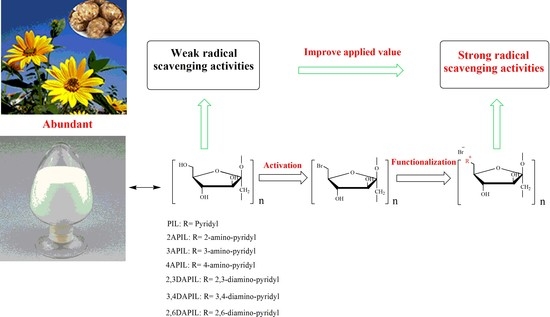
Radical Scavenging Activities of Novel Cationic Inulin Derivatives
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
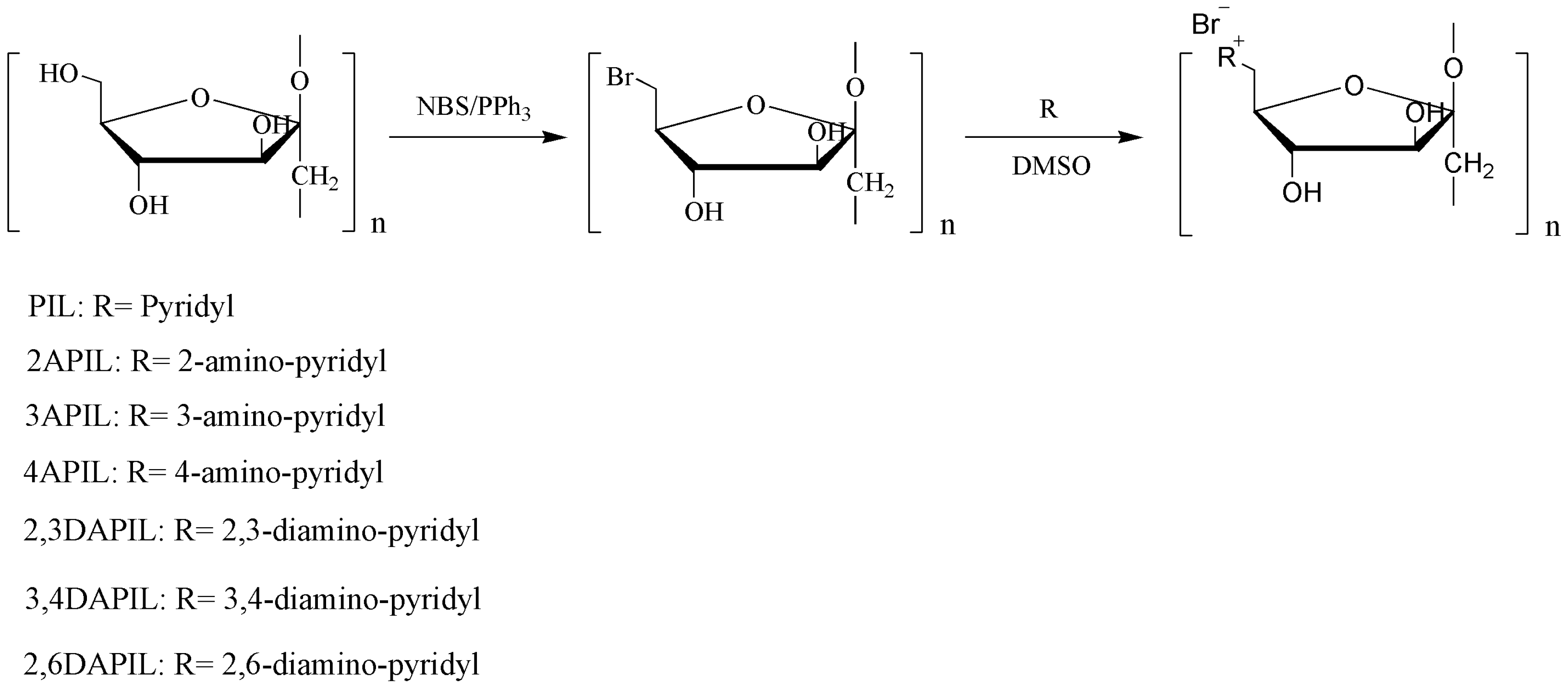
2.2. Synthesis of Inulin Derivatives
2.2.1. Synthesis of 6-Bromo-6-deoxyinulin
2.2.2. Synthesis of Cationic Inulin Derivatives Bearing Pyridine or Amino-Pyridine Groups
2.3. Analytical Methods
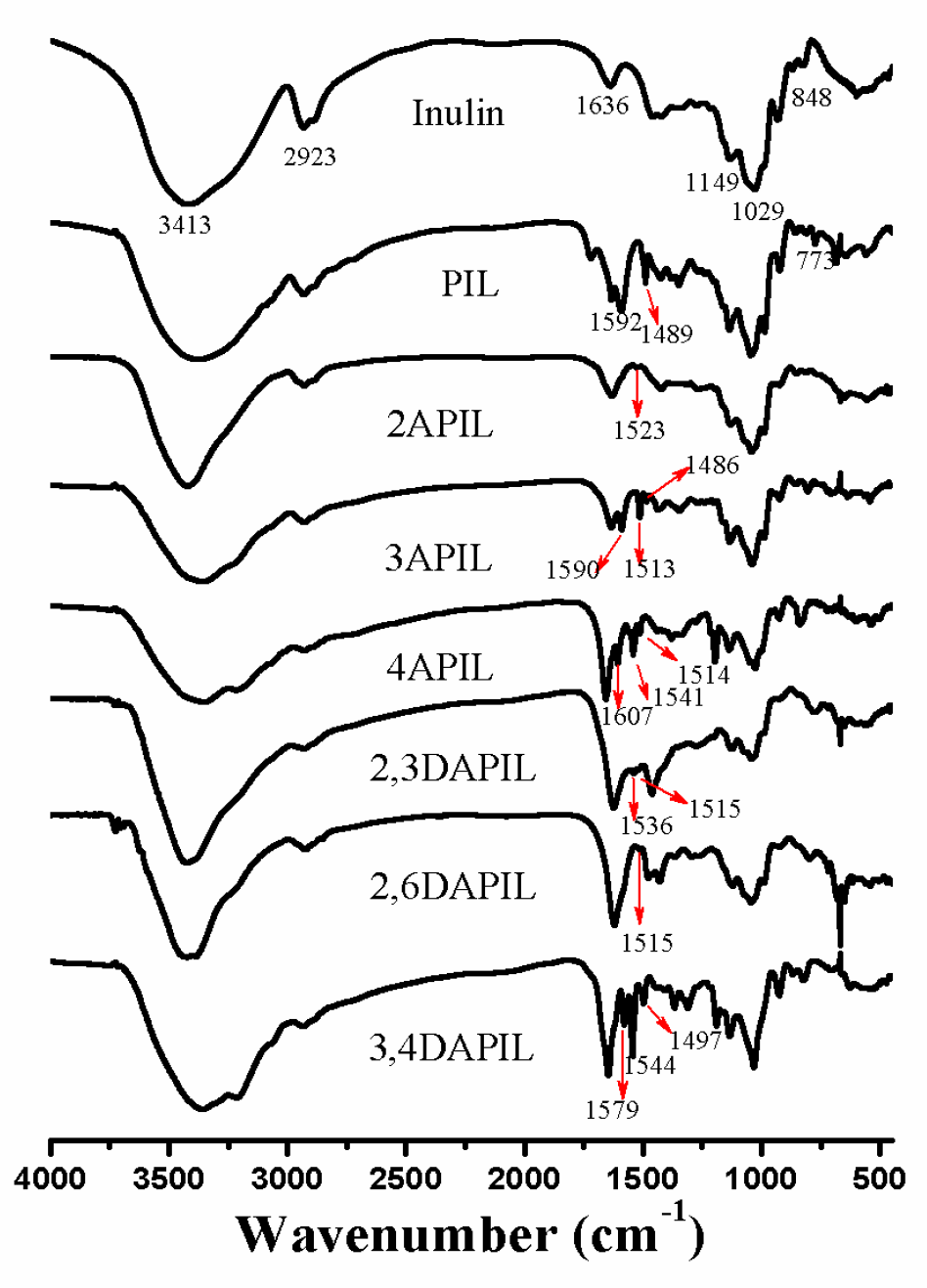
2.3.1. Fourier Transform Infrared Spectroscopy (FT-IR) Spectroscopy
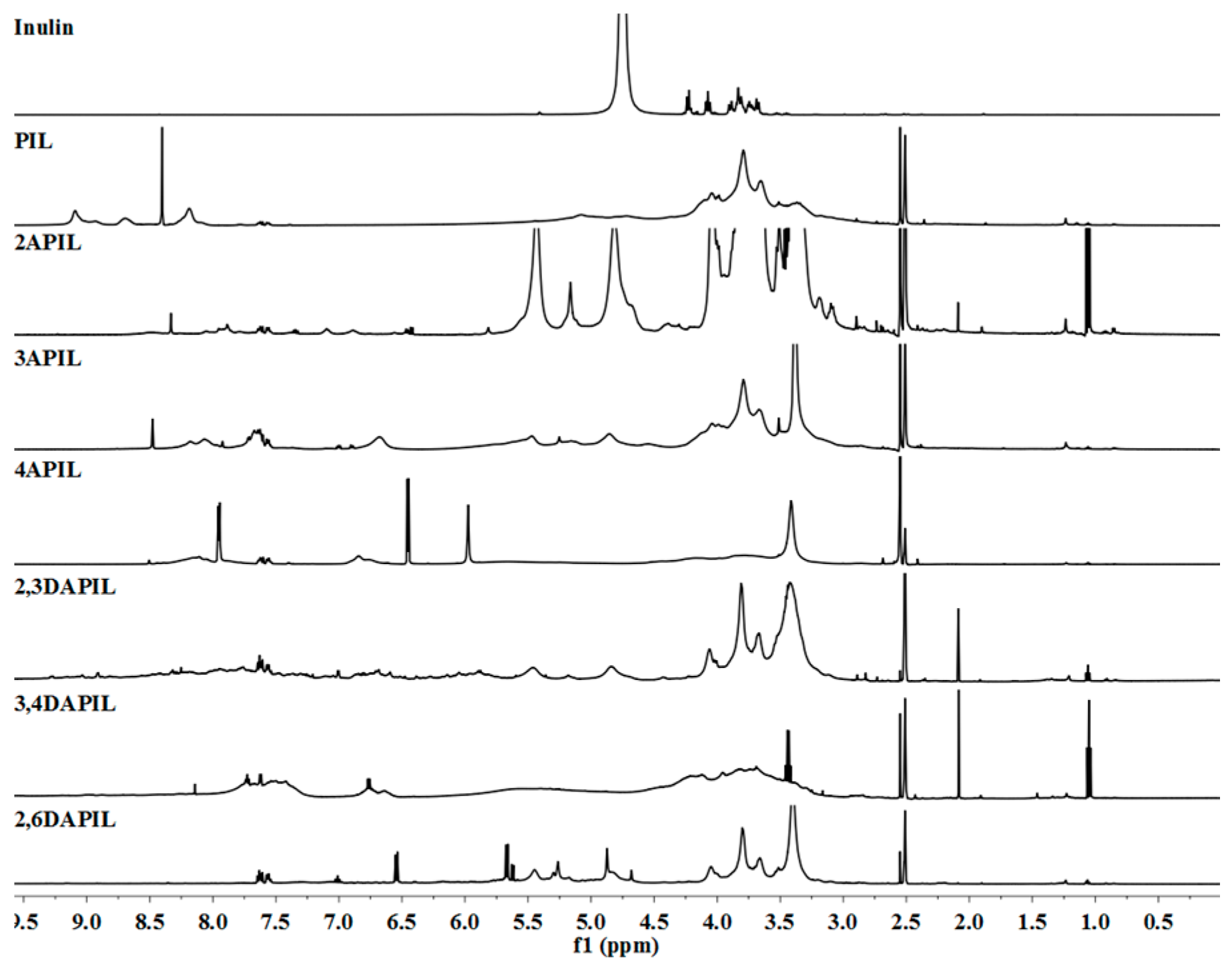
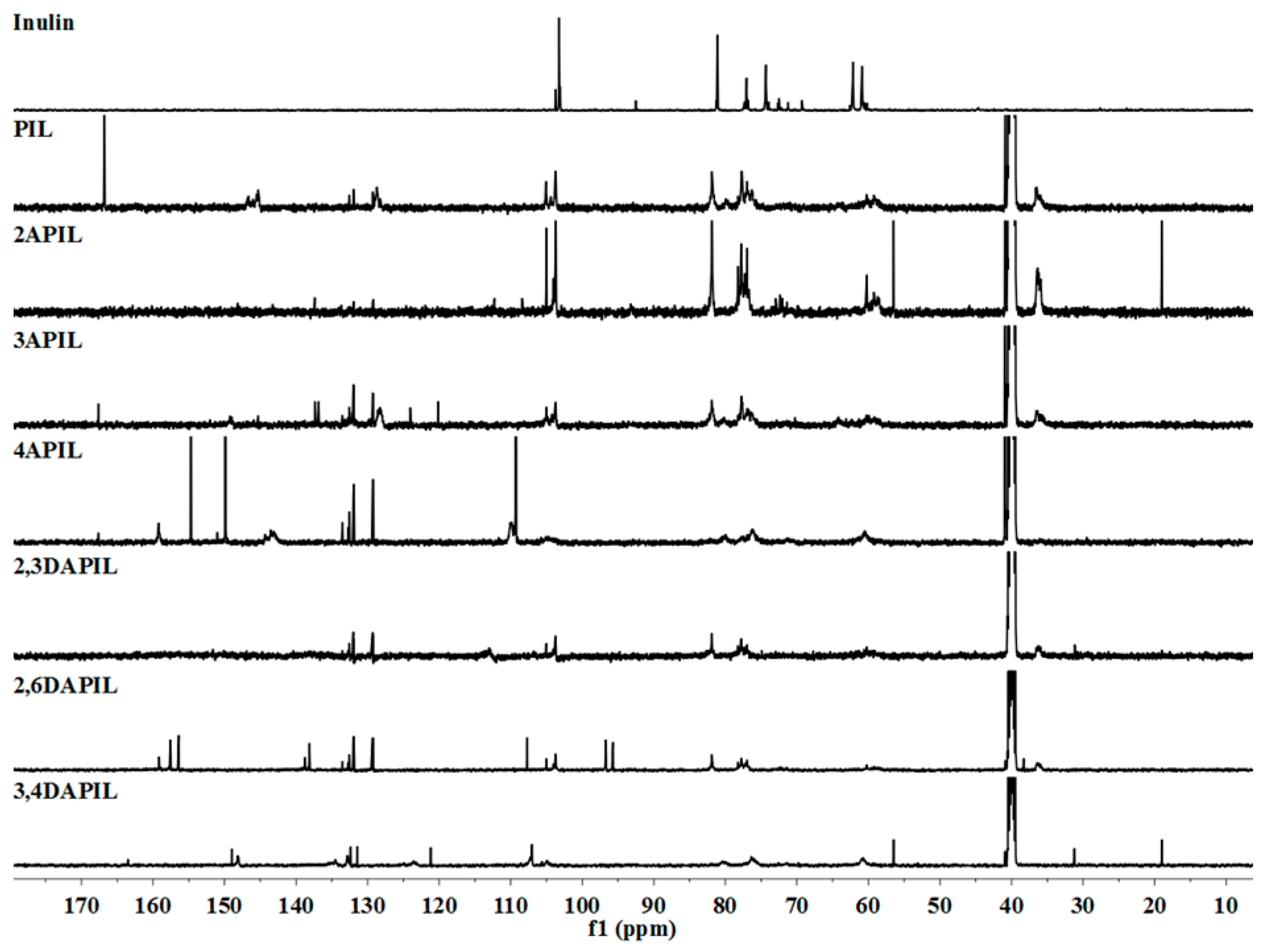
2.3.2. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.3.3. Ultraviolet (UV) Spectroscopy
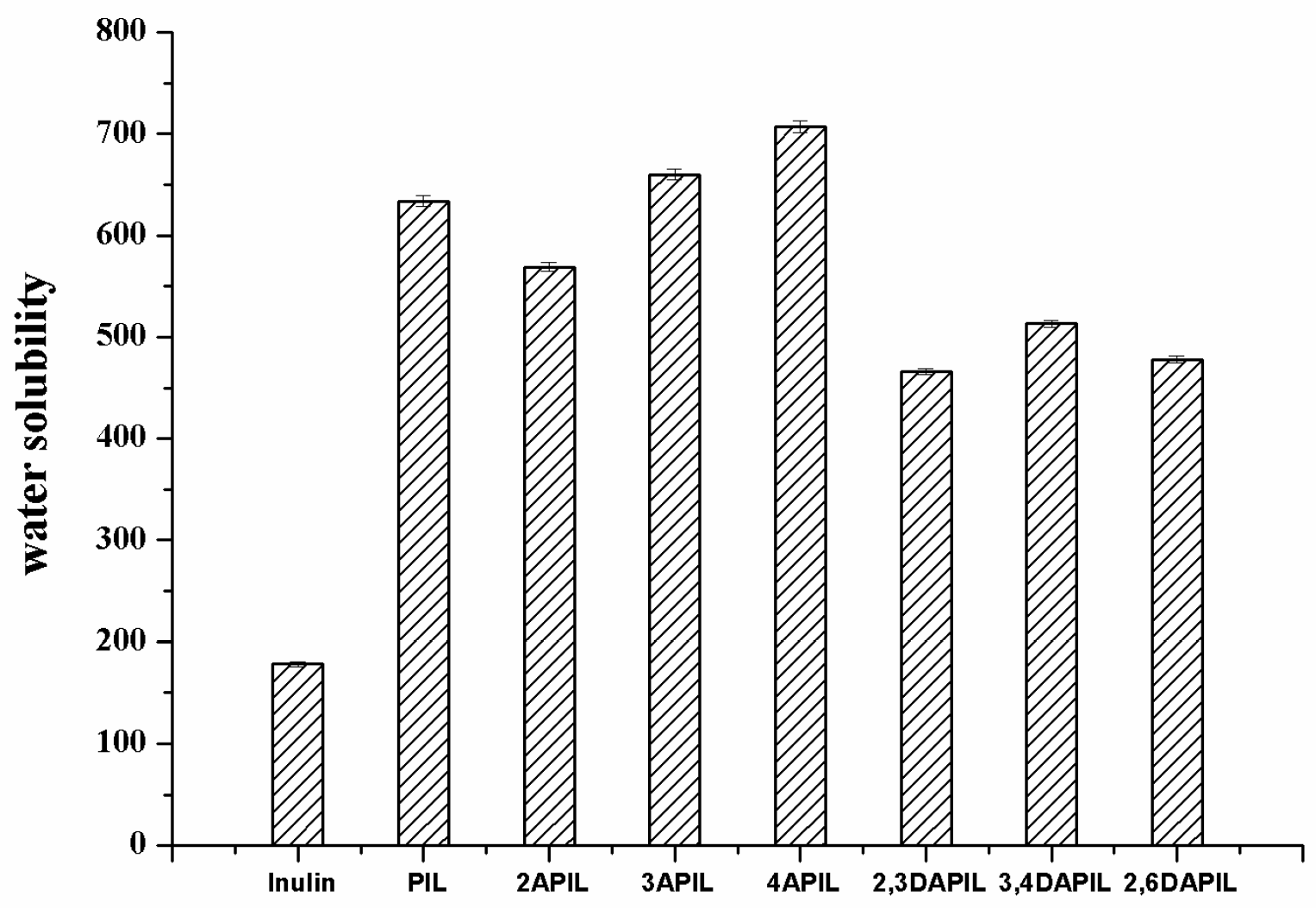
2.4. Water Solubility
2.5. Radical Scavenging Assays
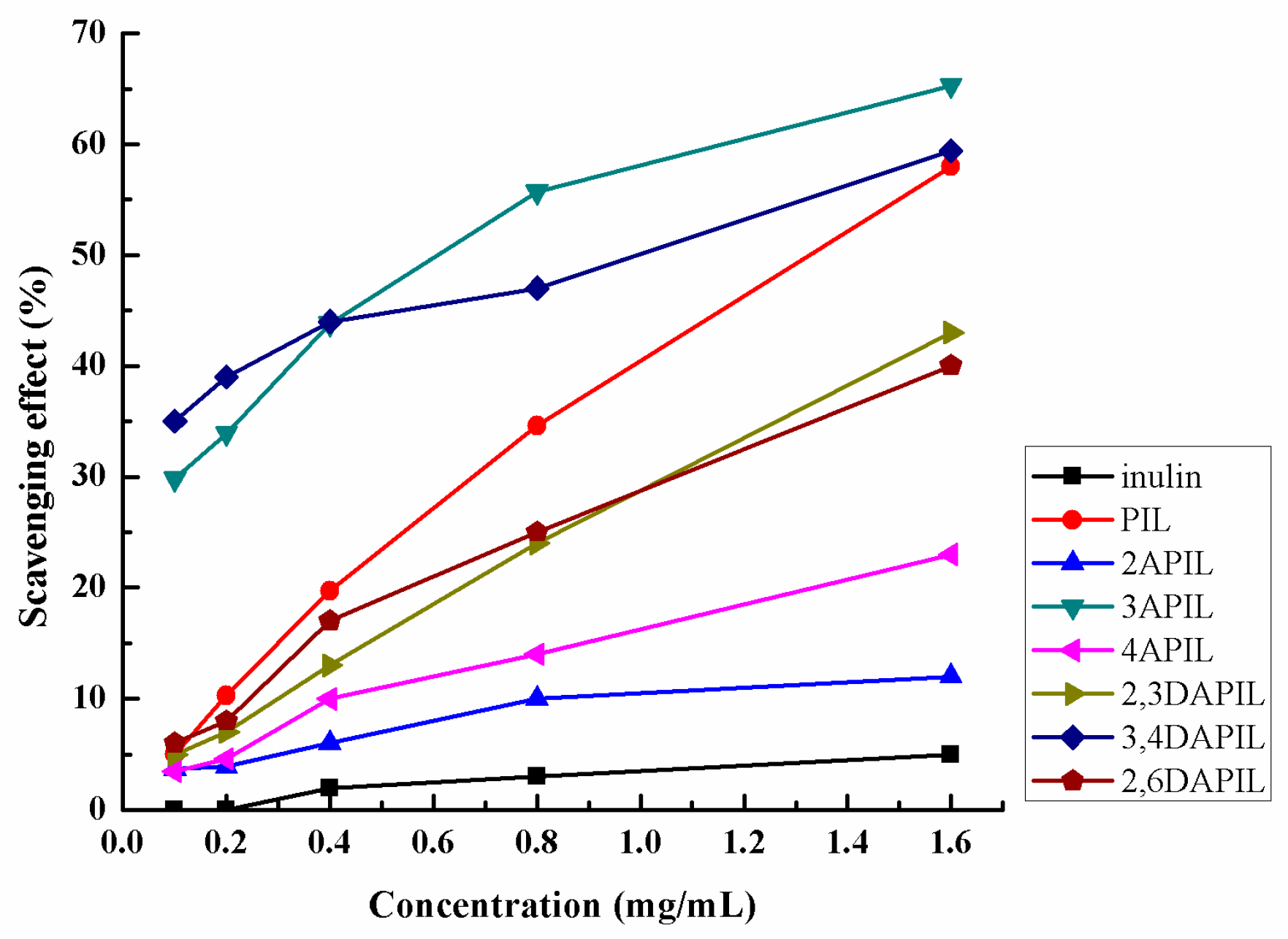
2.5.1. Hydroxyl Radicals’ Scavenging Capacities
2.5.2. DPPH Radicals’ Scavenging Activities
3. Results and Discussion
3.1. Structures of Inulin and Cationic Inulin Derivatives Bearing Pyridine or Amino-Pyridine Groups
3.2. Water-Solubilities of Inulin and Inulin Derivatives
3.3. Radical Scavenging Activity
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Anraku, M.; Gebicki, J.M.; Iohara, D.; Tomida, H.; Uekama, K.; Maruyama, T.; Hirayama, F.; Otagiri, M. Antioxidant activities of chitosans and its derivatives in in vitro and in vivo studies. Carbohydr. Polym. 2018, 199, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Min, L.; Zhu, C.; Rao, Z.; Liu, L.; Xu, W.; Luo, P.; Fan, L. Preparation, characterization and antioxidant activity of silk peptides grafted carboxymethyl chitosan. Int. J. Biol. Macromol. 