Isothermal Adsorption Properties for the Adsorption and Removal of Reactive Blue 221 Dye from Aqueous Solutions by Cross-Linked β-Chitosan Glycan as Acid-Resistant Adsorbent
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
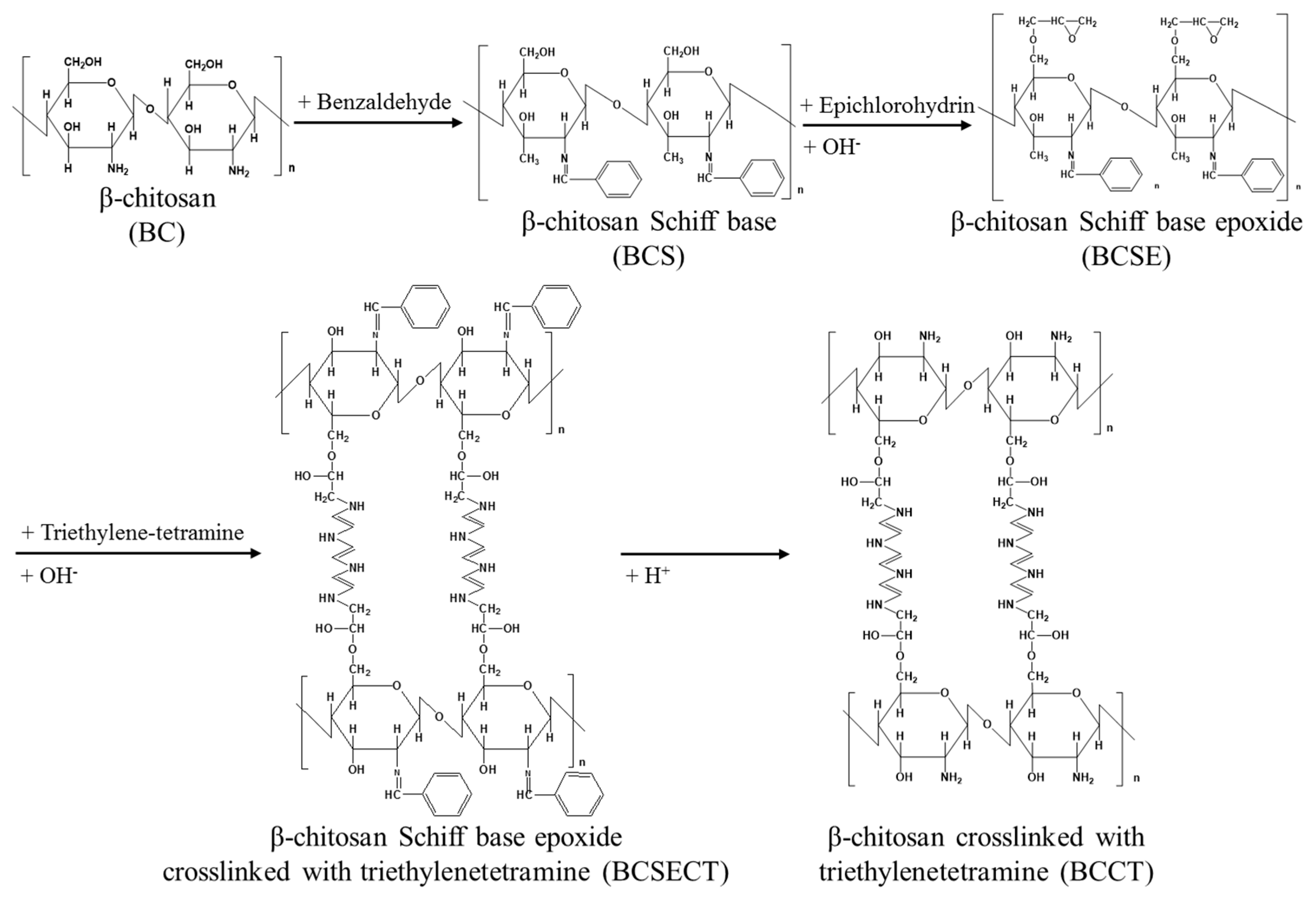
2.2. Synthesis of β-Chitosan Cross-Linked with Triethylenetetramine (BCCT)
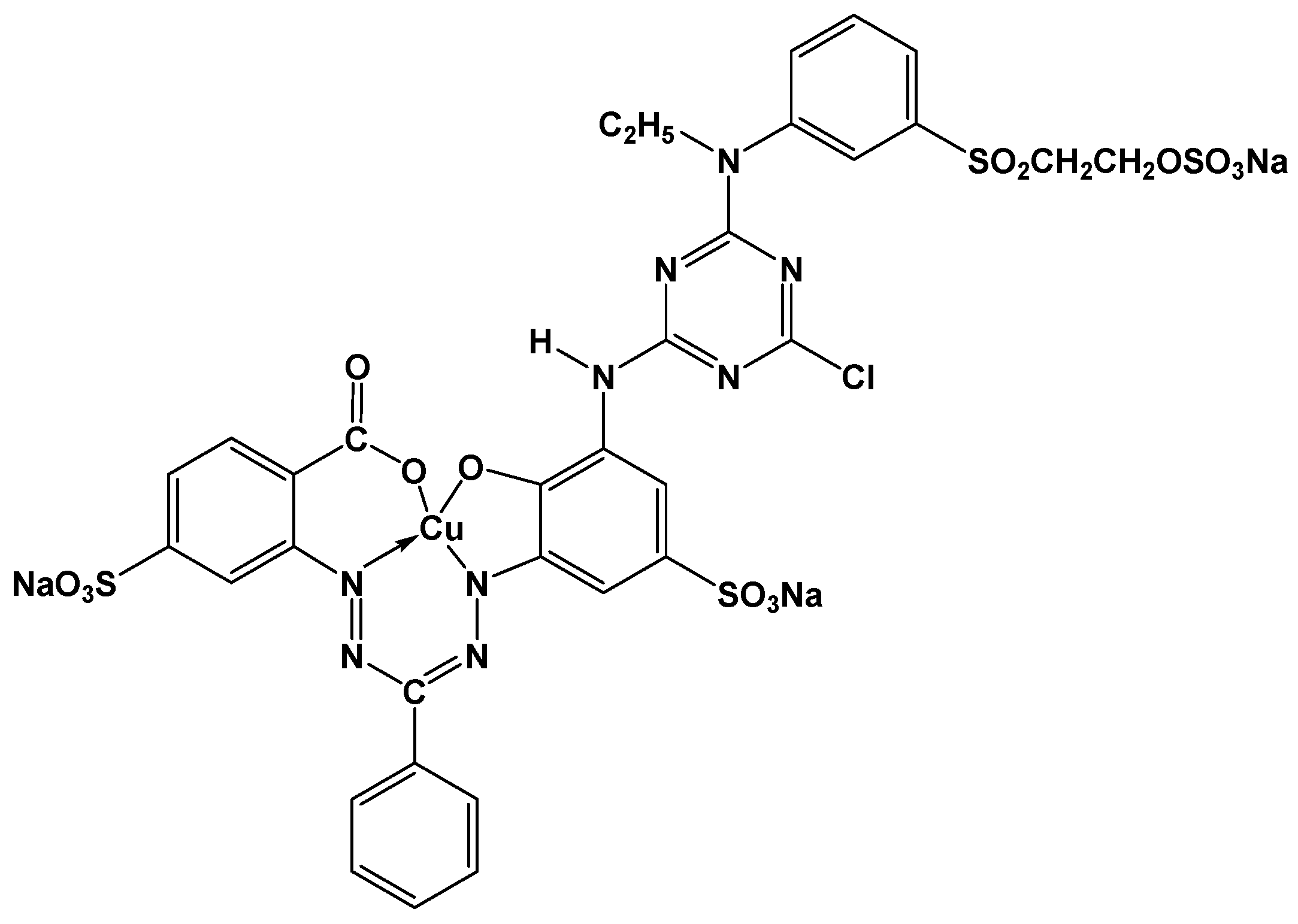
2.3. Adsorption of the Reactive Dye
2.4. Characterization and Measurements
2.4.1. Fourier-Transform Infrared Spectra (FTIR) Were Collected on Perkin Elmer, Waltham, MA, USA
