Effect of Chemical Composition Variant and Oxygen Plasma Treatments on the Wettability of PLGA Thin Films, Synthesized by Direct Copolycondensation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
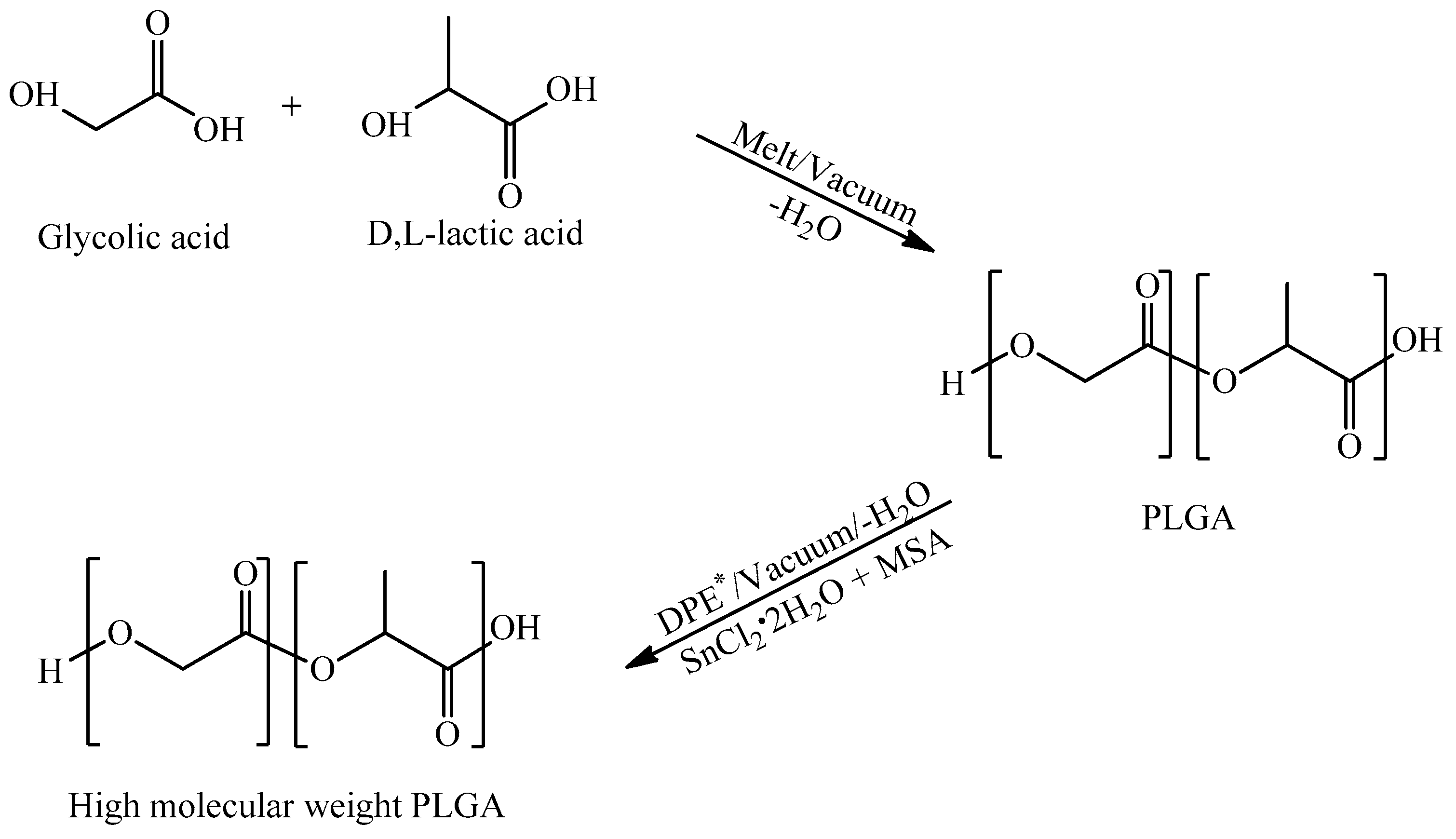
2.2. Synthesis of Copolymer
2.3. Characterization
2.4. Polymer Film Preparation
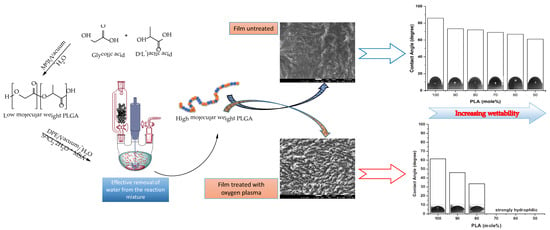
2.5. Oxygen Plasma Treatments
2.6. Water Contact Angle Measurement of PLGA Copolymer Films
2.7. Scanning Electron Microscopy of PLGA Films
2.8. Measurements of Specific Optical Rotation
3. Results and Discussion
3.1. Synthesis of PLA and PLGA Copolymers
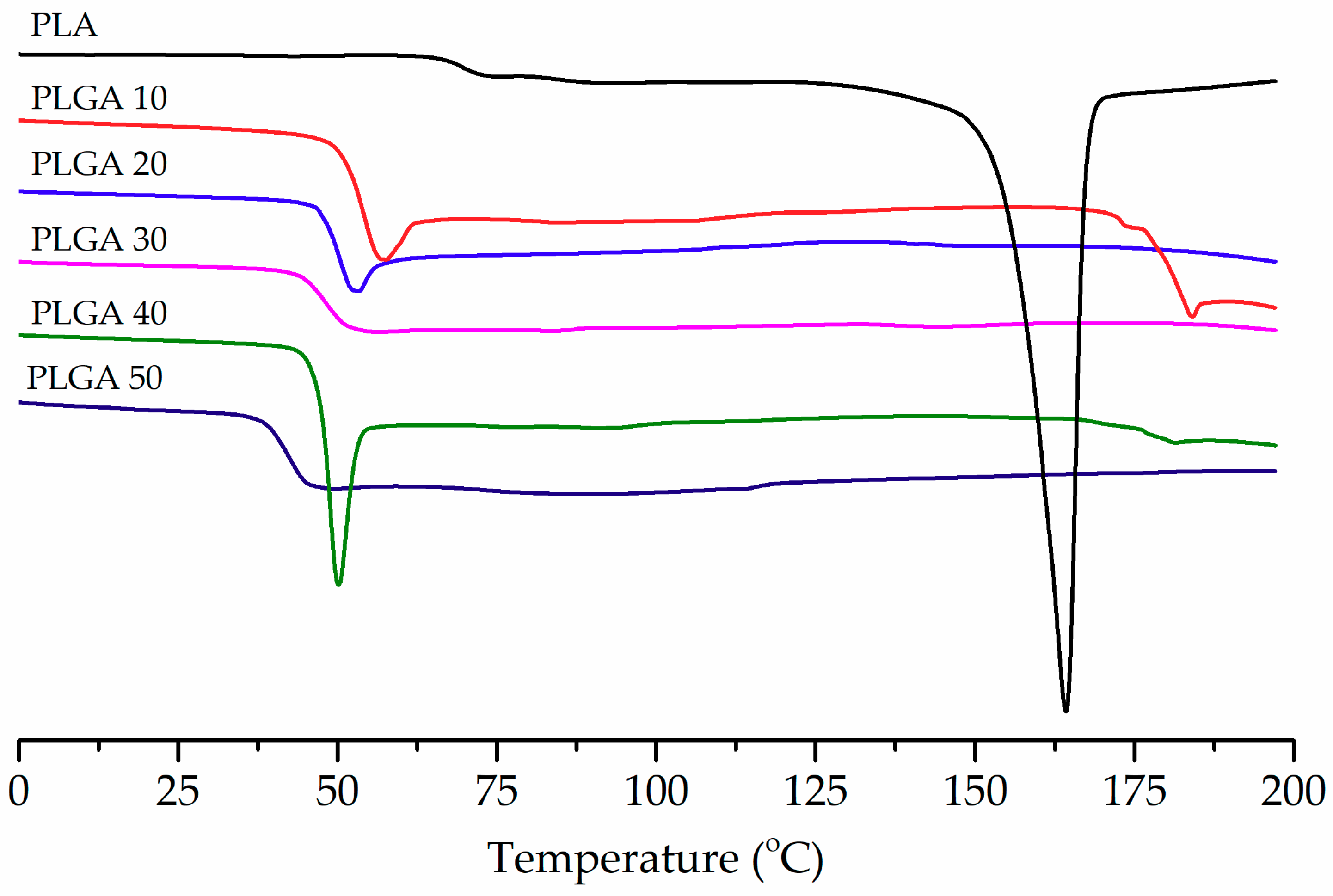
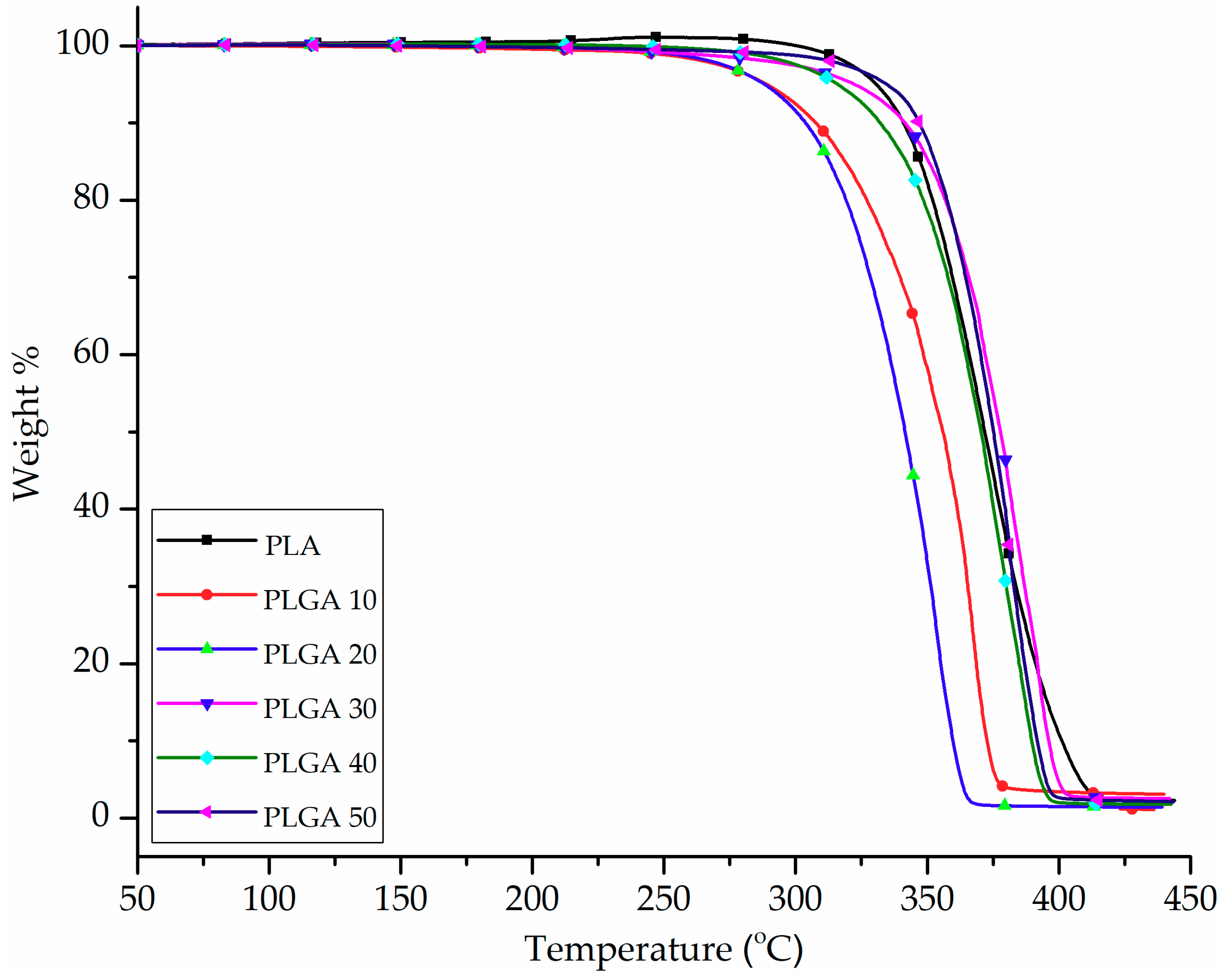
3.2. Thermal Properties of PLA and PLGA Copolymers
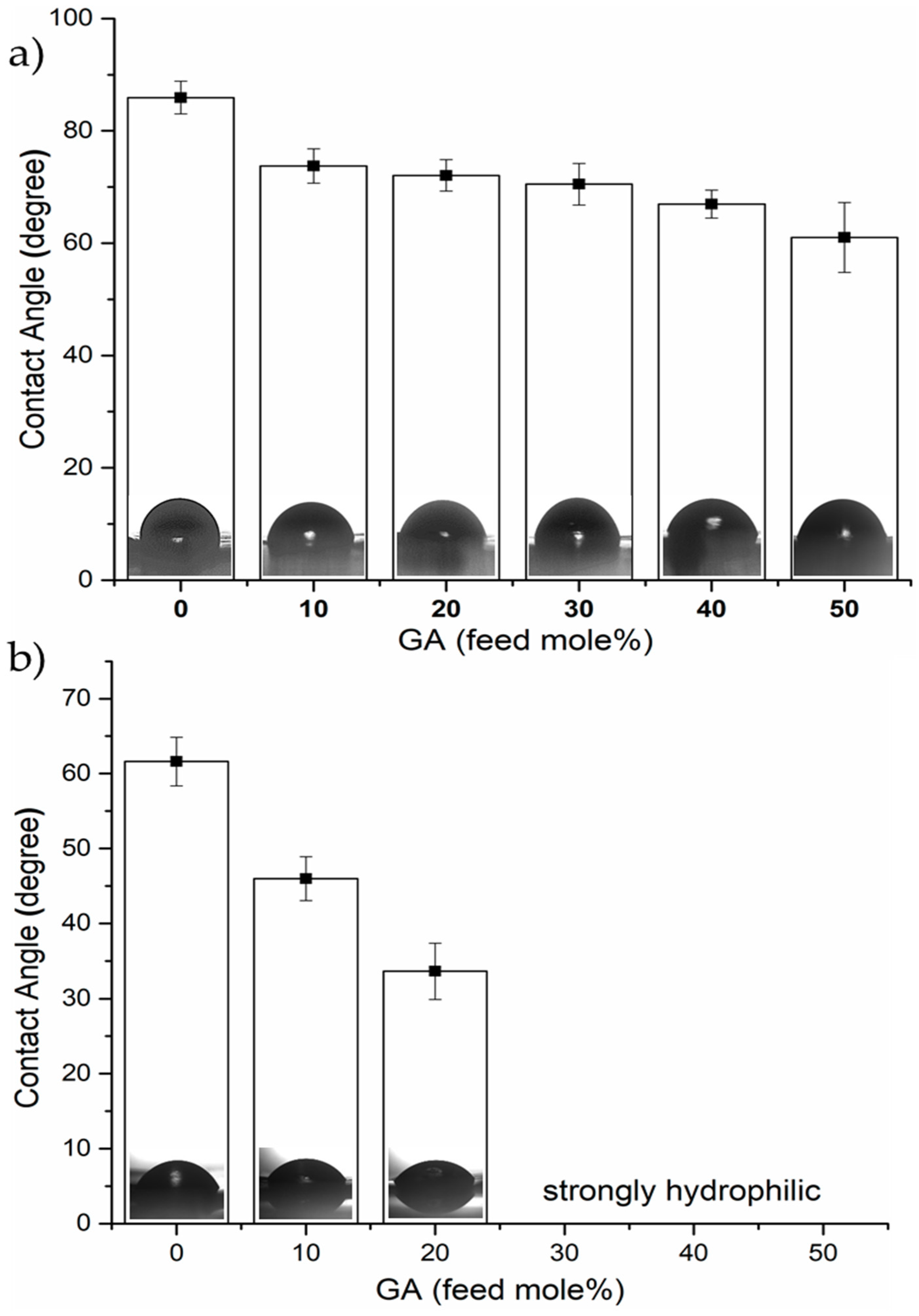
3.3. Surface Analysis and Wettability Results of Polymer Films
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Katti, D.; Lakshmi, S.; Langer, R.; Laurencin, C. Toxicity, biodegradation and elimination of polyanhydrides. Adv. Drug. Deliv. Rev. 2002, 54, 933–961. [Google Scholar] [CrossRef]
- Burg, K. Poly(α-ester)s. In Natural and Synthetic Biomedical Polymers, 1st ed.; Sangamesh, G., Kumbar, G.S., Laurencin, C.T., Deng, M., Eds.; Elsevier Inc.: San Diego, CA, USA, 2014; pp. 115–121. ISBN 978-0-12-396983-5. [Google Scholar]
- Singh, V.; Tiwari, M. Structure-processing-property relationship of poly(Glycolic Acid) for drug delivery systems 1: Synthesis and catalysis. Int. J. Polym. Sci. 2010, 2010, 536–543. [Google Scholar] [CrossRef]
- Ayyoob, M.; Lee, D.H.; Kim, J.H.; Nam, S.W.; Kim, Y.J. Synthesis of poly(glycolic acids) via solution polycondensation and investigation of their thermal degradation behaviors. Fiber. Polym. 2017, 18, 407–415. [Google Scholar] [CrossRef]
- De Oca, H.M.; Ward, I.M. Structure and mechanical properties of PGA crystals and fibres. Polymer 2006, 47, 7070–7077. [Google Scholar] [CrossRef]
