Effects of Calcium Alginate Submicroparticles on Seed Germination and Seedling Growth of Wheat (Triticum aestivum L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of CaAlg Submicroparticles
2.2. Characterization of CaAlg Submicroparticles
2.3. Adsorption of CaAlg Submicroparticles on Wheat
2.4. Wheat Growth Condition
2.5. Determination of Chlorophyll and Soluble Protein Content
2.6. Determination of IAA Content and IAA Oxidase Activity
2.7. Gene Expression Analysis of the Wheat by RT-qPCR
2.8. Statistical Analysis
3. Results
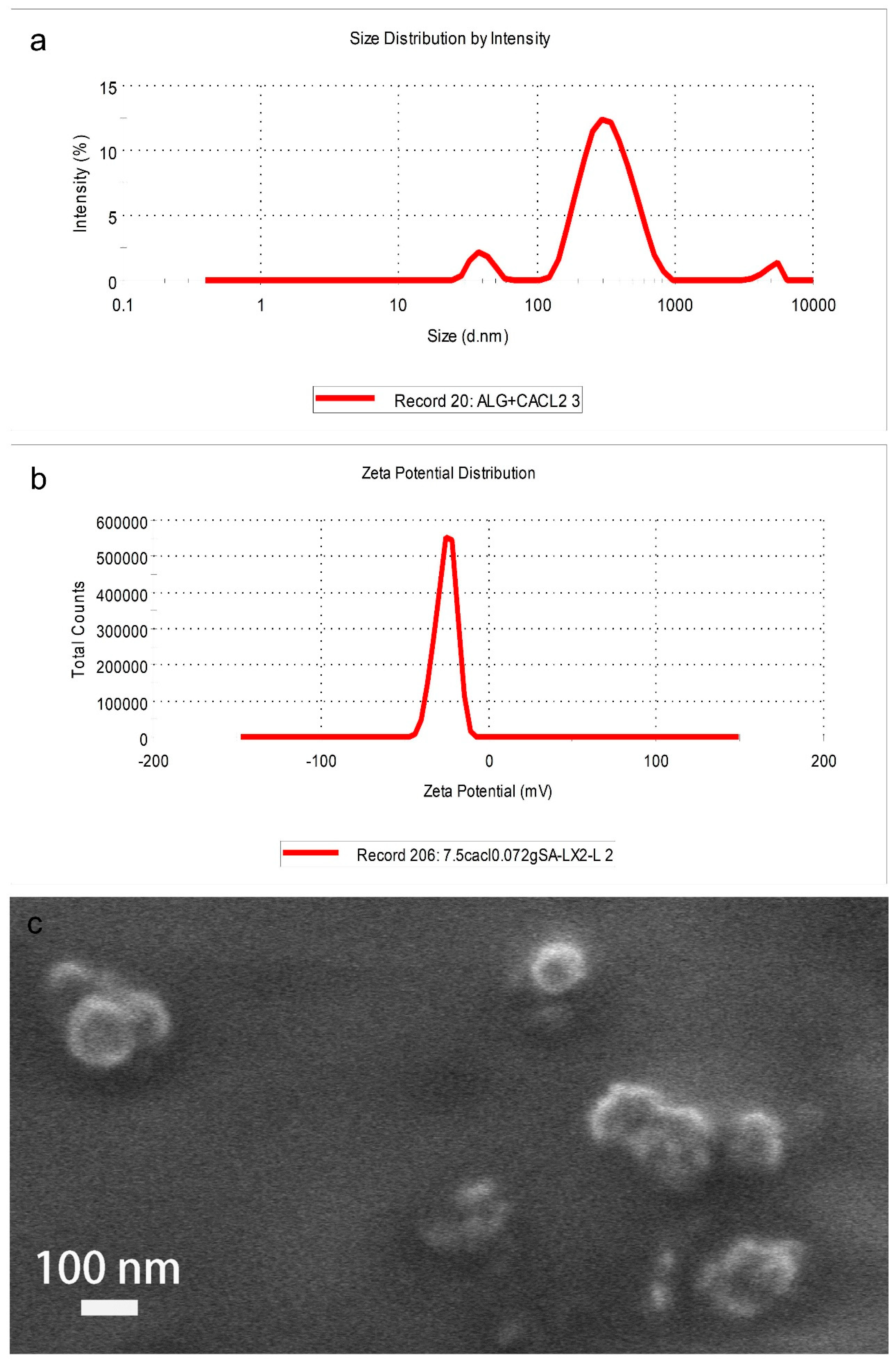
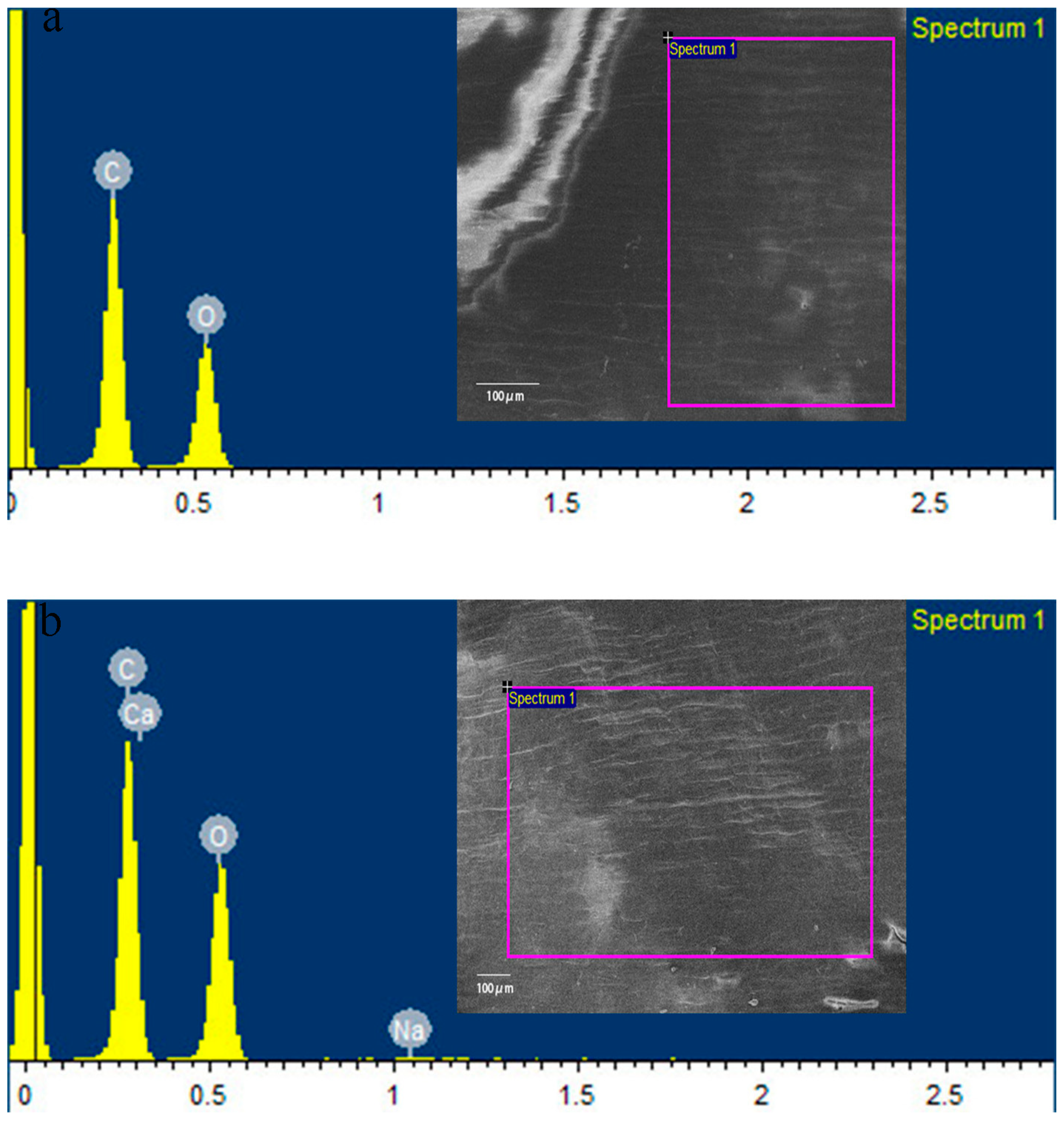
3.1. Characterization of CaAlg Submicroparticles
3.2. Experiment of CaAlg Submicroparticles Adsorption of Wheat Seeds
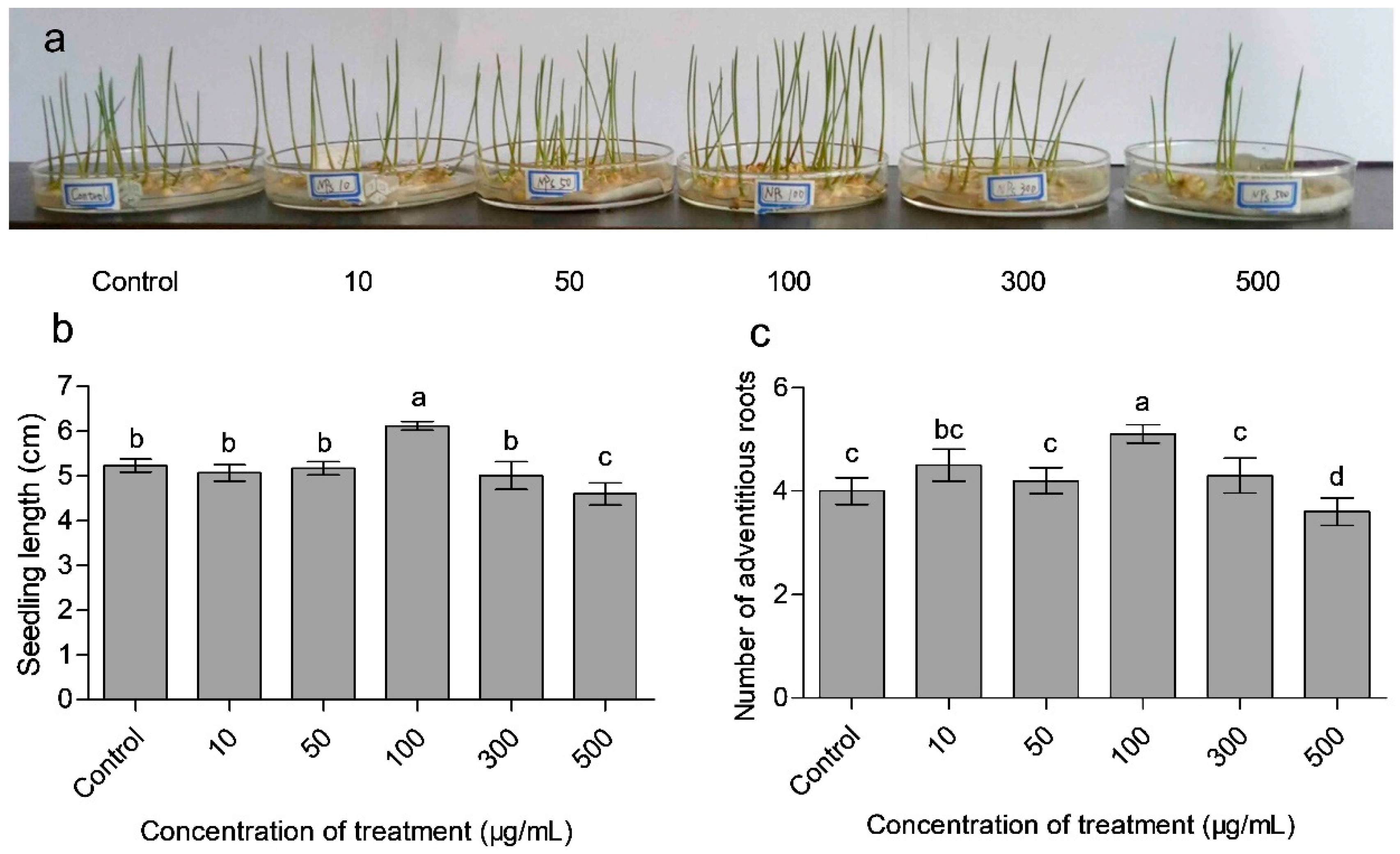
3.3. Effects of CaAlg Submicroparticles on Seed Germination and Plant Biomass
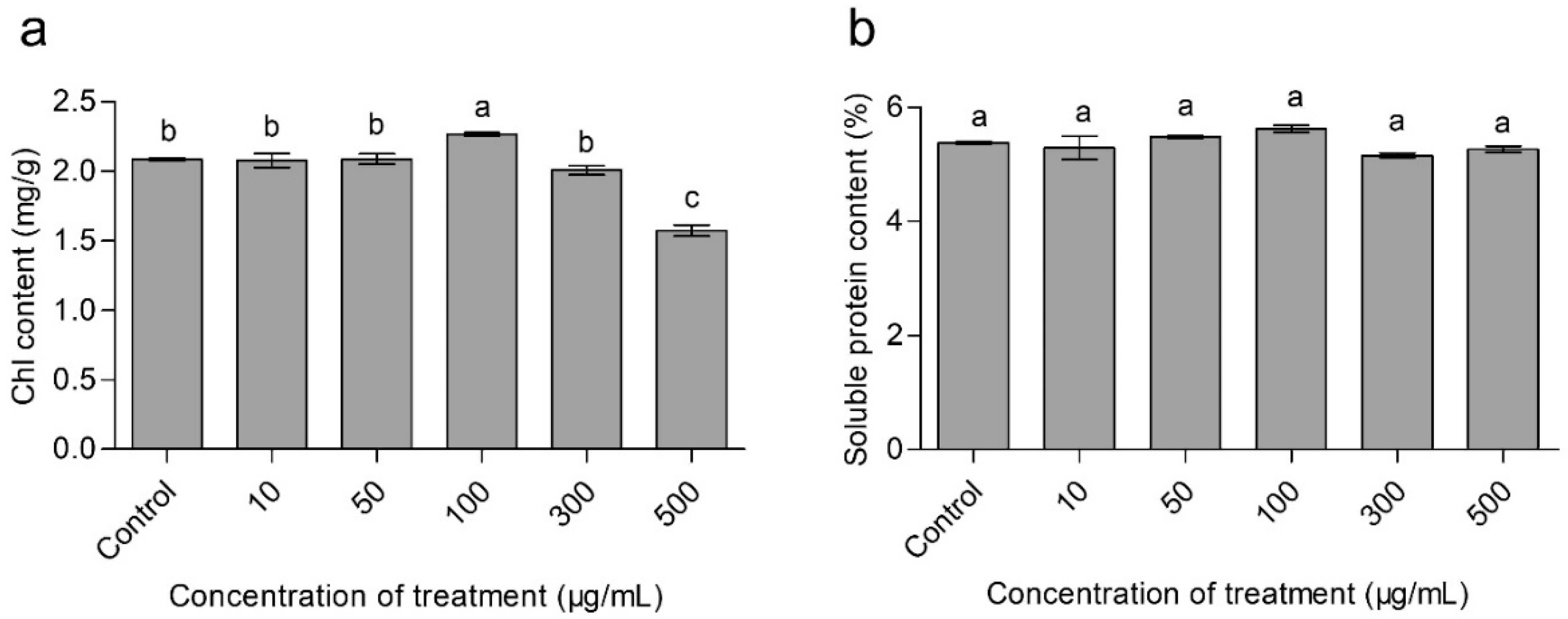
3.4. Effects of CaAlg Submicroparticles on Chorophyll Content of Wheat
3.5. Effects of CaAlg Submicroparticles on Soluble Protein Content of Wheat
3.6. Effects of CaAlg Submicroparticles on IAA Synthesis and Metabolism of Wheat
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yokose, T.; Nishikawa, T.; Yamamoto, Y.; Yamasaki, Y.; Yamaguchi, K.; Oda, T. Growth-promoting effect of alginate oligosaccharides on a unicellular marine microalga, nannochloropsis oculata. Biosci. Biotechnol. Biochem. 2009, 73, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, S.; Zhang, Y.; Chen, L. High-level expression of a thermally stable alginate lyase using pichia pastoris, characterization and application in producing brown alginate oligosaccharide. Mar. Drugs 2018, 16. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, C.-H.; Kumar, G.V.; Adhikary, S.; Velusamy, P.; Pandian, K.; Anbu, P. Preparation of cotton fabric using sodium alginate-coated nanoparticles to protect against nosocomial pathogens. Biochem. Eng. J. 2017, 117, 28–35. [Google Scholar] [CrossRef]