2.4.2. Ultraviolet–Visible (UV–VIS) Data Were Collected on V-670 Mode Spectrophotometer
2.4.3. Zeta Potential Analysis Was Conducted on Malvern Instruments Zetasizer Nano ZS90, Westborough, MA, USA
2.4.4. Elemental Analysis (EA) Was Conducted on Thermo Flash 2000, Waltham, MA, USA
2.4.5. Solid-State Nuclear Magnetic Resonance Spectroscopy Was Performed on Varian Inova AS500 MHz, Palo Alto, CA, USA
3. Results and Discussion
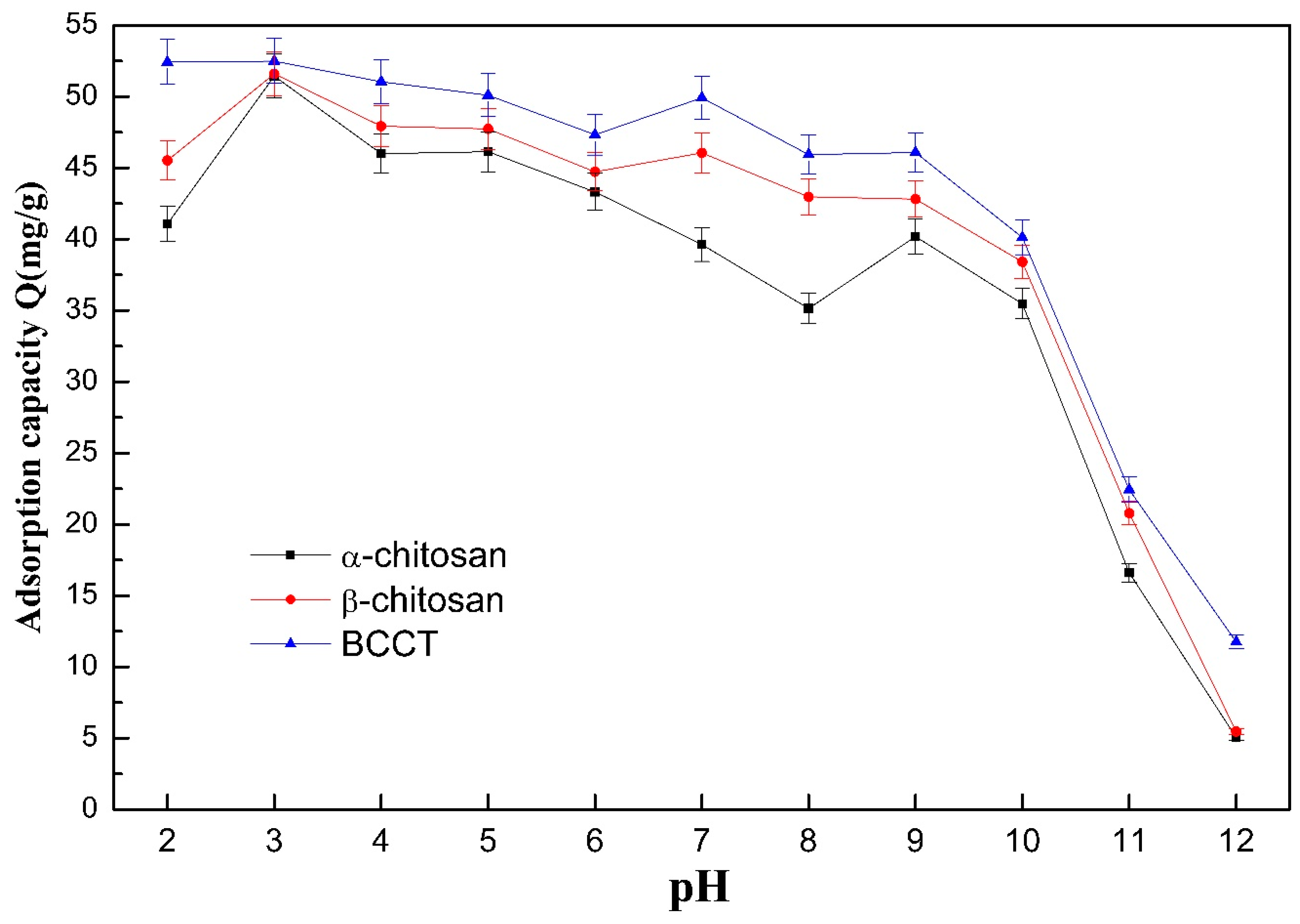
3.1. Effect of pH on the Adsorption Capability of the Various Adsorbents
