Effect of Potassium Permanganate Modification on Plasticized Spinning Polyacrylonitrile Fibers with Different Diameters
Abstract
:1. Introduction
2.Material and Methods
2.1. Materials
2.2. Preparation of Samples
2.3. Characterization
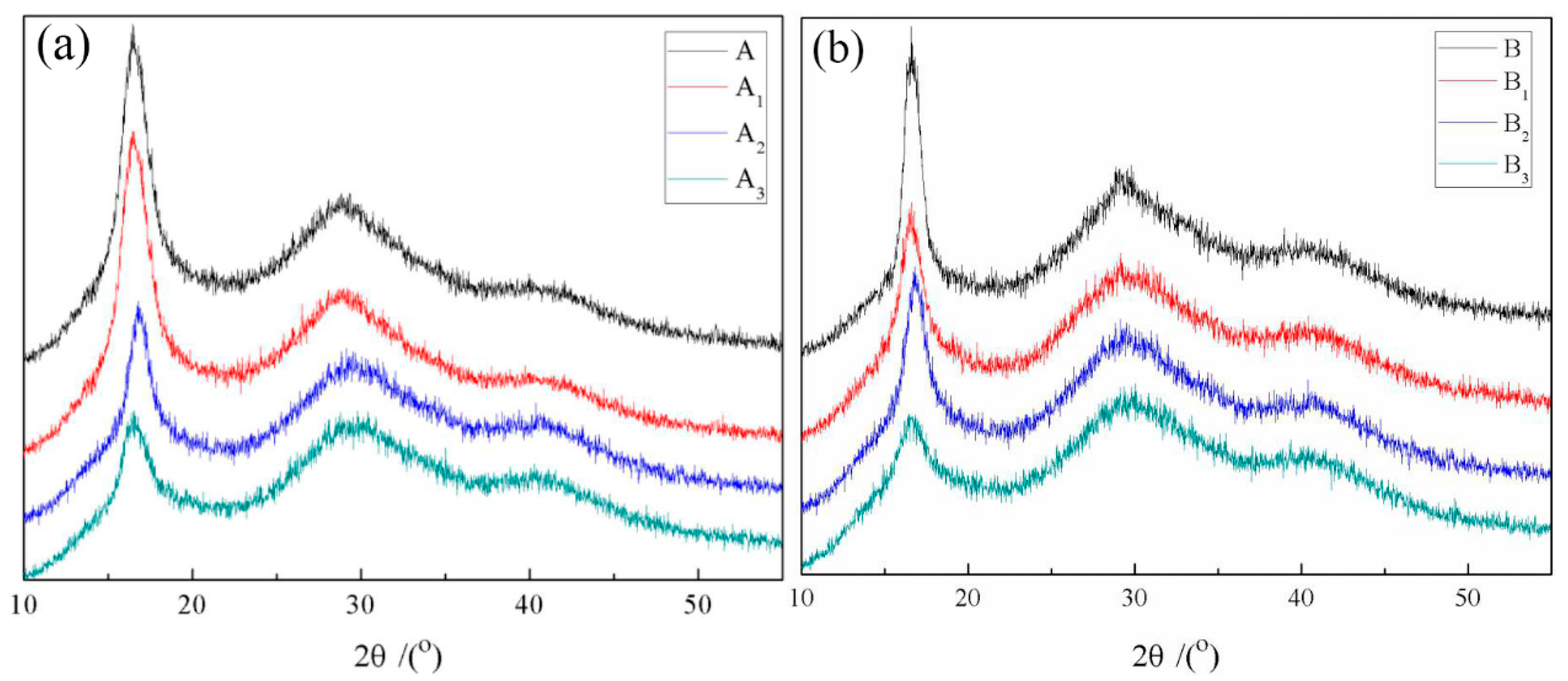
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, X.; Qin, A.W.; Zhao, X.Z.; Liu, D.P.; Wang, H.Y.; He, C.J. Drawing dependent structures, mechanical properties and cyclization behaviors of polyacrylonitrile and polyacrylonitrile/carbon nanotube composite fibers prepared by plasticized spinning. Phys. Chem. Chem. Phys. 2015, 7, 21856–21865. [Google Scholar] [CrossRef] [PubMed]
- Matandabuzo, M.; Ajibade, P.A. Synthesis and surface functionalization of multi-walled carbon nanotubes with imidazolium and pyridinium-based ionic liquids: Thermal stability, dispersibility and hydrophobicity characteristics. J. Mol. Lid. 2018, 268, 284–293. [Google Scholar] [CrossRef]