- Carneiro-da-Cunha, M.G.; Cerqueira, M.A.; Souza, B.W.S.; Carvalho, S.; Quintas, M.A.C.; Teixeira, J.A.; Vicente, A.A. Physical and thermal properties of a chitosan/alginate nanolayered pet film. Carbohydr. Polym. 2010, 82, 153–159. [Google Scholar] [CrossRef]
- Sarfaraz, A.; Naeem, M.; Nasir, S.; Idrees, M.; Aftab, T.; Hashmi, N.; Khan, M.M.A.; Moinuddin; Varshney, L. An evaluation of the effects of irradiated sodium alginate on the growth, physiological activities and essential oil production of fennel (Foeniculum vulgare mill.). J. Med. Plants Res. 2011, 5, 15–21. [Google Scholar]
- Xu, X.; Iwamoto, Y.; Kitamura, Y.; Oda, T.; Muramatsu, T. Root growth-promoting activity of unsaturated oligomeric uronates from alginate on carrot and rice plants. Biosci. Biotechnol. Biochem. 2003, 67, 2022–2025. [Google Scholar] [CrossRef] [PubMed]
- Abedini, F.; Ebrahimi, M.; Roozbehani, A.H.; Domb, A.J.; Hosseinkhani, H. Overview on natural hydrophilic polysaccharide polymers in drug delivery. Polym. Advan. Technol. 2018, 29, 2564–2573. [Google Scholar] [CrossRef]
- Douglas, K.L.; Tabrizian, M. Effect of experimental parameters on the formation of alginate-chitosan nanoparticles and evaluation of their potential application as DNA carrier. J. Biomater. Sci. Polym. Ed. 2005, 16, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.M.; Yang, T.Y.; Pan, W.M. Preparation and distribution of 5-fluorouracil i-125 sodium alginate-bovine serum albumin nanoparticles. World J. Gastroenterol. 1999, 5, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Guo, L.; Wang, S.; Liu, D.; Qin, X.; Zheng, L.; Tian, C.; Han, X.; Chen, R.; Yin, R. Preparation of self-assembled nanoparticles of epsilon-polylysine-sodium alginate: A sustained-release carrier for antigen delivery. Coll. Surf. B Biointerfaces 2018, 171, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, B.; Ribeiro, A.J.; Veiga, F.; Ferreira, D.C.; Neufeld, R.J. Insulin-loaded nanoparticles are prepared by alginate ionotropic pre-gelation followed by chitosan polyelectrolyte complexation. J. Nanosci. Nanotechnol. 