Morphologies Tuning of Polypyrrole and Thermoelectric Properties of Polypyrrole Nanowire/Graphene Composites
Abstract
:1. Introduction
2. Experimental
2.1. Materials
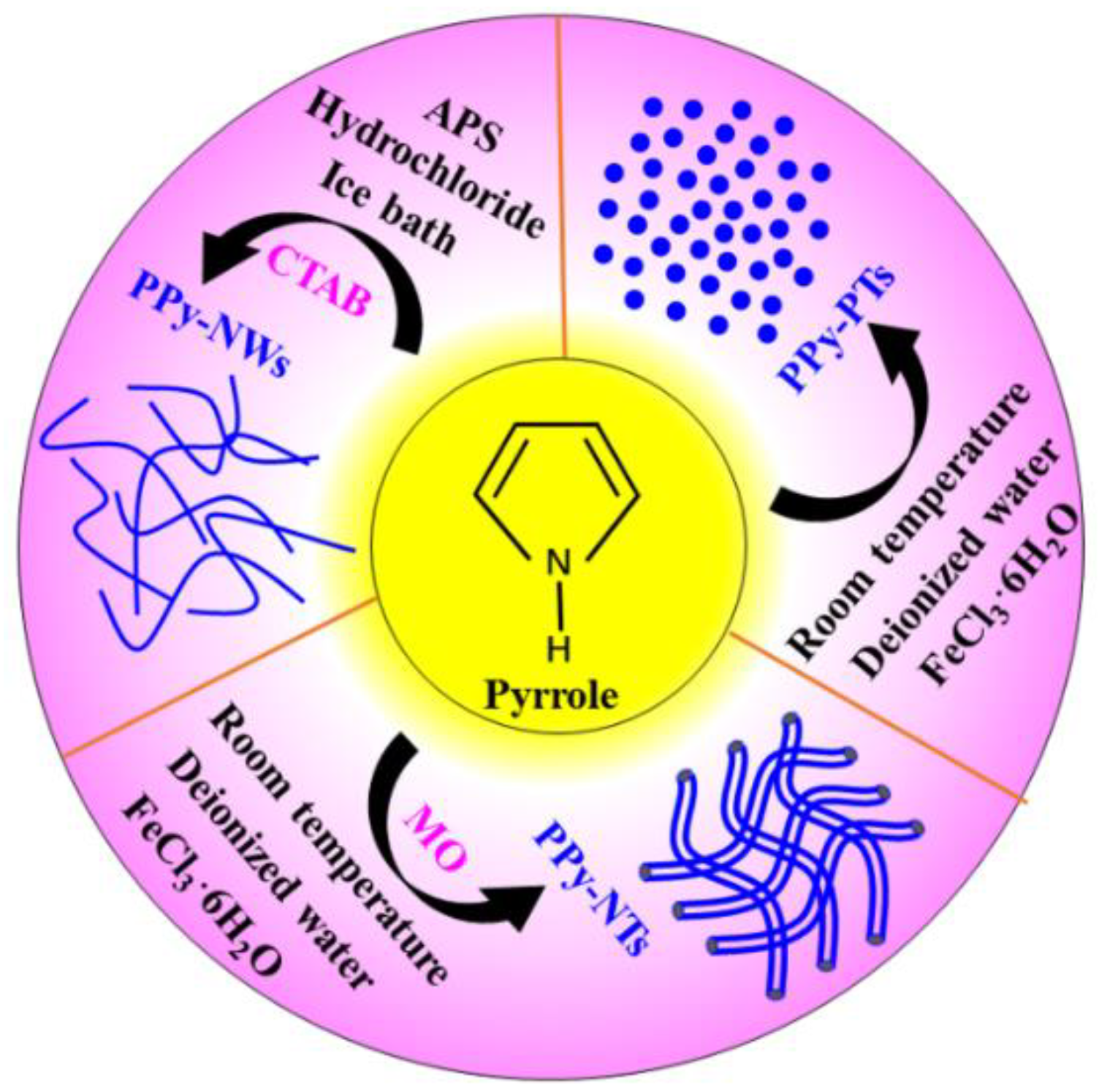
2.2. Preparation of the PPy Particles
2.3. Preparation of the PPy Nanotubes
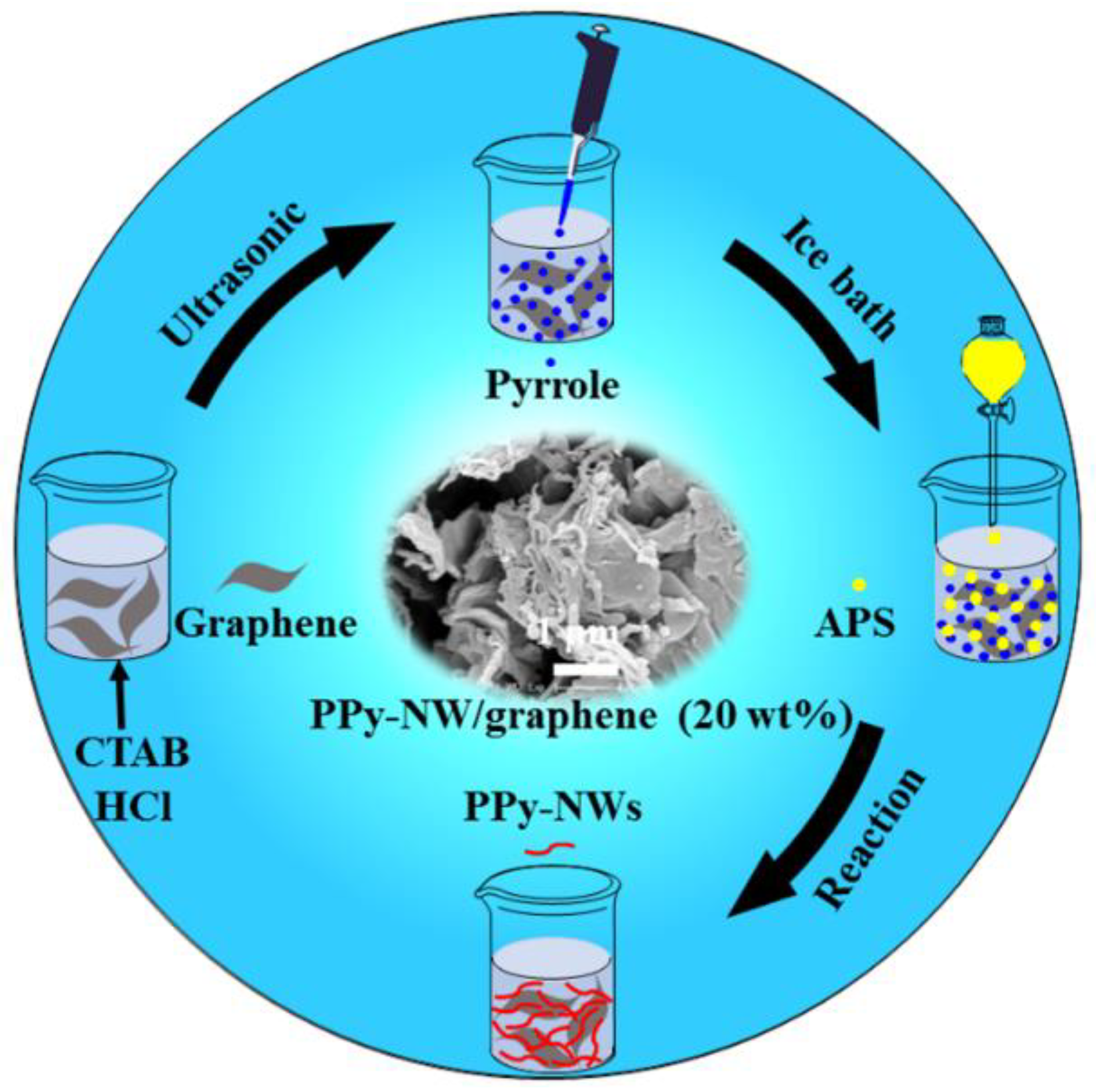
2.4. Preparation of the PPy-NWs and PPy-NW/Graphene Nanocomposites
2.5. Characterizations
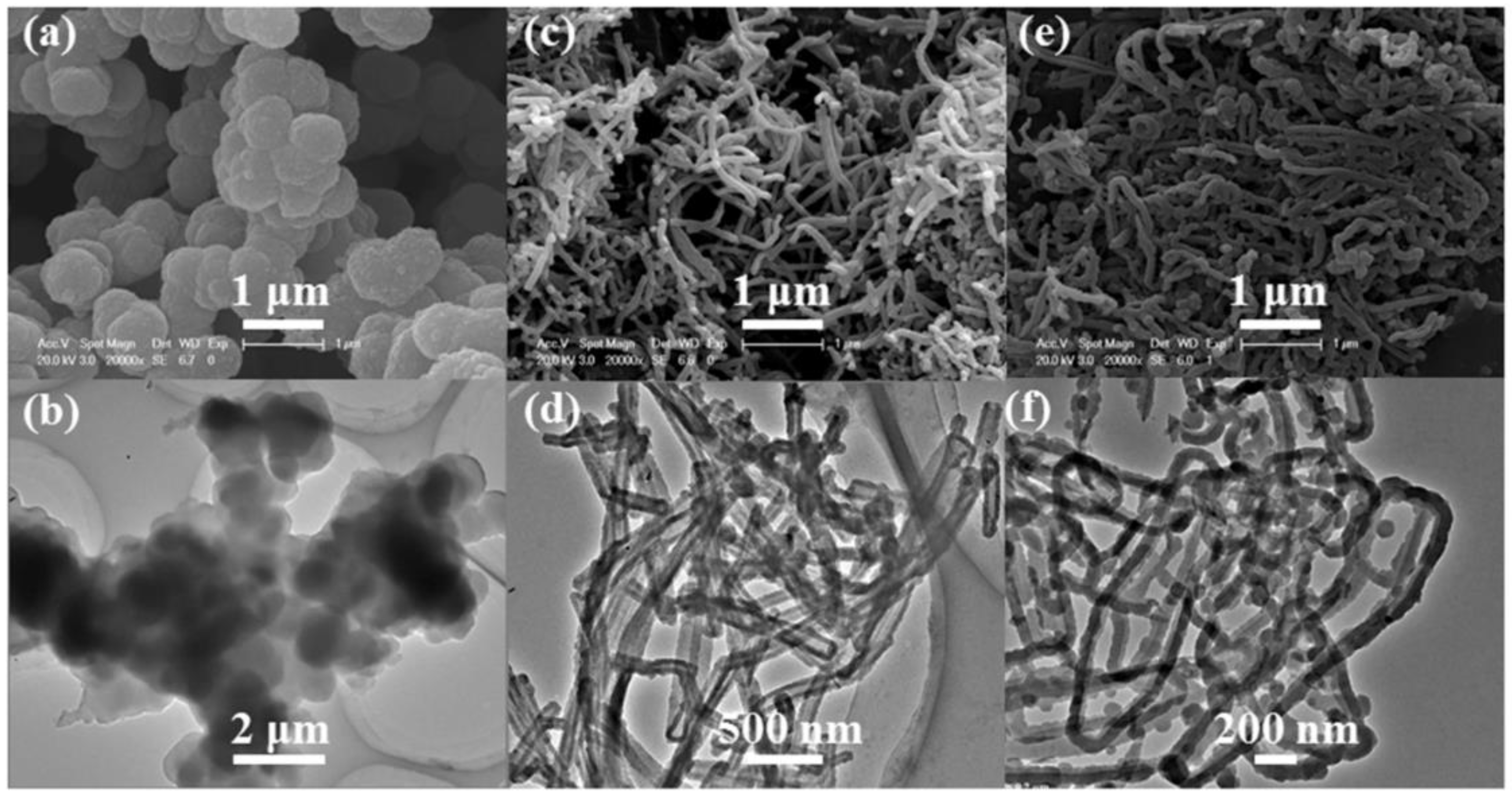
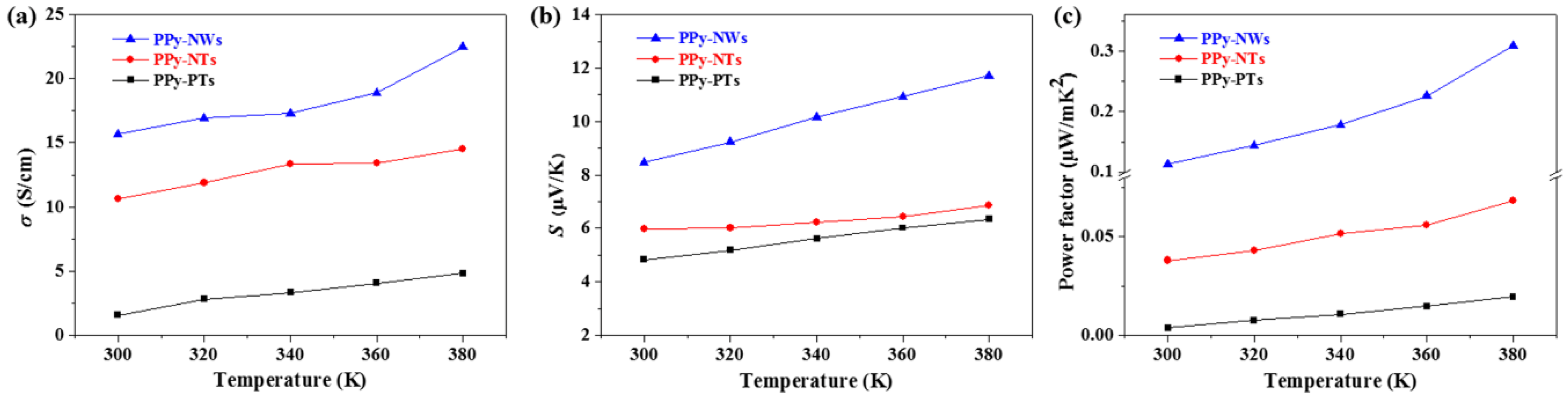
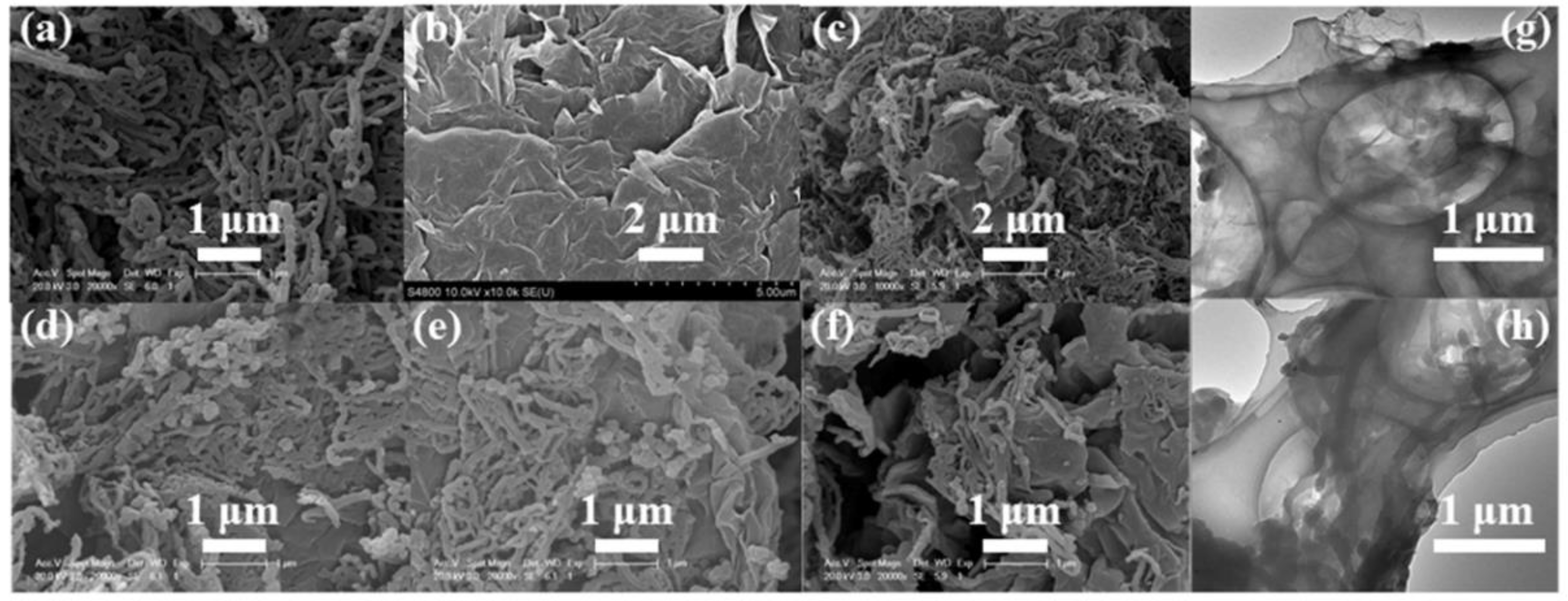
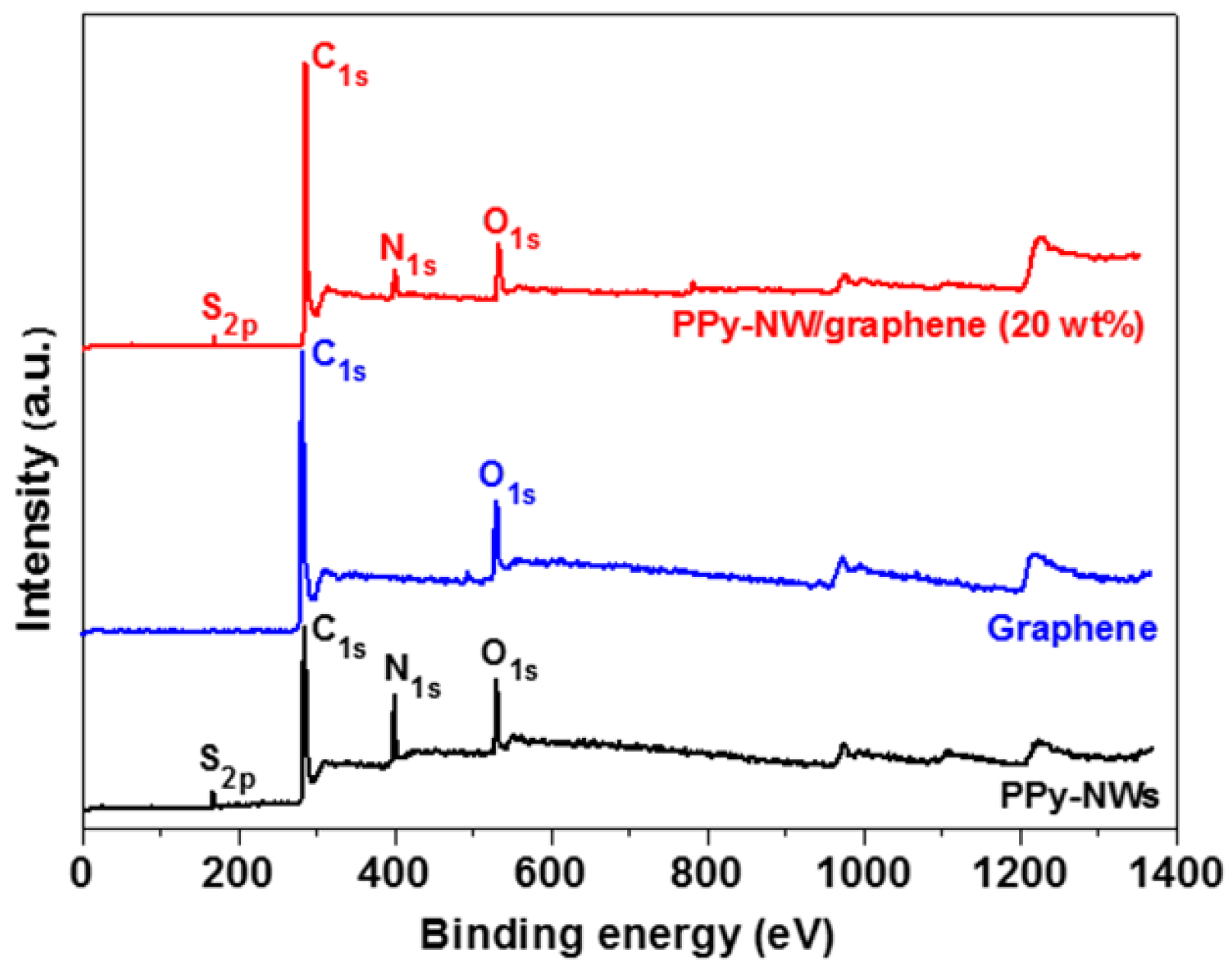
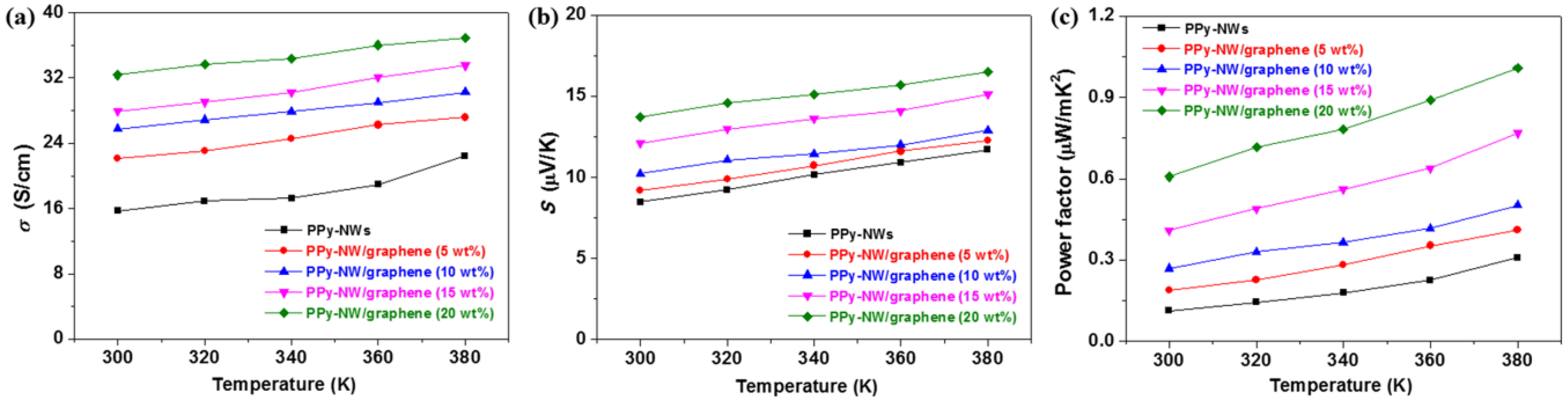
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Seebeck, T.J. Magnetische Polarisation der Metalle und erze Durch Temperatur-Differenz; Abh. Akad. Wiss.: Berlin, Germany, 1822; pp. 289–346. [Google Scholar]
- Du, Y.; Xu, J.Y.; Paul, B.; Eklund, P. Flexible thermoelectric materials and devices. Appl. Mater. Today 2018, 12, 366–388. [Google Scholar] [CrossRef]
