Effect of the Surface Hydrophobicity Degree on the In Vitro Release of Polar and Non-Polar Drugs from Polyelectrolyte Matrix Tablets
Abstract
:1. Introduction
2. Materials and Methods
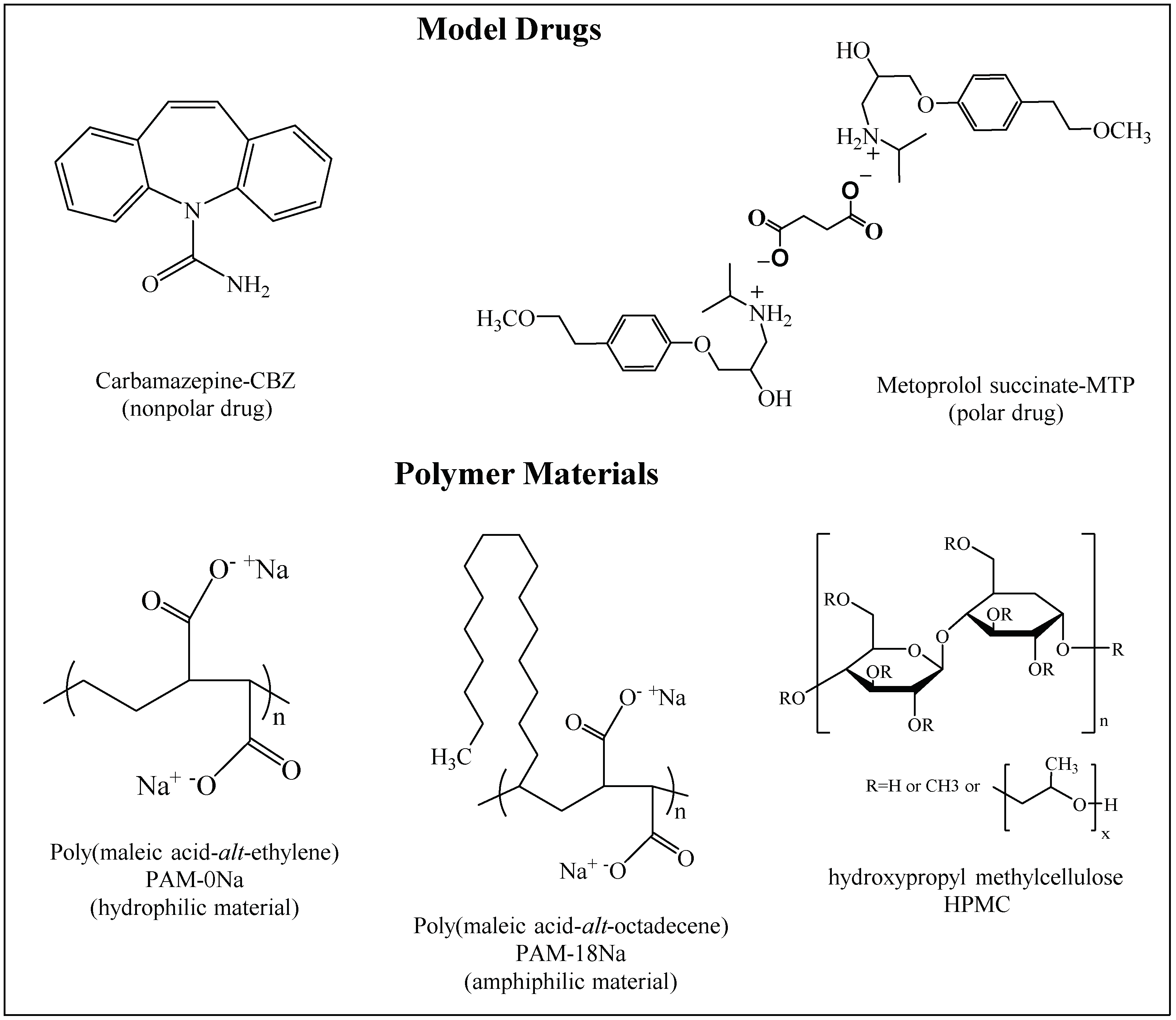
2.1. Materials
Collection and Characterization of the Polymeric Materials
2.2. Methods
2.2.1. Characterization of Powder Polymeric Materials
2.2.2. Thermal Characterization of Polymer-Drug Blends
2.2.3. Preparation of Tablets
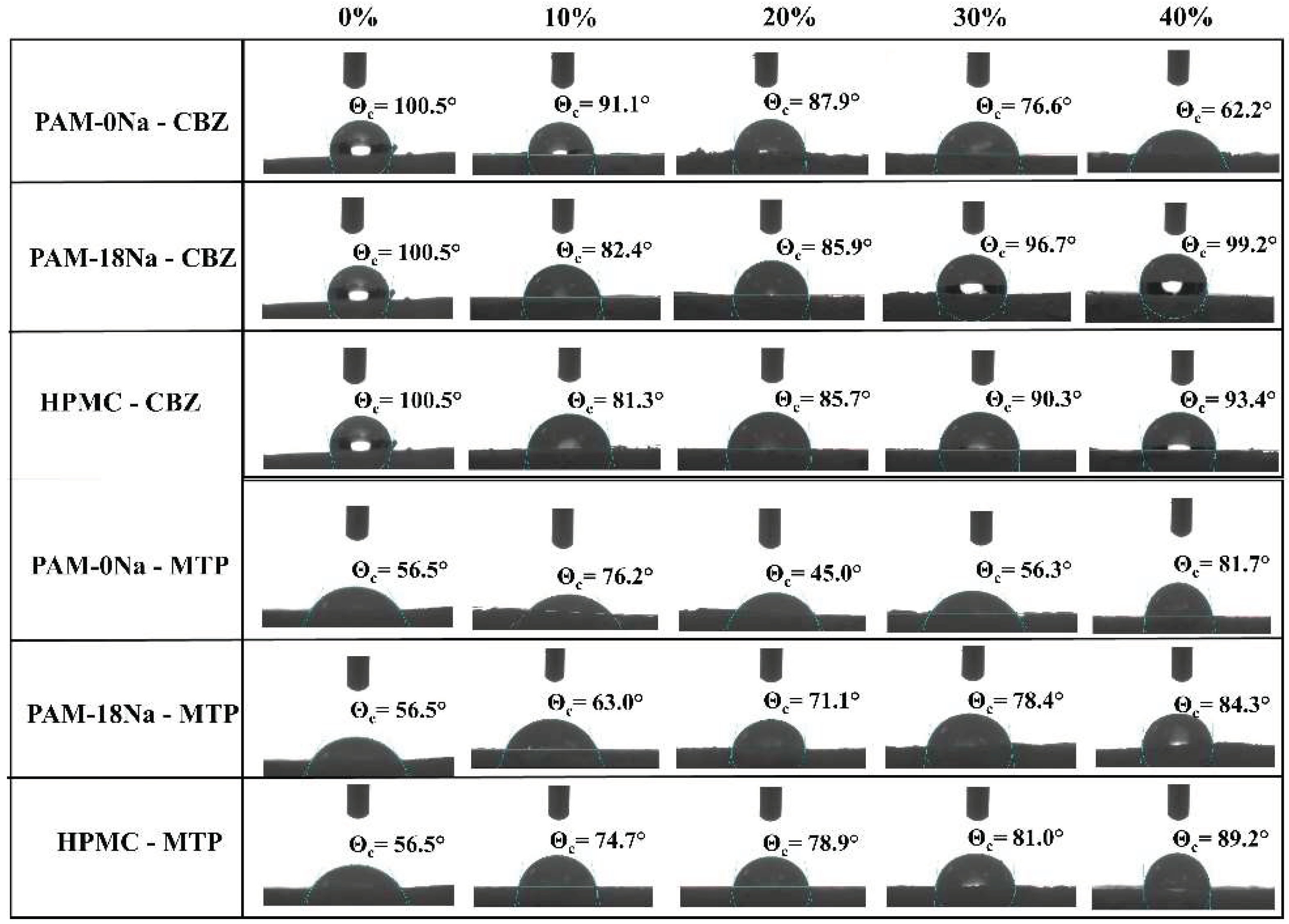
2.2.4. Contact Angle Measurements
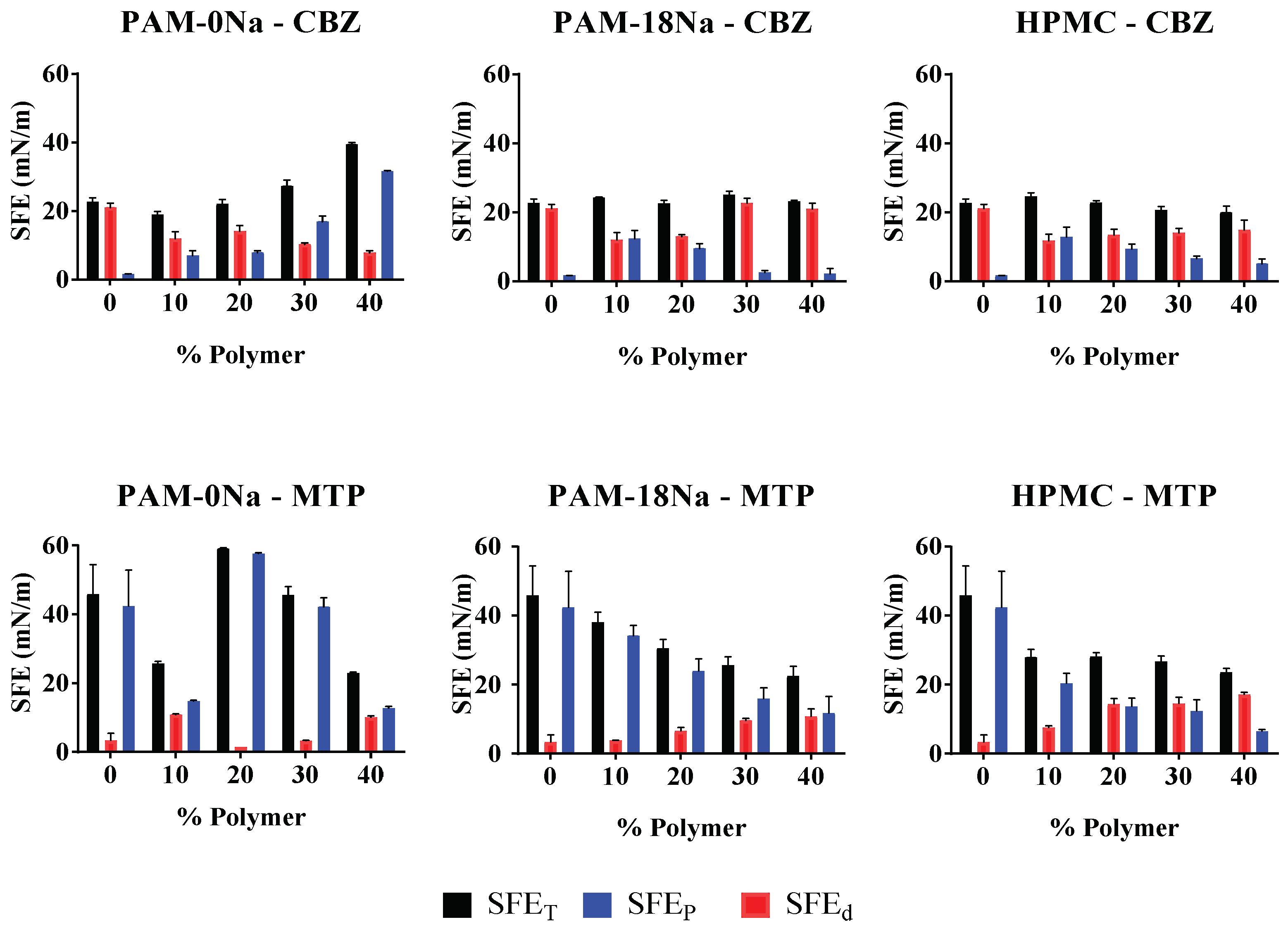
2.2.5. Determination Thermodynamics Surface Parameters
2.2.6. In Vitro Dissolution Test
2.2.7. Processing and Data Analysis
3. Results and Discussion
3.1. Collection and Characterization of the Polymeric Materials
3.2. Characterization of Powder Polymeric Materials
3.3. Thermal Characterization of Polymer-Drug Blends
3.4. Physical Characterization of the Binary Tablets
3.5. Contact Angle Measurements
3.6. Determination Thermodynamics Surface Parameters
3.7. In Vitro Dissolution of CBZ and MTP
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Trivedi, B.N.C. Polymers and Copolymers of Maleic Anhydride; Springer publisher: New York, NY, USA, 1982; Volume 102. [Google Scholar]
- Salamanca, C.H.; Yarce, C.J.; Roman, Y.; Davalos, A.F.; Rivera, G.R. Application of nanoparticle technology to reduce the anti-microbial resistance through β-lactam antibiotic-polymer inclusion nano-complex. Pharmaceuticals 