- Ramdhanie, L.I.; Aubuchon, S.R.; Boland, E.D.; Knapp, D.C.; Barnes, C.P.; Simpson, D.G.; Wnek, G.E.; Bowlin, G.L. Thermal and Mechanical Characterization of Electrospun Blends of Poly(lactic acid) and Poly(glycolic acid). Polym. J. 2006, 38, 1137–1145. [Google Scholar] [CrossRef] [Green Version]
- Ueda, H.; Tabata, Y. Polyhydroxyalkanonate derivatives in current clinical applications and trials. Adv. Drug Deliv. Rev. 2003, 55, 501–518. [Google Scholar] [CrossRef]
- Suuronen, R.; Pohjonen, T.; Hietanen, J.; Lindqvist, C. A 5-year in vitro and in vivo study of the biodegradation of polylactide plates. J. Oral Maxillofac. Surg. 1998, 56, 604–614. [Google Scholar] [CrossRef]
- François, S.; Chakfé, N.; Durand, B.; Laroche, G. A poly(l-lactic acid) nanofibre mesh scaffold for endothelial cells on vascular prostheses. Acta Biomater. 2009, 5, 2418–2428. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Yamaoka, H.; Nishizawa, S.; Nagata, S.; Ogasawara, T.; Asawa, Y.; Fujihara, Y.; Takato, T.; Hoshi, K. The optimization of porous polymeric scaffolds for chondrocyte/atelocollagen based tissue-engineered cartilage. Biomaterials 2010, 31, 4506–4516. [Google Scholar] [CrossRef] [PubMed]
- Schofer, M.D.; Fuchs-Winkelmann, S.; Gräbedünkel, C.; Wack, C.; Dersch, R.; Rudisile, M.; Wendorff, J.H.; Greiner, A.; Paletta, J.R.; Boudriot, U. Influence of poly(l-lactic acid) nanofibers and BMP-2-containing poly(l-lactic acid) nanofibers on growth and osteogenic differentiation of human mesenchymal stem cells. Sci. World J. 2008, 8, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.-C.; Liu, B.-Y.; Liu, C.-M.; Chou, H.-H.; Ho, M.-H.; Liu, H.-C.; Wang, D.-M.; Hou, L.-T. Bone tissue engineering with novel rhBMP2-PLLA composite scaffolds. J. Biomed. Mater. Res. Part A 2007, 81A, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Hu, J.; Ma, P.X. The engineering of patient-specific, anatomically shaped, digits. Biomaterials 2009, 30, 2735–2740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunatillake, P.A. Biodegradable synthetic polymers for tissue engineering. Eur. Cells Mater. 2003, 5, 1–16. [Google Scholar] [CrossRef]
