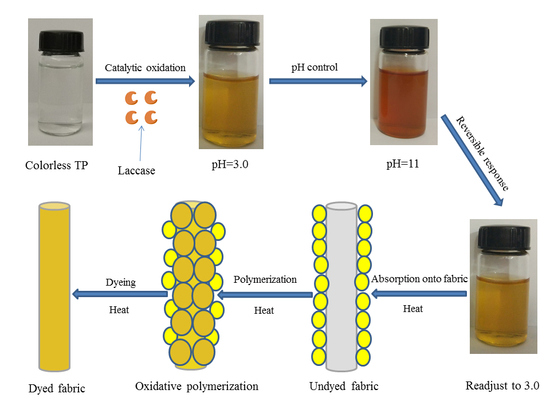
2.2. Preparation of Biopigment with Laccase
Tea polyphenols (5 g) were added to a buffer solution containing both 0.1 M citric acid and 0.2 M disodium hydrogen phosphate, and the pH value was adjusted to 4.5. Then, 1.0 g (120 LAMU) laccase was placed in that, and volume adjusted to 500 mL. Eventually, all shake flasks were cultivated in an incubator shaker (Shanghai Zhicheng Analytical Instrument Limited Company, Shanghai, China) at 60 °C and 180 rpm.
2.3. Dyeing Procedure