2017, 104, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Bourdon, E.; Blache, D. The importance of proteins in defense against oxidation. Antioxid. Redox Signal. 2001, 3, 293. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Holmgren, A.; Larsson, N.-G.; Halliwell, B.; Chang, C.J.; Kalyanaraman, B.; Rhee, S.G.; Thornalley, P.J.; Partridge, L.; Gems, D.; et al. Unraveling the biological roles of reactive oxygen species. Cell Metab. 2011, 13, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. The definition and measurement of antioxidants in biological systems. Free Radicals Biol. Med. 1995, 18, 125–126. [Google Scholar] [CrossRef]
- Martelli, G.; Giacomini, D. Antibacterial and antioxidant activities for natural and synthetic dual-active compounds. Eur. J. Med. Chem. 2018, 158, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A common Mechanism of cellular Death induced by bactericidal antibiotics. Cell 2007, 130, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik-Kulesza, K.A.; Oniszczuk, A.; Oniszczuk, T.; Waksmundzka-Hajnos, M. The influence of common free radicals and antioxidants on development of Alzheimer’s disease. Biomed. Pharmacother. 2016, 78, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Ezhilarasan, D. Oxidative stress is bane in chronic liver diseases: Clinical and experimental perspective. Arab J. Gastroenterol. 2018, 19, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Serviddio, G.; Bellanti, F.; Vendemiale, G. Free radical biology for medicine: learning from nonalcoholic fatty liver disease. Free Radical Biol. Med. 2013, 65, 952–968. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Perry, G. Free radical damage, iron, and Alzheimer’s disease. J. Neurol. Sci. 1995, 134, 92–94. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.S.; Gómez, B.; Barba, F.J.; Mora, L.; Pérez-Santaescolástica, C.; Toldrá, F. Bioactive peptides as natural antioxidants in food products–A review. Trends Food Sci. Technol. 2018, 79, 136–147. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, W.; Li, Q.; Dong, F.; Gu, G.; Guo, Z. Synthesis of inulin derivatives with quaternary phosphonium salts and their antifungal activity. Int. J. Biol. Macromol. 2018, 113, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Li, Q.; Wei, L.; Wang, P.; Gao, Z.; Chen, Y.; Dong, F.; Guo, Z. Synthesis, characterization, and antifungal property of starch derivatives modified with quaternary phosphonium salts. Mater. Sci. Eng. 2017, 76, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, M.; Shehzad, A.; Omar, M.; Rakha, A.; Raza, H.; Sharif, H.R.; Shakeel, A.; Ansari, A.; Niazi, S. Inulin: Properties, health benefits and food applications. Carbohydr. Polym. 2016, 147, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Karimi, R.; Azizi, M.H.; Ghasemlou, M.; Vaziri, M. Application of inulin in cheese as prebiotic, fat replacer and texturizer: A review. Carbohydr. Polym. 