- Miller, R.A.; Brady, J.M.; Cutright, D.E. Degradation rates of oral resorbable implants (polylactates and polyglycolates): Rate modification with changes in PLA/PGA copolymer ratios. J. Biomed. Mater. Res. 1977, 11, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Jain, R.A. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials 2000, 21, 2475–2490. [Google Scholar] [CrossRef]
- Reul, G.J. Use of vicryl (polyglactin 910) sutures in general surgical and cardiothoracic procedures. Am. J. Surg. 1977, 134, 297–299. [Google Scholar] [CrossRef]
- Conn, J.; Oyasu, R.; Welsh, M.; Beal, J.M. Vicryl (polyglactin 910) synthetic absorbable sutures. Am. J. Surg. 1974, 128, 19–23. [Google Scholar] [CrossRef]
- Heya, T.; Okada, H.; Ogawa, Y.; Toguchi, H. Factors influencing the profiles of TRH release from copoly(d,l-lactic/glycolic acid) microspheres. Int. J. Pharm. 1991, 12, 199–205. [Google Scholar]
- Phua, K.K.L.; Roberts, E.R.H.; Leong, K.W. Degradable polymers. In Comprehensive Biometrials; Ducheyne, P., Ed.; Elsevier: Oxford, UK, 2011; pp. 381–415. [Google Scholar]
- Gilding, D.K.; Reed, A.M. Biodegradable polymers for use in surgery—Polyglycolic/poly(actic acid) homo- and copolymers: 1. Polymer 1979, 20, 1459–1464. [Google Scholar] [CrossRef]
- Amass, W.; Amass, A.; Tighe, B. A review of biodegradable polymers: Uses, current developments in the synthesis and characterization of biodegradable polyesters, blends of biodegradable polymers and recent advances in biodegradation studies. Polym. Int. 1998, 47, 89–144. [Google Scholar] [CrossRef]