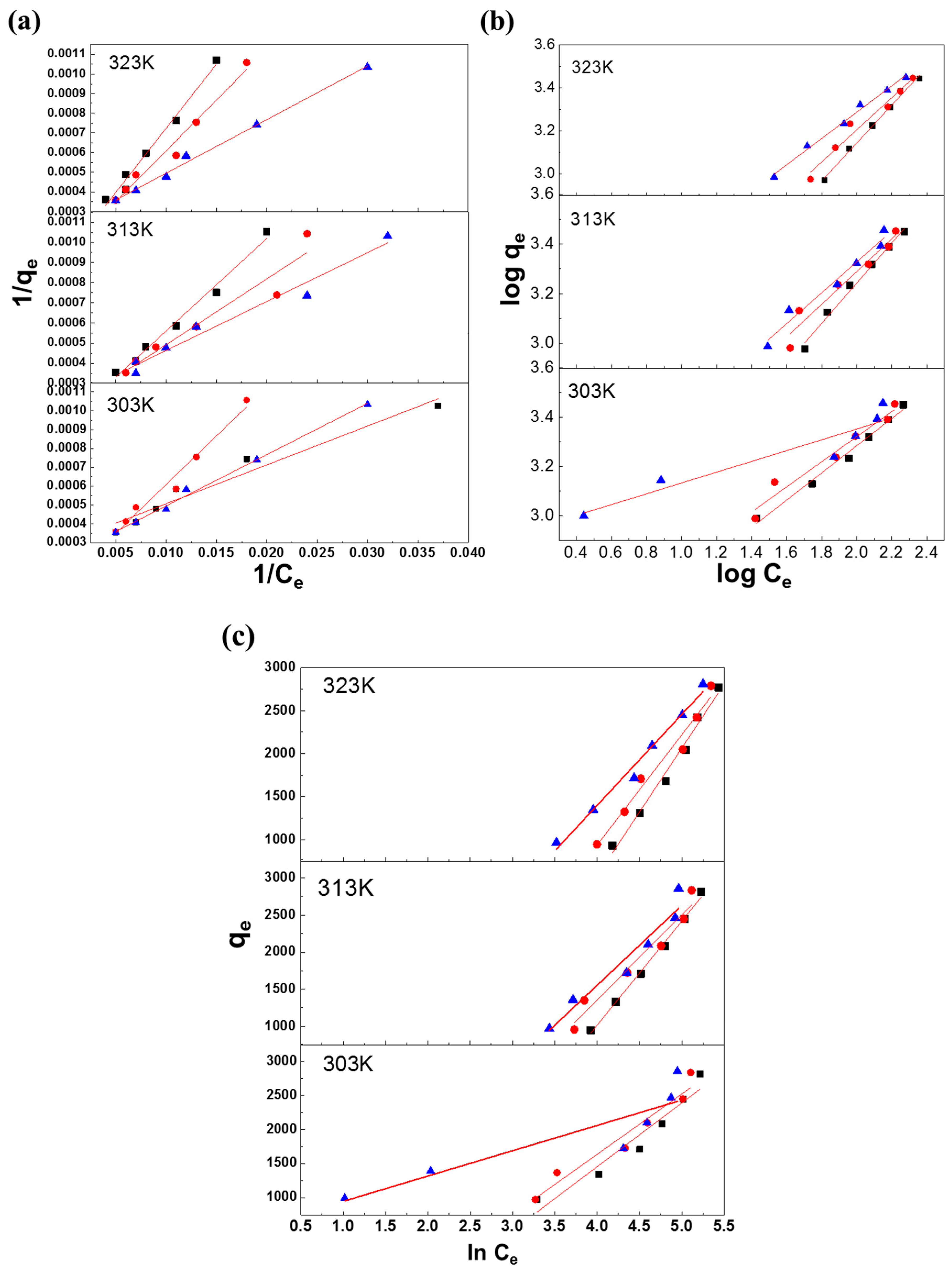
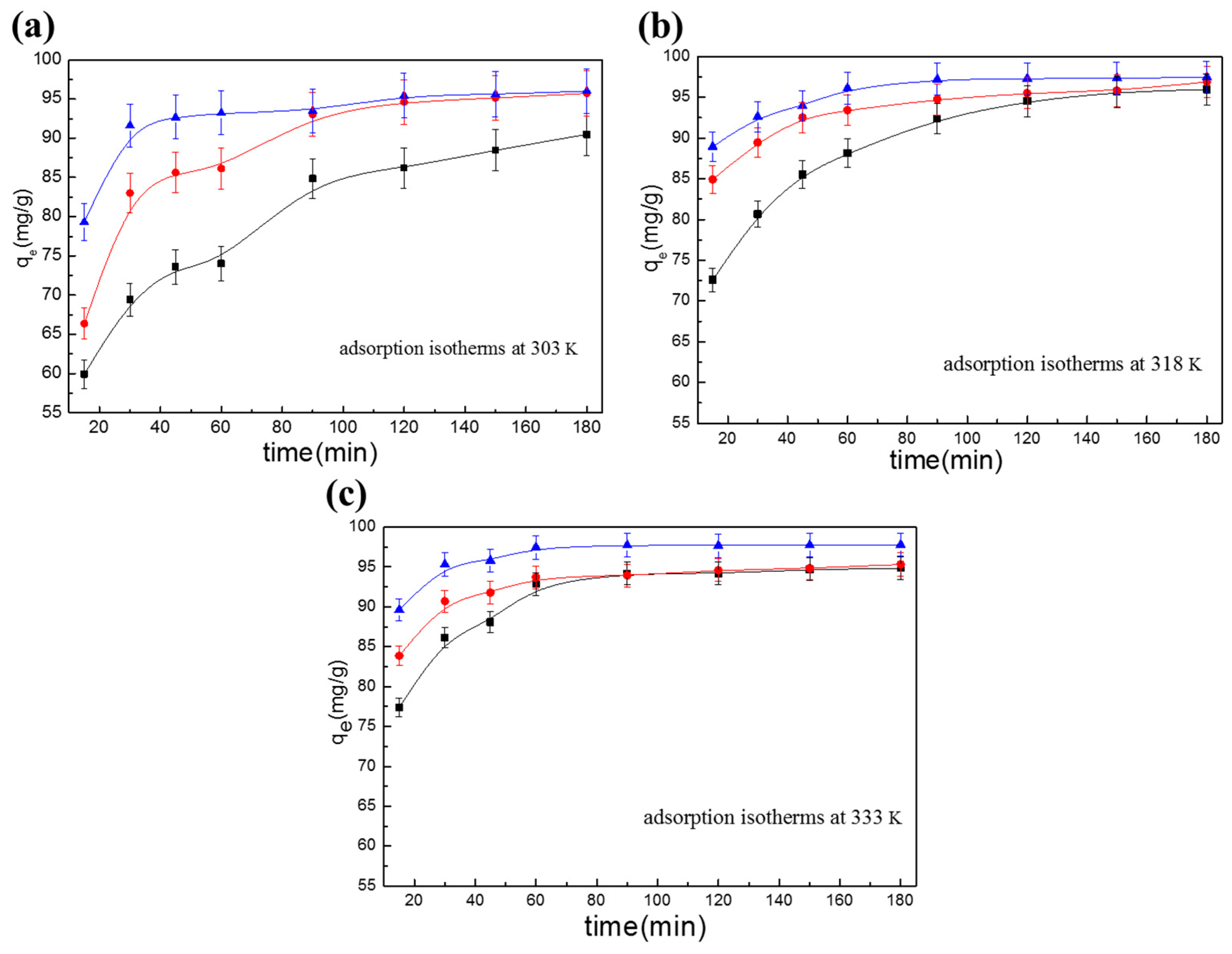
3.2. Discussion of Isothermal Adsorption Models
3.2.1. Langmuir adsorption isotherm
3.2.2. Freundlich Adsorption Isotherm
3.2.3. Temkin Adsorption Isotherm
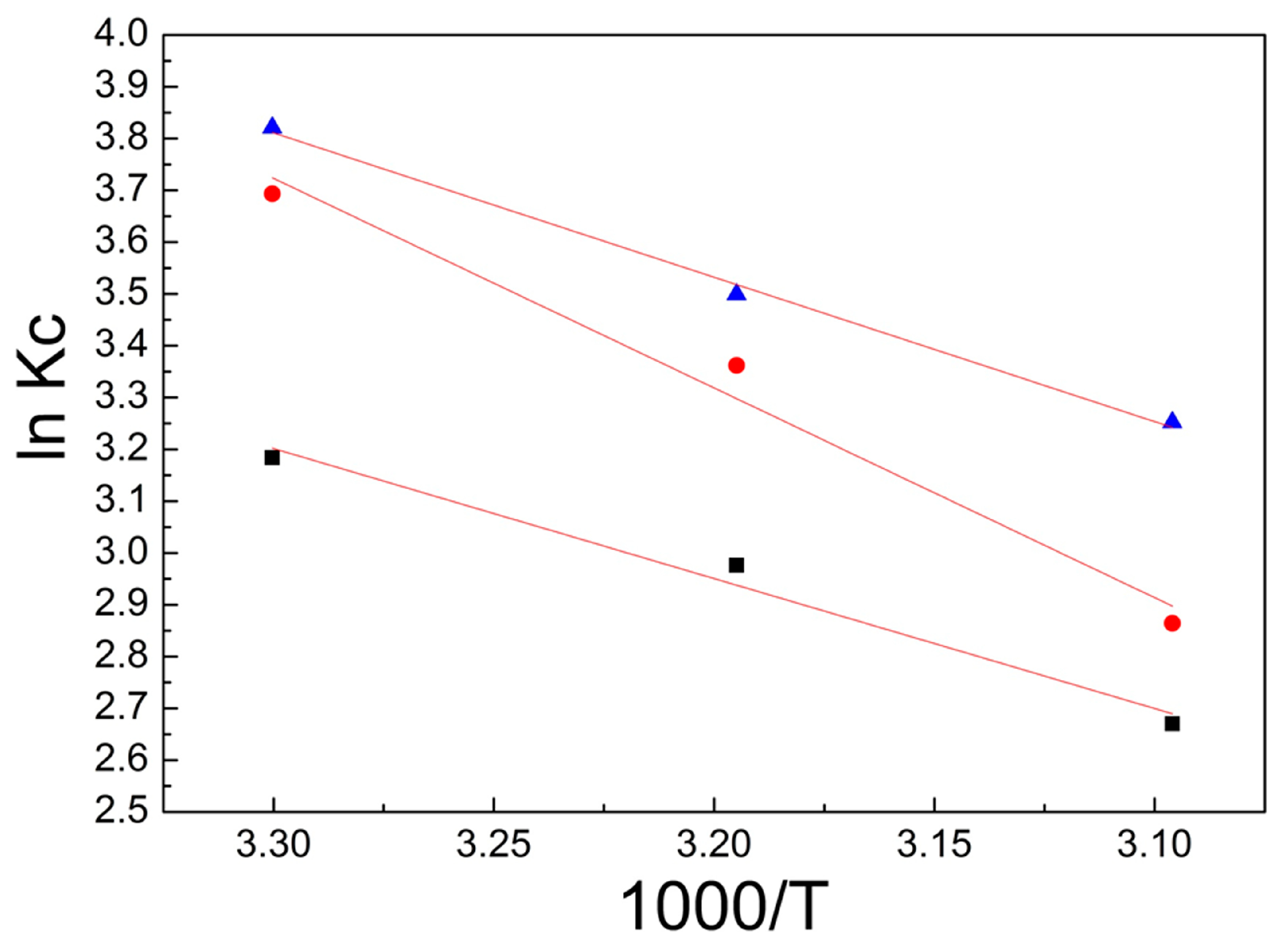
3.3. Adsorption Thermodynamics
- During the adsorption of the RB221 dye in the system by the different adsorbents, as the temperature increased from 303 to 323 K, both the ΔG0 and ΔH0 were negative, indicating the spontaneity of the process, as no energy needed to be supplied from the outside.