2007, 7, 2833–2841. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Van, S.; Dinh Minh, H.; Nguyen Anh, D. Study on chitosan nanoparticles on biophysical characteristics and growth of Robusta coffee in green house. Biocatal. Agric. Biotechnol. 2013, 2, 289–294. [Google Scholar] [CrossRef]
- Corradini, E.; de Moura, M.R.; Mattoso, L.H.C. A preliminary study of the incorparation of NPK fertilizer into chitosan nanoparticles. Express. Polym. Lett. 2010, 4, 509–515. [Google Scholar] [CrossRef]
- Rajaonarivony, M.; Vauthier, C.; Couarraze, G.; Puisieux, F.; Couvreur, P. Development of a new drug carrier made from alginate. J. Pharm. Sci. 1993, 82, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Hu, J.; Zhang, Y.; Xie, X.J.; Knapp, A. Seed priming with brassinolide improves lucerne (Medicago sativa L.) seed germination and seedling growth in relation to physiological changes under salinity stress. Aust. J. Agr. Res. 2007, 58, 811. [Google Scholar] [CrossRef]
- Kaya, M.; Kaya, G.; Kaya, M.D.; Atak, M.; Saglam, S.; Khawar, K.M.; Ciftci, C.Y. Interaction between seed size and NaCl on germination and early seedling growth of some Turkish cultivars of chickpea (Cicer arietinum L.). J. Zhejiang Univ. Sci. B 2008, 9, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Y.H.; Yin, H.; Wang, W.X.; Zhao, X.M.; Du, Y.G. Alginate oligosaccharides enhanced Triticum aestivum L. tolerance to drought stress. Plant Physiol. Biochem. 2013, 62, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, X.; Yin, H.; He, A.; Sun, K. Effects of seed soaking with oligosaccharides on seed germination and seedling growth of wheat (Triticum aestivum L.). J. Henan Agric. Sci. 2014, 43, 16–21. [Google Scholar]