- Daniels, A.U.; Chang, M.K.O.; Andriano, K.P.; Heller, J. Mechanical properties of biodegradable polymers and composites proposed for internal fixation of bone. J. Appl. Biomater. 1990, 1, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, K. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials 1996, 17, 93–102. [Google Scholar] [CrossRef]
- Tsuji, H.; Tashiro, K.; Bouapao, L.; Narita, J. Polyglycolide as a biodegradable nucleating agent for poly(l-lactide). Macromol. Mater. Eng. 2008, 293, 947–951. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Stereocomplex formation between enantiomeric poly(lactic acid)s. X. Binary blends from poly(d-lactide-CO-glycolide) and poly(l-lactide-CO-glycolide). J. Appl. Polym. Sci. 1994, 53, 1061–1071. [Google Scholar] [CrossRef]
- Karahaliloğlu, Z.; Ercan, B.; Chung, S.; Taylor, E.; Denkbaş, E.B.; Webster, T.J. Nanostructured anti-bacterial poly-lactic-co-glycolic acid films for skin tissue engineering applications. J. Biomed. Mater. Res. Part A 2014, 102, 4598–4608. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Sato, T.; Ohgushi, H.; Ushida, T.; Tateishi, T.; Tanaka, J. Culturing of skin fibroblasts in a thin PLGA–collagen hybrid mesh. Biomaterials 2005, 26, 2559–2566. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi-Avalshahr, A.; Nokhasteh, S.; Molavi, A.M.; Khorsand-Ghayeni, M.; Mahdavi-Shahri, M. Synthesis and characterization of collagen/PLGA biodegradable skin scaffold fibers. Regen. Biomater. 2017, 4, 309–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacelli, S.; Manoharan, V.; Desalvo, A.; Lomis, N.; Jodha, K.S.; Prakash, S.; Paul, A. Tailoring biomaterial surface properties to modulate host-implant interactions: Implication in cardiovascular and bone therapy. J. Mater. Chem. B 2016, 4, 1586–1599. [Google Scholar] [CrossRef] [PubMed]