- A negative ΔH0 showed that the adsorption was an exothermic process. Alkan et al. [27,28,29] noticed a standard enthalpy change of 40–120 KJ/mol caused by chemisorption, which was larger than that by physisorption. In this sense, the enthalpy of the adsorption found in this study is below that of the chemisorption, which indicates that the adsorption in this case may be due to physical interactions.
- The final concentrations of the RB221 dye removed by the adsorbents at different temperatures were found. It was noticed that the maximum adsorption capacities of α-chitosan, β-chitosan, and BCCT decreased from 1344.29, 1365.99, and 1369.98 mg/g to 1309.34, 1324.48, and 1347.83 mg/g, respectively. This could be attributed to the adsorption of the RB221 dye being exothermic, because of the Coulombic electrostatic force between the sulfonates of the RB221 molecules and the protonated amines on the chitosan. In addition, hydrogen bonding between the adsorbents and dye molecules could be a reason.
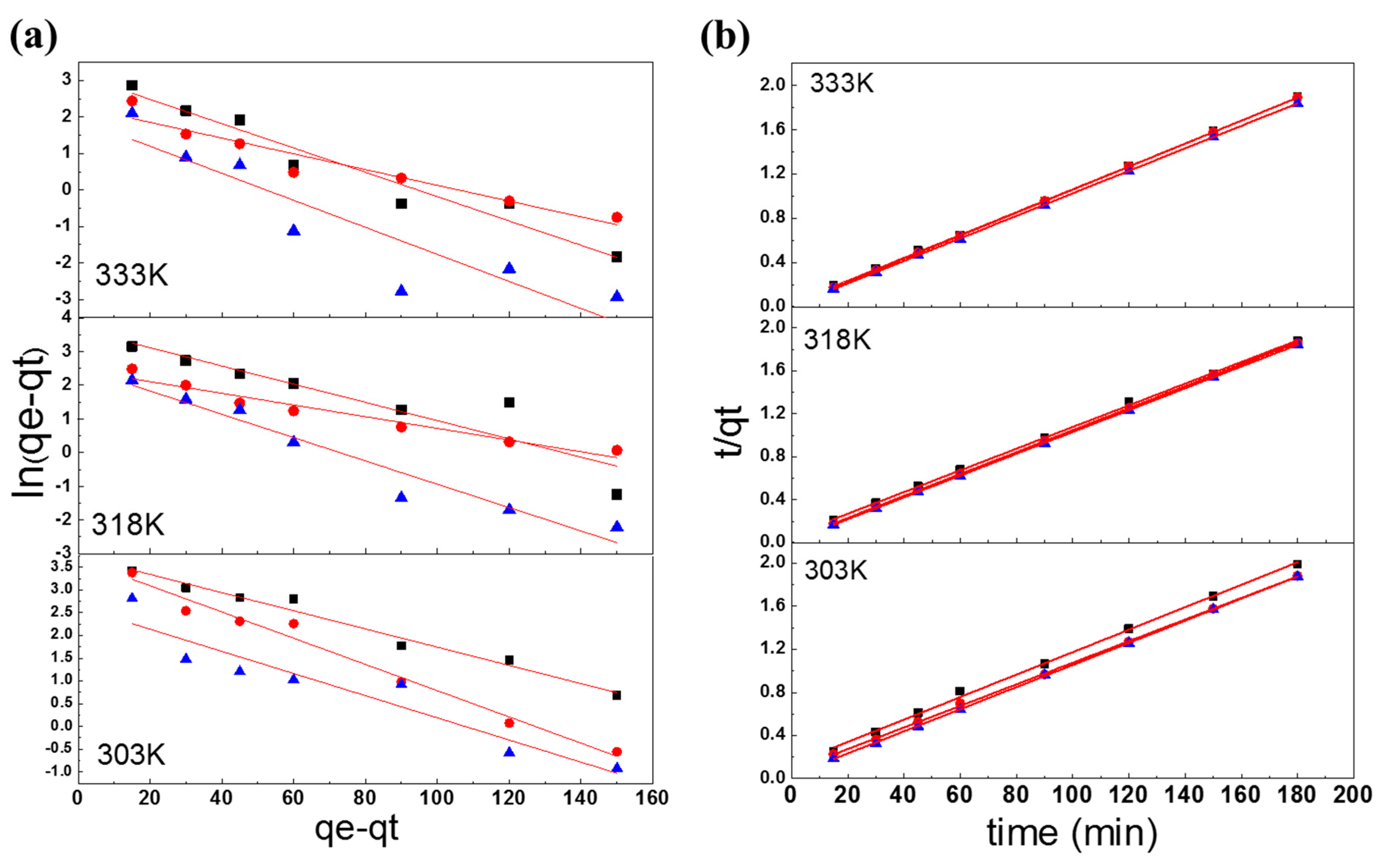
3.4. Adsorption Kinetics
3.4.1. Pseudo-First-Order Kinetics Model
3.4.2. Pseudo-Second-Order Kinetics Model
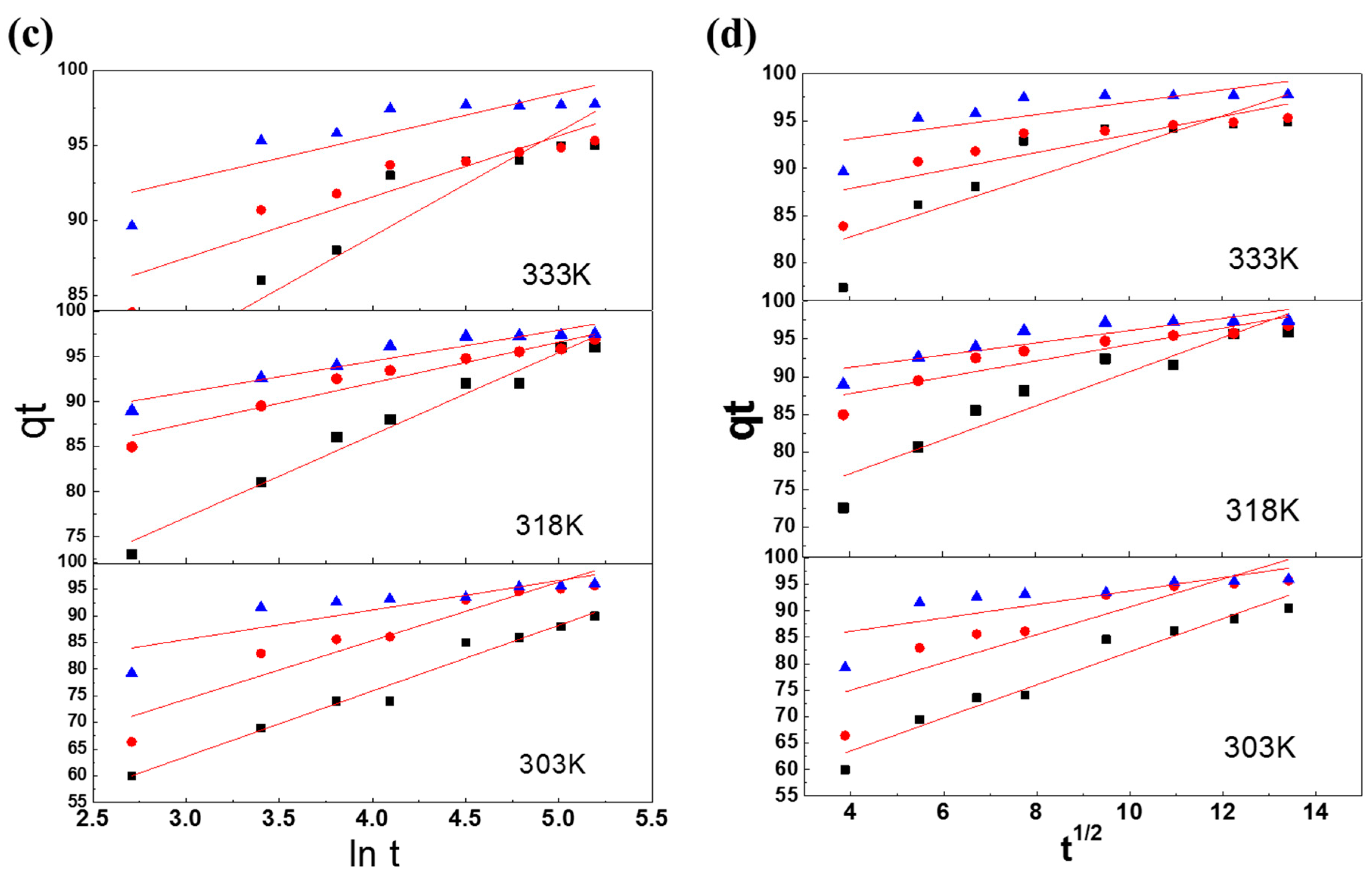
3.4.3. Elovich Model
3.4.4. Weber–Morris model
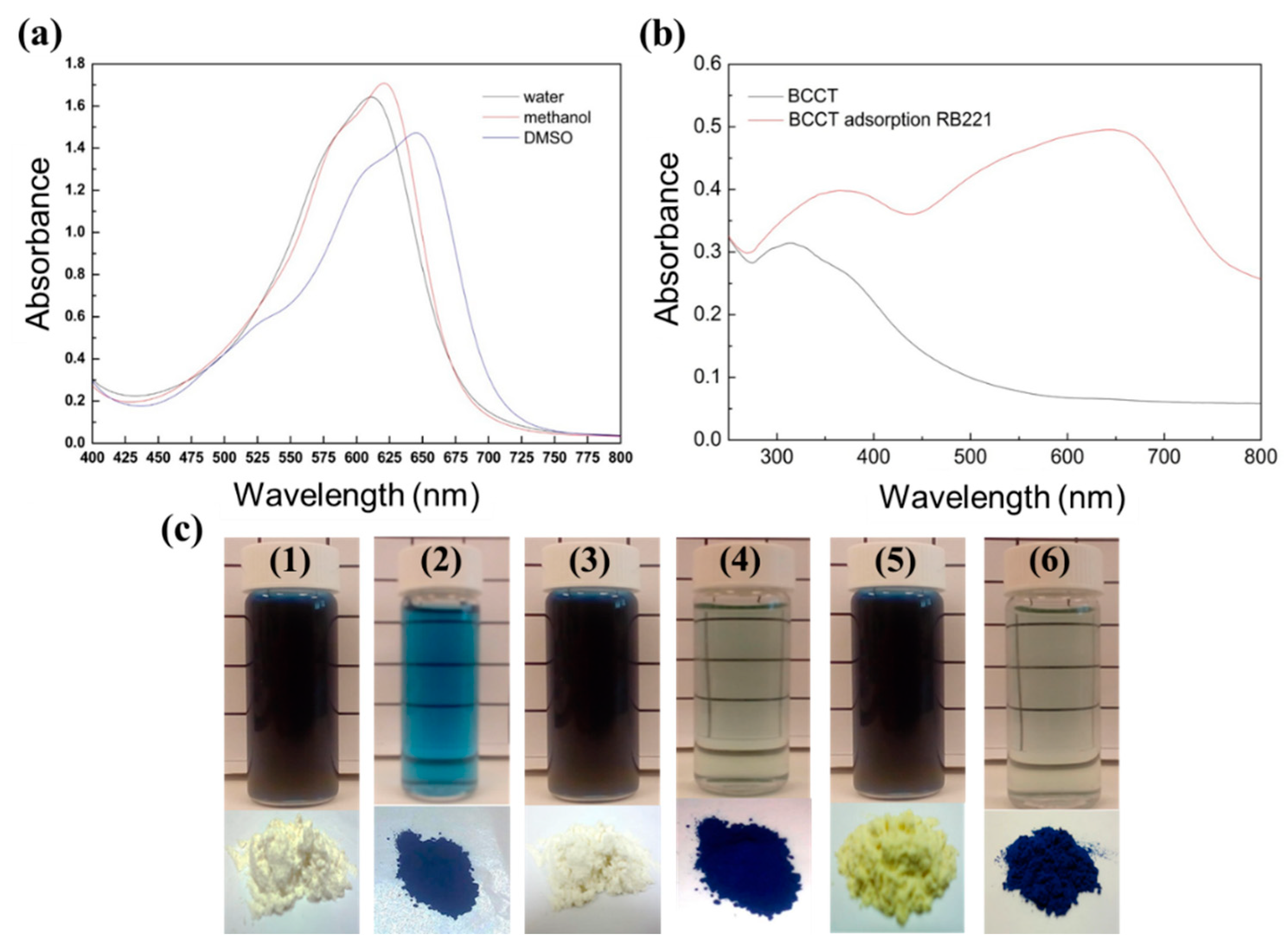
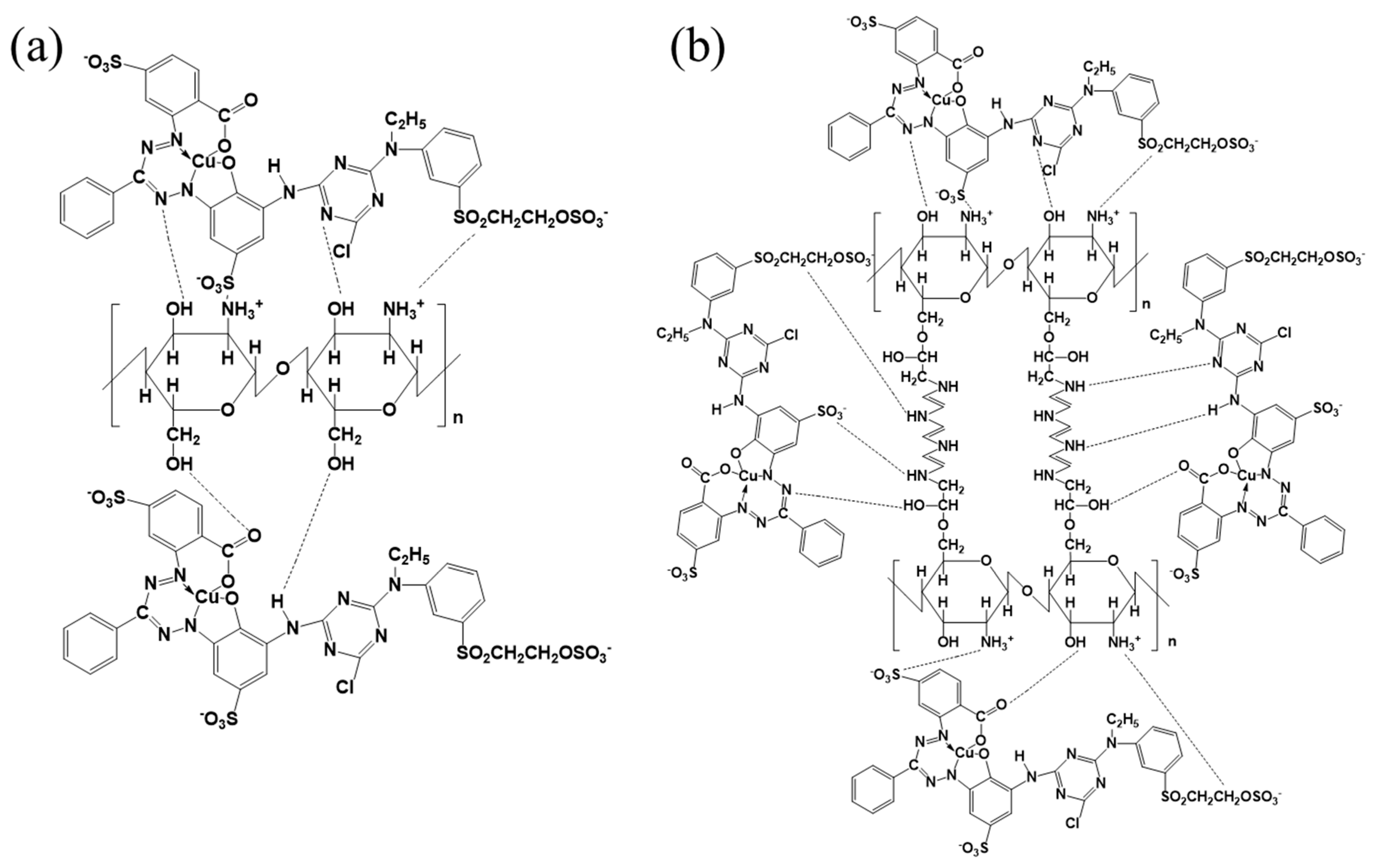
3.5. Adsorption Mechanism of BCCT for Reactive Blue 221 Dye at pH 2
3.5.1. Action of Hydrogen Bonding
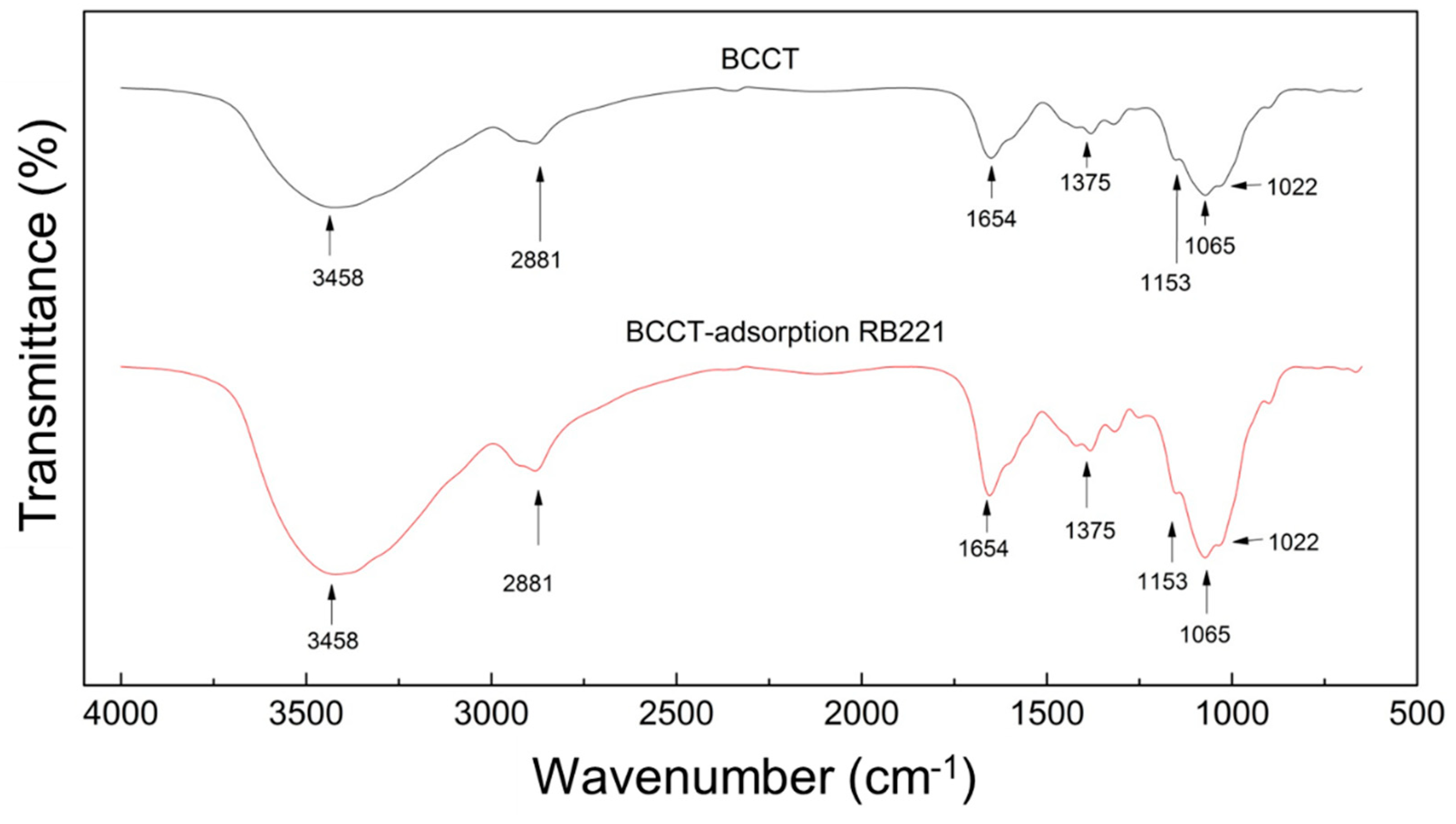
3.5.2. FTIR Spectroscopy
3.5.3. Elemental Analysis
3.5.4. Zeta Potential Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, L.; Xu, K.; Cheng, X.; Xu, Y.; Jia, Q. Study on optimizing production scheduling for water-saving in textile dyeing industry. J. Clean Prod. 2017, 141, 721–727. [Google Scholar] [CrossRef]
- Zhou, L.; Peng, B.; Xiao, K. Role of micronutrients on dyeing effluent treatment in activated sludge process. Water Environ. Res. 2017, 89, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Yukseler, H.; Uzal, N.; Sahinkaya, E.; Kitis, M.; Dilek, F.B.; Yetis, U. Analysis of the best available techniques for effluents from a denim manufacturing textile mill. J. Environ. Manag. 2017, 203, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, S. Impacts of operating parameters on oxidation–reduction potential and pretreatment efficacy in the pretreatment of printing and dyeing effluent by Fenton process. J. Hazard. Mater. 2012, 243, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Rosales, E.; Meijidea, J.; Tavares, T.; Pazosa, M.; Sanromán, M.A. Grapefruit peelings as a promising biosorbent for the removal of leather dyes and hexavalentchromium. Process Saf. Environ. Protect. 2016, 101, 61–71. [Google Scholar] [CrossRef]
- Monsalvea, S.O.; Dornelles, J.; Polla, E.; Castrillón, M.R.; Valentec, P.; Gutterresa, M. Biodecolourisation and biodegradation of leather dyes by a native isolate of Trametes villosa. Process Saf. Environ. Protect. 2017, 109, 437–451. [Google Scholar] [CrossRef]
- Şenay, R.H.; Gökalp, S.M.; Türker, E.; Feyzioğlu, E.; Aslan, A.; Akgöl, S. A new morphological approach for removing acid dye from leather waste water: Preparation and characterization of metal-chelated spherical particulated membranes (SPMs). J. Environ. Manag. 2015, 151, 295–302. [Google Scholar] [CrossRef]