Dyeing of Protein Fabric under Different pH
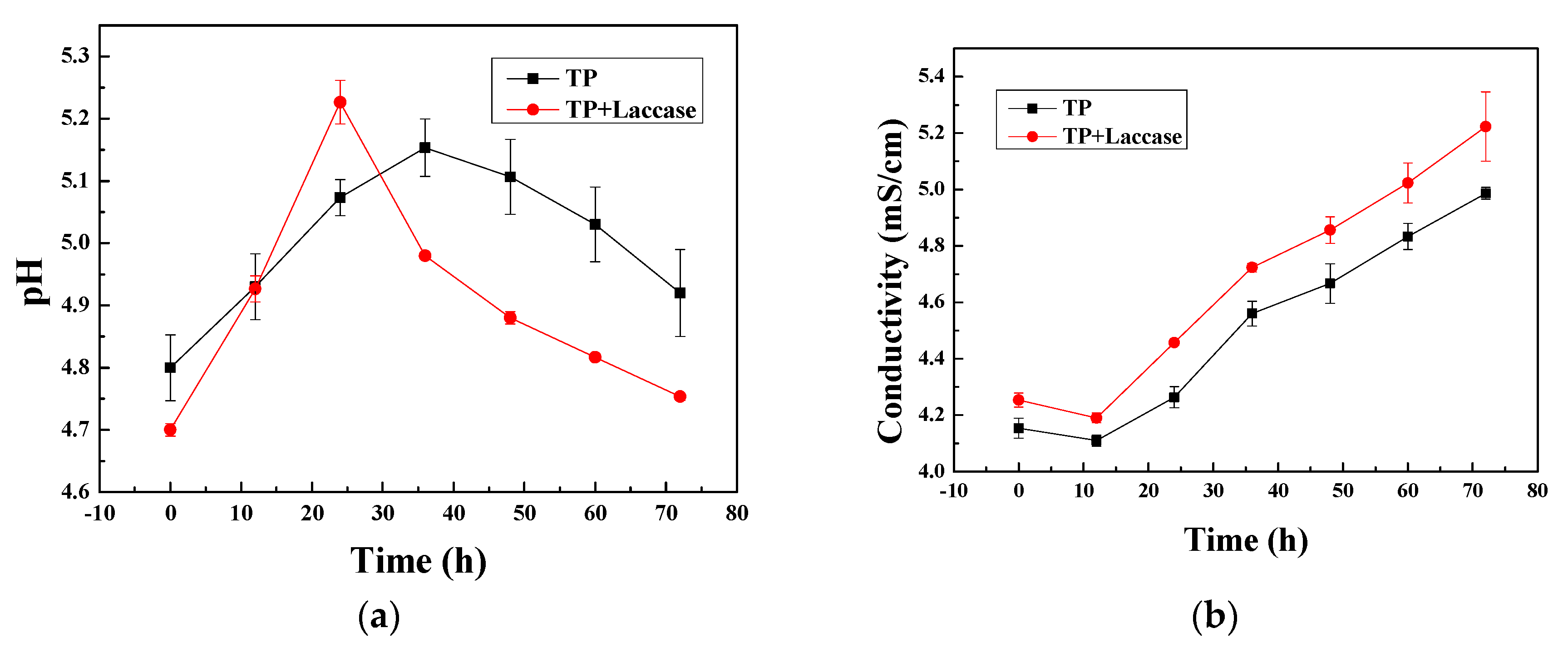
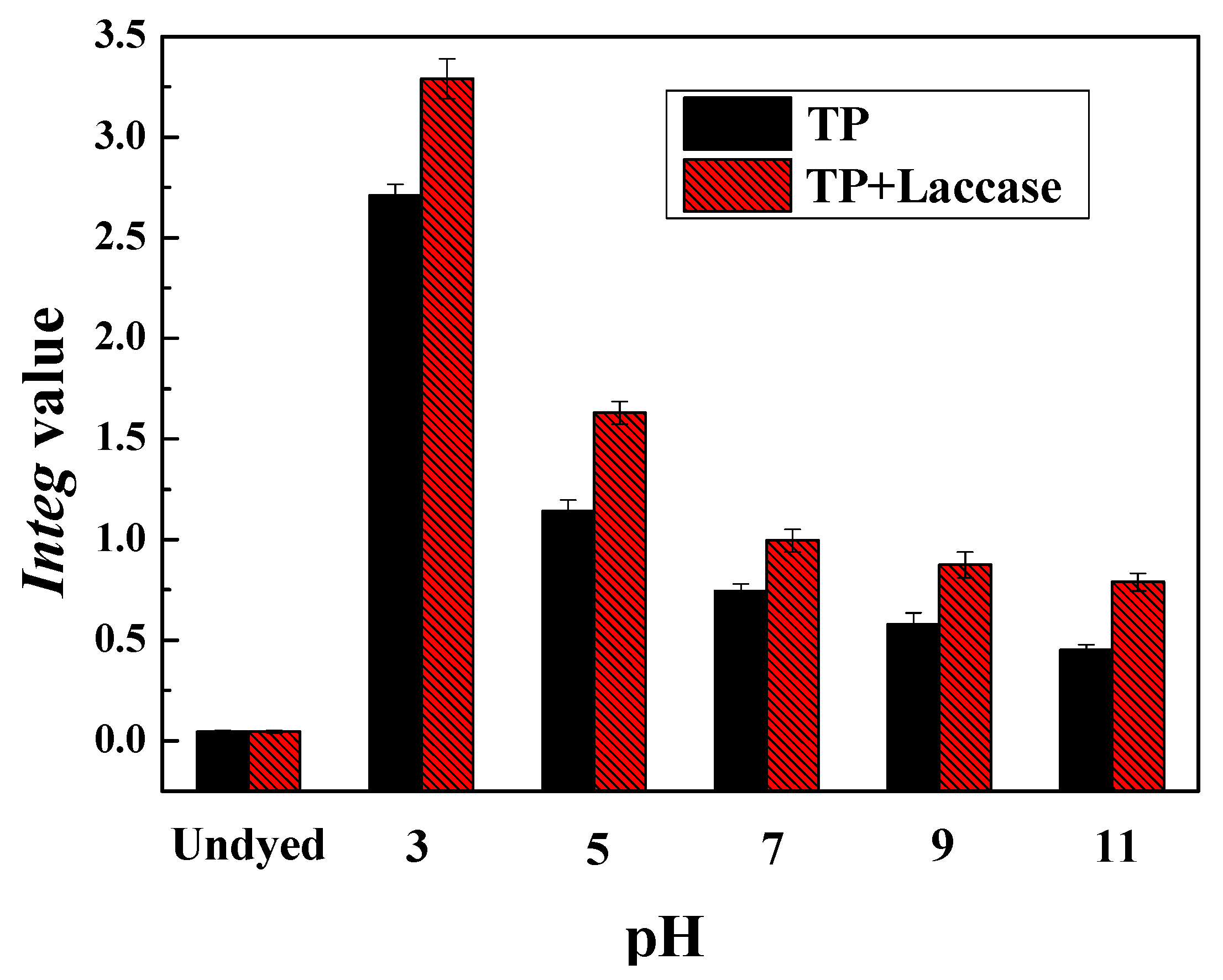
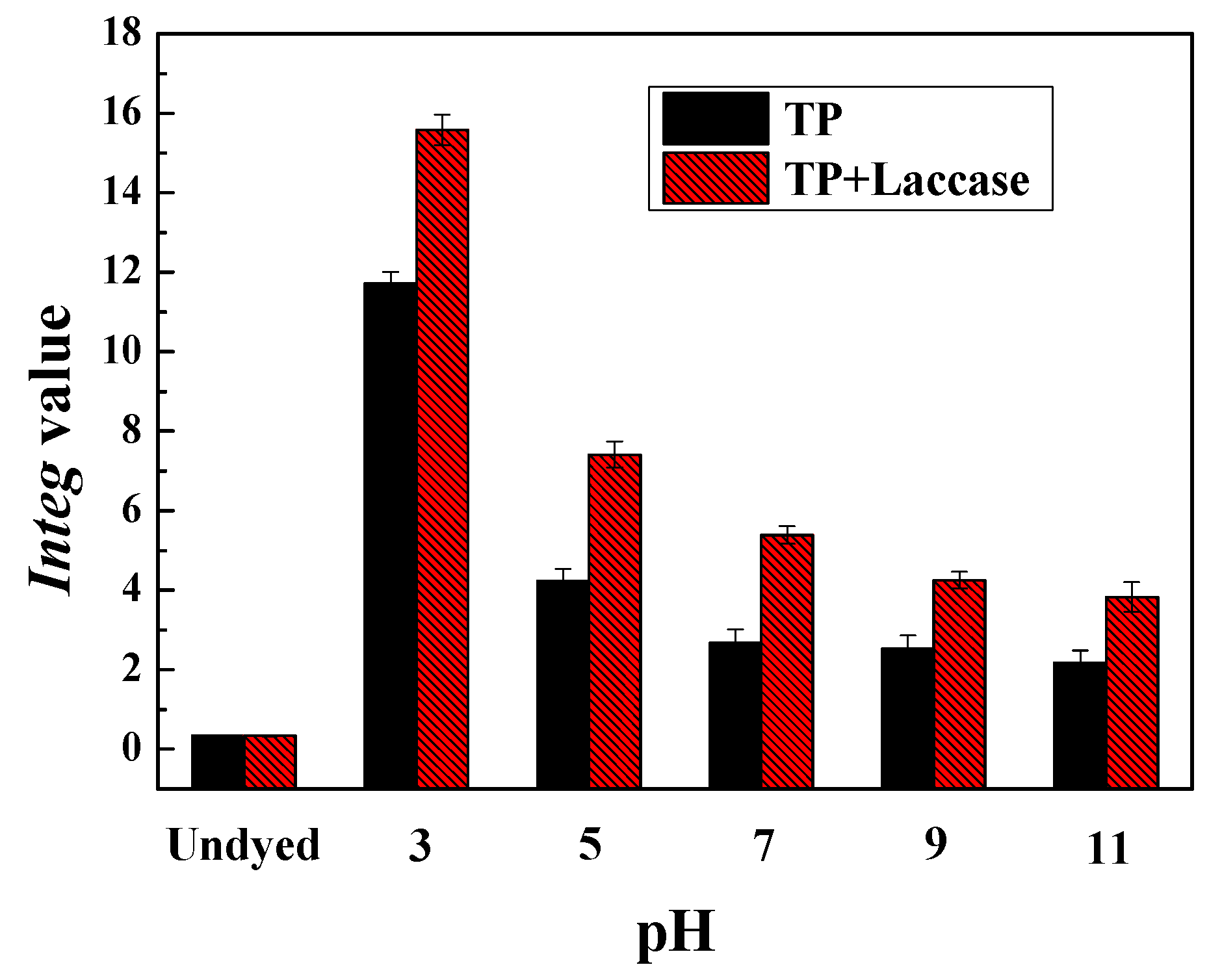
The pH value of theaflavin, based on laccase-catalyzed oxidative polymerization for tea polyphenols, was adjusted to 3, 5, 7, 9, as well as 11, respectively, and then silk and wool fabrics were placed in dyeing tanks according to liquor ratio 1:50. Dyeing experiment was carried out in an infrared dyeing equipment (Data color corporation, State of New jersey, USA). The dyeing temperature was 100 °C and soaking time was 60 min, which started from indoor temperature 30 °C with a heating rate of 3 °C/min. At the end of dyeing process, dyed fabrics were washed under running water, and also, were carried out via employing 2 g/L neutral soap flakes at 80 °C for 10 min to wash away residual uncombined pigment from fabrics. After soaping, fabrics were washed with water at 80 °C twice, and washed under running water, followed by drying in a drying oven.
