Preparation of Crystallites for Oriented Poly(Lactic Acid) Films Using a Casting Method under a Magnetic Field
Abstract
:1. Introduction
2. Experimental Method
2.1. Materials
2.2. Synthesis of PLLA
2.3. Synthesis of [bmini]dbp
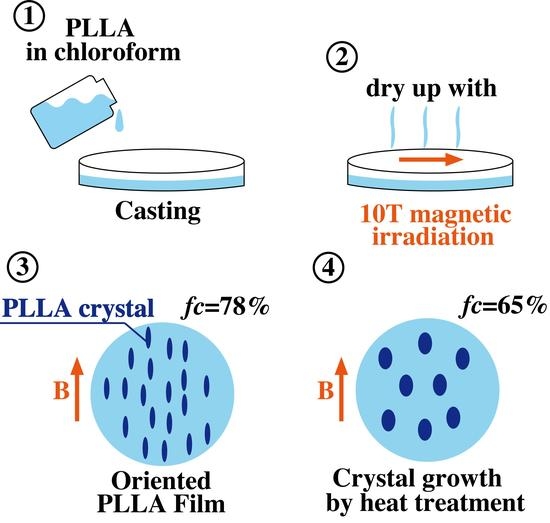
2.4. Preparation of Oriented PLLA Films
3. Characterization
4. Results and Discussion
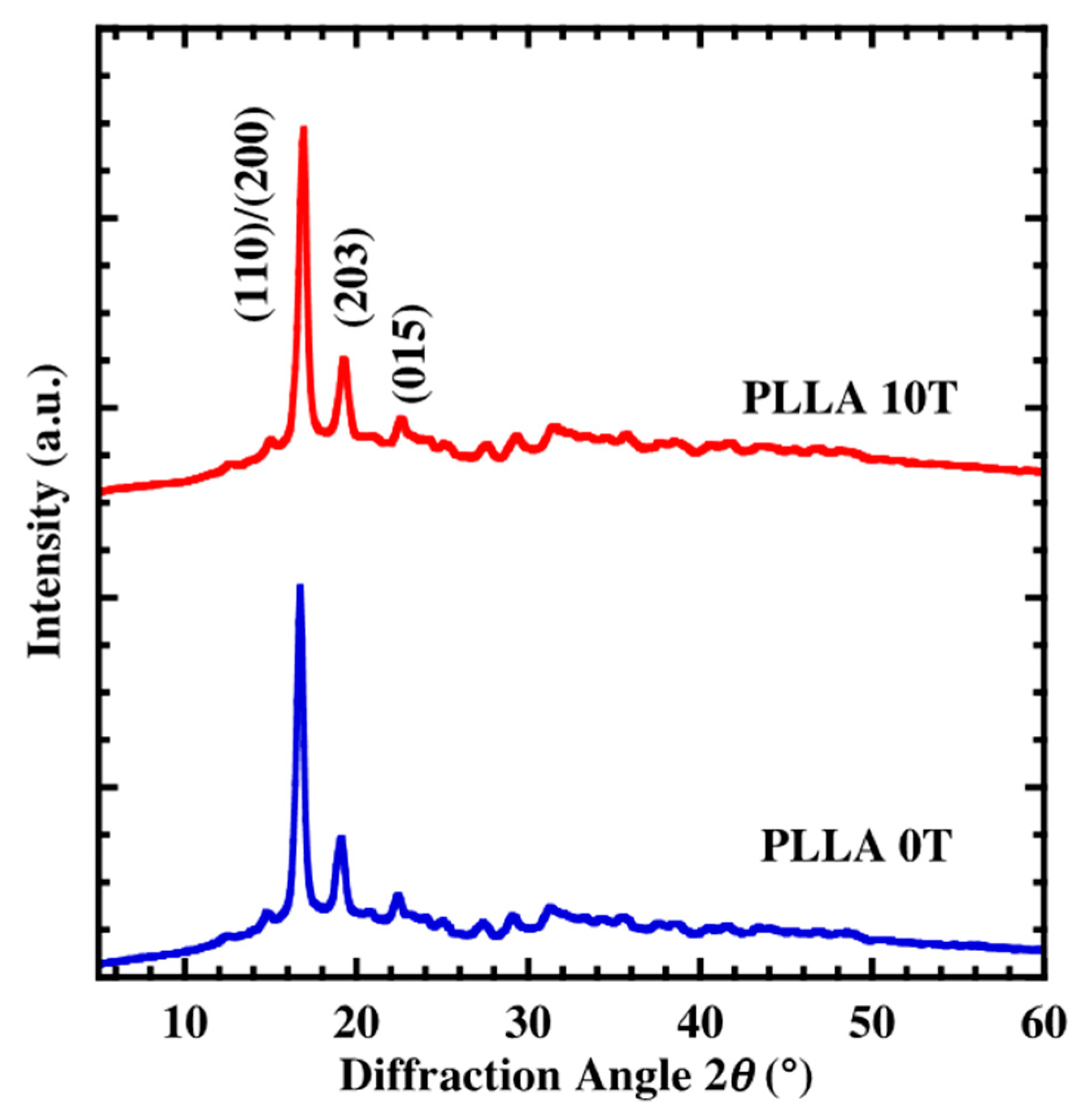
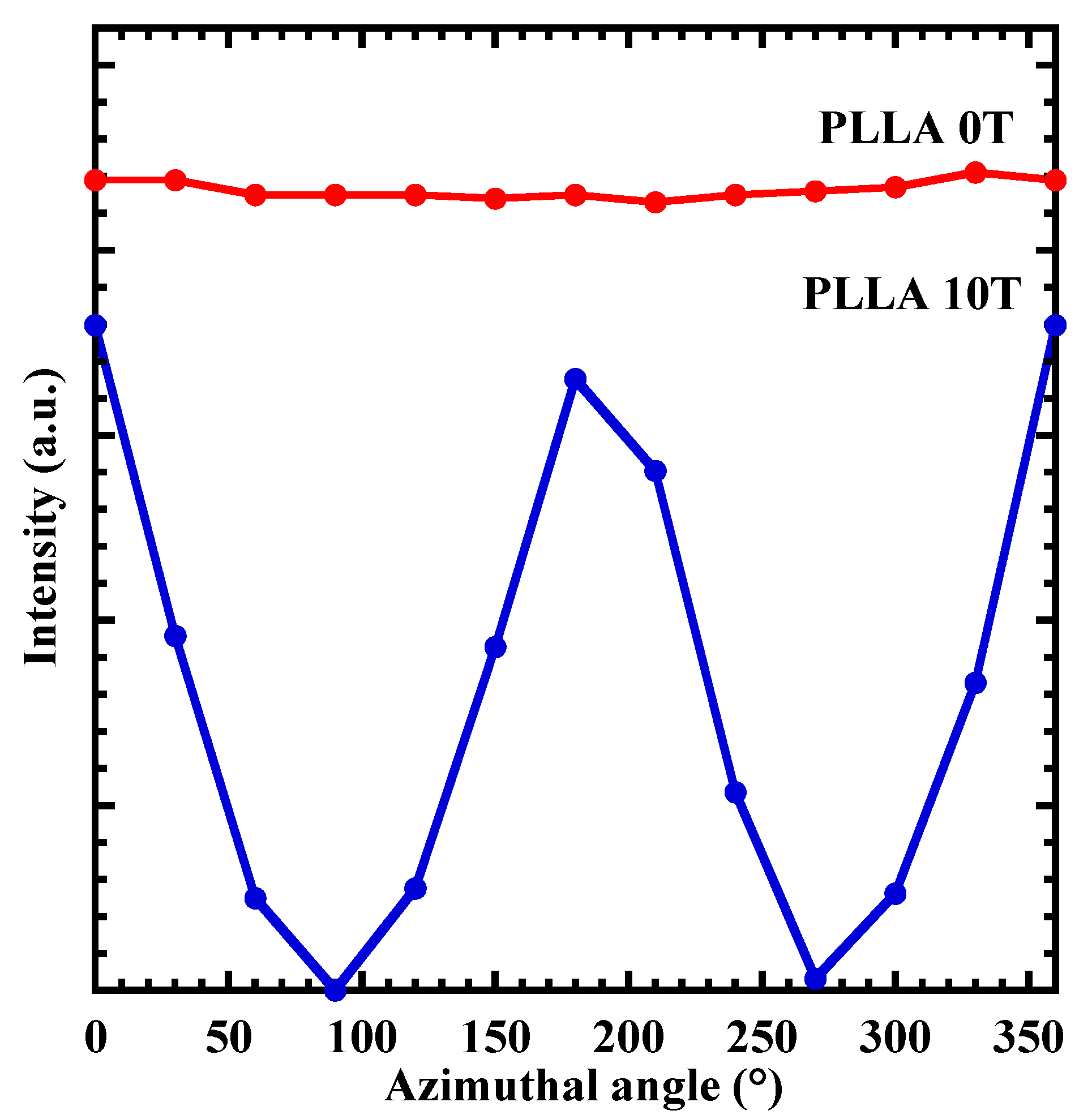
4.1. Characterization of Oriented PLLA Film
4.2. Crystal Growth of Oriented Film
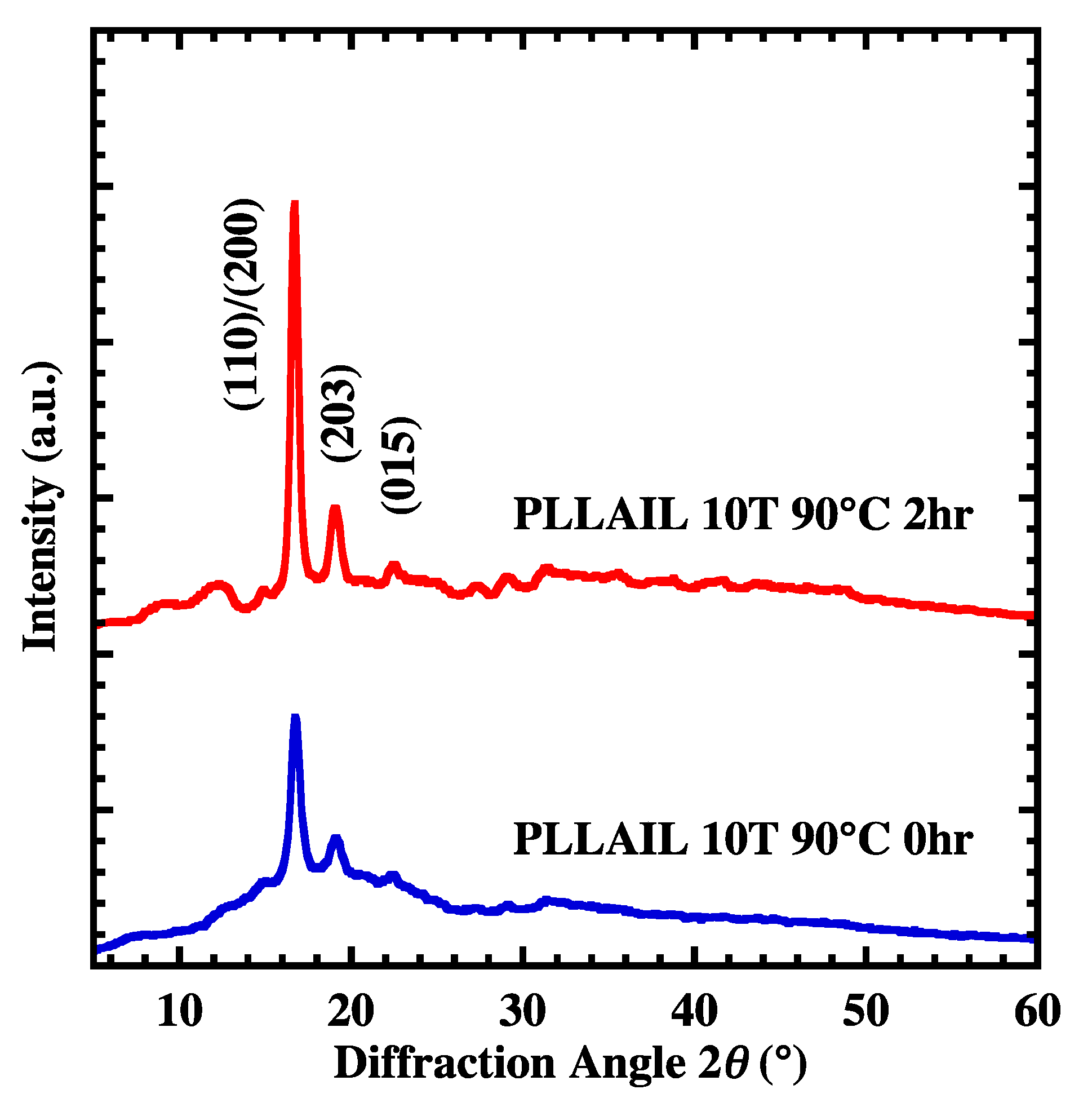
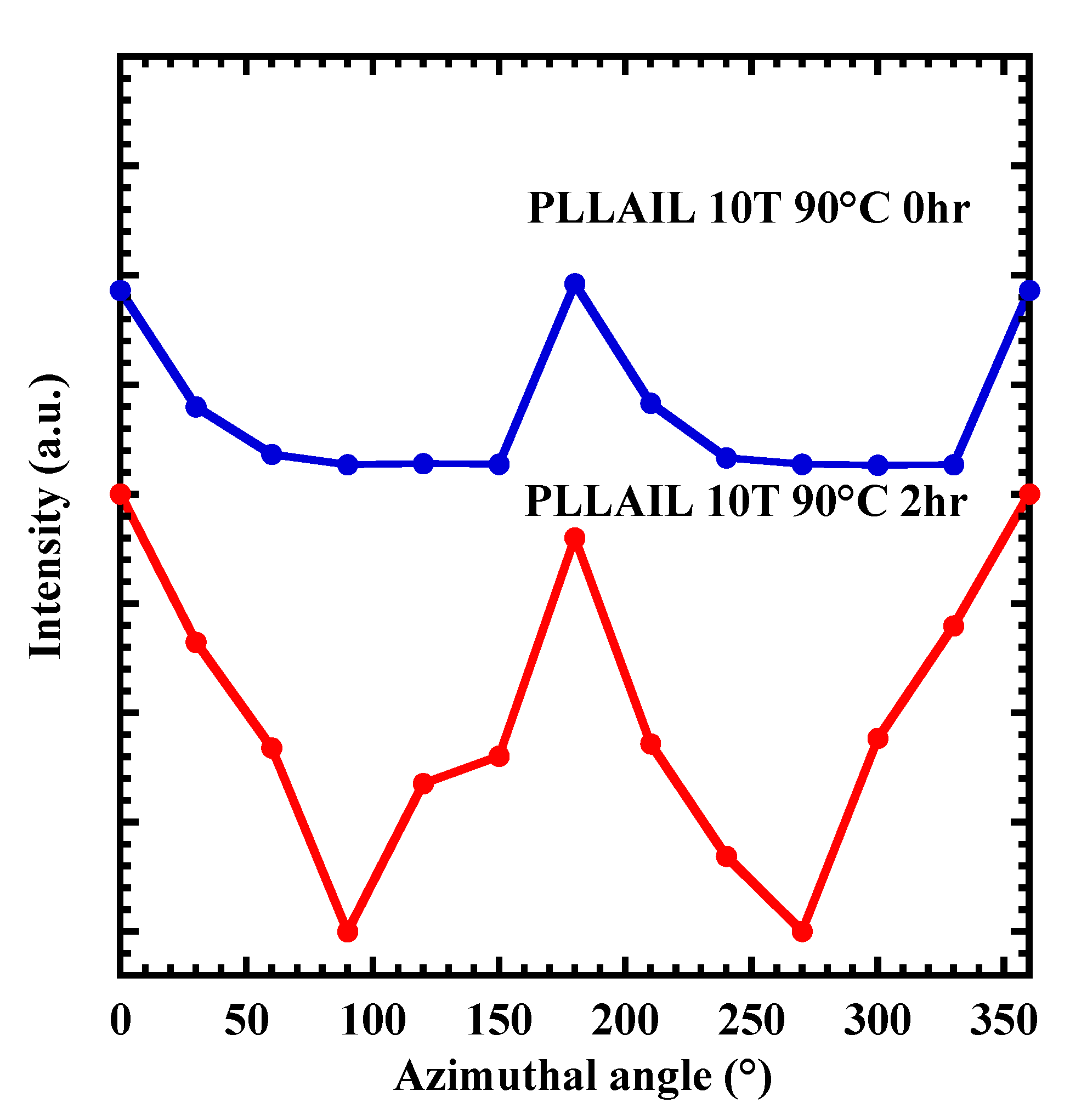
4.3. Crystal Growth and Orientation of [bmim]dbp Composite PLLA
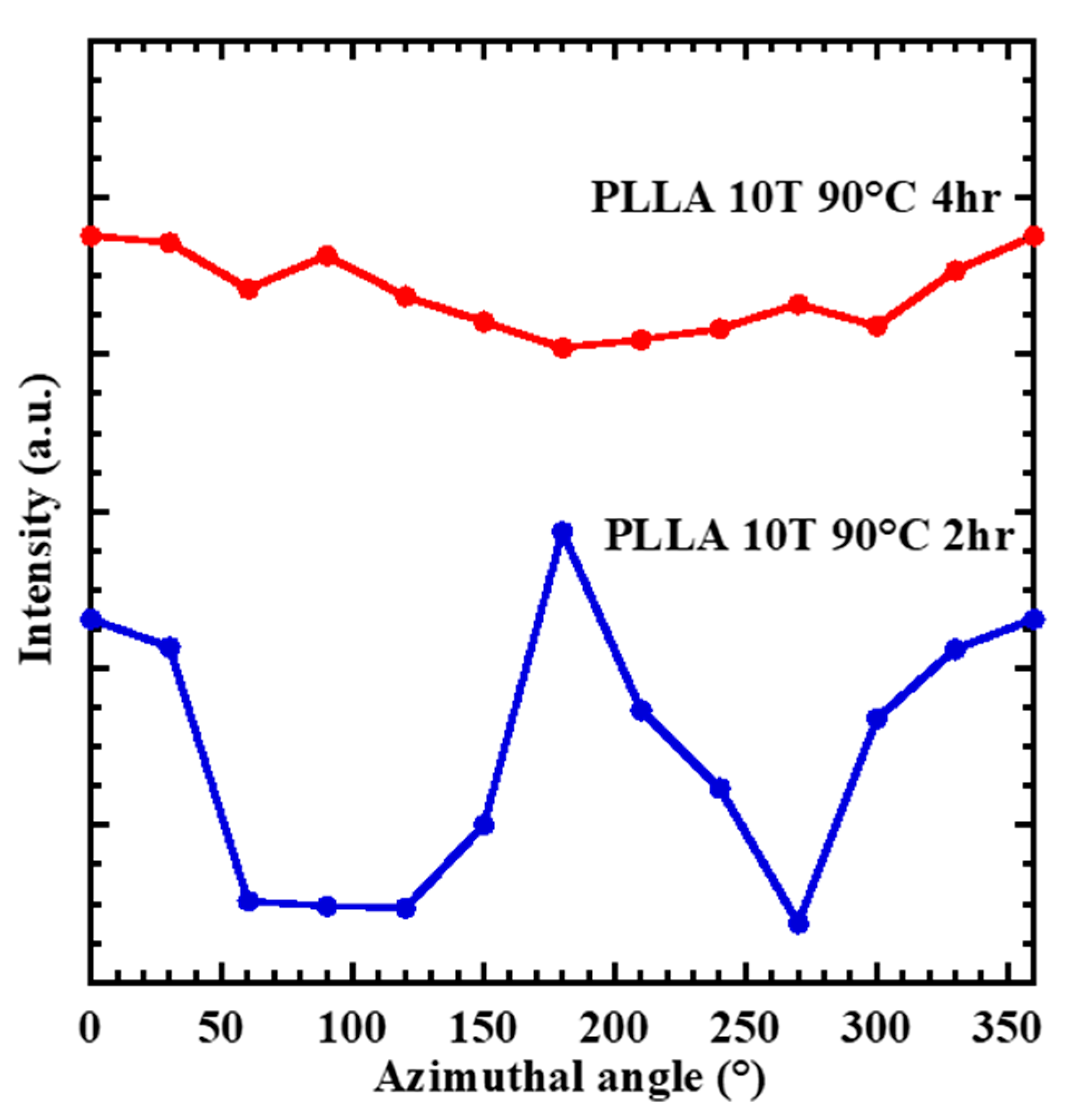
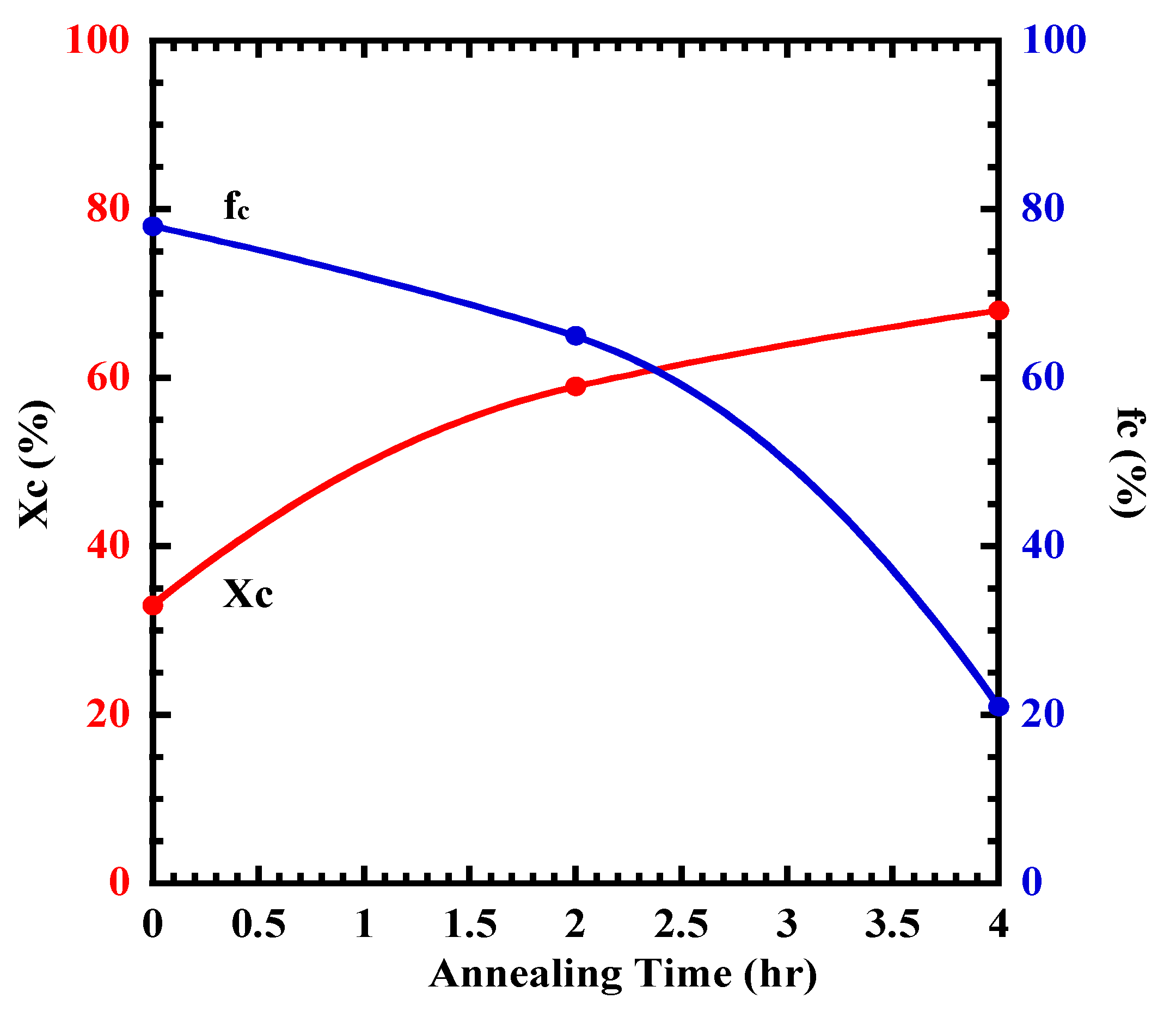