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Salamanca, C.H.; Yarce, C.J.; Zapata, C.A.; Giraldo, J.A. Relationship between the polymeric ionization degree and powder and surface properties in materials derived from poly(maleic anhydride-alt-octadecene). Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Yarce, C.J.; Pineda, D.; Correa, C.E.; Salamanca, C.H. Relationship between surface properties and in Vitro drug release from a compressed matrix containing an amphiphilic polymer material. Pharmaceuticals 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Yarce, C.; Echeverri, J.; Palacio, M.; Rivera, C.; Salamanca, C. Relationship between Surface Properties and In Vitro Drug Release from Compressed Matrix Containing Polymeric Materials with Different Hydrophobicity Degrees. Pharmaceuticals 2017, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Gad, S.C. United States Pharmacopoeia (USP). In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 881–882. ISBN 978-0-12-386455-0. [Google Scholar]
- Saw, H.Y.; Davies, C.E.; Paterson, A.H.J.; Jones, J.R. Correlation between powder flow properties measured by shear testing and Hausner ratio. Procedia Eng. 2015, 102, 218–225. [Google Scholar] [CrossRef]
- Grey, R.O.; Beddow, J.K. On the Hausner Ratio and its relationship to some properties of metal powders. Powder Technol. 1969, 2, 323–326. [Google Scholar] [CrossRef]