2.4. Measurements
2.4.1. Color Characteristics
The CIE L*, a*, b*, C*, h, and
Integ values, were measured by employing Data color 600 spectrophotometer (Data color corporation, NJ, USA) under photosource D65, 10° visual angle. The measured results were an averaged value from four different locations. The
Integ value could be calculated according to the following Equation (1):
where F(
X), F(
Y) as well as F(
Z) are pseudo tristimulus values.
2.4.2. Color Fastness
The rubbing, soaping, as well as light fastness of dyed fabrics were measured on the basis of ISO 105-C01, ISO 105-X12, as well as ISO 105-B02, respectively.
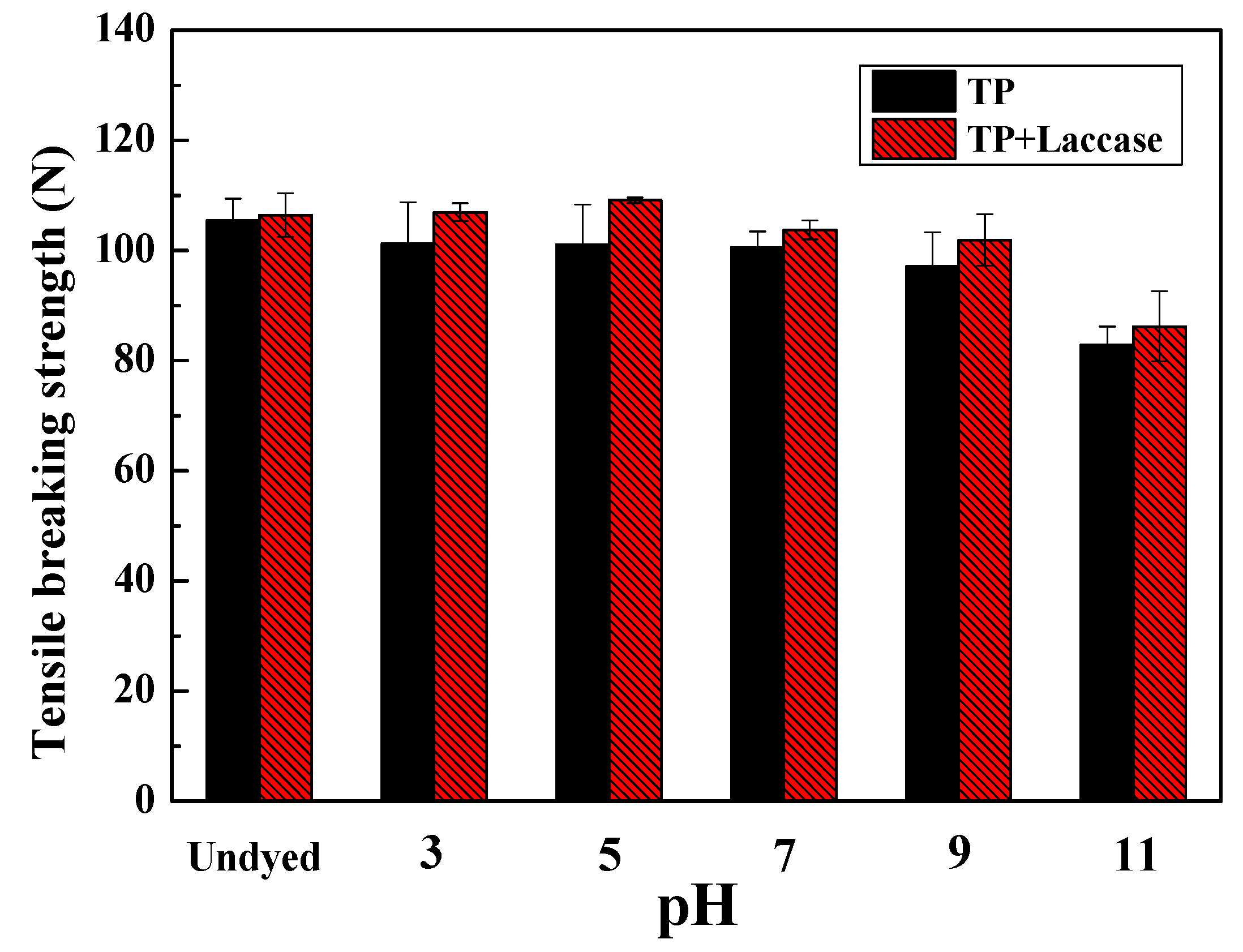
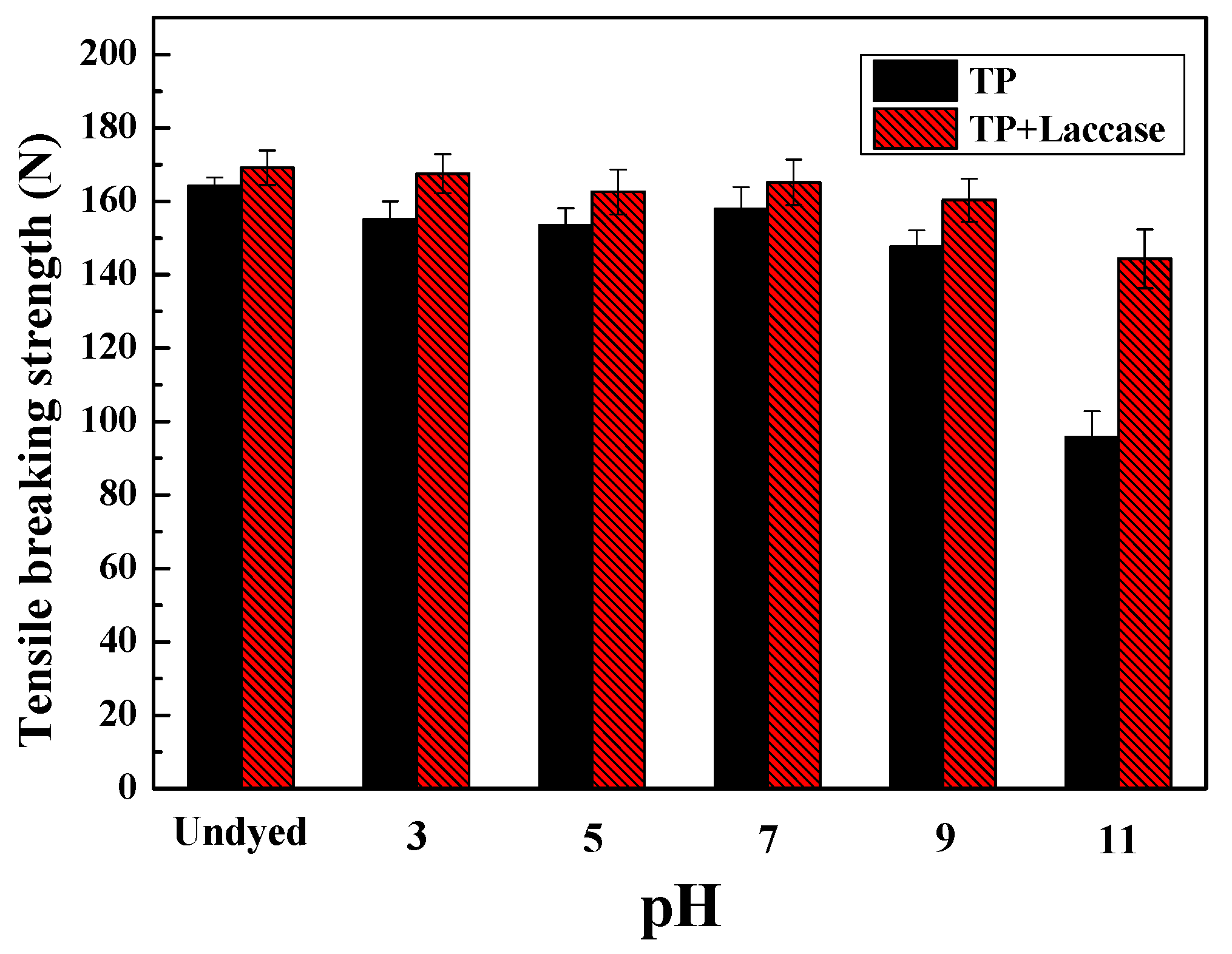
2.4.3. Breaking Strength
The breaking strength of dyed protein fabrics was measured by the YG065 electronic fabric strength tester (Changzhou No. 1 Textile Equipment Co. Limited, Changzhou, China) according to GB/T 3923.1-2013: Textiles-Tensile properties of fabrics—Part1. The length and width were 100 mm and 25 mm, respectively, and the tensile velocity was 100 mm/min.