4.4. Changing the Crystal Morphology of Oriented PLLA and PLLAIL with Heating Treatment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ikada, Y.; Tsuji, H. Biodegradable polyesters for medical and ecological applications. Macromol. Rapid Commun. 2000, 21, 117–132. [Google Scholar] [CrossRef]
- Curiy, E.J.; Ke, K.; Chorsi, M.T.; Wrobel, K.S.; Miller, A.N.; Patel, A.; Kim, I.; Feng, J.; Yue, L.; Wu, Q.; et al. Biodegradable Piezoelectric Force Sensor. Proc. Natl. Acad. Sci. USA 2018, 115, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Talemi, P.; Delaigue, M.; Murphy, P.; Fabretto, M. Flexible Polymer-on-Polymer Architecture for Piezo/Pyroelectric Energy Harvesting. ACS Appl. Mater. Interfaces 2015, 7, 8465–8471. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Kawamura, H.; Kageyama, K.; Tajitsu, Y. Film Sensor Device Fabricated by a Piezoelectric Poly(l-lactic acid) Film. Jpn. J. Appl. Phys. 2012, 51, 09LD14. [Google Scholar] [CrossRef]
- Hoogsteen, W.; Postema, A.R.; Pennings, A.J.; Brinke, G.T.; Zugenmaier, P. Crystal structure, conformation and morphology of solution-spun poly(l-lactide) fibers. Macromolecules 1990, 31, 634–642. [Google Scholar] [CrossRef]
- Puiggali, J.; Ikada, Y.; Tsuji, H.; Cartier, L.; Okihara, T.; Lotz, B. The frustrated structure of poly(l-lactide). Polymer (Guildf) 2000, 47, 8921–8930. [Google Scholar] [CrossRef]
- Wasanasuk, K.; Tashiro, K.; Hanesaka, M.; Ohhara, T.; Kurihara, K.; Kuroki, R.; Tamada, T.; Ozeki, T.; Kanamoto, T. Crystal Structure Analysis of Poly(l-lactic Acid) r Form On the basis of the 2-Dimensional Wide-Angle Synchrotron X-ray and Neutron Diffraction Measurements. Macromolecules 2011, 44, 6441–6452. [Google Scholar] [CrossRef]
