Efficient Adsorption of Pb(II) from Aqueous Solutions by Metal Organic Framework (Zn-BDC) Coated Magnetic Montmorillonite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
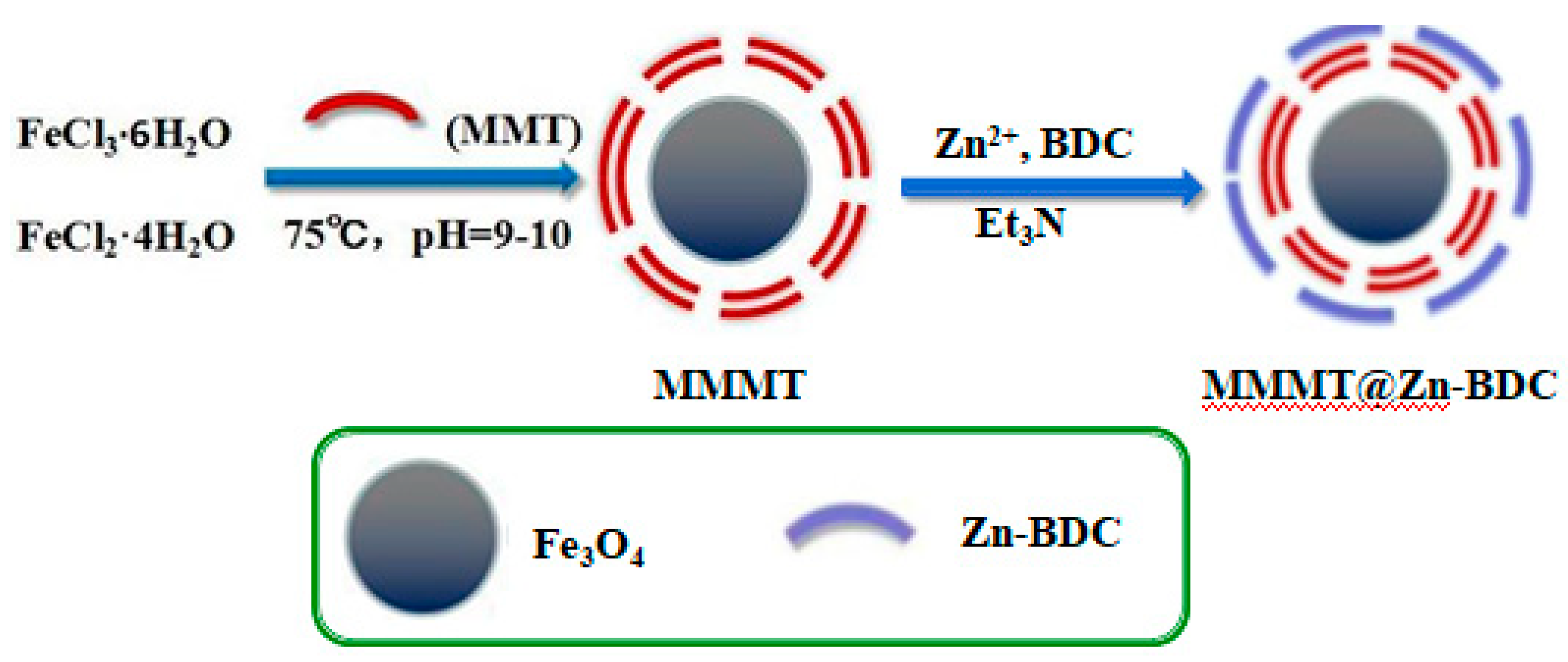
2.2. Preparation of MMMT@Zn-BDC
2.2.1. Preparation of Zn-BDC
2.2.2. Preparation of MMMT
2.2.3. Preparation of MMMT@Zn-BDC
2.3. Characterization
2.4. Adsorption Experiment
2.5. Reutilization Test
3. Results and Discussion
3.1. Optimize the Preparation Conditions of MMMT@Zn-BDC
3.2. Characterization
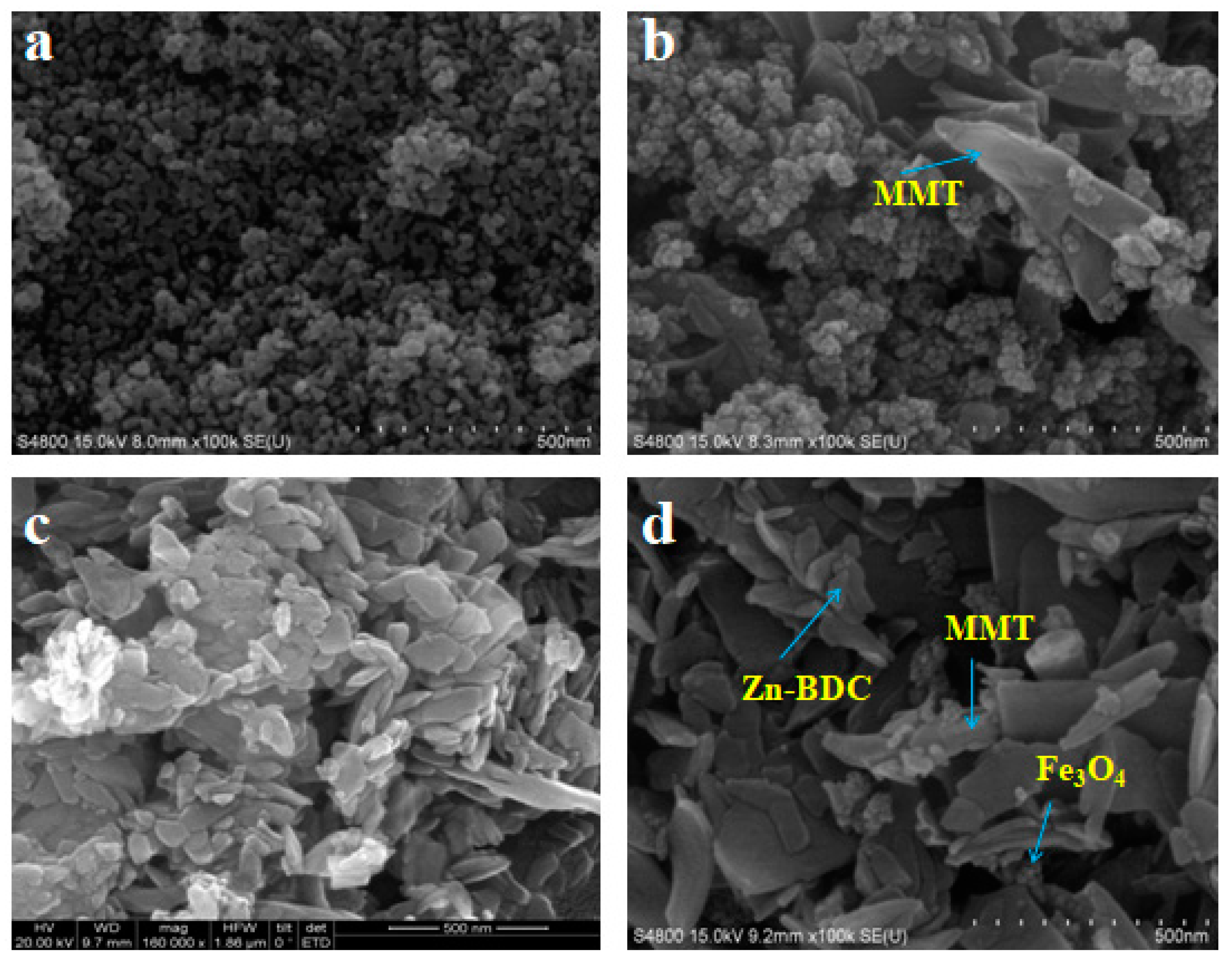
3.2.1. SEM Observation
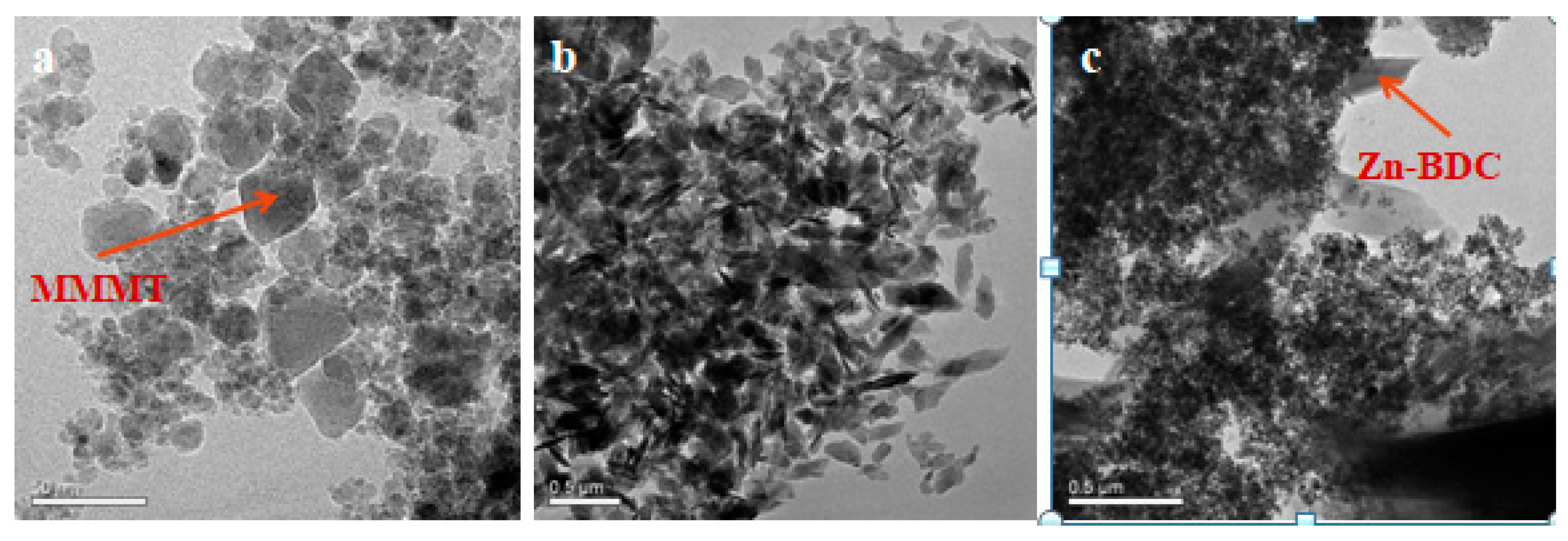
3.2.2. TEM Analysis
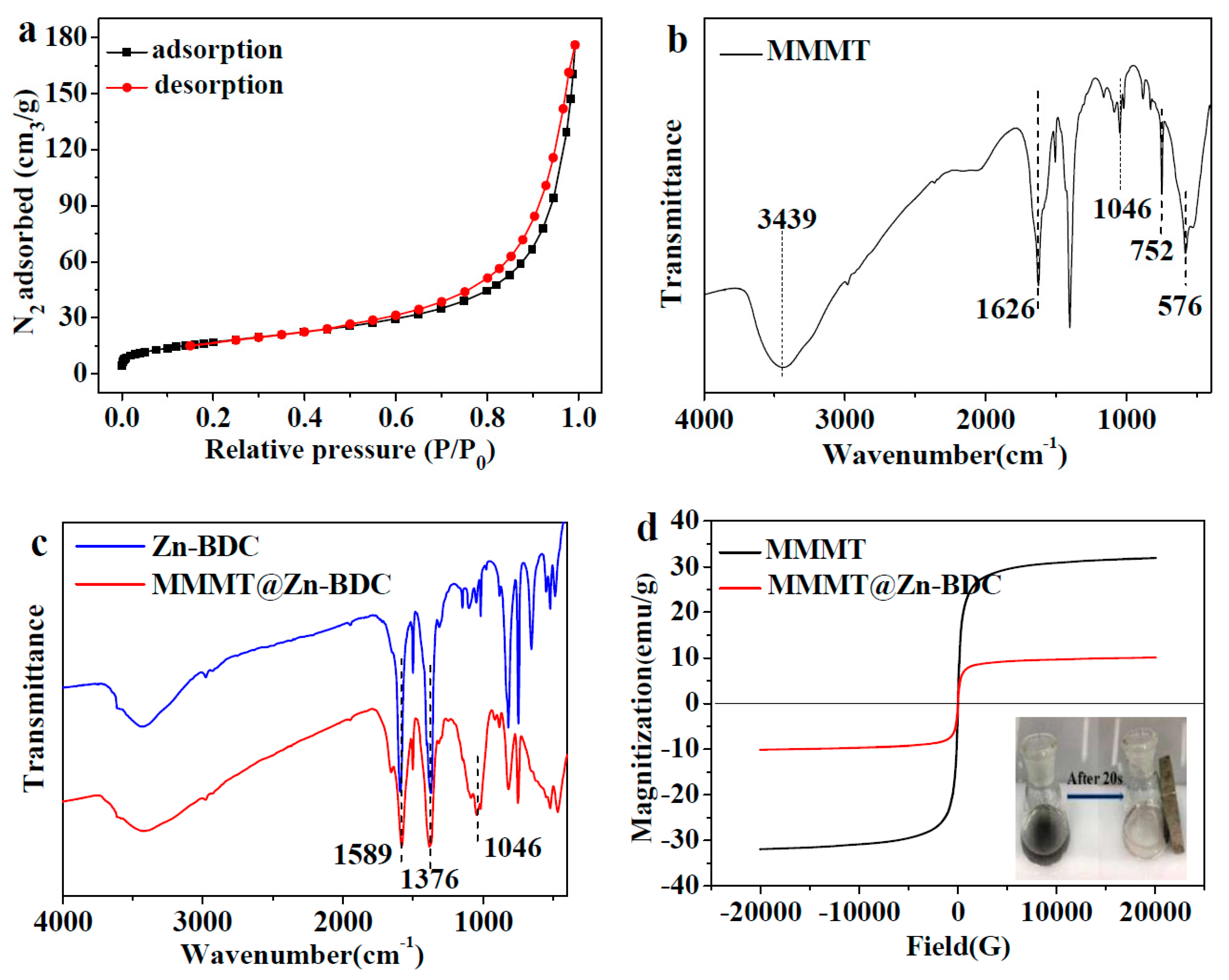
3.2.3. N2 Adsorption and Desorption Analysis
3.2.4. FTIR Analysis
3.2.5. VSM Analysis
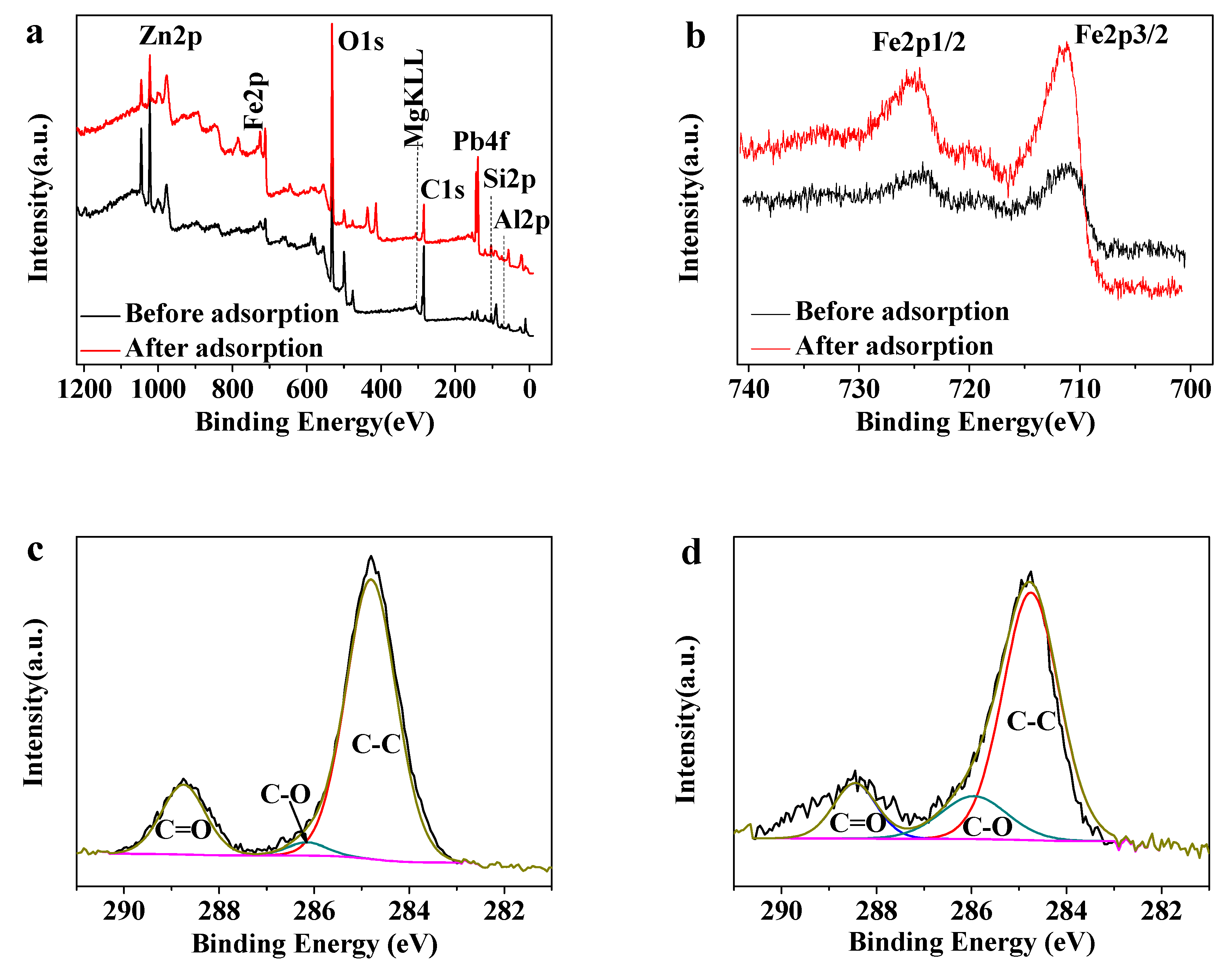
3.2.6. XPS Analysis
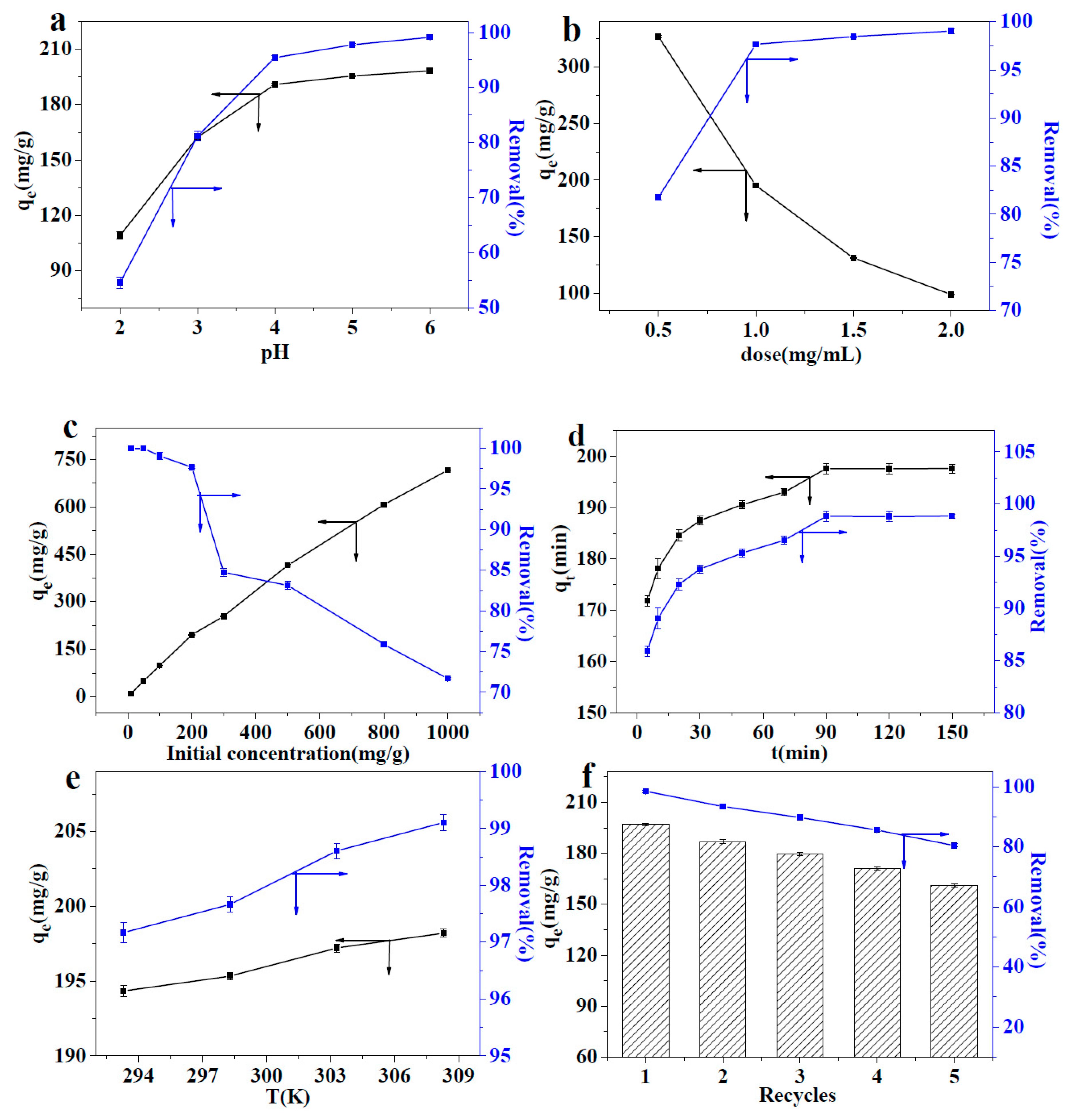
3.3. Optimization of the Adsorption Conditions
3.3.1. Effect of the pH of Pb(II) Solution
3.3.2. Effect of the Adsorbent Dose
3.3.3. Effect of the Pb(II) Concentration
3.3.4. Effect of Adsorption Time
3.3.5. Effect of the Adsorption Temperature
3.3.6. Effect of the Recycling Process
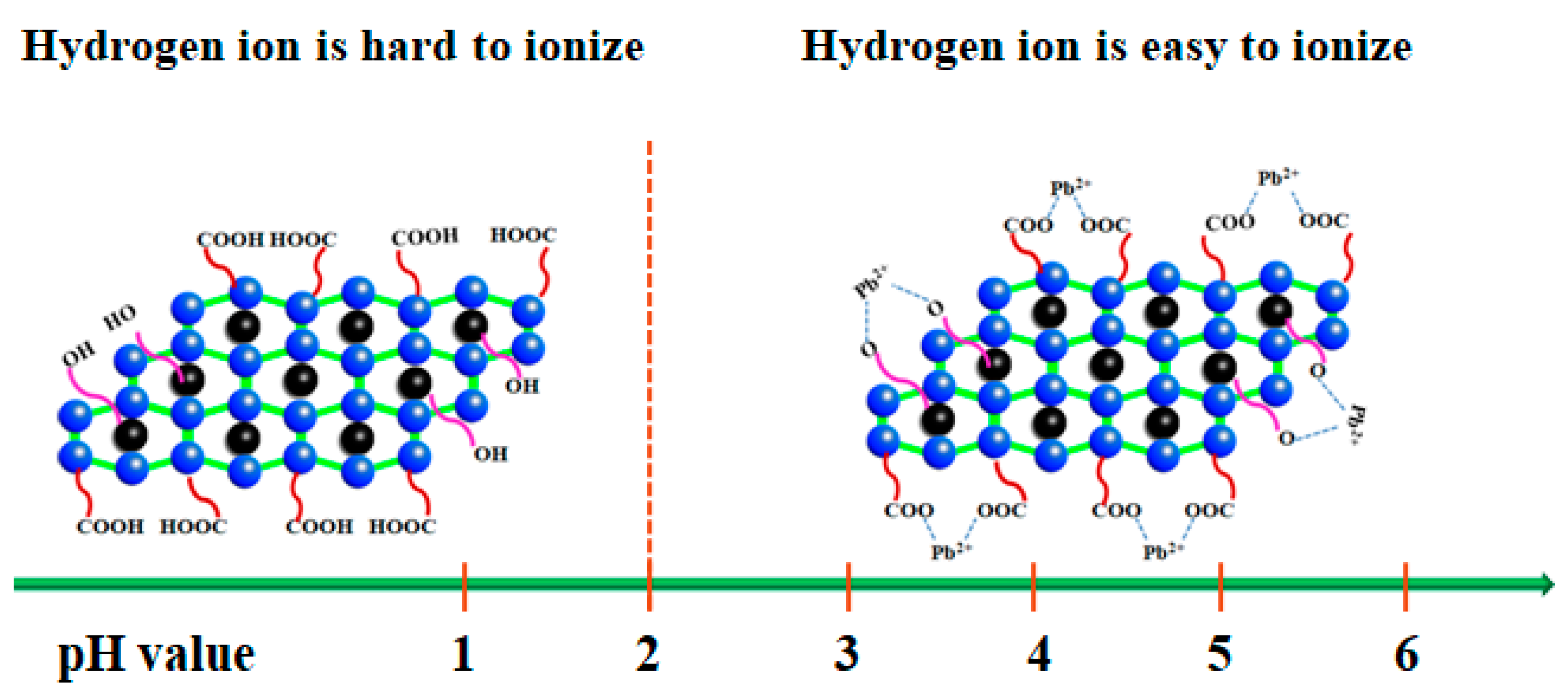
3.4. Adsorption Mechanism
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Yang, J.; Meng, X.Z.; Duan, Y.P.; Liu, L.Z.; Chen, L.; Cheng, H. Spatial distributions and sources of heavy metals in sediment from public park in shanghai, the yangtze river delta. Appl. Geochem. 2014, 44, 54–60. [Google Scholar] [CrossRef]
- Awasthi, A.K.; Zeng, X.; Li, J. Environmental pollution of electronic waste recycling in india: A critical review. Environ. Pollut. 2016, 211, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Lemtiri, A.; Liénard, A.; Alabi, T.; Brostaux, Y.; Cluzeau, D.; Francis, F.; Colinet, G. Earthworms eisenia fetida affect the uptake of heavy metals by plants vicia faba and zea mays in metal-contaminated soils. Appl. Soil Ecol. 2016, 104, 67–78. [Google Scholar] [CrossRef]