- Ilari, J.L. Flow properties of industrial dairy powders. Lait 2002, 82, 383–399. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Nakamura, K.; Hatakeyama, T.; Hatakeyama, H. Studies on Bound Water of Cellulose by Differential Scanning Calorimetry. Text. Res. J. 1981, 51, 607–613. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Nakamura, K.; Hatakeyama, H. Determination of bound water content in polymers by DTA, DSC and TG. Thermochim. Acta 1988, 123, 153–161. [Google Scholar] [CrossRef]
- Ping, Z.H.; Nguyen, Q.T.; Chen, S.M.; Zhou, J.Q.; Ding, Y.D. States of water in different hydrophilic polymers—DSC and FTIR studies. Polymer 2001, 42, 8461–8467. [Google Scholar] [CrossRef]
- Liu, Y.; Huglin, M.B. Observations by DSC on bound water structure in some physically crosslinked hydrogels. Polym. Int. 1995, 37, 63–67. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Tanaka, M.; Kishi, A.; Hatakeyama, H. Comparison of measurement techniques for the identification of bound water restrained by polymers. Thermochim. Acta 2012, 532, 159–163. [Google Scholar] [CrossRef]
- Grzesiak, A.L.; Lang, M.; Kim, K.; Matzger, A.J. Comparison of the Four Anhydrous Polymorphs of Carbamazepine and the Crystal Structure of Form I. J. Pharm. Sci. 2003, 92, 2260–2271. [Google Scholar] [CrossRef] [PubMed]
- Porter, W.W.; Elie, S.C.; Matzger, A.J. Polymorphism in carbamazepine cocrystals. Cryst. Growth Des. 2008, 8, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Ao, J.; Liu, S.; Wu, C.; Lai, A.; Gao, H.; Zhang, G. A new polymorphic form of metoprolol succinate. Pharm. Dev. Technol. 2017, 22, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, K.; Nishinari, K. Rheological and Dsc Studies of Chemically Modified Starch. Hydrocolloids 2000, 43, 319–324. [Google Scholar] [CrossRef]