- Hiraga, Y.; Koyama, K.; Sato, Y.; Smith, R.L., Jr. High pressure densities for mixed ionic liquids having different functionalities: 1-butyl-3-methylimidazolium chloride and 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide. J. Chem. Thermodyn. 2017, 108, 7–17. [Google Scholar] [CrossRef]
- Li, X.; Qin, A.W.; Zhao, X.Z.; He, C.J. The plasticized spinning and cyclization behaviors of polyacrylonitrile/functionalized carbon nanotube (PAN/CNT) fibers. RSC Adv. 2015, 5, 52226–522234. [Google Scholar] [CrossRef]
- Liu, S.P.; Han, K.Q.; Chen, L.; Zheng, Y.; Yu, M.H.; Li, J.Q.; Yang, Z. Influence of External Tension on the Structure and Properties of Melt-Spun PAN Precursor Fibers during Thermal Oxidation. Macromol. Mater. Eng. 2015, 300, 1001–1009. [Google Scholar] [CrossRef]
- Liu, S.P.; Han, K.Q.; Chen, L.; Zheng, Y.; Yu, M.H. Structure and properties of partially cyclized polyacrylonitrile-based carbon fiber—Precursor fiber prepared by melt-spun with ionic liquid as the medium of processing. Polym. Eng. Sci. 2015, 55, 2722–2728. [Google Scholar] [CrossRef]