- He, Y.F.; Zhang, L.; Wang, R.M.; Li, H.R.; Wang, Y. Loess clay based copolymer for removing Pb (II) ions. J. Hazard. Mater. 2012, 227–228, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, K.; Joshi, U.M. Chicken eggshells remove Pb (II) ions from synthetic wastewater. Environ. Eng. Sci. 2013, 30, 67–73. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Qian, Q.; Machida, M.; Tatsumoto, H. Effect of zno loading to activated carbon on Pb (II) adsorption from aqueous solution. Carbon 2006, 44, 195–202. [Google Scholar] [CrossRef]
- Collins, J.; Li, X.; Pletcher, D.; Tangirala, R.; Stratton-Campbell, D.; Walsh, F.C.; Zhang, C. A novel flow battery: A lead acid battery based on an electrolyte with soluble lead (II). Part ix: Electrode and electrolyte conditioning with hydrogen peroxide. J. Power Sources 2010, 195, 2975–2978. [Google Scholar] [CrossRef]
- Wang, N.; Ouyang, X.K.; Yang, L.Y. Fabrication of a magnetic cellulose nanocrystal/metal-organic framework composite for removal of Pb (II) from water. Acs Sustainable Chem. Eng. 2017, 5, 10447–10458. [Google Scholar] [CrossRef]
- Wang, N.; Jin, R.; Omer, A.M.; Ouyang, X. Adsorption of Pb (II) from fish sauce using carboxylated cellulose nanocrystal: Isotherm, kinetics, and thermodynamic studies. Int. J. Biol. Macromol. 2017, 102, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Choi, W.S.; Nam, B.; Yoon, C.; Lee, H.J. Needle-like iron oxide@CaCO3 adsorbents for ultrafast removal of anionic and cationic heavy metal ions. Chem. Eng. J. 2016, 307, 208–219. [Google Scholar] [CrossRef]