- Zhang, Y.; Yin, H.; Zhao, X.; Wang, W.; Du, Y.; He, A.; Sun, K. The promoting effects of alginate oligosaccharides on root development in Oryza sativa L. mediated by auxin signaling. Carbohydr. Polym. 2014, 113, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.M.; Liu, E.H. Inhibition of peroxidase and indole-3-acetic-acid oxidase activity by British anti-lewisite. Arch. Biochem. Biophys. 1978, 186, 317–323. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Takato, S.; Kakei, Y.; Mitsui, M.; Ishida, Y.; Suzuki, M.; Yamazaki, C.; Hayashi, K.I.; Ishii, T.; Nakamura, A.; Soeno, K.; et al. Auxin signaling through SCFTIR1/AFBs mediates feedback regulation of IAA biosynthesis. Biosci. Biotechnol. Biochem. 2017, 81, 1320–1326. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.-J.; Hu, J.; Wang, X.-J.; Shao, C.-X. Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress. J. Zhejiang Univ. Sci. B 2009, 10, 427–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saharan, V.; Kumaraswamy, R.V.; Choudhary, R.C.; Kumari, S.; Pal, A.; Raliya, R.; Biswas, P. Cu-chitosan nanoparticle mediated sustainable approach to enhance seedling growth in maize by mobilizing reserved food. J. Agric. Food Chem. 2016, 64, 6148–6155. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.E.S.; Sandoval-Herrera, I.E.; Zavala-Betancourt, S.A.; Oliveira, H.C.; Ledezma-Perez, A.S.; Romero, J.; Fraceto, L.F. Gamma-polyglutamic acid/chitosan nanoparticles for the plant growth regulator gibberellic acid: Characterization and evaluation of biological activity. Carbohydr. Polym. 2017, 157, 1862–1873. [Google Scholar] [CrossRef] [PubMed]
- Terry, N. Limiting factors in photosynthesis: 4. Iron stress-mediated changes in light-harvesting and electron-transport capacity and its effects on photosynthesis invivo. Plant Physiol. 1983, 71, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Sharma, P.; Kumar, S.; Nayan, R.; Khanna, P.K.; Zaidi, M.G.H. Gold-nanoparticle induced enhancement in growth and seed yield of Brassica juncea. Plant Growth Regul. 2012, 66, 303–310. [Google Scholar] [CrossRef]