- Du, Y.; Shen, S.Z.; Cai, K.F.; Casey, P.S. Research progress on polymer-inorganic thermoelectric nanocomposite materials. Prog. Polym. Sci. 2012, 37, 820–841. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.M.; Xu, W.; Zhu, D.B. Organic thermoelectric materials: Emerging green energy materials converting heat to electricity directly and efficiently. Adv. Mater. 2014, 26, 6829–6851. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.Y.; Chen, G.M. Conducting polymer/carbon particle thermoelectric composites: Emerging green energy materials. Compos. Sci. Technol. 2016, 124, 52–70. [Google Scholar] [CrossRef]
- Culebras, M.; Uriol, B.; Gomez, C.M.; Cantarero, A. Controlling the thermoelectric properties of polymers: Application to PEDOT and polypyrrole. Phys. Chem. Chem. Phys. 2015, 17, 15140–15145. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.X.; Liu, T.X.; Li, H.; Yang, D.W.; Chen, L.J.; Tang, X.F. Notably enhanced thermoelectric properties of lamellar polypyrrole by doping with β-naphthalene sulfonic acid. RSC Adv. 2017, 7, 20192–20200. [Google Scholar] [CrossRef] [Green Version]
- Song, H.J.; Cai, K.F.; Wang, J.; Shen, S. Influence of polymerization method on the thermoelectric properties of multi-walled carbon nanotubes/polypyrrole composites. Synth. Met. 2016, 211, 58–65. [Google Scholar] [CrossRef]
- Du, Y.; Xu, J.Y.; Lin, T. Single-Walled Carbon Nanotube/Polypyrrole Thermoelectric Composite Materials; IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2017; Volume 108, p. 022040. [Google Scholar]
- Han, S.B.; Zhai, W.T.; Chen, G.M.; Wang, X. Morphology and thermoelectric properties of graphene nanosheets enwrapped with polypyrrole. RSC Adv. 2014, 4, 29281–29285. [Google Scholar] [CrossRef]
- Kim, G.H.; Shao, L.; Zhang, K.; Pipe, K.P. Engineered doping of organic semiconductors for enhanced thermoelectric efficiency. Nat. Mater. 2013, 12, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.C.; Chen, G.M.; Wang, X.; Wang, H.F. Tuning thermoelectric performance by nanostructure evolution of a conducting polymer. J. Mater. Chem. A 2015, 3, 20896–20902. [Google Scholar] [CrossRef]