- Sen, T.K.; Afroze, S.; Ang, H.M. Equilibrium, kinetics and mechanism of removal of methylene blue from aqueous solution by adsorption onto pine cone biomass of pinus radiate. Water Air Soil Pollut. 2011, 218, 499–515. [Google Scholar] [CrossRef]
- Paz, A.; Carballo, J.; Pérez, M.J.; Domínguez, J.M. Biological treatment of model dyes and textile effluents. Chemosphere 2017, 181, 168–177. [Google Scholar] [CrossRef]
- Nitayaphat, W. Chitosan/coffee residue composite beads for removal of reactive dye. Mater. Today Proc. 2017, 4, 6274–6283. [Google Scholar] [CrossRef]
- Belpaire, C.; Reyns, T.; Geeraerts, C.; Loco, J.V. Toxic textile dyes accumulate in wild European eel Anguilla Anguilla. Chemosphere 2015, 138, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Letašiová, S.; Medveďová, A.; Šovčíková, A.; Dušinská, M.; Volkovová, K.; Mosoiu, C.; Bartonová, A. Bladder cancer, a review of the environmental risk factors. Environ. Health 2012, 11, S11. [Google Scholar] [CrossRef] [PubMed]
- Kadirvelu, K.; Kavipriya, M.; Karthika, C.; Radhika, M.; Vennilamani, N.; Pattabhi, S. Utilization of various agricultural wastes for activated carbon preparation and application for the removal of dyes and metal ions from aqueous solutions. Bioresour. Technol. 2003, 87, 129–132. [Google Scholar] [CrossRef]
- Suryavathi, V.; Sharma, S.; Sharma, S.; Saxena, P.; Pandey, S.; Grover, R.; Kumar, S.; Sharma, K.P. Acute toxicity of textile dye effluents (untreated and treated) of Sanganer on male reproductive systems of albino rats and mice. Reprod. Toxicol. 2005, 19, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Deng, S.; Wu, R.; Hong, S.; Wang, B.; Huang, J.; Wang, Y.; Yu, G. Highly efficient removal of hexavalent chromium from electroplating effluent using aminated wheat straw. RSC Adv. 2016, 6, 8797–8805. [Google Scholar] [CrossRef]
- Su, E.C.; Huang, B.S.; Wey, M.Y. Sustainable hydrogen production from electroplating effluent over a solar light responsive photocatalyst. RSC Adv. 2016, 6, 71273–71281. [Google Scholar] [CrossRef]
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A.W.A.; Idris, A. Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, S.; Guo, X.; Huang, H. Adsorption of chromium(III) on lignin. Bioresour. Technol. 2008, 99, 7709–7715. [Google Scholar] [CrossRef] [PubMed]
- Aydin, Y.A.; Aksoy, N.D. Adsorption of chromium on chitosan: Optimization, kinetics and thermodynamics. Chem. Eng. J. 2009, 151, 188–194. [Google Scholar] [CrossRef]
- Peng, S.; Meng, H.; Ouyang, Y.; Chang, J. Nanoporous magnetic cellulose-chitosan composite microspheres: Preparation, characterization, and application for Cu(II) adsorption. Ind. Eng. Chem. Res. 2014, 53, 2106–2113. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. Applications of chitin- and chitosan-derivatives for the detoxification of water and effluent-A short review. Adv. Colloid Interface Sci. 2009, 152, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.C.; Szeto, Y.S.; Cheung, W.H.; McKay, G. Adsorption of acid dyes on chitosan-equilibrium isotherm analyses. Process Biochem. 2004, 39, 693–702. [Google Scholar] [CrossRef]
- Madill, E.A.W.; Garcia-Valdez, O.; Champagne, P.; Cunningham, M.F. CO2-responsive graft modified chitosan for heavy metal (nickel) recovery. Polymers 2017, 9, 394. [Google Scholar] [CrossRef]