- Qin, P.; Kong, Z.; Liao, X.; Liu, Y. Effects of accelerated aging on physiological and biochemical characteristics of waxy and non-waxy wheat seeds. J. Northeast Agric. Univ. (Engl. Ed.) 2011, 18, 7–12. [Google Scholar] [CrossRef]
- Mukherjee, A.; Sun, Y.; Morelius, E.; Tamez, C.; Bandyopadhyay, S.; Niu, G.; White, J.C.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Differential toxicity of bare and hybrid ZnO nanoparticles in green pea (Pisum sativum L.): A life cycle study. Front. Plant Sci. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, P.; Priyanka, N.; Manikandan, K.; Ganeshbabu, I.; Indiraarulselvi, P.; Geetha, N.; Muralikrishna, K.; Bhattacharya, R.C.; Tiwari, M.; Sharma, N.; et al. Enhanced plant growth promoting role of phycomolecules coated zinc oxide nanoparticles with p supplementation in cotton (Gossypium hirsutum L.). Plant Physiol. Bioch. 2017, 110, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Peng, B.; Hernandez-Viezcas, J.A.; Rico, C.; Sun, Y.; Peralta-Videa, J.R.; Tang, X.; Niu, G.; Jin, L.; Varela-Ramirez, A.; et al. Stress response and tolerance of Zea mays to CeO2 nanoparticles: Cross talk among H2O2, heat shock protein, and lipid peroxidation. ACS Nano 2012, 6, 9615–9622. [Google Scholar] [CrossRef] [PubMed]
- Hentrich, M.; Boettcher, C.; Duechting, P.; Cheng, Y.; Zhao, Y.; Berkowitz, O.; Masle, J.; Medina, J.; Pollmann, S. The jasmonic acid signaling pathway is linked to auxin homeostasis through the modulation of YUCCA8 and YUCCA9 gene expression. Plant J. 2013, 74, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Otori, K.; Tamoi, M.; Tanabe, N.; Shigeoka, S. Enhancements in sucrose biosynthesis capacity affect shoot branching in Arabidopsis. Biosci. Biotechnol. Biochem. 2017, 81, 1470–1477. [Google Scholar] [CrossRef] [PubMed]