- Huang, B.; Lu, M.; Wang, D.; Song, Y.; Zhou, L. Versatile magnetic gel from peach gum polysaccharide for efficient adsorption of Pb2+ and Cd2+ ions and catalysis. Carbohydr. Polym. 2018, 181, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.H.; Omer, A.M.; Ouyang, X.K.; Yu, D. Fabrication of carboxylated cellulose nanocrystal/sodium alginate hydrogel beads for adsorption of Pb (II) from aqueous solution. Int. J. Biol. Macromol. 2018, 108, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Jin, R.N.; Liu, C.; Wang, Y.F.; Ouyang, X.K. Magnetic carboxylated cellulose nanocrystals as adsorbent for the removal of Pb (II) from aqueous solution. Int. J. Biol. Macromol. 2016, 93, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Wang, Y.G.; Ouyang, X.K.; Yang, L.Y. Surface-imprinted magnetic carboxylated cellulose nanocrystals for the highly selective extraction of six fluoroquinolones from egg samples. ACS Appl. Mater. Interfaces 2017, 9, 1759–1769. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.B.; Zhang, P.; Zhou, H.C.; Sharma, V.K. Water-stable metal-organic frameworks for aqueous removal of heavy metals and radionuclides: A review. Chemosphere 2018, 209, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.L.; Afsar, A.; Harwood, L.M.; Jiang, T.; Laventine, D.M.; Shaw, L.J.; Hodson, M.E. Adsorption of Pb and Zn from binary metal solutions and in the presence of dissolved organic carbon by dtpa-functionalised, silica-coated magnetic nanoparticles. Chemosphere 2017, 183, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.-P.; Zheng, B.; Qin, Y.; Yang, D.; Liao, D.-D.; Xu, X.-K.; Zhang, X.-H.; Zhu, J.-H. A superparamagnetic Fe3O4-graphene oxide nanocomposite for enrichment of nuciferine in the extract of nelumbinis folium (lotus leaf). Appl. Surf. Sci. 2016, 364, 332–339. [Google Scholar] [CrossRef]
- Nikje, M.M.A.; Moghaddam, S.T.; Noruzian, M.; Nejad, M.A.F.; Shabani, K.; Haghshenas, M.; Shakhesi, S. Preparation and characterization of flexible polyurethane foam nanocomposites reinforced by magnetic core-shell Fe3O4@apts nanoparticles. Colloid Polym. Sci. 2014, 292, 627–633. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S.S. Calcined tetrabutylammonium kaolinite and montmorillonite and adsorption of Fe (II), Co (II) and Ni (II) from solution. Appl. Clay Sci. 2009, 46, 216–221. [Google Scholar] [CrossRef]