- Wu, M.T.; Tsai, Y.L.; Chiu, C.W.; Cheng, C.C. Synthesis, characterization, and highly acid-resistant properties of crosslinking β-chitosan with polyamines for heavy metal ion adsorption. RSC Adv. 2016, 6, 104–754. [Google Scholar] [CrossRef]
- Staron, P.; Chwastowski, J.; Banach, M. Sorption and desorption studies on silver ions from aqueous solution by coconut fiber. J. Clean Prod. 2017, 149, 290–301. [Google Scholar] [CrossRef]
- Schuurmann, G.; Timofei, S.F. Multilinear regression and comparative molecular field analysis (CoMFA) of azo dye-fiber affinities. 2. inclusion of solution-phase molecular orbital descriptors. J. Chem. Inf. Comput. Sci. 2003, 43, 1502–1512. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Ibrahim, M.H.; Abdullah, A.Z. Elimination of reactive blue 4 from aqueous solutions using 3-aminopropyl triethoxysilane modified chitosan beads. Carbohydr. Polym. 2015, 132, 89–96. [Google Scholar] [CrossRef]
- Alkan, M.; Demirbas, O.; Celikcapa, S.; Dogan, M. Sorption of acid red 57 from aqueous solution onto sepiolite. J. Hazard. Mater. 2004, 116, 135–145. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem. Eng. J. 2009, 153, 1–8. [Google Scholar] [CrossRef]
- Haitham, K.; Razak, S.; Nawi, M.A. Kinetics and isotherm studies of methyl orange adsorption by a highly recyclable immobilized polyaniline on a glass plate. Arab. J. Chem. 2014. [Google Scholar] [CrossRef]
- Cestari, A.R.; Vieira, E.F.; Dos Santos, A.G.; Mota, J.A.; de Almeida, V.P. Adsorption of anionic dyes on chitosan beads. 1. The influence of the chemical structures of dyes and temperature on the adsorption kinetics. J. Colloid Interface Sci. 2004, 280, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Guibal, E.; McCarrick, P.; Tobin, J.M. Comparison of the sorption of anionic dyes on activated carbon and chitosan derivatives from dilute solutions. Sep. Sci. Technol. 2003, 38, 3049–3073. [Google Scholar] [CrossRef]
- Xing, Y.; Sun, X.; Li, B. Poly(methacrylic acid)-modified chitosan for enhancement adsorption of water-soluble cationic dyes. Polym. Eng. Sci. 2009, 49, 272–280. [Google Scholar] [CrossRef]
- Cestari, A.R.; Vieira, E.F.; Tavares, A.M.; Bruns, R.E. The removal of the indigo carmine dye from aqueous solutions using cross-linked chitosan: Evaluation of adsorption thermodynamics using a full factorial design. J. Hazard Mater. 2008, 153, 566–574. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Jiang, R.; Xiao, L.; Li, W. A novel magnetically separable gamma-Fe2O3/crosslinked chitosan adsorbent: Preparation, characterization and adsorption application for removal of hazardous azo dye. J. Hazard Mater. 2010, 179, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Salehi, R.; Arami, M.; Mahmoodi, N.M.; Bahrami, H.; Khorramfar, S. Novel biocompatible composite (Chitosan-zinc oxide nanoparticle): Preparation, characterization and dye adsorption properties. Colloids Surf. B 2010, 80, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Cinar, S.; Kaynar, U.H.; Aydemir, T.; Cam Kaynar, S.; Ayvacikli, M. An efficient removal of RB5 from aqueous solution by adsorption onto nano-ZnO/Chitosan composite beads. Int. J. Biol. Macromol. 2017, 96, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Yamaki, S.B.; Barros, D.S.; Garcia, C.M.; Socoloski, P.; Oliveira, O.N.; Atvars, T.D.Z. Spectroscopic studies of the intermolecular interactions of congo red and tinopal CBS with modified cellulose fibers. Langmuir 2005, 21, 5414–5420. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xiang, B.; Cheng, R.; Li, Y. Behaviors and mechanism of acid dyes sorption onto diethylenetriamine-modified native and enzymatic hydrolysis starch. J. Hazard. Mater. 2010, 183, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, C.; Lievenbrück, M.; Ritter, H. Polymers and dyes: Developments and applications. Polymers 2015, 7, 717–746. [Google Scholar] [CrossRef]