4. Conclusions
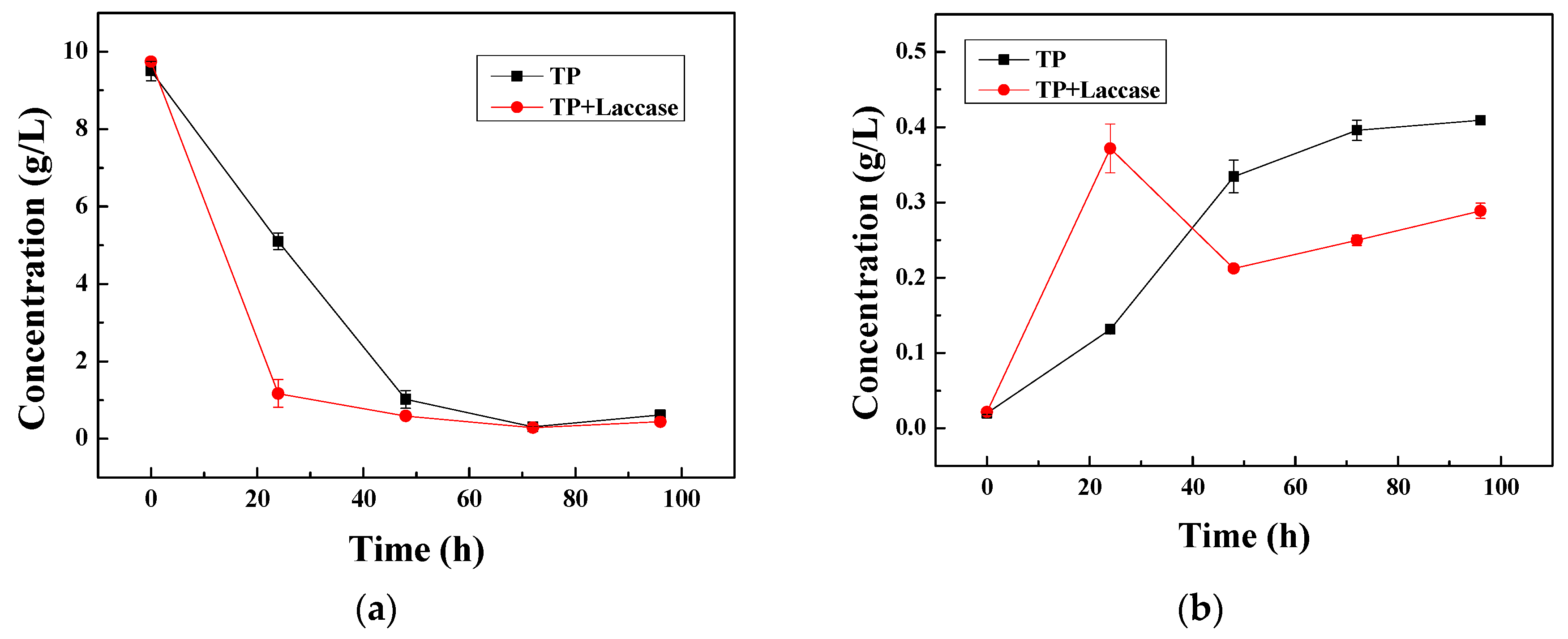
The reduction rate of tea polyphenols with non-enzymatic oxidation was 46.30%, and the increasing rate of theaflavin was 84.75% during the first 24 h. Additionally, the reduction rate of tea polyphenols with laccase was 77.99%, and the increasing rate of theaflavin was 94.22% during the first 24 h. Accordingly, the reduction rate of tea polyphenols and the increasing rate of theaflavin were enhanced 41.69% and 19.47%, respectively, compared to non-enzymatic oxidation.
The preparation of theaflavin from tea polyphenols with laccase was carried out, and dyeing of protein fabric was achieved from acidity to alkalinity, in this investigation. Experimental results demonstrated dyeing properties were better under acidic conditions compared to alkalinity, and both dyeing property and fixation rate were the best when pH value was 3. In addition, the dyeing property of wool fabric was better than silk when dyed by identical dye liquor.
Nowadays, natural products especially derived from plants, are gaining popularity around the globe for their application in textiles, by virtue of abundant availability, biocompatibility, low toxicity, compatibility with green approaches, and eco-friendly nature. Tea is the predominant plant resource in China, and large amounts of tea stem and other waste will be produced during the processing period, which provides rich raw material for the extraction of natural functional substances.