- Wu, J.S.; Sun, Y.M.; Xu, W.; Zhang, Q.C. Investigating thermoelectric properties of doped polyaniline nanowires. Synth. Met. 2014, 189, 177–182. [Google Scholar] [CrossRef]
- Wu, J.S.; Sun, Y.M.; Pei, W.B.; Ling, H.; Xu, W.; Zhang, Q.C. Polypyrrole nanotube film for flexible thermoelectric application. Synth. Met. 2014, 196, 173–177. [Google Scholar] [CrossRef]
- Liang, L.R.; Chen, G.M.; Guo, C.Y. Polypyrrole nanostructures and their thermoelectric performance. Mater. Chem. Front. 2017, 1, 380–386. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, C.E.; Lin, Z.Q.; Gu, P.Y.; Zhang, Q.C. Nanostructured conjugated polymers for energy-related applications beyond solar cells. Chem. Asian J. 2016, 11, 1489–1511. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Kuila, T.; Uddin, M.E.; Kim, N.H.; Lau, A.K.T.; Lee, J.H. In-situ synthesis and characterization of electrically conductive polypyrrole/graphene nanocomposites. Polymer 2010, 51, 5921–5928. [Google Scholar] [CrossRef]
- Zhao, Y.C.; Lu, Z.; Tian, J.N.; Nie, S.L.; Zhen, N. Enhanced electrocatalytic oxidation of methanol on Pd/polypyrrole–graphene in alkaline medium. Electrochim. Acta 2011, 56, 1967–1972. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, C.Y.; Yue, B.B.; Gambhir, S.; Too, C.O.; Wallace, G.G. Electrochemically synthesized polypyrrole/graphene composite film for lithium batteries. Adv. Energy Mater. 2012, 2, 266–272. [Google Scholar] [CrossRef]
- Oliveira, H.P.D.; Sydlik, S.A.; Swager, T.M. Supercapacitors from free-standing polypyrrole/graphene nanocomposites. J. Phys. Chem. C 2013, 117, 10270–10276. [Google Scholar] [CrossRef]
- Gao, Y.S.; Xu, J.K.; Lu, L.M.; Wu, L.P.; Zhang, K.X.; Nie, T.; Zhu, X.F.; Wu, Y. Overoxidized polypyrrole/graphene nanocomposite with good electrochemical performance as novel electrode material for the detection of adenine and guanine. Biosens. Bioelectron. 