- Wu, P.; Wu, W.; Li, S.; Xing, N.; Zhu, N.; Li, P.; Wu, J.; Yang, C.; Dang, Z. Removal of Cd2+ from aqueous solution by adsorption using fe-montmorillonite. J. Hazard. Mater. 2009, 169, 824–830. [Google Scholar] [CrossRef]
- Hu, B.; Luo, H. Adsorption of hexavalent chromium onto montmorillonite modified with hydroxyaluminum and cetyltrimethylammonium bromide. Appl. Surf. Sci. 2010, 257, 769–775. [Google Scholar] [CrossRef]
- Liu, C.; Omer, A.M.; Ouyang, X.K. Adsorptive removal of cationic methylene blue dye using carboxymethyl cellulose/k-carrageenan/activated montmorillonite composite beads: Isotherm and kinetic studies. Int. J. Biol. Macromol. 2018, 106, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhu, P.; Cai, M.; Hu, H.; Fu, Q. Comparative adsorption of Pb (II), Cu (II) and Cd (II) on chitosan saturated montmorillonite: Kinetic, thermodynamic and equilibrium studies. Appl. Clay Sci. 2017, 143, 320–326. [Google Scholar] [CrossRef]
- Ma, Z.Y.; Shan, C.; Liang, J.L.; Tong, M.P. Efficient adsorption of selenium (IV) from water by hematite modified magnetic nanoparticles. Chemosphere 2018, 193, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic design of pore size and functionality in isoreticular mofs and their application in methane storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef]
- Liu, D.; Lu, K.; Poon, C.; Lin, W. Metal–organic frameworks as sensory materials and imaging agents. Inorg. Chem. 2014, 53, 1916. [Google Scholar] [CrossRef] [PubMed]
- Sikdar, N.; Bhogra, M.; Waghmare, U.V.; Maji, T.K. Oriented attachment growth of anisotropic meso/nanoscale mofs: Tunable surface area and CO2 separation. J. Mater. Chem. A 2017, 5. [Google Scholar] [CrossRef]