- Hasjim, J.; Jane, J.L. Production of resistant starch by extrusion cooking of acid-modified normal-maize starch. J. Food Sci. 2009, 74. [Google Scholar] [CrossRef] [PubMed]
- Wilpiszewska, K.; Spychaj, T. Chemical modification of starch with hexamethylene diisocyanate derivatives. Carbohydr. Polym. 2007, 70, 334–340. [Google Scholar] [CrossRef]
- Sandoval, A.; Rodriguez, E.; Fernandez, A. Application of analysis by differential scanning calorimetry (DSC) for the characterization of the modifications of the starch. DYNA 2005, 72, 45–53. [Google Scholar]
- Aggarwal, P.; Dollimore, D. The effect of chemical modification on starch studied using thermal analysis. Thermochim. Acta 1998, 324, 1–8. [Google Scholar] [CrossRef]
- Pygall, S.R.; Griffiths, P.C.; Wolf, B.; Timmins, P.; Melia, C.D. Solution interactions of diclofenac sodium and meclofenamic acid sodium with hydroxypropyl methylcellulose (HPMC). Int. J. Pharm. 2011, 405, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Loh, G.O.K.; Tan, Y.T.F.; Peh, K.K. Effect of HPMC concentration on β-cyclodextrin solubilization of norfloxacin. Carbohydr. Polym. 2014, 101, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Djemai, A.; Gendron, C.M.; Xi, H.; Smith, M.; Kogan, J.; Li, L. Development of a HPMC-based controlled release formulation with hot melt extrusion (HME). Drug Dev. Ind. Pharm. 2013, 39, 1070–1083. [Google Scholar] [CrossRef] [PubMed]