- Liu, S.P.; Han, K.Q.; Chen, L.; Zheng, Y.; Yu, M.H. Influence of air circulation on the structure and properties of melt-spun PAN precursor fibers during thermal oxidation. RSC Adv. 2015, 5, 37669–37674. [Google Scholar] [CrossRef]
- Mittal, J.; Mathur, R.B.; Bahl, O.P.; Inagaki, M. Post spinning treatment of PAN fibers using succinic acid to produce high performance carbon fibers. Carbon 1998, 36, 893–897. [Google Scholar] [CrossRef]
- Mathur, R.B.; Mittal, J.; Bahl, O.P.; Sandle, N.K. Characteristics of KMnO4-modified PAN fibres—Its influence on the resulting carbon fibres’ properties. Carbon 1994, 32, 71–77. [Google Scholar] [CrossRef]
- Bahl, D.P.; Mathur, R.B.; Dhami, T.L. Modification of polyacrylonitrile fibres to make them suitable for conversion into high performance carbon fibres. Mater. Sci. Eng. 1985, 73, 105–112. [Google Scholar] [CrossRef]
- Zhang, W.X.; Wang, Y.Z. Manufacture of carbon fibers from polyacrylonitrile precursors treated with CoSO4. J. Appl. Polym. Sci. 2002, 85, 153–158. [Google Scholar] [CrossRef]
- Yusof, N.; Ismail, A.F. Post spinning and pyrolysis processes of polyacrylonitrile (PAN)-based carbon fiber and activated carbon fiber: A review. J. Anal. Appl. Pyrol. 2002, 93, 1–13. [Google Scholar] [CrossRef]