- Marchant, A.; Bhalerao, R.; Casimiro, I.; Eklof, J.; Casero, P.J.; Bennett, M.; Sandberg, G. AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell 2002, 14, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Teale, W.D.; Paponov, I.A.; Palme, K. Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 2006, 7, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Mironova, V.; Teale, W.; Shahriari, M.; Dawson, J.; Palme, K. The systems biology of auxin in development emoryos. Trends Plant Sci. 2017, 22, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.G.; Lim, E.K.; Li, Y.; Kowalczyk, M.; Sandberg, G.; Hoggett, J.; Ashford, D.A.; Bowles, D.J. Identification and biochemical characterization of an Arabidopsis indole-3-acetic acid glucosyltransferase. J. Biol. Chem. 2001, 276, 4350–4356. [Google Scholar] [CrossRef] [PubMed]
- Mellor, N.; Band, L.R.; Pencik, A.; Novak, O.; Rashed, A.; Holman, T.; Wilson, M.H.; Voss, U.; Bishopp, A.; King, J.R.; et al. Dynamic regulation of auxin oxidase and conjugating enzymes AtDAO1 and GH3 modulates auxin homeostasis. Proc. Natl. Acad. Sci. USA 2016, 113, 11022–11027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porco, S.; Pencik, A.; Rashed, A.; Voss, U.; Casanova-Saez, R.; Bishopp, A.; Golebiowska, A.; Bhosale, R.; Swarup, R.; Swarup, K.; et al. Dioxygenase-encoding AtDAO1 gene controls IAA oxidation and homeostasis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2016, 113, 11016–11021. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Castro, J.; Vera, J.; Moenne, A. Seaweed oligosaccharides stimulate plant growth by enhancing carbon and nitrogen assimilation, basal metabolism, and cell division. J. Plant Growth Regul. 2012, 32, 443–448. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, H.; Yin, H.; Wang, W.; Zhao, X.; Du, Y. Nitric oxide mediates alginate oligosaccharides-induced root development in wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2013, 71, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Anis, M.; Zaki, M.J.; Dawar, S. Development of a Na-alginate-based bioformulation and its use in the management of charcoal rot of sunflower (Helianthus annuus L.). Pak. J. Bot. 2012, 44, 1167–1170. [Google Scholar]