- Sujja-Areevath, J.; Munday, D.L.; Cox, P.J.; Khan, K.A. Relationship between swelling, erosion and drug release in hydrophillic natural gum mini-matrix formulations. Eur. J. Pharm. Sci. 1998, 6, 207–217. [Google Scholar] [CrossRef]
- Kale, T.S.; Klaikherd, A.; Popere, B.; Thayumanavan, S. Supramolecular assemblies of amphiphilic homopolymers. Langmuir 2009, 25, 9660–9670. [Google Scholar] [CrossRef] [PubMed]
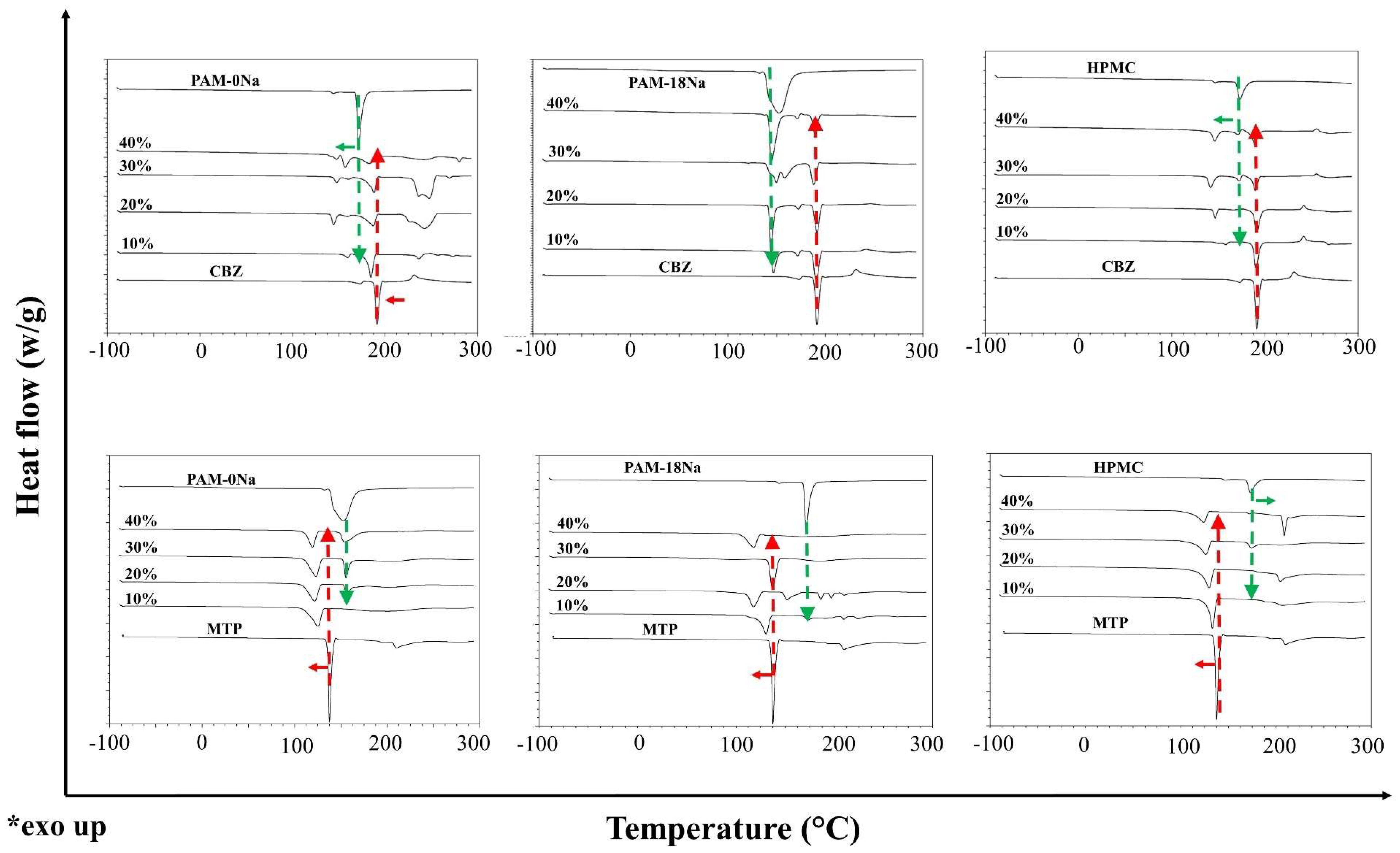
 = 10%,
= 10%,  = 20%,
= 20%,  = 30% and
= 30% and  = 40% and two media of physiological simulation.
= 40% and two media of physiological simulation.
 = 10%,
= 10%,  = 20%,
= 20%,  = 30% and
= 30% and  = 40% and two media of physiological simulation.
= 40% and two media of physiological simulation.
| Parameter | PAM-0Na | PAM-18Na | HPMC | CBZ | MTP |
|---|---|---|---|---|---|
| Bulk density g/mL | 0.54 ± 0.02 | 0.24 ± 0.02 | 0.36 ± 0.01 | 0.42 ± 0.02 | 0.46 ± 0.01 |
| Tapped Density g/mL | 0.60 ± 0.01 | 0.28 ± 0.15 | 0.47 ± 0.01 | 0.52 ± 0.01 | 0.50 ± 0.01 |
| Carr Index (%) | 10.10 ± 2.3 | 12.7 ± 1.2 | 21.6 ± 2.0 | 18.5 ± 0.8 | 7.2 ± 2.0 |
| Hausner Index (%) | 1.10 ± 0.02 | 1.2 ± 0.01 | 1.3 ± 0.03 | 1.2 ± 0.01 | 1.1 ± 0.02 |
| Angle of repose (°) | 23.50 ± 2.8 | 36.3 ± 2.3 | 26.9 ± 1.4 | 27.7 ± 2.0 | 40.4 ± 2.2 |
| Drug | Polymer Material | % Polymer | Hardness (kp) | Disintegration Time (min: s ± s) |