- Spriano, S.; Sarath, C.V.; Cochis, A.; Uberti, F.; Rimondini, L.; Bertone, E.; Vitale, E.; Scolaro, C.; Farrari, M.; Crisano, F.; et al. How do wettability, zeta potential and hydroxylation degree affect the biological response of biomaterials? Mater. Sci. Eng. C 2017, 74, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Kang, S.G.; Lee, J.H. Degradation behavior of hydrophilized PLGA scaffolds prepared by melt-molding particulate-leaching method: Comparison with control hydrophobic one. J. Mater. Sci. Mater. Med. 2006, 17, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Janorkar, A.V.; Metters, A.T.; Hirt, D.E. Modification of Poly(lactic acid) Films: Enhanced Wettability from Surface-Confined Photografting and Increased Degradation Rate Due to an Artifact of the Photografting Process. Macromolecules 2004, 37, 9151–9159. [Google Scholar] [CrossRef]
- Therin, M.; Christel, P.; Li, S.; Garreau, H.; Vert, M. In vivo degradation of massive poly(α-hydroxy acids): Validation of In vitro findings. Biomaterials 1992, 13, 594–600. [Google Scholar] [CrossRef]
- Paragkumar, N.T.; Edith, D.; Six, J.-L. Surface characteristics of PLA and PLGA films. Appl. Surf. Sci. 2006, 253, 2758–2764. [Google Scholar] [CrossRef]
- Kiss, É.; Bertóti, I.; Vargha-Butler, E.I. XPS and wettability characterization of modified poly(lactic acid) and poly(lactic/glycolic acid) films. J. Colloid. Interface Sci. 2002, 245, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, Y.; Wang, F.; Wang, J. Syntheses of Poly(lactic acid-co-glycolic acid) Serial Biodegradable Polymer Materials via Direct Melt Polycondensation and Their Characterization. J. Appl. Polym. Sci. 2005, 99, 244–252. [Google Scholar] [CrossRef]
- Ouyang, C.; Ma, G.; Zhao, S.; Wang, L.; Wu, L.; Wang, Y.; Song, C.; Zhang, Z. Preparation and characterization of the molecular weight controllable poly(lactide-co-glycolide). Polym. Bull. 2010, 67, 793–803. [Google Scholar] [CrossRef]
- Kenley, R.A.; Lee, M.O.; Mahoney, T.R.; Sanders, L.M. Poly(lactide-co-glycolide) decomposition kinetics in vivo and in vitro. Macromolecules 1987, 20, 2398–2403. [Google Scholar] [CrossRef]