- Liu, R.; Jiang, X.; Guan, H.; Li, X.; Du, Y.; Wang, P.; Mou, H. Promotive effects of alginate-derived oligosaccharides on the inducing drought resistance of tomato. J. Ocean Univ. China 2009, 8, 303–311. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Lin, X.; Yan, G.; Liu, L.; Zheng, H.; Zhao, B.; Tang, J.; Guo, Y.D. Alginate-derived oligosaccharides promote water stress tolerance in cucumber (Cucumis sativus L.). Plant Physiol. Biochem. 2018, 130, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.K.; Jiang, X.L.; Hwang, H.M.; Liu, S.L.; Guan, H.S. Promotive effects of alginate-derived oligosaccharide on maize seed germination. J. Appl. Phycol. 2004, 16, 73–76. [Google Scholar] [CrossRef]
- Liang, J.; Yan, H.; Puligundla, P.; Gao, X.; Zhou, Y.; Wan, X. Applications of chitosan nanoparticles to enhance absorption and bioavailability of tea polyphenols: A review. Food Hydrocoll. 2017, 69, 286–292. [Google Scholar] [CrossRef]
- Wang, W.X.; Li, S.G.; Zhao, X.M.; Du, Y.G.; Lin, B.C. Oligochitosan induces cell death and hydrogen peroxide accumulation in tobacco suspension cells. Pestic. Biochem. Physiol. 2008, 90, 106–113. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Wang, W.X.; Yin, H.; Zhao, X.M.; Du, Y.G. Oligochitosan induces programmed cell death in tobacco suspension cells. Carbohydr. Polym. 2012, 87, 2270–2278. [Google Scholar] [CrossRef] [Green Version]

| Gene | Primer Sequence (Forward 5′–3′) | Primer Sequence (Reverse 5′–3′) |
|---|---|---|
| β-tubulin | CATGCTATCCCTCGTCTCGACCT | CGCACTTCATGATGGAGTTGTAT |
| YUCCA9 | GATTCTGGGCATCTCAACA | GGGACCACCTTTATTTCG |
| AUX1 | CCGTCATTCCACAACTACC | ACATCGCGTGCATTATCT |
| ARF | AGCCAAAAGCAGAACTACC | AGCCATCCCAAGCACTAT |