- Tian, Y.C.; Han, K.G.; Zhang, W.H.; Zhang, J.J.; Rong, H.P.; Wang, D.; Yan, B.; Liu, S.P.; Yu, M.H. Influence of residence time on the structure of polyacrylonitrile in ionic liquids during melt spinning process. Mater. Lett. 2003, 92, 119–121. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.L.; Dang, X.N.; Luan, D.; Wang, F.; Chen, H.Y.; Wang, C.T. Structural and thermal property changes of plasticized spinning polyacrylonitrile fibers under different spinning speeds. J. Appl. Polym. Sci. 2017, 134, 45267. [Google Scholar] [CrossRef]
- Gupta, V.B.; Kumar, S. The effect of heat setting on the structure and mechanical properties of poly(ethylene terephthalate) fiber. I. Structural changes. J. Appl. Polym. Sci. 1981, 26, 1865–1876. [Google Scholar] [CrossRef]
- Ju, A.Q.; Guang, S.Y.; Xu, H.Y. Effect of comonomer structure on the stabilization and spinnability of polyacrylonitrile copolymers. Carbon 2013, 54, 323–335. [Google Scholar] [CrossRef]
- Li, X.; Qin, A.W.; Zhao, X.Z.; Ma, B.M.; He, C.J. The plasticization mechanism of polyacrylonitrile/1-butyl-3-methylimidazolium chloride system. Polymer 2014, 55, 5773–5780. [Google Scholar] [CrossRef]
- Li, X.; Liu, C.Y.; Qin, A.W.; Zhao, X.Z.; He, C.J. Effect of processing conditions on properties of KMnO4-treated PAN fibers. Adv. Mater. Res. 2013, 821–822, 23–27. [Google Scholar]
- Morris, E.A.; Weisenberger, M.C.; Abdallah, M.G.; Vautard, F.; Grappe, H.; Ozcan, S.; Paulauskas, F.L.; Eberle, C.; Jackson, D.; Mecham, S.J.; et al. High performance carbon fibers from very high molecular weight polyacrylonitrile precursors. Carbon 2016, 245, 245–252. [Google Scholar] [CrossRef]
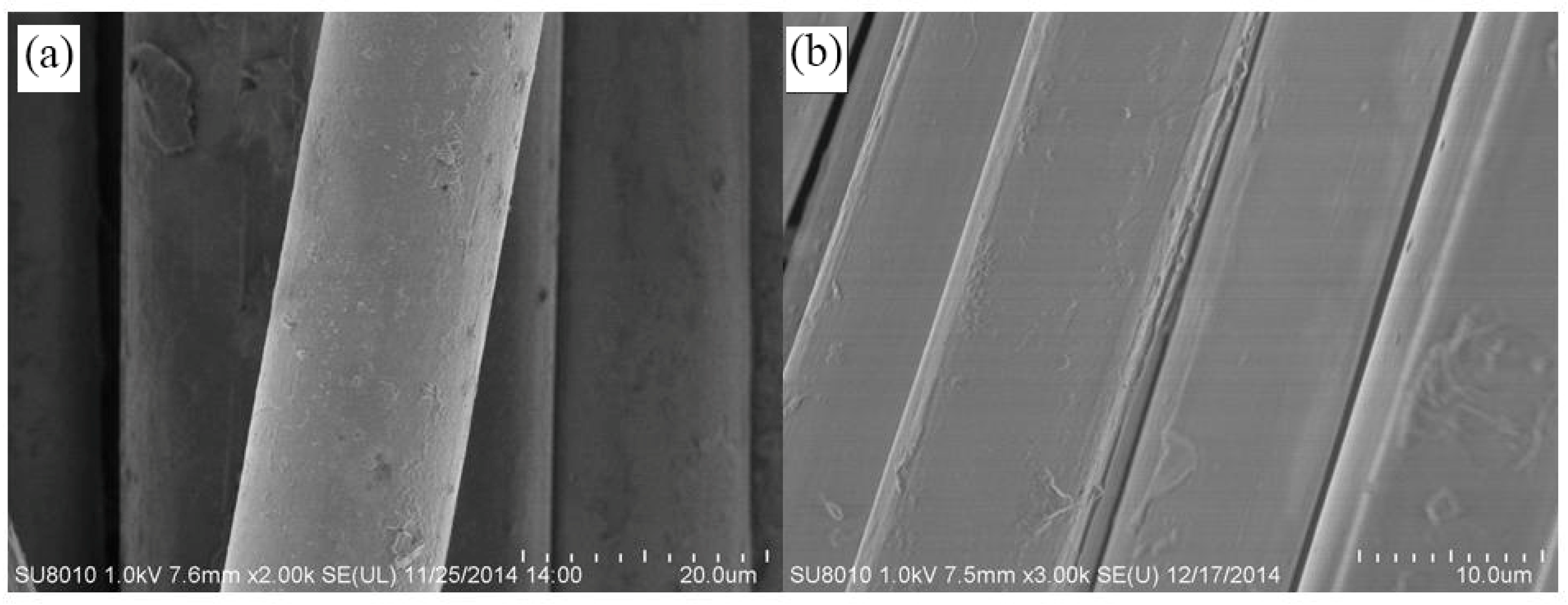
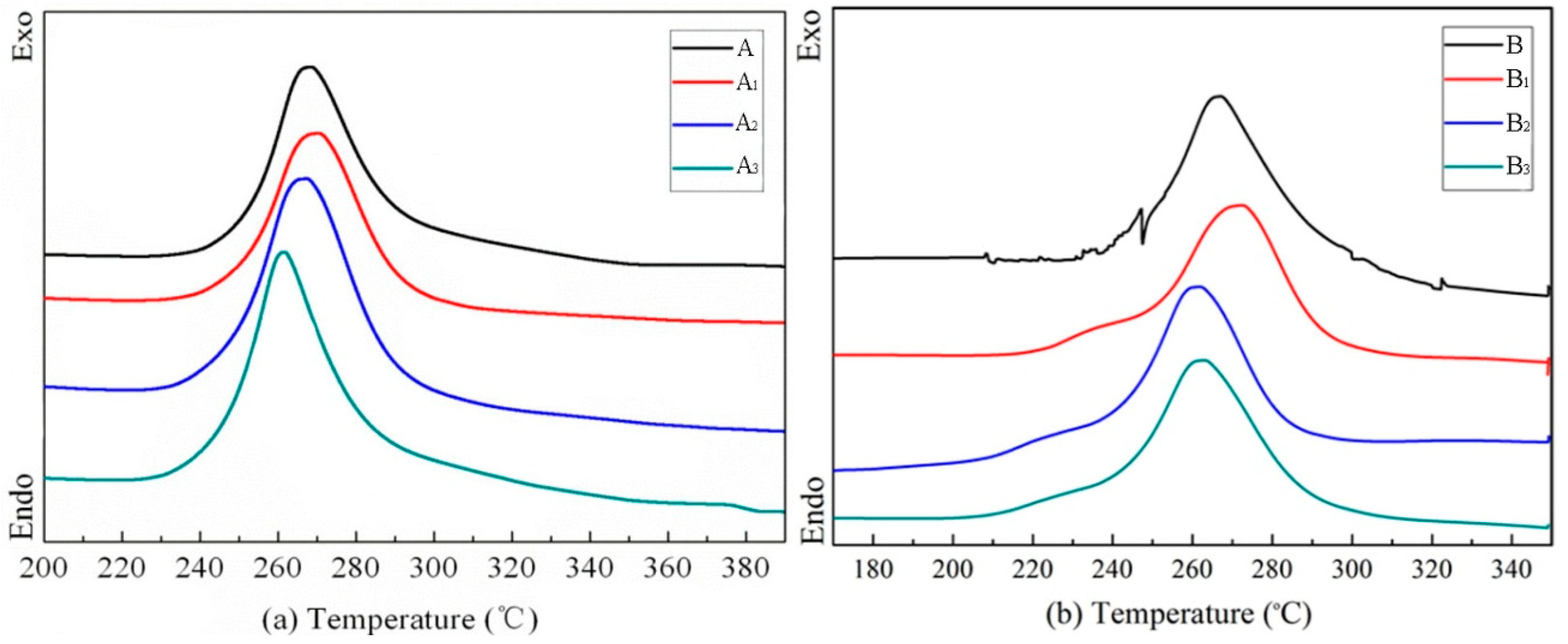
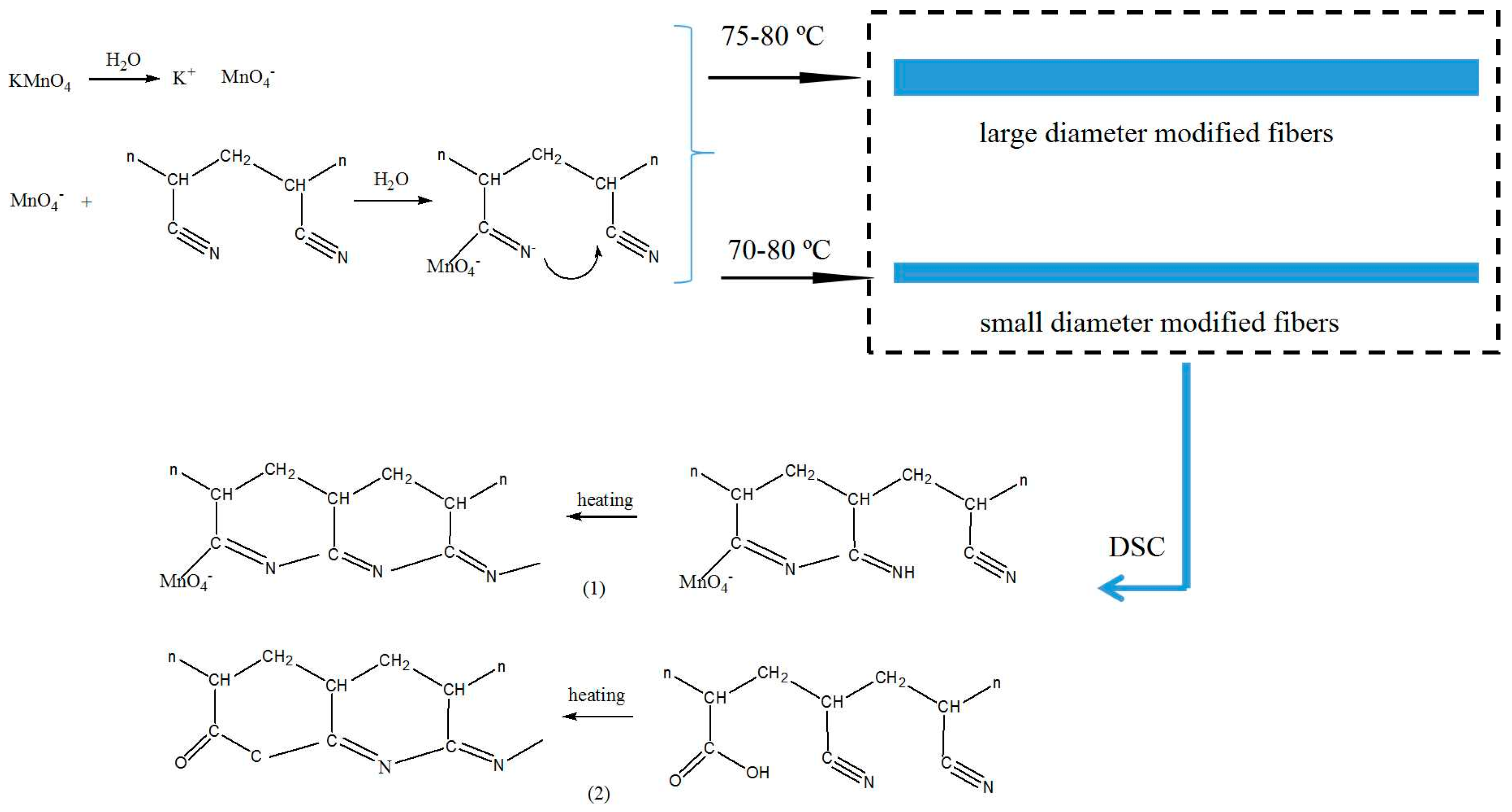
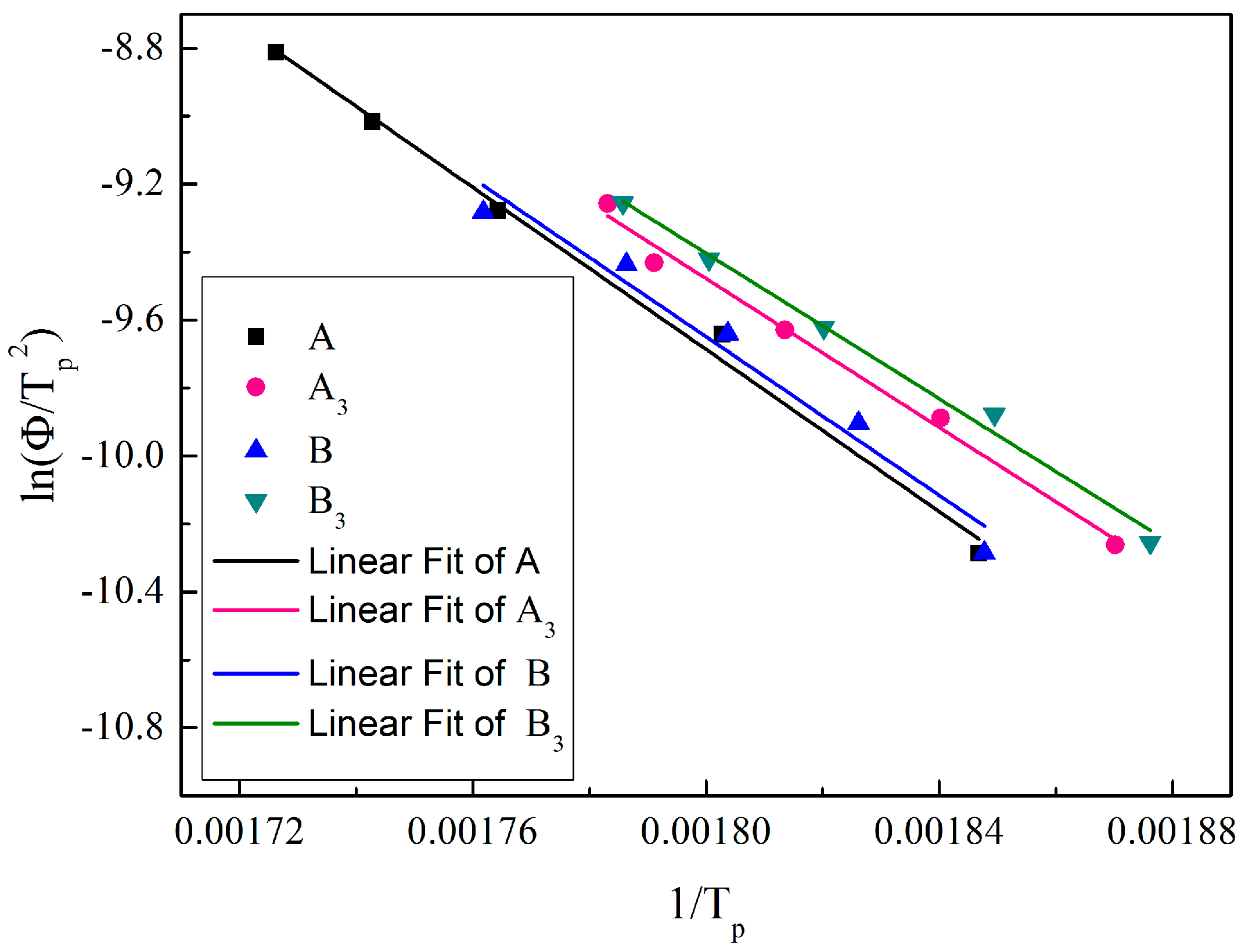
| Fiber Type | Fiber Fineness/dtex | Fiber Diameter/μm | Elongation at Break/% | Breaking Strength/(cN/dtex) | Initial Modulus/(cN/dtex) |
|---|---|---|---|---|---|
| Large diameter | 2.33 | 16.6 | 9.26 | 5.95 | 100.92 |