- Park, P.I.P.; Jonnalagadda, S. Predictors of glass transition in the biodegradable polylactide and poly-lactide-co-glycolide polymers. J. Appl. Polym. Sci. 2006, 100, 1983–1987. [Google Scholar] [CrossRef]
- Nieuwenhuis, J. Synthesis of polylactides, polyglycolides and their copolymers. Clin. Mater. 1992, 10, 59–67. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Stereocomplex Formation between Enantiomeric Poly(lactic acid)s. 6. Binary Blends from Copolymers. Macromolecules 1992, 25, 5719–5723. [Google Scholar] [CrossRef]
- Tsuji, H.; Kikkawa, K.; Arakawa, Y. Cocrystallization of monomer units of biobased and biodegradable Poly(l-lactic acid-co-glycolic acid) random copolymers. Polym. J. 2018. [Google Scholar] [CrossRef]
- Brostow, W.; Chiu, R.; Kalogeras, I.M.; Vassilikou-Dova, A. Prediction of glass transition temperatures: Binary blends and copolymers. Mater. Lett. 2008, 62, 3152–3155. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Yang, H.W.; Deri, F.; Ko, Y.G. Properties and medical applications of polylactic acid: A review. Express Polym. Lett. 2015, 9, 435–455. [Google Scholar] [CrossRef]
- Ljungberg, N.; Wesslén, B. The effects of plasticizers on the dynamic mechanical and thermal properties of poly(lactic acid). J. Appl. Polym. Sci. 2002, 86, 1227–1234. [Google Scholar] [CrossRef]
- Engelberg, I.; Kohn, J. Physico-mechanical properties of degradable polymers used in medical applications: A comparative study. Biomaterials 1991, 12, 292–304. [Google Scholar] [CrossRef]
- Wang, L.; Feng, C.; Zhou, D.; Shao, J.; Hou, H.; Li, G. The crystallization and phase transition behaviors of asymmetric PLLA/PDLA blends: From the amorphous state. Polym. Cryst. 2018, 1, e10006. [Google Scholar] [CrossRef]