2015, 119, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Walz, M.; Hagemann, D.; Trentzsch, M.; Weber, A.; Henle, T. Degradation studies of modified inulin as potential encapsulation material for colon targeting and release of mesalamine. Carbohydr. Polym. 2018, 199, 102–108. [Google Scholar] [CrossRef] [PubMed]
- López-Molina, D.; Chazarra, S.; How, C.W.; Pruidze, N.; Navarro-Perán, E.; García-Cánovas, F.; García-Ruiz, P.A.; Rojas-Melgarejo, F.; Rodríguez-López, J.N. Cinnamate of inulin as a vehicle for delivery of colonic drugs. Int. J. Pharm. 2015, 479, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Rahul, R.; Jha, U.; Sen, G.; Mishra, S. Carboxymethyl inulin: A novel flocculant for wastewater treatment. Int. J. Biol. Macromol. 2014, 63, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Yuan, W.; Kong, L.; Xiang, R.; Zhong, S. Efficient ethanol production from inulin by two-stage aerate strategy. Biomass and Bioenergy 2015, 80, 10–16. [Google Scholar] [CrossRef]
- Gholipourmalekabadi, M.; Zhao, S.; Harrison, B.S.; Mozafari, M.; Seifalian, A.M. Oxygen-generating biomaterials: A new, viable paradigm for tissue engineering? Trends Biotechnol. 2016, 34, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Alessia Meriggi, A.; Booten‡, K. Chemical modification of inulin, a valuable renewable resource, and its industrial applications. Biomacromolecules 2001, 2, 1. [Google Scholar]