- Koopmans, C.; Ritter, H. Color change of N-isopropylacrylamide copolymer bearing reichardts dye as optical sensor for lower critical solution temperature and for host−guest interaction with β-cyclodextrin. J. Am. Chem. Soc. 2007, 12, 3502–3503. [Google Scholar] [CrossRef] [PubMed]
| Adsorbent Type | α-Chitosan | β-Chitosan | BCCT | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Temperature (K) | 303 | 313 | 323 | 303 | 313 | 323 | 303 | 313 | 323 |
| Langmuir isotherm | |||||||||
| Q0 (mg/g) | 49.02 | 21.14 | 15.31 | 54.35 | 31.45 | 20.45 | 625.00 | 41.49 | 36.36 |
| b (L/mg) | 68.00 | 525.56 | 1088.33 | 61.33 | 159.00 | 489.00 | 4.00 | 120.50 | 137.50 |
| RL | 1.5 × 10−5 | 1.9 × 10−6 | 9.2 × 10−7 | 1.6 × 10−5 | 6.3 × 10−6 | 2.0 × 10−6 | 2.5 × 10−4 | 8.3 × 10−6 | 7.3 × 10−6 |
| R2 | 0.9552 | 0.9917 | 0.9972 | 0.9515 | 0.9210 | 0.9749 | 0.8922 | 0.9676 | 0.9954 |
| Freundlich isotherm | |||||||||
| N | 1.64 | 1.23 | 1.14 | 1.96 | 1.52 | 1.34 | 4.56 | 1.61 | 1.64 |
| 1/n | 0.61 | 0.81 | 0.88 | 0.51 | 0.66 | 0.75 | 0.22 | 0.62 | 0.61 |
| kf (mg/g) | 116.17 | 41.91 | 24.22 | 200.45 | 93.78 | 51.49 | 819.03 | 121.53 | 116.17 |
| R2 | 0.9886 | 0.9893 | 0.9939 | 0.9613 | 0.9472 | 0.9675 | 0.8936 | 0.9688 | 0.9925 |
| Temkin isotherm | |||||||||
| Kt (dm3/g) | 1.38 | 1.36 | 1.32 | 1.59 | 1.43 | 1.36 | 1.91 | 1.48 | 1.45 |
| b (J/mol) | 0.87 | 0.57 | 0.50 | 1.32 | 0.80 | 0.64 | 4.37 | 0.95 | 0.92 |
| R2 | 0.9854 | 0.995 | 0.9837 | 0.9485 | 0.9569 | 0.9729 | 0.8131 | 0.9416 | 0.9854 |
| Adsorbent Type | Final Concentration Ce (mg/L) | T (K) | Final Dye Removal CAe (mg/L) | Kc | Thermodynamic Parameter | ||
|---|---|---|---|---|---|---|---|
| ΔG0 (KJ/mol) | ΔH0 (KJ/mol) | ΔS0 (J/mol k) | |||||
| α-chitosan | 55.70 | 303 | 1344.29 | 24.133 | −8.020 | −20.837 | 42.145 |
| 67.92 | 313 | 1332.07 | 19.610 | −7.745 | |||
| 90.65 | 323 | 1309.34 | 14.443 | −7.171 | |||
| β-chitosan | 34.00 | 303 | 1365.99 | 40.172 | −9.304 | −33.633 | 80.038 |
| 46.90 | 313 | 1353.09 | 28.848 | −8.749 | |||
| 75.51 | 323 | 1324.48 | 17.539 | −7.692 | |||
| BCCT | 30.01 | 303 | 1369.98 | 45.643 | −9.625 | −23.174 | 44.792 |
| 41.09 | 313 | 1358.91 | 33.072 | −9.105 | |||
| 52.16 | 323 | 1347.83 | 25.838 | −8.733 | |||
| Adsorbent Type | α-Chitosan | β-Chitosan | BCCT | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Temperature (K) | 303 | 318 | 333 | 303 | 318 | 333 | 303 | 318 | 333 |
| Pseudo-first-order model | |||||||||
| k1 (1/min) | 0.9802 | 0.9734 | 0.9672 | 0.9716 | 0.9828 | 0.9786 | 0.9760 | 0.9660 | 0.9636 |
| R2 | 0.9796 | 0.8496 | 0.9519 | 0.9812 | 0.9514 | 0.9263 | 0.9027 | 0.9503 | 0.8372 |
| Pseudo-second-order-model | |||||||||
| h = k2 × qe2 (mg/g min) | 96.1538 | 99.0099 | 97.0874 | 97.0874 | 98.0392 | 96.1538 | 100.0000 | 98.0392 | 98.0392 |
| k2 (g/mg min) | 1.6577 | 0.4755 | 0.1336 | 0.9997 | 0.0769 | 0.0436 | 0.0761 | 0.0297 | 0.0114 |
| R2 | 0.9981 | 0.9994 | 0.9999 | 0.9997 | 0.9999 | 1.0000 | 0.9999 | 1.0000 | 1.0000 |