- Song, J.Y.; Jhung, S.H. Adsorption of pharmaceuticals and personal care products over metal-organic frameworks functionalized with hydroxyl groups: Quantitative analyses of h-bonding in adsorption. Chem. Eng. J. 2017, 322, 366–374. [Google Scholar] [CrossRef]
- Zhuo, N.; Lan, Y.; Yang, W.; Yang, Z.; Li, X.; Zhou, X.; Liu, Y.; Shen, J.; Zhang, X. Adsorption of three selected pharmaceuticals and personal care products (PPCPs) onto mil-101(Cr)/natural polymer composite beads. Sep. Purif. Technol. 2016, 177, 272–280. [Google Scholar] [CrossRef]
- Jabbari, V.; Veleta, J.M.; Zarei-Chaleshtori, M.; Gardea-Torresdey, J.; Villagran, D. Green synthesis of magnetic MOF@GO and MOF@CNT hybrid nanocomposites with high adsorption capacity towards organic pollutants. Chem. Eng. J. 2016, 304, 774–783. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Tian, Z.; Yin, N. Significantly enhancing Cu (II) adsorption onto zr-mofs through novel cross-flow disturbance of ceramic membrane. Ind. Eng. Chem. Res. 2018, 57, 3773–3780. [Google Scholar] [CrossRef]
- Du, X.D.; Yi, X.H.; Wang, P.; Zheng, W.W.; Deng, J.G.; Wang, C.C. Robust photocatalytic reduction of cr(II) on UIO-66-NH2(Zr/HF) metal-organic framework membrane under sunlight irradiation. Chem. Eng. J. 2019, 356, 393–399. [Google Scholar] [CrossRef]
- Khanjani, S.; Morsali, A. Ultrasound-promoted coating of MOF-5 on silk fiber and study of adsorptive removal and recovery of hazardous anionic dye "congo red. Ultrason. Sonochem. 2014, 21, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, Y.; Wang, S.; Zhou, Y. Synthesis of montmorillonite/Fe3O4-OTAB composite capable of using as anisotropic nanoparticles. Appl. Surf. Sci. 2017, 402, 384–391. [Google Scholar] [CrossRef]