| Small diameter | 1.16 | 10.2 | 7.58 | 5 | 83.56 |
| Diameter/μm | Temperature/°C | Abbreviation | Ti/°C | Tf/°C | ΔT | T/°C | ΔH/J g−1 |
|---|---|---|---|---|---|---|---|
| 16.6 | - | A | 229.5 | 353.2 | 123.72 | 268.52 | 403 |
| 70 | A1 | 229.8 | 364.17 | 139.39 | 270.43 | 335.2 | |
| 75 | A2 | 226.1 | 371.79 | 145.67 | 267.39 | 454.5 | |
| 80 | A3 | 224 | 382.81 | 158.82 | 261.69 | 480.5 | |
| 10.2 | - | B | 229.8 | 346.8 | 117 | 268.23 | 451.2 |
| 70 | B1 | 213.6 | 325.84 | 112.23 | 272.39 | 411.8 | |
| 75 | B2 | 207.5 | 313.82 | 106.31 | 261.52 | 404.4 | |
| 80 | B3 | 199 | 312.37 | 113.37 | 260 | 400.6 |
| Heating Rate/(°C/min) | 16.6 μm | 10.2 μm | ||
|---|---|---|---|---|
| A | A3 | B | B3 | |
| 10 | 268.5 | 261.7 | 268.2 | 260 |
| 15 | 281.7 | 270.4 | 274.6 | 267.7 |
| 20 | 293.8 | 278.4 | 281.4 | 276.4 |
| 25 | 300.8 | 285.3 | 286.8 | 282.4 |
| 30 | 306.3 | 287.8 | 294.6 | 287 |
| Fiber Type | Crystallinity/% | Crystal Size/nm | Orientation Degree/% |
|---|---|---|---|
| A | 67.4 | 4.71 | 85.3 |
| A1 | 67.1 | 4.68 | 85.5 |
| A2 | 63.04 | 4.45 | 79.6 |