| UGT | CTCACCTTTAGTGGCTTTTG | CTCACCATCGCATCTCAG |
| Elements | ddH2O (%) | CaAlg Submicroparticles (%) |
|---|---|---|
| C | 57.41 | 50.06 |
| O | 42.59 | 49.12 |
| Na | - | 0.15 |
| Ca | - | 0.67 |
| Total | 100 | 100 |
| Treatment | Fresh Weight (mg explant −1) | Root/Shoot | Seedling | Germination Percentage | Germination | |
|---|---|---|---|---|---|---|
| (μg/mL) | Aboveground Part | Underground Part | Ratio | Index | (%) | Index |
| Control | 35.78 b | 21.16 b | 0.591 bc | 33.67 c | 74.44 b | 25.10 b |
| 10 | 35.54 b | 21.98 b | 0.618 b | 35.57 b | 63.33 c | 20.83 d |
| 50 | 35.29 b | 21.15 b | 0.599 bc | 33.83 a | 72.22 b | 23.05 c |
| 100 | 42.36 a | 29.73 a | 0.702 a | 50.60 a | 81.11 a | 27.02 a |
| 300 | 35.53 b | 21.89 b | 0.616 b | 35.38 b | 63.33 c | 21.40 d |
| 500 | 33.39 c | 19.36 c | 0.580 c | 30.59 d | 54.44 d | 16.86 e |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Li, R.; Sun, X.; Wang, W.; Hu, J.; Xie, H.; Yin, H. Effects of Calcium Alginate Submicroparticles on Seed Germination and Seedling Growth of Wheat (Triticum aestivum L.). Polymers 2018, 10, 1154. https://doi.org/10.3390/polym10101154
He J, Li R, Sun X, Wang W, Hu J, Xie H, Yin H. Effects of Calcium Alginate Submicroparticles on Seed Germination and Seedling Growth of Wheat (Triticum aestivum L.). Polymers. 2018; 10(10):1154. https://doi.org/10.3390/polym10101154
Chicago/Turabian StyleHe, Jinxia, Ruixin Li, Xue Sun, Wenxia Wang, Jianen Hu, Hongguo Xie, and Heng Yin. 2018. "Effects of Calcium Alginate Submicroparticles on Seed Germination and Seedling Growth of Wheat (Triticum aestivum L.)" Polymers 10, no. 10: 1154. https://doi.org/10.3390/polym10101154
APA StyleHe, J., Li, R., Sun, X., Wang, W., Hu, J., Xie, H., & Yin, H. (2018). Effects of Calcium Alginate Submicroparticles on Seed Germination and Seedling Growth of Wheat (Triticum aestivum L.). Polymers, 10(10), 1154. https://doi.org/10.3390/polym10101154