- Li, J.; Cheng, S.; Zhao, Q.; Long, P.; Dong, J. Synthesis and hydrogen-storage behavior of metal–organic framework MOF-5. Int. J. Hydrog. Energy 2009, 34, 1377–1382. [Google Scholar] [CrossRef]
- Fu, W.; Huang, Z.Q. Magnetic dithiocarbamate functionalized reduced graphene oxide for the removal of Cu (II), Cd (II), Pb (II), and Hg (II) ions from aqueous solution: Synthesis, adsorption, and regeneration. Chemosphere 2018, 209, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Adzmi, F.; Meon, S.; Musa, M.H.; Yusuf, N.A. Preparation, characterisation and viability of encapsulated trichoderma harzianum upm40 in alginate-montmorillonite clay. J. Microencapsul. 2012, 29, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, Y.F.; Omer, A.M.; Ouyang, X.K. Fabrication of novel surface-imprinted magnetic graphene oxide-grafted cellulose nanocrystals for selective extraction and fast adsorption of fluoroquinolones from water. Anal. Bioanal. Chem. 2017, 409, 6643–6653. [Google Scholar] [CrossRef] [PubMed]
- Arjmandi, M.; Pakizeh, M. Mixed matrix membranes incorporated with cubic-MOF-5 for improved polyetherimide gas separation membranes: Theory and experiment. J. Ind. Eng. Chem. 2014, 20, 3857–3868. [Google Scholar] [CrossRef]
- Mravčáková, M.; Omastová, M.; Olejníková, K.; Pukánszky, B.; Chehimi, M.M. The preparation and properties of sodium and organomodified-montmorillonite/polypyrrole composites: A comparative study. Synth. Met. 2007, 157, 347–357. [Google Scholar] [CrossRef]
- Feng, X.; Li, W.; Fang, L.; Wang, D. Synthesis of akageneite (beta-Feooh)/reduced graphene oxide nanocomposites for oxidative decomposition of 2-chlorophenol by fenton-like reaction. J. Hazard. Mater. 2016, 308, 11–20. [Google Scholar]
- Jiang, L.H.; Liu, Y.G.; Zeng, G.M.; Xiao, F.Y.; Hu, X.J.; Hu, X.; Wang, H.; Li, T.T.; Zhou, L.; Tan, X.F. Removal of 17β-estradiol by few-layered graphene oxide nanosheets from aqueous solutions: External influence and adsorption mechanism. Chem. Eng. J. 2016, 284, 93–102. [Google Scholar] [CrossRef]