- Marubayashi, H.; Asai, S.; Hikima, T.; Takata, M.; Iwata, T. Biobased Copolymers Composed of l-Lactic Acid and Side-Chain-Substituted Lactic Acids: Synthesis, Properties, and Solid-State Structure. Macromol. Chem. Phys. 2013, 214, 2546–2561. [Google Scholar] [CrossRef]
- Lan, P.; Zhang, Y.; Gao, Q.; Shao, H.; Hu, X. Studies on the Synthesis and Thermal Properties of Copoly(l-lactic acid/glycolic acid) by Direct Melt Polycondensation. J. Appl. Polym. Sci. 2004, 92, 2163–2168. [Google Scholar] [CrossRef]
- Palacios, J.; Albano, C.; González, G.; Castillo, R.V.; Karam, A.; Covis, M. Characterization and thermal degradation of poly(d,l-lactide-co-glycolide) composites with nanofillers. Polym. Eng. Sci. 2013, 53, 1414–1429. [Google Scholar] [CrossRef]
- Yin, H.; Wang, R.; Ge, H.; Zhang, X.; Zhu, Z. Synthesis and structure control of l-lactic acid-glycolic acid copolymer by homo-copolymerization. J. Appl. Polym. Sci. 2015, 132, 41566. [Google Scholar] [CrossRef]
- Min, B.; Kim, S.H.; Kim, S.H.; Kwon, S.; Kim, H.J.; Kim, W.G. Free acid effect and NMR study of glycolide. Bull. Korean Chem. Soc. 2000, 21, 635–637. [Google Scholar]
- Mader, M.; Jérôme, V.; Freitag, R.; Agarwal, S.; Greiner, A. Ultraporous, compressible, wettable polylactide/polycaprolactone sponges for tissue engineering. Biomacromolecules 2018, 19, 1663–1673. [Google Scholar] [CrossRef] [PubMed]

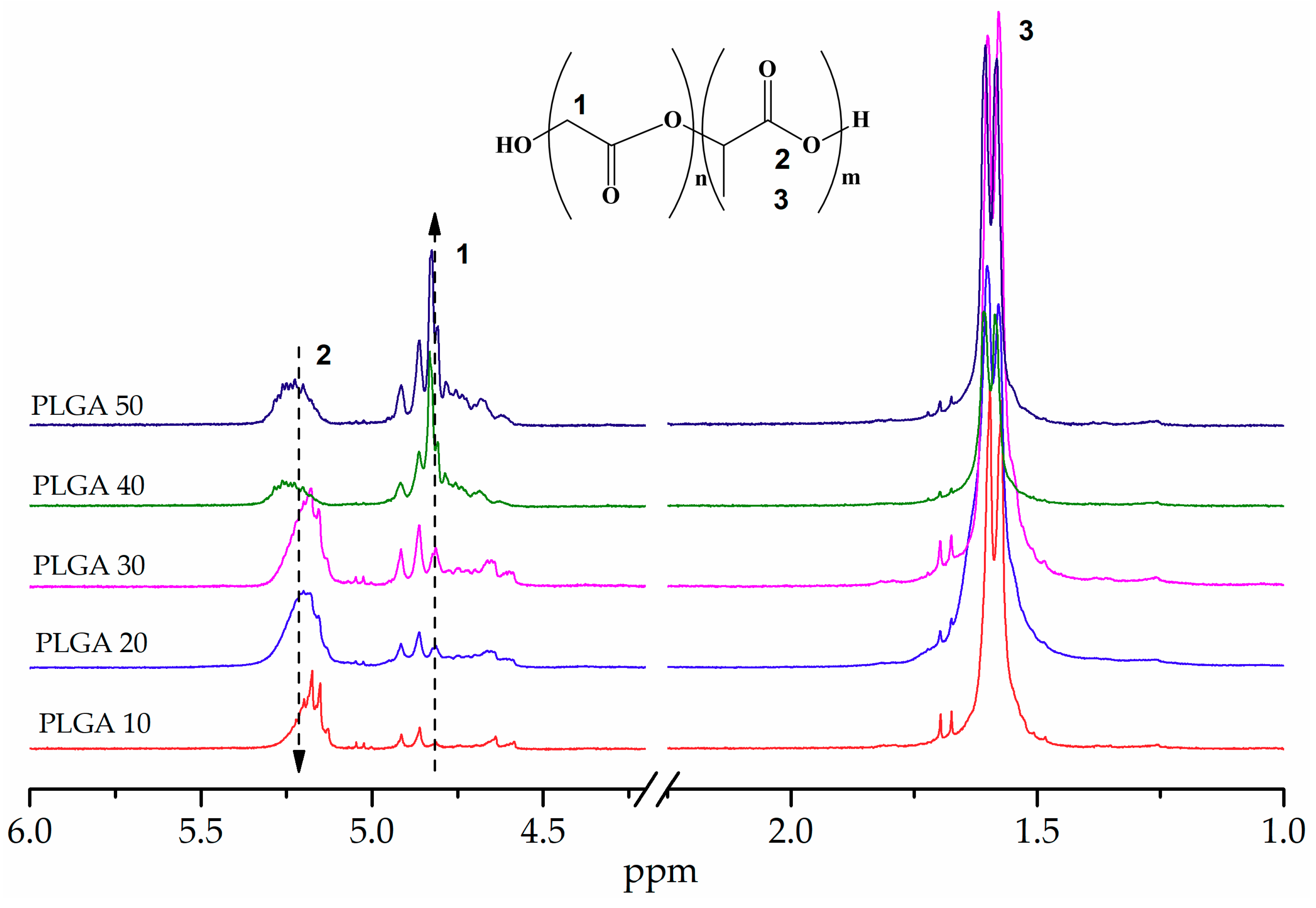


| Lactic Acid/Glycolic Acid (mol. ratio) | η * (30 °C/CHCl3) | Solubility (chloroform) | |
|---|---|---|---|
| PLA 100/0 | 100/0 | 0.11 | ++++ |
| PLGA 99/01 | 99/01 | 0.12 | ++++ |
| PLGA 98/02 | 98/02 | 0.11 | ++++ |
| PLGA 95/05 | 95/05 | 0.11 | ++++ |
| PLGA 90/10 | 90/10 | 0.11 | ++++ |
| PLGA 80/20 | 80/20 | 0.12 | ++++ |
| PLGA 65/35 | 65/35 | 0.12 | +++ |
| PLGA 50/50 | 50/50 | 0.11 | +++ |
| PLGA 35/65 | 35/65 | Not soluble | ++ |
| PLGA 20/80 | 20/80 | Not soluble | + |
| PLGA 10/90 | 10/90 | Not soluble | − |
| PLGA 05/95 | 05/95 | Not soluble | − |
| PLGA 02/98 | 02/98 | Not soluble | − |