|---|---|---|---|---|
| Carbamazepine (CBZ) | 0 | 2.48 ± 0.44 | >4 h | |
| PAM-0Na | 10 | 1.41 ± 0.29 | 24:40 ± 1.53 | |
| 20 | 2.11 ± 0.69 | 37:00 ± 1.73 | ||
| 30 | 4.29 ± 0.34 | 44:30 ± 0.58 | ||
| 40 | 8.82 ± 0.31 | 65:40 ± 1.15 | ||
| PAM-18Na | 10 | 1.56 ± 0.73 | 28:40 ± 1.53 | |
| 20 | 1.55 ± 0.45 | 47:30 ± 2.52 | ||
| 30 | 1.62 ± 0.11 | 56:10 ± 1.03 | ||
| 40 | 1.89 ± 0.05 | 67:30 ± 2.52 | ||
| HPMC | 10 | 3.59 ± 1.01 | >4 h | |
| 20 | 5.92 ± 0.97 | >4 h | ||
| 30 | 5.81 ± 1.58 | >4 h | ||
| 40 | 8.24 ± 0.90 | >4 h | ||
| Metoprolol succinate (MTP) | 0 | 1.97 ± 0.37 | 16:40 ± 1.52 | |
| PAM-0Na | 10 | 1.56 ± 0.15 | 14:20 ± 1.15 | |
| 20 | <0.60 | 19:40 ± 1.53 | ||
| 30 | <0.60 | 24:40 ± 0.58 | ||
| 40 | <0.60 | 29:30 ± 1.15 | ||
| PAM-18Na | 10 | 0.84 ± 0.28 | 33:40 ± 0.58 | |
| 20 | 0.59 ± 0.14 | 54:30 ± 1.15 | ||
| 30 | 0.64 ± 0.06 | 54:40 ± 0.58 | ||
| 40 | 0.64 ± 0.31 | 59:40 ± 1.53 | ||
| HPMC | 10 | 1.26 ± 0.07 | 19:20 ± 1.15 | |
| 20 | 0.93 ± 0.13 | 24:40 ± 0.58 | ||
| 30 | 0.67 ± 0.16 | 64:40 ± 1.53 | ||
| 40 | 1.48 ± 0.98 | 106:20 ± 1.15 |
| Drug | Media | % Polymer | Dissolution Efficiency Percentage (%) | ||
|---|---|---|---|---|---|
| PAM-0Na | PAM-18Na | HPMC | |||
| CBZ | Gastric | 0 | 7.5 | 7.5 | 7.5 |
| 10 | 49.8 | 15.6 | 37.1 | ||
| 20 | 46.1 | 8.5 | 38.1 | ||
| 30 | 48.7 | 9.9 | 21.1 | ||
| 40 | 63.3 | 5.1 | 13.0 | ||
| Duodenal | 0 | 9.4 | 9.4 | 9.4 | |
| 10 | 12.7 | 48.9 | 81.3 | ||
| 20 | 19.6 | 36.6 | 45.6 | ||