- Wu, F.C.; Ruling, T.; Rueyshin, J. A review and experimental verification of using chitosan and its derivatives as adsorbents for selected heavy metals. J. Environ. Manag. 2010, 91, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; Ofomaja, A.E. Pseudo-second-order model for lead ion sorption from aqueous solutions onto palm kernel fiber. J. Hazard. Mater. 2006, 129, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, Y.; Zhou, X.; Ren, J.; Zhong, C. Magnetic multi-porous bio-adsorbent modified with amino siloxane for fast removal of Pb (II) from aqueous solution. Appl. Surf. Sci. 2018, 427, 976–985. [Google Scholar] [CrossRef]
- Huang, G.; Wang, D.; Ma, S.; Chen, J.; Jiang, L.; Wang, P. A new, low-cost adsorbent: Preparation, characterization, and adsorption behavior of Pb (II) and Cu (II). J. Colloid Interface Sci. 2015, 445, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fu, S.; Zhang, L.; Zhan, H.; Levit, M.V. Use of carboxylated cellulose nanofibrils-filled magnetic chitosan hydrogel beads as adsorbents for Pb (II). Carbohydr. Polym. 2014, 101, 75–82. [Google Scholar] [CrossRef]
- Wang, K.; Gu, J.; Yin, N. Efficient removal of Pb (II) and Cd (II) using NH2-functionalized zr-mofs via rapid microwave-promoted synthesis. Ind. Eng. Chem. Res. 2017, 56, 1880–1887. [Google Scholar] [CrossRef]
- Wang, N.; Yang, L.Y.; Wang, Y.G.; Ouyang, X.K. Fabrication of composite beads based on calcium alginate and tetraethylenepentamine-functionalized mil-101 for adsorption of Pb (II) from aqueous solutions. Polymers 2018, 10, 750. [Google Scholar] [CrossRef]
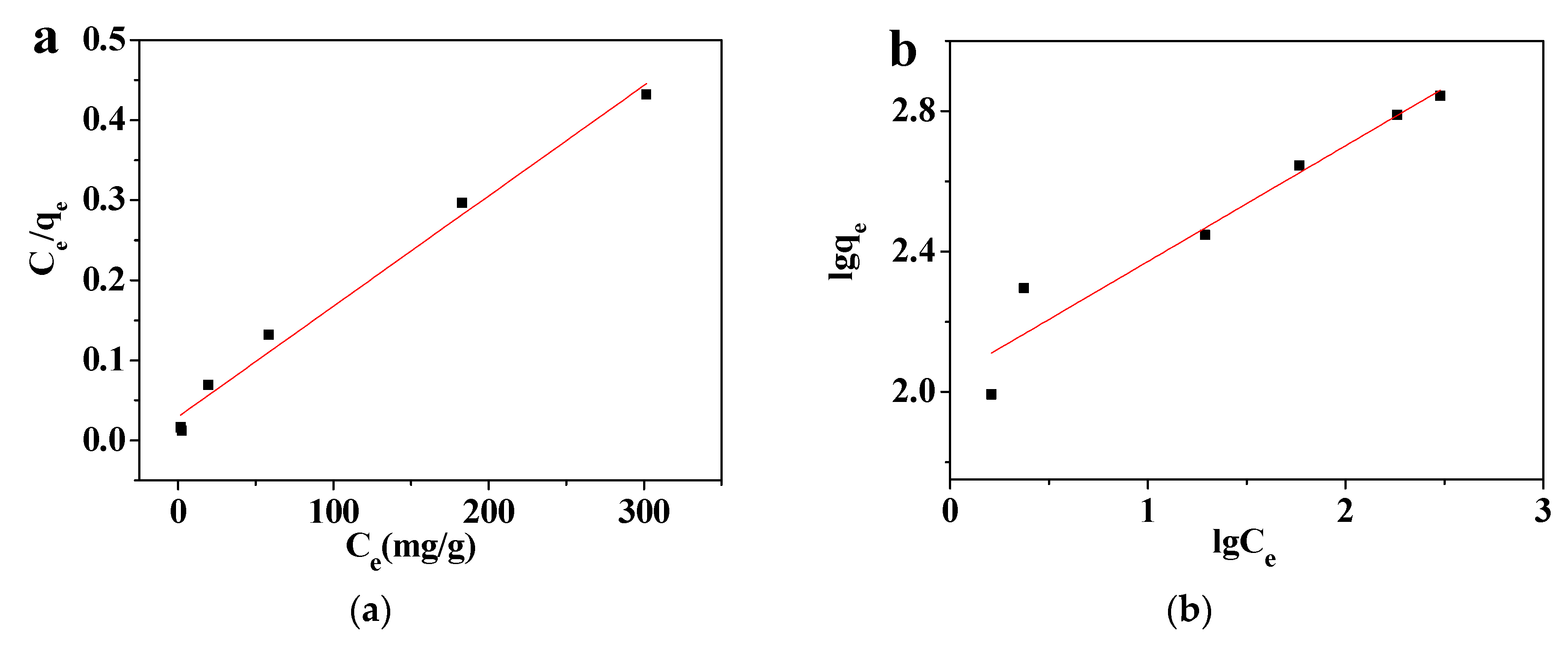

| Fe3O4:MMT (g/g) | BR (%) | qe (mg/g) | MMMT: Zn-BDC (g/g) | BR (%) | qe (mg/g) |
|---|---|---|---|---|---|
| 2:1 | 97.26 ± 0.32 | 71.36 ± 0.23 | 2:1 | 69.12 ± 0.28 | 224.66 ± 0.18 |