- Liu, J.; Lu, J.-f.; Wen, X.-y.; Kan, J.; Jin, C.-h. Antioxidant and protective effect of inulin and catechin grafted inulin against CCl4-induced liver injury. Int. J. Biol. Macromol. 2015, 72, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Mittal, H.; Ray, S.S.; Kaith, B.S.; Bhatia, J.K.; Sukriti; Sharma, J.; Alhassan, S.M. Recent progress in the structural modification of chitosan for applications in diversified biomedical fields. Eur. Polym. J. 2018, 109, 402–434. [Google Scholar] [CrossRef]
- Yang, R.; Li, H.; Huang, M.; Yang, H.; Li, A. A review on chitosan-based flocculants and their applications in water treatment. Water Res. 2016, 95, 59–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.-W.; Zhang, H.; Yang, Q.; Wang, H.; Zhang, G. Preparation, characterization and antibacterial activity of octenyl succinic anhydride modified inulin. Int. J. Biol. Macromol. 2015, 78, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Spizzirri, U.G.; Parisi, O.I.; Iemma, F.; Cirillo, G.; Puoci, F.; Curcio, M.; Picci, N. Antioxidant–polysaccharide conjugates for food application by eco-friendly grafting procedure. Carbohydr. Polym. 2010, 79, 333–340. [Google Scholar] [CrossRef]
- Jiang, Y.; Zi, W.; Pei, Z.; Liu, S. Characterization of polysaccharides and their antioxidant properties from Plumula nelumbinis. Saudi. Pharm. J. 2018, 26, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Li, Q.; Dong, F.; Zhang, J.; Luan, F.; Wei, L.; Chen, Y.; Guo, Z. Novel cationic chitosan derivative bearing 1,2,3-triazolium and pyridinium: Synthesis, characterization, and antifungal property. Carbohydr. Polym. 