| 30 | 25.5 | 55.4 | 20.6 | ||
| 40 | 32.6 | 34.9 | 13.4 | ||
| MTP | Gastric | 0 | 86.0 | 86.0 | 86.0 |
| 10 | 72.8 | 80.7 | 93.1 | ||
| 20 | 73.5 | 68.1 | 84.2 | ||
| 30 | 88.8 | 57.5 | 95.5 | ||
| 40 | 79.1 | 37.5 | 90.0 | ||
| Duodenal | 0 | 83.4 | 83.4 | 83.4 | |
| 10 | 88.7 | 70.2 | 99.4 | ||
| 20 | 96.9 | 71.7 | 102.0 | ||
| 30 | 94.2 | 71.5 | 97.7 | ||
| 40 | 87.7 | 46.3 | 89.0 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yarce, C.J.; Echeverri, J.D.; Salamanca, C.H. Effect of the Surface Hydrophobicity Degree on the In Vitro Release of Polar and Non-Polar Drugs from Polyelectrolyte Matrix Tablets. Polymers 2018, 10, 1313. https://doi.org/10.3390/polym10121313
Yarce CJ, Echeverri JD, Salamanca CH. Effect of the Surface Hydrophobicity Degree on the In Vitro Release of Polar and Non-Polar Drugs from Polyelectrolyte Matrix Tablets. Polymers. 2018; 10(12):1313. https://doi.org/10.3390/polym10121313
Chicago/Turabian StyleYarce, Cristhian J., Juan D. Echeverri, and Constain H. Salamanca. 2018. "Effect of the Surface Hydrophobicity Degree on the In Vitro Release of Polar and Non-Polar Drugs from Polyelectrolyte Matrix Tablets" Polymers 10, no. 12: 1313. https://doi.org/10.3390/polym10121313
APA StyleYarce, C. J., Echeverri, J. D., & Salamanca, C. H. (2018). Effect of the Surface Hydrophobicity Degree on the In Vitro Release of Polar and Non-Polar Drugs from Polyelectrolyte Matrix Tablets. Polymers, 10(12), 1313. https://doi.org/10.3390/polym10121313