| PLGA 01/99 | 01/99 | Not soluble | − |
| PLGA 0/100 | 0/100 | Not soluble | − |
| Sample Code | Yield (%) | [η] * (dL/g) | Mv (g/mol.) | Tg (°C) | WCA a (degrees) | Film Thickness (μm) | Glycolic Acid Contents (mol.%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Untreated | Treated | In the Feed | In the Copolymer | |||||||
| PLA | 91.3 | −138 | 0.83 | 130,000 | 70 | 85.9 | 61.6 | 130 | 0 | 0 |
| PLGA 10 | 82.6 | −135 | 0.65 | 94,000 | 55 | 73.3 | 46.0 | 120 | 10 | 16.7 |
| PLGA 20 | 88.4 | −124 | 0.62 | 88,000 | 51 | 72.0 | 33.6 | 130 | 20 | 22.1 |
| PLGA 30 | 89.2 | −108 | 0.81 | 125,000 | 48 | 69.0 | N/D | 120 | 30 | 31.9 |
| PLGA 40 | 83.8 | −85 | 0.78 | 120,000 | 48 | 66.8 | N/D | 150 | 40 | 47.9 |
| PLGA 50 | 84.7 | −54 | 0.75 | 113,000 | 44 | 61.0 | N/D | 170 | 50 | 55.5 |
| PLGA 50 | 81.6 | N/D | 0.86 | 133,000 | 46 | 62.1 | N/D | 160 | 50 | 61.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayyoob, M.; Kim, Y.J. Effect of Chemical Composition Variant and Oxygen Plasma Treatments on the Wettability of PLGA Thin Films, Synthesized by Direct Copolycondensation. Polymers 2018, 10, 1132. https://doi.org/10.3390/polym10101132
Ayyoob M, Kim YJ. Effect of Chemical Composition Variant and Oxygen Plasma Treatments on the Wettability of PLGA Thin Films, Synthesized by Direct Copolycondensation. Polymers. 2018; 10(10):1132. https://doi.org/10.3390/polym10101132
Chicago/Turabian StyleAyyoob, Muhammad, and Young Jun Kim. 2018. "Effect of Chemical Composition Variant and Oxygen Plasma Treatments on the Wettability of PLGA Thin Films, Synthesized by Direct Copolycondensation" Polymers 10, no. 10: 1132. https://doi.org/10.3390/polym10101132
APA StyleAyyoob, M., & Kim, Y. J. (2018). Effect of Chemical Composition Variant and Oxygen Plasma Treatments on the Wettability of PLGA Thin Films, Synthesized by Direct Copolycondensation. Polymers, 10(10), 1132. https://doi.org/10.3390/polym10101132