| A3 | 59.67 | 4.25 | 75.4 |
| B | 66.6 | 4.63 | 83.4 |
| B1 | 62.15 | 4.14 | 74.5 |
| B2 | 56.52 | 3.98 | 67.1 |
| B3 | 53.12 | 3.46 | 62.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Dang, X. Effect of Potassium Permanganate Modification on Plasticized Spinning Polyacrylonitrile Fibers with Different Diameters. Polymers 2018, 10, 1330. https://doi.org/10.3390/polym10121330
Li X, Dang X. Effect of Potassium Permanganate Modification on Plasticized Spinning Polyacrylonitrile Fibers with Different Diameters. Polymers. 2018; 10(12):1330. https://doi.org/10.3390/polym10121330
Chicago/Turabian StyleLi, Xiang, and Xiaonan Dang. 2018. "Effect of Potassium Permanganate Modification on Plasticized Spinning Polyacrylonitrile Fibers with Different Diameters" Polymers 10, no. 12: 1330. https://doi.org/10.3390/polym10121330
APA StyleLi, X., & Dang, X. (2018). Effect of Potassium Permanganate Modification on Plasticized Spinning Polyacrylonitrile Fibers with Different Diameters. Polymers, 10(12), 1330. https://doi.org/10.3390/polym10121330