| Elovich model | |||||||||
| α (mg/g min) | 1.01 × 102 | 1.84 × 103 | 5.98 × 104 | 4.74 × 102 | 5.65 × 107 | 4.23 × 108 | 1.48 × 106 | 5.12 × 1010 | 1.52 × 1013 |
| β (g/mg) | 0.0802 | 0.1080 | 0.1470 | 0.0909 | 0.2209 | 0.2452 | 0.1810 | 0.2903 | 0.3483 |
| R2 | 0.9795 | 0.9713 | 0.8883 | 0.9100 | 0.9500 | 0.8537 | 0.7583 | 0.9162 | 0.7645 |
| Weber–Morris model | |||||||||
| kdif (mg/g min) | 3.1238 | 2.2639 | 1.6022 | 2.6255 | 2.2639 | 1.0810 | 1.2708 | 0.8187 | 0.6522 |
| C | 51.0390 | 68.0210 | 76.2880 | 64.5030 | 68.0210 | 83.3890 | 81.0540 | 87.9630 | 90.4380 |
| R2 | 0.9519 | 0.8985 | 0.7625 | 0.8036 | 0.8985 | 0.8529 | 0.6214 | 0.8012 | 0.6110 |
| Adsorbents | Dye | Adsorption Capacity Qmax (mg/g) | Temperature (°C) | pH | Reference |
|---|---|---|---|---|---|
| Chitosan beads | Reactive yellow | 9 | 50 | 2 | [32] |
| Reactive blue | 10 | 25 | 2 | ||
| Reactive red | 7.5 | 50 | 2 | ||
| Cross-linked chitosan | Direct blue 71 | 14 | -- | 4 | [33] |
| Acid black 1 | 37 | -- | 4 | ||
| Glutaraldehyde-cross-linked chitosan (GLA-CTS) | Methyl green | 33.7 | 25 | 4 | [34] |
| Cross-linked chitosan | Indigo carmine | 0.6 | 35 | 4 | [35] |
| Magnetic γ-Fe2O3/crosslinked chitosan composite | Methyl orange | 29.8 | 25 | 4 | [36] |
| Zinc oxide nanoparticles/chitosan | Direct blue 78 | 34.5 | 21 | 2 | [37] |
| Nano-ZnO/chitosan composite beads (Nano-ZnO/CT-CB) | Reactive black 5 | 189.4 | 40 | 4 | [38] |
| β-chitosan cross-linked with Triethylenetetramine (BCCT) | Reactive blue 221 | 52.4 | 25 | 2 | Our work |
| 625.00 a | 30 | 5 |
| Sample | Element (%) | ||||||
|---|---|---|---|---|---|---|---|
| C | N | H | O | S | Total | S/C | |
| α-chitosan | 41.85 | 7.60 | 7.99 | 40.79 | - | 98.24 | - |
| β-chitosan | 41.72 | 7.77 | 8.06 | 40.63 | - | 98.18 | - |
| BCCT | 42.06 | 7.78 | 8.08 | 40.40 | - | 98.32 | - |
| α-chitosan adsorption of RB221 | 41.34 | 8.04 | 7.05 | 41.27 | 0.33 | 98.03 | 0.0079 |
| β-chitosan adsorption of RB221 | 41.45 | 8.15 | 7.07 | 41.55 | 0.34 | 98.55 | 0.0082 |
| BCCT adsorption of RB221 | 42.98 | 8.20 | 6.98 | 40.04 | 0.44 | 98.63 | 0.0102 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, C.-W.; Wu, M.-T.; Lee, J.C.-M.; Cheng, T.-Y. Isothermal Adsorption Properties for the Adsorption and Removal of Reactive Blue 221 Dye from Aqueous Solutions by Cross-Linked β-Chitosan Glycan as Acid-Resistant Adsorbent. Polymers 2018, 10, 1328. https://doi.org/10.3390/polym10121328
Chiu C-W, Wu M-T, Lee JC-M, Cheng T-Y. Isothermal Adsorption Properties for the Adsorption and Removal of Reactive Blue 221 Dye from Aqueous Solutions by Cross-Linked β-Chitosan Glycan as Acid-Resistant Adsorbent. Polymers. 2018; 10(12):1328. https://doi.org/10.3390/polym10121328
Chicago/Turabian StyleChiu, Chih-Wei, Ming-Tsung Wu, Jimmy Chi-Min Lee, and Ting-Yu Cheng. 2018. "Isothermal Adsorption Properties for the Adsorption and Removal of Reactive Blue 221 Dye from Aqueous Solutions by Cross-Linked β-Chitosan Glycan as Acid-Resistant Adsorbent" Polymers 10, no. 12: 1328. https://doi.org/10.3390/polym10121328
APA StyleChiu, C.-W., Wu, M.-T., Lee, J. C.-M., & Cheng, T.-Y. (2018). Isothermal Adsorption Properties for the Adsorption and Removal of Reactive Blue 221 Dye from Aqueous Solutions by Cross-Linked β-Chitosan Glycan as Acid-Resistant Adsorbent. Polymers, 10(12), 1328. https://doi.org/10.3390/polym10121328