- Lai, W.C. Thermal Behavior and Crystal Structure of Poly(l-lactic acid) with 1,3:2,4-Dibenzylidene-d-sorbitol. J. Phys. Chem. B 2011, 115, 11029–11037. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, R.; Ikake, H.; Kurita, K.; Shimizu, S.; Kurumi, S.; Suzuki, K.; Takahashi, K.; Watanabe, K. Preparation of Poly(lactic acid) Films in a Magnetic Field and their Microstructure. Kobunshi Ronbunshu 2015, 72, 661–666. [Google Scholar] [CrossRef]
- Nakayama, R.; Ikake, H.; Kurita, K.; Shimizu, S.; Kurumi, S.; Suzuki, K. Effect of Amorphous Region on Magnetic Orientation of Poly(lactic acid) Blend Films with Different Molecular Weight. J. Magn. Soc. Jpn. 2017, 41, 66–69. [Google Scholar] [CrossRef] [Green Version]
- Wei, T.; Pang, S.; Xu, N.; Pan, L.; Zhang, Z.; Xu, R.; Ma, N.; Lin, Q. Crystallization behavior and isothermal crystallization kinetics of PLLA blended with ionic liquid, 1-butyl-3-methylimidazolium dibutylphosphate. J. Appl. Polym. Sci. 2015, 132, 1–11. [Google Scholar] [CrossRef]
- Nishida, H.; Mori, T.; Hoshihara, S.; Fan, Y.; Shirai, Y.; Lndo, T. Effect of tin on poly(l-lactic acid) pyrolysis. Polym. Degrad. Stab. 2003, 81, 515–523. [Google Scholar] [CrossRef]
- Mori, T.; Nishida, H.; Shirai, Y.; Lndo, T. Effects of chain end structures on pyrolysis of poly(l-lactic acid) containing Tin atoms. Polym. Degrad. Stab. 2004, 84, 243–251. [Google Scholar] [CrossRef]
- Nie, Y.; Li, C.X.; Sun, A.; Meng, H.; Wang, Z.H. Extractive Desulfurization of Gasoline Using Imidazolium-Based Phosphoric Ionic Liquids. Energy Fuels 2006, 20, 2083–2087. [Google Scholar] [CrossRef]