2018, 182, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Adhikary, P.; Karmakar, N.C.; Gupta, S.; Singh, R.P.; Krishnamoorthi, S. Synthesis, characterization and application of novel cationic and amphoteric flocculants based on amylopectin. Carbohydr. Polym. 2015, 127, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Narain, R. 4–Synthetic cationic glycopolymers for gene delivery. In Polymers and Nanomaterials for Gene Therapy; Woodhead Publishing: Cambridge, England, 2016; pp. 81–98. [Google Scholar]
- Zhang, R.; Liu, S.; Edgar, K.J. Regioselective synthesis of cationic 6-deoxy-6-(N,N,N-trialkylammonio)curdlan derivatives. Carbohydr. Polym. 2016, 136, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Xing, R.; Liu, S.; Yu, H.; Wang, P.; Li, C.; Li, P. The synthesis and antioxidant activity of the Schiff bases of chitosan and carboxymethyl chitosan. Bioorg. Med. Chem. Lett. 2005, 15, 4600–4603. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Liu, H.; Chen, X.; Ji, X.; Li, P. Hydroxyl radicals scavenging activity of N-substituted chitosan and quaternized chitosan. Bioorg. Med. Chem. Lett. 2006, 16, 6348–6350. [Google Scholar] [CrossRef] [PubMed]
- Izawa, K.; Hasegawa, T. Tosylated and azidated inulins as key substrates for further chemical modifications to access inulin-based advanced materials: An inulin-based glycocluster. Bioorg. Med. Chem. Lett. 2012, 22, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Meng, X.; Xing, R.; Liu, S.; Chen, X.; Qin, Y.; Yu, H.; Li, P. Design, synthesis and antimicrobial activity of 6-N-substituted chitosan derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 4548–4551. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Edgar, K.J. Water-soluble co-polyelectrolytes by selective modification of cellulose esters. Carbohydr. Polym. 2017, 162, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pohar, A.; Likozar, B.; Tsai, S.T. Dissolution, nucleation, crystal growth, crystal aggregation, and particle breakage of amlodipine salts: Modeling crystallization kinetics and thermodynamic equilibrium, scale-up, and optimization. Ind. Eng. Chem. Res. 2014, 53, 10762–10774. [Google Scholar] [CrossRef]
- Likozar, B.; Senica, D.; Pavko, A. Interpretation of experimental results for vancomycin adsorption on polymeric resins in a fixed Bed column by mathematical modeling with independently estimated parameters. Ind. Eng. Chem. Res. 2013, 52, 9247–9258. [Google Scholar] [CrossRef]
- Grom, M.; Stavber, G.; Drnovšek, P.; Likozar, B. Modelling chemical kinetics of a complex reaction network of active pharmaceutical ingredient (API) synthesis with process optimization for benzazepine heterocyclic compound. Chem. Eng. J. 2016, 283, 703–716. [Google Scholar] [CrossRef]
- Dong, F.; Zhang, J.; Yu, C.; Li, Q.; Ren, J.; Wang, G.; Gu, G.; Guo, Z. Synthesis of amphiphilic aminated inulin via ‘click chemistry’ and evaluation for its antibacterial activity. Bioorg. Med. Chem. Lett. 2014, 24, 4590–4593. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Liu, J.; Dong, F.; Guo, Z. Synthesis and hydroxyl radicals scavenging activity of N-(aminoethyl)inulin. Carbohydr. Polym. 2011, 85, 268–271. [Google Scholar] [CrossRef]