2014, 62, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Shen, S.Z.; Yang, W.D.; Donelson, R.; Cai, K.F.; Casey, P.S. Simultaneous increase in conductivity and Seebeck coefficient in a polyaniline/graphene nanosheets thermoelectric nanocomposite. Synth. Met. 2012, 161, 2688–2692. [Google Scholar] [CrossRef]
- Kim, G.H.; Hwang, D.H.; Woo, S.I. Thermoelectric properties of nanocomposite thin films prepared with poly(3,4-ethylenedioxythiophene) poly(styrenesulfonate) and graphene. Phys. Chem. Chem. Phys. 2012, 14, 3530–3536. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Du, Y.; Jia, R.P.; Xu, J.Y.; Shen, S.Z. Thermoelectric properties of flexible PEDOT: PSS/polypyrrole/paper nanocomposite Films. Materials 2017, 10, 780. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Du, Y.; Jia, R.P.; Xu, J.Y.; Shen, S.Z. Flexible thermoelectric composite films of polypyrrole nanotubes coated paper. Coatings 2017, 7, 211. [Google Scholar] [CrossRef]
- Sapurina, I.; Li, Y.; Alekseeva, E.; Bober, P.; Trchová, M.; Morávková, Z.; Stejskal, J. Polypyrrole nanotubes: The tuning of morphology and conductivity. Polymer 2017, 113, 247–258. [Google Scholar] [CrossRef]
- Kopecká, J.; Kopecký, D.; Vrňata, M.; Fitl, P.; Stejskal, J.; Trchová, M.; Bober, P.; Morávková, Z.; Prokeš, J.; Sapurina, I. Polypyrrole nanotubes: Mechanism of formation. RSC Adv. 2014, 4, 1551–1558. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, G.M.; Wang, H.F.; Zhai, W.T. Enhanced thermoelectric property by the construction of a nanocomposite 3D interconnected architecture consisting of graphene nanolayers sandwiched by polypyrrole nanowires. J. Mater. Chem. C 2015, 3, 1649–1654. [Google Scholar] [CrossRef]
- Zhang, X.T.; Zhang, J.; Liu, Z.F.; Robinson, C. Inorganic/organic mesostructure directed synthesis of wire/ribbon-like polypyrrole nanostructures. Chem. Commun. 2004, 1852–1853. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.H.; Sun, J.; Gao, L. Synthesis of novel hierarchical graphene/polypyrrole nanosheet composites and their superior electrochemical performance. J. Mater. Chem. 2011, 21, 11253–11258. [Google Scholar] [CrossRef]
- Zhang, X.T.; Zhang, J.; Song, W.H.; Liu, Z.F. Controllable synthesis of conducting polypyrrole nanostructures. J. Phys. Chem. B 2006, 110, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Niu, H.; Li, J.; Dou, Y.; Shen, S.Z.; Jia, R.; Xu, J. Morphologies Tuning of Polypyrrole and Thermoelectric Properties of Polypyrrole Nanowire/Graphene Composites. Polymers 2018, 10, 1143. https://doi.org/10.3390/polym10101143
Du Y, Niu H, Li J, Dou Y, Shen SZ, Jia R, Xu J. Morphologies Tuning of Polypyrrole and Thermoelectric Properties of Polypyrrole Nanowire/Graphene Composites. Polymers. 2018; 10(10):1143. https://doi.org/10.3390/polym10101143
Chicago/Turabian StyleDu, Yong, Hao Niu, Jun Li, Yunchen Dou, Shirley Z. Shen, Runping Jia, and Jiayue Xu. 2018. "Morphologies Tuning of Polypyrrole and Thermoelectric Properties of Polypyrrole Nanowire/Graphene Composites" Polymers 10, no. 10: 1143. https://doi.org/10.3390/polym10101143