| 1:1 | 96.83 ± 0.41 | 98.67 ± 0.19 | 1:1 | 66.39 ± 0.57 | 280.51 ± 0.23 |
| 2:3 | 65.94 ± 0.29 | 99.06 ± 0.22 | 2:3 | 32.94 ± 0.69 | 281.48 ± 0.35 |
| 1:2 | 49.61 ± 0.30 | 98.92 ± 0.47 | 1:2 | 19.97 ± 0.64 | 282.04 ± 0.44 |
| T (K) | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|
| qm (mg/g) | b (L/mg) | R2 | KF | n | R2 | |
| 298.2 | 724.64 | 0.047 | 0.9850 | 110.07 | 3.03 | 0.9239 |
| C0(mg/L) | 10 | 50 | 100 | 200 | 300 | 500 | 800 | 1000 |
| RL | 0.68 | 0.30 | 0.18 | 0.096 | 0.066 | 0.041 | 0.026 | 0.021 |
| qe,exp | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|
| qe,cal | k1 | R2 | qe,cal | k2 | R2 | |
| 197.63 | 8.71 | 0.02390 | 0.9566 | 194.93 | 0.02521 | 0.9999 |
| Δ G (kJ/mol) T (K) | Δ H (kJ/mol) | Δ S (J/mol∙K) | |||
| 293.2 | 298.2 | 303.2 | 308.2 | ||
| −8.62 | −9.26 | −10.74 | −12.06 | 60.779 | 0.2359 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, J.; Wang, N.; Wang, Y.G.; Yu, D.; Ouyang, X. Efficient Adsorption of Pb(II) from Aqueous Solutions by Metal Organic Framework (Zn-BDC) Coated Magnetic Montmorillonite. Polymers 2018, 10, 1383. https://doi.org/10.3390/polym10121383
Shen J, Wang N, Wang YG, Yu D, Ouyang X. Efficient Adsorption of Pb(II) from Aqueous Solutions by Metal Organic Framework (Zn-BDC) Coated Magnetic Montmorillonite. Polymers. 2018; 10(12):1383. https://doi.org/10.3390/polym10121383
Chicago/Turabian StyleShen, Jian, Nan Wang, Yang Guang Wang, Di Yu, and Xiao–kun Ouyang. 2018. "Efficient Adsorption of Pb(II) from Aqueous Solutions by Metal Organic Framework (Zn-BDC) Coated Magnetic Montmorillonite" Polymers 10, no. 12: 1383. https://doi.org/10.3390/polym10121383
APA StyleShen, J., Wang, N., Wang, Y. G., Yu, D., & Ouyang, X. (2018). Efficient Adsorption of Pb(II) from Aqueous Solutions by Metal Organic Framework (Zn-BDC) Coated Magnetic Montmorillonite. Polymers, 10(12), 1383. https://doi.org/10.3390/polym10121383