- Ren, J.; Wang, P.; Dong, F.; Feng, Y.; Peng, D.; Guo, Z. Synthesis and antifungal properties of 6-amino-6-deoxyinulin, a kind of precursors for facile chemical modifications of inulin. Carbohydr. Polym. 2012, 87, 1744–1748. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Tan, W.; Wang, G.; Dong, F.; Li, Q.; Guo, Z. Antioxidant activity of inulin derivatives with quaternary ammonium. Starch Stärke 2017, 69, e201700046. [Google Scholar] [CrossRef]
- Kim, H.-S.; Jadhav, J.R.; Jung, S.-J.; Kwak, J.-H. Synthesis and antimicrobial activity of imidazole and pyridine appended cholestane-based conjugates. Bioorg. Med. Chem. Lett. 2013, 23, 4315–4318. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Mi, Y.; Zhang, J.; Dong, F.; Li, Q.; Ji, N.; Guo, Z. Radical Scavenging Activities of Novel Cationic Inulin Derivatives. Polymers 2018, 10, 1295. https://doi.org/10.3390/polym10121295
Chen Y, Mi Y, Zhang J, Dong F, Li Q, Ji N, Guo Z. Radical Scavenging Activities of Novel Cationic Inulin Derivatives. Polymers. 2018; 10(12):1295. https://doi.org/10.3390/polym10121295
Chicago/Turabian StyleChen, Yuan, Yingqi Mi, Jingjing Zhang, Fang Dong, Qing Li, Naiyun Ji, and Zhanyong Guo. 2018. "Radical Scavenging Activities of Novel Cationic Inulin Derivatives" Polymers 10, no. 12: 1295. https://doi.org/10.3390/polym10121295
APA StyleChen, Y., Mi, Y., Zhang, J., Dong, F., Li, Q., Ji, N., & Guo, Z. (2018). Radical Scavenging Activities of Novel Cationic Inulin Derivatives. Polymers, 10(12), 1295. https://doi.org/10.3390/polym10121295