- Nie, Y.; Li, C.X.; Wang, Z.H. Extractive Desulfurization of Fuel Oil Using Alkylimidazole and Its Mixture with Dialkylphosphate Ionic Liquids. Ind. Eng. Chem. Res. 2007, 46, 5108–5112. [Google Scholar] [CrossRef]
- Schindler, A.; Harper, D. Polylactide. II. Viscosity–molecular weight relationships and unperturbed chain dimensions. J. Polym. Sci. Polym. Chem. Ed. 1979, 17, 2593–2599. [Google Scholar] [CrossRef]
- Pan, P.; Kai, W.; Zhu, B.; Dong, T.; Inoue, Y. Polymorphous Crystallization and Multiple Melting Behavior of Poly(l-lactide): Molecular Weight Dependence. Macromolecules 2007, 40, 6898–6905. [Google Scholar] [CrossRef]
- Hermans, J.J.; Hermans, P.H.; Vermaas, D.; Weidinger, A.P. Quantitative evaluation of orientation in cellulose fibres from the X-ray fibre diagram. Rec. Trav. Chim. Pays-Bas 1946, 65, 427–447. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Blends of isotactic and atactic poly (lactide) s: 2. Molecular-weight effects of atactic component on crystallization and morphology of equimolar blends from the melt. Polymer 1996, 37, 595–602. [Google Scholar] [CrossRef]
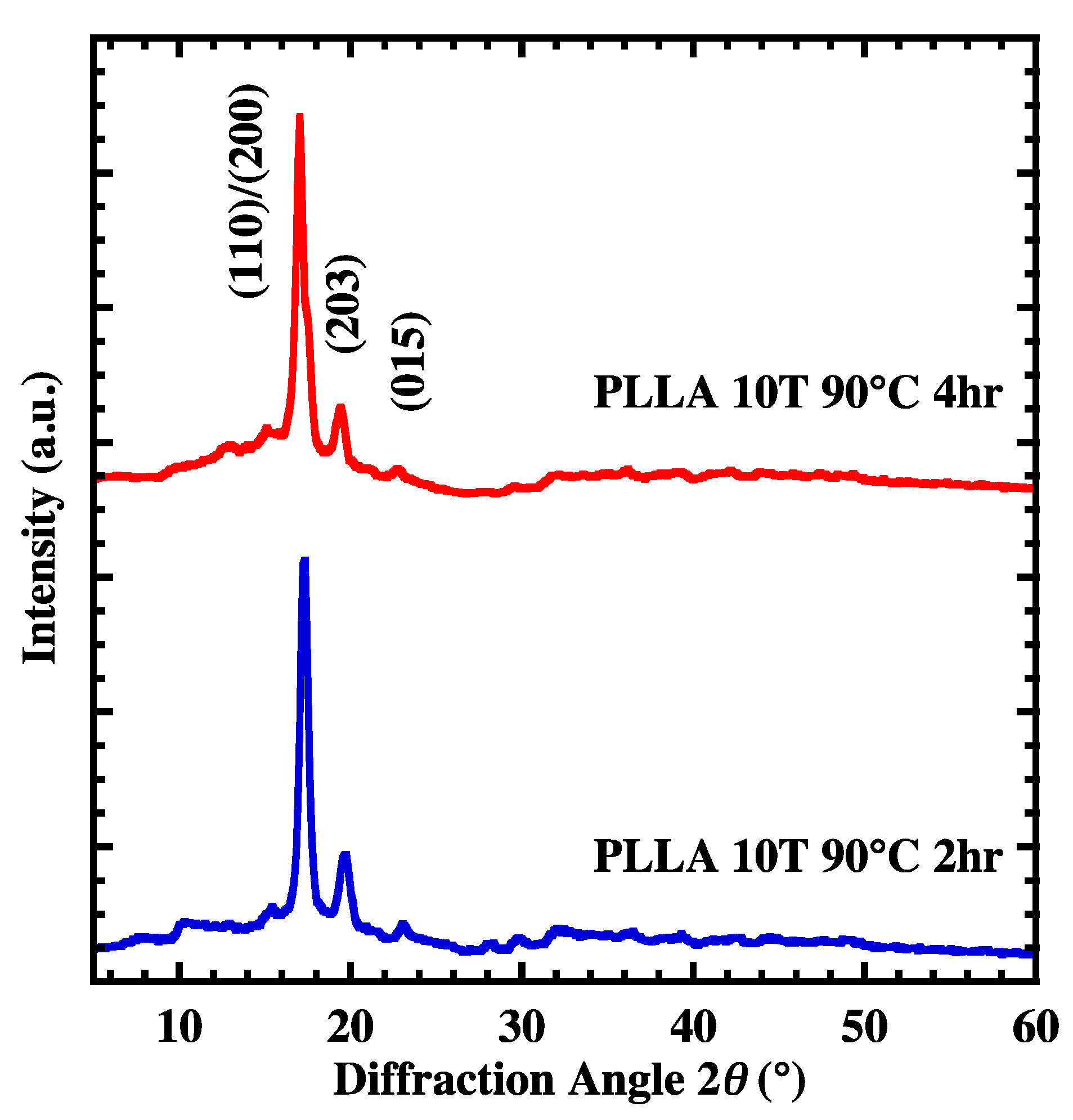
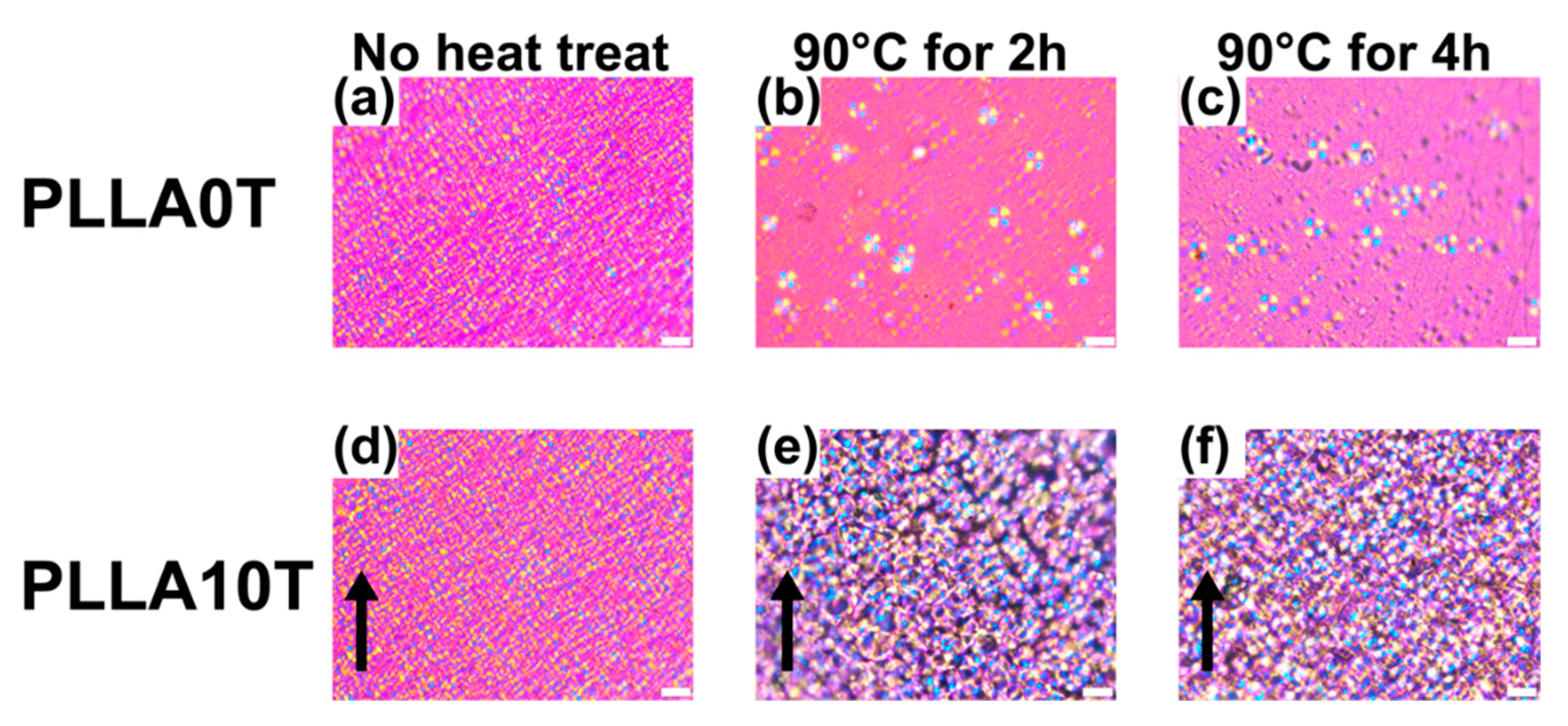
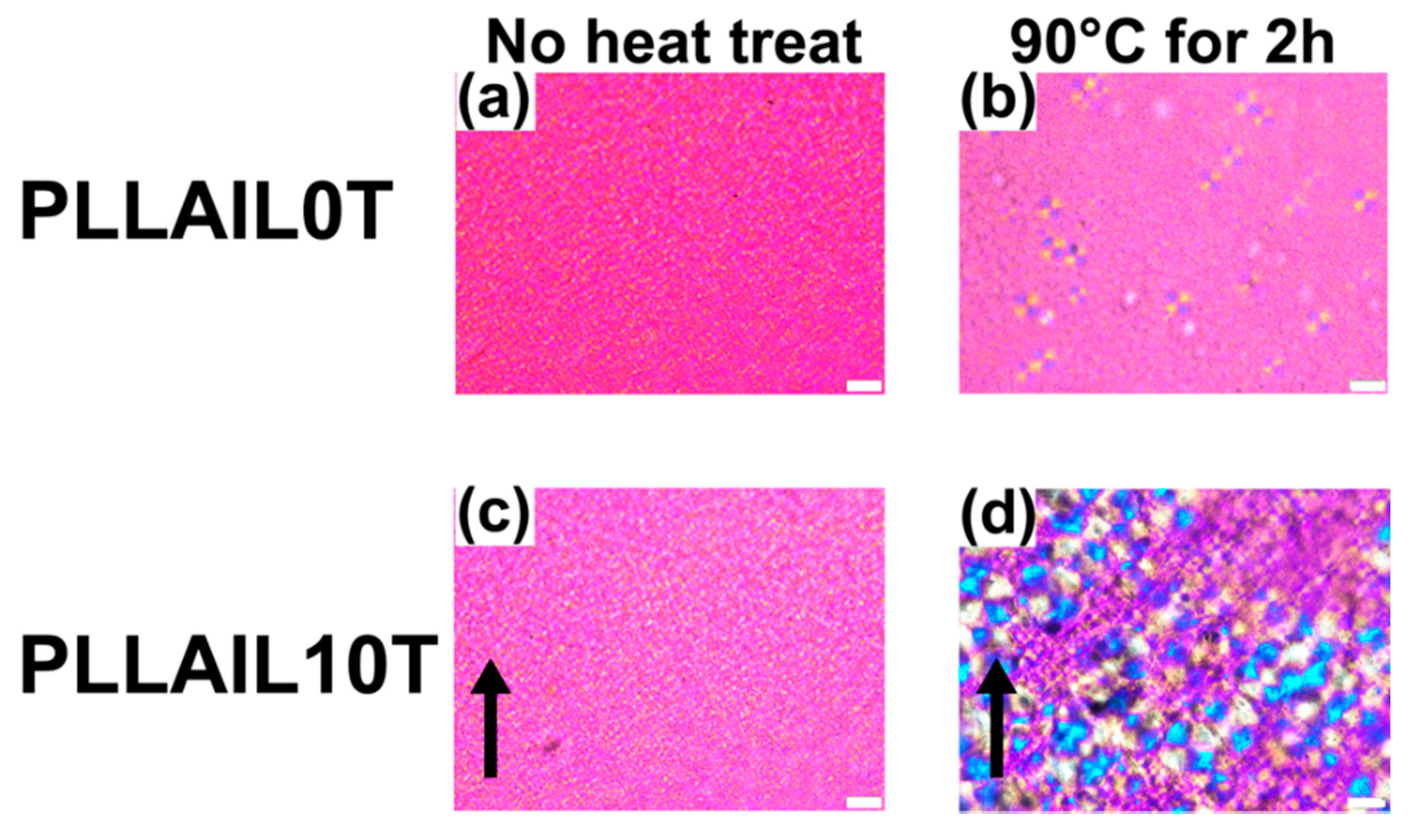
| Sample Name | PLLA 10 T | PLLA 10 T2 | PLLA 10 T4 |
|---|---|---|---|
| Xc | 31 | 57 | 66 |
| Crystallite size (nm) | 14.86 | 22.95 | 24.64 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hara, S.; Watanabe, S.; Takahashi, K.; Shimizu, S.; Ikake, H. Preparation of Crystallites for Oriented Poly(Lactic Acid) Films Using a Casting Method under a Magnetic Field. Polymers 2018, 10, 1083. https://doi.org/10.3390/polym10101083
Hara S, Watanabe S, Takahashi K, Shimizu S, Ikake H. Preparation of Crystallites for Oriented Poly(Lactic Acid) Films Using a Casting Method under a Magnetic Field. Polymers. 2018; 10(10):1083. https://doi.org/10.3390/polym10101083
Chicago/Turabian StyleHara, Shuta, Shuto Watanabe, Kohki Takahashi, Shigeru Shimizu, and Hiroki Ikake. 2018. "Preparation of Crystallites for Oriented Poly(Lactic Acid) Films Using a Casting Method under a Magnetic Field" Polymers 10, no. 10: 1083. https://doi.org/10.3390/polym10101083
APA StyleHara, S., Watanabe, S., Takahashi, K., Shimizu, S., & Ikake, H. (2018). Preparation of Crystallites for Oriented Poly(Lactic Acid) Films Using a Casting Method under a Magnetic Field. Polymers, 10(10), 1083. https://doi.org/10.3390/polym10101083